Abstract
Robot-assistance is being increasingly used for radical cystectomy (RC). Fifteen years of surgical evolution might be considered a short period for a radical procedure to be established as the treatment of choice, but robot assisted radical cystectomy (RARC) is showing promising results when compared with the current gold standard, open RC (ORC). In this review, we describe the current status of RARC and continue the discussion on the on-going RARC versus ORC debate.
INTRODUCTION
Bladder cancer is a lethal disease. A global overview of 2012 showed an incidence of 430,000 cases and a death toll of more than 160,000 cases.[1] The highest mortality rates were seen in Europe, around eight deaths per 100,000 patients.[1] Despite these numbers, the oncological efficacy of any available treatment for bladder cancer has not significantly improved the surivival rates over the past 30 years.[2] RC is a surgical procedure with high postoperative complication rates and there is need for minimizing surgical morbidity. In addition, RC should aim to provide good functional outcomes. The challenge for bladder cancer surgeons is to extirpate the disease and deliver an acceptable postoperative quality of life. In this effort, minimally invasive surgery, and especially robot-assisted RC (RARC) has emerged as an alternative to open surgery.
RARC has been adopted globally, and its use has increased more than 25-fold, from 0.7% to 18.5% in 2012.[3] Recent data from high-volume centers and registries are reporting promising results. In this review, we discuss the current knowledge on RARC, namely its oncological efficacy, functional outcomes, and safety. Moreover, we will discuss the available evidence comparing RARC versus open RC (ORC).
COMPLICATIONS
Even the most experienced high-volume institutions exhibit high rates of overall complications of RC reaching up to 64%,[4] while the rates of Clavien >3 complications can be as high as 41%.[5] These figures reflect the necessity of centralization of RC in dedicated centers. Another critical factor is to optimize the surgical and clinical pathway of a patient undergoing RC. The use of enhanced recovery protocols (ERP) with RARC utilizing totally intracorporeal techniques for urinary diversion aim to improve patient recovery compared to traditional perioperative management.[6] ERPs have been shown to reduce the length of stay by 1–2 days, while minimizing postoperative ileus, complications, and risk for readmission at 30 days.[7] The ERP concept was introduced by the open colorectal surgeons[8] more than 30 years ago. An effort has been made for ERP to be adopted globally, especially by robotic surgeons, but it remains underutilized,[6] this despite the fact that minimally invasive surgery is included as one of the 22 elements of an ERP for RC.[9]
Concerning the totally intracorporeal technique, results published by the international RC consortium (IRCC), have shown that shifting to the intracorporeal technique is safe and advantageous.[10] In a multi-center, retrospective study of 167 patients undergoing RARC with intracorporeal diversion (ileal conduit: 106; neobladder: 61), and 768 patients undergoing RARC with extracorporeal diversion (ileal conduit: 570; neobladder: 198), the intracorporeal patients had a lower risk of postoperative complication at 90 days postoperatively. (32%) (odds ratio: 0.68; 95% confidence interval, 0.50–0.94; P = 0.02).[10] In addition, gastrointestinal and infectious complications were significantly lower in pateints in whom an intracorporeal approach was used.[10] The obvious advantages of the totally intracorporeal technique are the protection of bowel inside the abdomen, no hypothermia or loss of fluids through osmosis, less bleeding, no need for extensive ureteral dissection, which may lead to ureteral strictures, and minimal surgical trauma.[11]
The lack of ERP and underutilization of the intracorporeal approach might jeopardize an accurate interpretation of the results of RC studies. Several studies including published randomized control trials (RCTs), are limited by these factors. In these studies, significant heterogeneity also exists in the types of urinary diversion used.
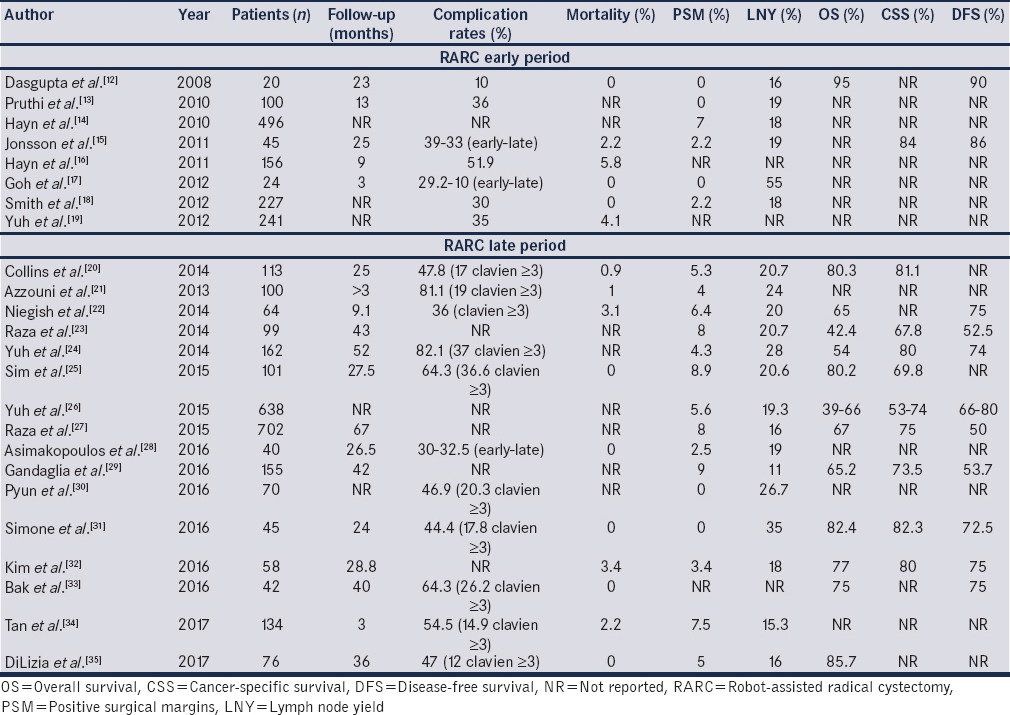
Table 1 shows a chronological evolution of the results of RARC, by looking at two time-periods.(Early period (2008–2012) and late period (2012–2017)).[12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35] All the included studies may overlap and may also include periods of surgeons’ learning curve. The fact that in the late period, we see more “mature” studies, does not result in significant improvements in the perioperative or oncological outcomes.
Table 1.
Individual robot-assisted radical cystectomy series in the early (2008-2012) and late (2013-2017) period
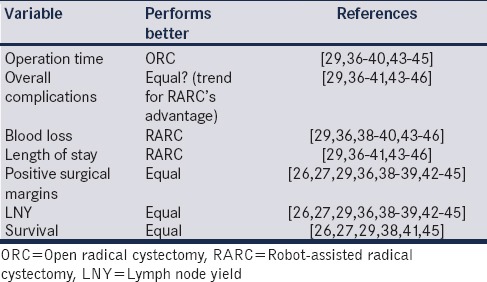
Apart from individual RARC series, we have identified 12 systematic analyses[26,27,29,36,37,38,39,40,41,42] and two meta-analyses of the 4 published RCTs[43,44] since 2013. Table 2 summarises which approach to RC performs significantly better in important surgical and oncological parameters. Apart from operation time, blood loss, positive surgical margins, and survival, other parameters remain debatable with some showing a trend toward RARC's superiority. As an example, as regards overall complications, five studies favor RARC[36,38,39,40,45] and six studies state that both procedures are equal.[29,37,41,43,44,46] As for the length of stay, seven studies are in favor of RARC[36,37,38,39,40,41,45] and three report equal results,[43,44,46] and as for lymph node yields, three studies show an advantage of RARC[36,39,41] and eight indicate equivalence between the two.[26,27,29,38,42,43,44,45] It is however clear that RARC performs as expected; as a minimally invasive procedure, providing less blood loss and transfusions, less length of stay (LOS) and probably less overall complications, although the latter aspect is not yet verified. In the recently announced results of RAZOR study, which is a 1:1 prospective, randomized, noninferiority trial comparing RARC to ORC, the intraoperative and postoperative transfusion rates as well as mean blood loss were significantly lower for RARC (42% versus 91% and 277.5 cc versus 558.8 cc, respectively).[47] It is noteworthy that in the same study, no other difference was recorded in postoperative complications. RARC had 58% and ORC had 56% overall complications with similar results in minor and major complications. Clavien 3–5 complications were 18.7% for RARC and 17.6% for ORC.[47]
Table 2.
Robot-assisted (robot-assisted radical cystectomy) versus open radical cystectomy performance in major surgical and oncological variables based on systematic analyses and meta-analysis of published studies
Blood loss and transfusion are considered as minor complications, by the Clavien-Dindo classification system, but it seems that they have a significantly negative impact on the oncological outcome. A possible explanation lies in the induced immunosuppression and the association of blood compatibility with infections.
Six retrospective studies comprising more than 7000 patients reported that transfusion during RC was associated with increased overall mortality, cancer-specific mortality and disease recurrence (hazard ratios: 1.19 (95% confidence interval [CI]: 1.11–1.27, P < 0.00001), 1.17 (95% CI: 1.06–1.30, P = 0.002), 1.14 (95% CI: 1.03–1.27), respectively).[48] Similarly, Abel et al. reported that intraoperative, and not postoperative transfusion, was linked to worse survival and a higher possibility for recurrence.[49] Whereas, Buchner et al. recorded a negative effect of transfusion in a cohort of 722 patients after 26-month follow-up irrespective its timing[50] and after adjusting for cancer stage. In the same fashion, Siemens et al. reported the negative impact of blood transfusions, by showing that it also increases LOS and re-admission rates.[51] On the other hand, a two-center retrospective study of 1060 patients, did not concur with the above reports.[52]
When looking at the four published prospective randomized trials and the recent meta-analysis by Tan et al., we see several variables, which limit us from drawing “conclusive findings.”[44] These differences consist of patient and tumor characteristics, the level of surgical experience, the surgical volume, the clinical pathway used, the types of urinary diversion (neobladder or ileal conduits), and the application of the extra- or intracorporeal approach. The small number of patients, the short follow-up and the nonmulticenter character of many of the published articles add further to their limitations. The conclusion is that RARC is better compared to open surgery regarding blood loss and wound complications and worse in operative time.[44] RARC and ORC performed equally in postoperative complications, positive surgical margins (PSM), resected lymph nodes and LOS.
The Memorial Sloan Kettering Cancer Center has published the largest RCT to date.[53] However, in this study, there are also apparent limitations: It is a single-center trial and low-powered (58 ORC-60 RARC). All RARCs were performed extracorporeally, almost 50% of the patients in both arms received a neobladder, and most notably, the study was designed to detect a statistical difference of 20% in Clavien grade 2–5 complications. The authors acknowledged the fact that if the difference was set to 10% or 15%, the outcome might have been different. The same limitations are seen in the other three RCTs. Thus, we have to await the results from the RAZOR study, which will be the largest noninferiority RCT with more than 320 patients, from 15 institutions.[54]
SURVIVAL OUTCOME MEASURES
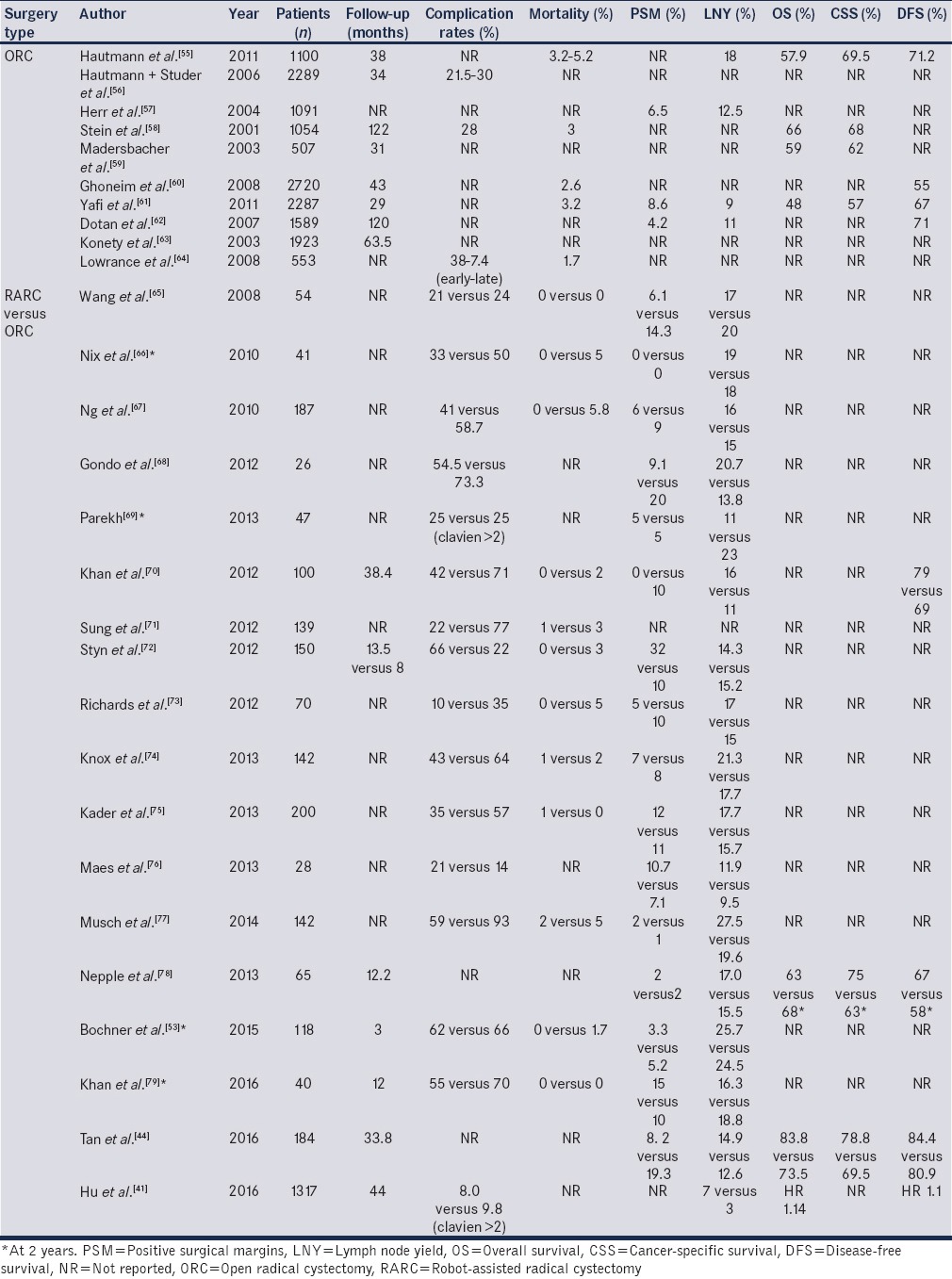
Table 3 summarizes the oncological end-points from traditional ORC series from high-volume centers, as well as RARC versus ORC series, including the 4 RCTs and meta-analyses.[41,44,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79] An overall conclusion that can be drawn from the above studies, further supported by the fact that long-term data are now available, is that RARC is equivalent to ORC in terms of oncological efficacy.
Table 3.
Summary of large open radical cystectomy series and comparative studies of robot-assisted radical cystectomy versus open radical cystectomy
Concerning lymph node dissection, which is a crucial part of the procedure from an oncological viewpoint, it has been shown that RARC can achieve the same or even better lymph node yields than ORC. Li et al. have reviewed nine comparative RARC versus ORC studies with 874 patients,[36] concluding that RARC removed at least two nodes more than ORC (WMD: 2.25; 95% CI, 0.57–3.94; P = 0.009). Four systematic analyses, between 2013 and 2017 were in agreement with the above results. On the other hand, the RCTs and their meta-analysis did not show any difference.
Another critical oncological variable is PSM. The first meta-analysis confirmed that PSM have a statistically significant negative effect on the survival outcomes.[80]
The systematic reviews and the meta-analysis of the RCTs show that RARC and ORC have comparable margin rates. A systematic review for RARC estimated a 5.6% (0%–26%) positive surgical margin rate.[26] However, when adjusted for surgeon experience, the margin rates ranged between 4% and 9%. pT2 and pT3-4 disease are associated with positive margins in 1%–1.5% and 0%–25%, respectively. The IRCC database (n = 939) showed an 8% positive margin rate.[27] Similar margin rates are seen in ORC studies from high-volume centers (4.2%–8.6%) [Table 3]. In the newly announced results of the RAZOR study, overall margin rates were similar for RARC and ORC, but RARC had a higher positive bladder margin rate (11% versus 5%, P = 0.05).[47]
When looking at survival end-points, the results of RARC are promising and appear equivalent to the results of ORC. A systematic review of the oncological outcomes of RARC, using the IRCC dataset[26] reported that 5-year disease-free survival, cancer-specific survival (CSS), and overall survival (OS) rates ranged between 53%–74%, 66%–80%, and 39%–66%, respectively. There was no statistically significant difference bwteen ORC and RARC. The results of the RAZOR study confirmed the oncological equivalence of the two approaches, considering that the noninferiority design had the 2-year progression-free survival as the end-point.[47] However, significant heterogeneity and overlapping between the included studies creates issues in the interpretation of the results.
The IRCC provided an updated analysis of the oncological outcomes of RARC.[27] In a median follow-up of 44 months, the 5-year recurrence-free survival (RFS), CSS, and OS were 67%, 75%, and 50%, respectively. While acknowledging that 38% of the cohort (n = 702) had advanced disease (pT3-4) and 21% were lymph-node positive, the published survival rates are encouraging.
Snow-Lisy et al. reported outcomes for both RARC and laparoscopic RC patients with the current longest published follow-up of 12 years.[81] The 5-year CSS rate was 70% for this cohort.
In the Karolinska Institute series of 113 consecutive totally intracorporeal RARC, with a median follow-up of 25 months, cancer-specific survival was 81% at 3 years and 67% at 5 years.[82]
Tan et al. published one of the few studies comparing ORC to RARC with totally intracorporeal urinary diversion.[83] In this study, a total of 184 patients, equally distributed, with 33.8 months of follow-up, found no difference in the recurrence-free survival and the recurrence sites.
There has been debate as to whether RARC negatively impacts early recurrence patterns due to inadequate resection or pneumoperitoneum; so far, there is no good evidence to support this viewpoint. A comparison of extracorporeal RARC versus ORC concluded that RARC exhibited different recurrence patterns, suggestive of higher rates of extrapelvic and peritoneal carcinomatosis.[84] However, the study was shown to have no statistical evidence to support these views.[85] Recurrence following RC often occurs early, with >80% of recurrences occurring within the first 2 years.[86] The ERUS Scientific Working Group reported on early recurrence patterns among 717 patients who underwent RARC with intracorporeal urinary diversion. RFS at 3, 12, and 24 months was 95.9%, 80.2%, and 74.6%, respectively. Distant recurrences most frequently occurred in the bones, lungs, and liver, and pelvic lymph nodes were the most common site of local recurrence. This multi-center series identified five patients (0.7%) with peritoneal carcinomatosis and two patients (0.3%) with metastasis at the port site (wound site) concluding that “unusual” recurrence patterns were not identified and that recurrence patterns appear similar to those in ORC series.[86]
The oncological equivalence of RARC and ORC approaches further highlights the urgent necessity for improved neoadjuvant and adjuvant chemotherapeutic regimens, which aim to augment the efficacy of surgery and improve patient survival outcomes.
FUNCTIONAL OUTCOMES
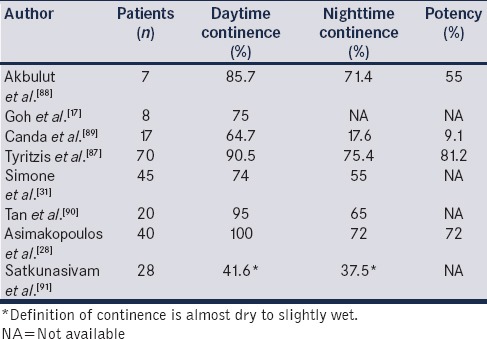
Continence and potency are the most important quality indicators for the neobladder patients. Unfortunately, there is a lack of data, which makes the comparison for RARC and ORC problematic. Looking at ORCs performance from the 2012 EAU International Consultation on Bladder Cancer, reviewing cases between 1970 and 2012, day-time and night-time continence was achieved in 85%–90% and 60%–80%, respectively.[4] Tyritzis et al. published the Karolinska Institute series which included functional outcomes from 70 RARCs with neobladder diversion.[87] Nineteen males (90.5%) and two out of three (66.7%) females were continent (0–1 pad/day) at 12 months. Sixteen patients that received a nerve-sparing RARC were potent with or without medication at 12 months. In Table 4, the published RARC studies of neobladders with functional outcomes are shown.[17,28,31,87,88,89,90,91]
Table 4.
Functional outcomes of studies of robot-assisted radical cystectomy with neobladder diversion
Since many techniques for the creation of neobladders have been described, urodynamic data would be an interesting indicator of functional continence outcomes. Satkunasivam et al. compared RARC with intracorporeal neobladder with ORC, stating that the RARC neobladder had similar urodynamic characteristics, but with inferior daytime continence.[91] Patient urinary bother scores were similar between the two procedures. The limitations of this study include its retrospective nature, its low power in patient numbers (28 RARC, 79 ORC) and a short follow-up for RARC (9.4 months) compared to the 62.1 months for ORC.[91]
COST COMPARISON
Cost analysis can be a challenging task due to differences between the individual health-systems, public insurance systems, and other parameters. In addition, indirect costs are not taken into account, as these are reflected by readmissions and the high acquisition and maintenance fees of the robot, limits further our ability to make a precise evaluation. Table 5 provides a summary of the available cost studies to date.
Table 5.
Available publications on cost comparative studies between robot-assisted radical cystectomy-open radical cystectomy
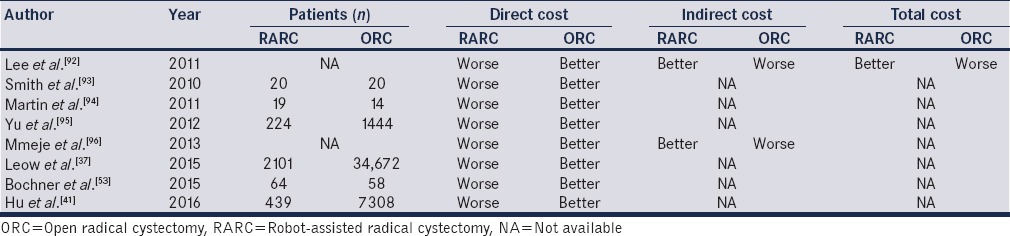
Lee et al. estimated that the costs of RARC with ileal conduits, cutaneous continent diversion and neobladders were $20,659, $22,102, and $22,685, respectively, compared to $25,505, $22,697 and $20,719 for ORC.[92] The dominant cost driver in the study was hospital stay, showing that RARC could be less expensive than ORC with a reduced hospital stay. Similarly, Leow et al. concluded that RARC could become cost-efficient if the operation time was <6 h and the hospital stay <1 week.[37] This was the first study that calculated all 90-day direct costs, including supplies. In this study, RARC had less major postoperative complications, which decreased the overall costs.[37]
Another study based on the surveillance, epidemiology, and end results program-Medicare linked data concluded that RARC was more expensive when looking at perioperative, 30- and 90-day costs.[41] The statistically significant difference ranged between $3000 and 4000 ($24051 [interquartile range (IQR) $15332–$32078] vs. $21 637 [IQR $12567–$32 460], P = 0.08). Finally, the most recent cost analysis by Bansal and associates suggested that RARC was more expensive than ORC by 18.9%. The key cost drivers were operative time, hospitalization time, and annual surgical volume.[97] None of the current publications on cost have taken into account the time for patients to get back to “normal activities,” following discharge from the hospital. The need for home-care is an important cost and the impact of minimally invasive surgery on this aspect of health economics has to date been under-investigated.
An update on health economics for muscle-invasive bladder cancer showed that the economic burden would be decreased if the surgical complications could be reduced and if neoadjuvant chemotherapy could be utilized more since it improves the quality of life and survival of the patients.[98]
CONCLUSION
RC is one of the most challenging and morbid surgical procedures, in which the surgeon has to provide the best outcome in terms of oncological control, complications, and functional outcomes. Experience of the surgeon and the center, has shown to positively impact outcomes. However, the procedure itself might not be enough for cure, since we are dealing with an aggressive cancer. Survival may be improved in the future by optimal neoadjuvant or adjuvant chemotherapy protocols. Apart from extirpating completely the disease, the complications of surgery need to be reduced. The current evidence indicates that RARC achieves better results in terms of blood loss, transfusion rates and hospital stay with an equivalent oncological outcome compared to ORC. On the other hand, the cost of RARC is a significant drawback. Future RCTs are awaited along with the further refinement of surgical technique and peri-operative patient management.
Footnotes
Financial support and sponsorship: Nil.
Conflicts of interest: There are no conflicts of interest.
REFERENCES
- 1.Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F, et al. Bladder cancer incidence and mortality: A Global overview and recent trends. Eur Urol. 2017;71:96–108. doi: 10.1016/j.eururo.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Zehnder P, Studer UE, Skinner EC, Thalmann GN, Miranda G, Roth B, et al. Unaltered oncological outcomes of radical cystectomy with extended lymphadenectomy over three decades. BJU Int. 2013;112:E51–8. doi: 10.1111/bju.12215. [DOI] [PubMed] [Google Scholar]
- 3.Menon M, Hemal AK, Tewari A, Shrivastava A, Shoma AM, El-Tabey NA, et al. Nerve-sparing robot-assisted radical cystoprostatectomy and urinary diversion. BJU Int. 2003;92:232–6. doi: 10.1046/j.1464-410x.2003.04329.x. [DOI] [PubMed] [Google Scholar]
- 4.Hautmann RE, Abol-Enein H, Davidsson T, Gudjonsson S, Hautmann SH, Holm HV, et al. ICUD-EAU international consultation on bladder cancer 2012: Urinary diversion. Eur Urol. 2013;63:67–80. doi: 10.1016/j.eururo.2012.08.050. [DOI] [PubMed] [Google Scholar]
- 5.Shabsigh A, Korets R, Vora KC, Brooks CM, Cronin AM, Savage C, et al. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur Urol. 2009;55:164–74. doi: 10.1016/j.eururo.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 6.Collins JW, Patel H, Adding C, Annerstedt M, Dasgupta P, Khan SM, et al. Enhanced recovery after robot-assisted radical cystectomy: EAU robotic urology section scientific working group consensus view. Eur Urol. 2016;70:649–60. doi: 10.1016/j.eururo.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 7.Tyson MD, Chang SS. Enhanced recovery pathways versus standard care after cystectomy: A Meta-analysis of the effect on perioperative outcomes. Eur Urol. 2016;70:995–1003. doi: 10.1016/j.eururo.2016.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth. 1997;78:606–17. doi: 10.1093/bja/78.5.606. [DOI] [PubMed] [Google Scholar]
- 9.Cerantola Y, Valerio M, Persson B, Jichlinski P, Ljungqvist O, Hubner M, et al. Guidelines for perioperative care after radical cystectomy for bladder cancer: Enhanced recovery after surgery (ERAS(®)) society recommendations. Clin Nutr. 2013;32:879–87. doi: 10.1016/j.clnu.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed K, Khan SA, Hayn MH, Agarwal PK, Badani KK, Balbay MD, et al. Analysis of intracorporeal compared with extracorporeal urinary diversion after robot-assisted radical cystectomy: Results from the international robotic cystectomy consortium. Eur Urol. 2014;65:340–7. doi: 10.1016/j.eururo.2013.09.042. [DOI] [PubMed] [Google Scholar]
- 11.Chan KG, Collins JW, Wiklund NP. Robot-assisted radical cystectomy: Extracorporeal vs intracorporeal urinary diversion. J Urol. 2015;193:1467–9. doi: 10.1016/j.juro.2015.02.042. [DOI] [PubMed] [Google Scholar]
- 12.Dasgupta P, Rimington P, Murphy D, Challacombe B, Hemal A, Elhage O, et al. Robotic assisted radical cystectomy: Short to medium-term oncologic and functional outcomes. Int J Clin Pract. 2008;62:1709–14. doi: 10.1111/j.1742-1241.2008.01858.x. [DOI] [PubMed] [Google Scholar]
- 13.Pruthi RS, Nielsen ME, Nix J, Smith A, Schultz H, Wallen EM, et al. Robotic radical cystectomy for bladder cancer: Surgical and pathological outcomes in 100 consecutive cases. J Urol. 2010;183:510–4. doi: 10.1016/j.juro.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 14.Hayn MH, Hussain A, Mansour AM, Andrews PE, Carpentier P, Castle E, et al. The learning curve of robot-assisted radical cystectomy: Results from the international robotic cystectomy consortium. Eur Urol. 2010;58:197–202. doi: 10.1016/j.eururo.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 15.Jonsson MN, Adding LC, Hosseini A, Schumacher MC, Volz D, Nilsson A, et al. Robot-assisted radical cystectomy with intracorporeal urinary diversion in patients with transitional cell carcinoma of the bladder. Eur Urol. 2011;60:1066–73. doi: 10.1016/j.eururo.2011.07.035. [DOI] [PubMed] [Google Scholar]
- 16.Hayn MH, Hellenthal NJ, Hussain A, Stegemann AP, Guru KA. Defining morbidity of robot-assisted radical cystectomy using a standardized reporting methodology. Eur Urol. 2011;59:213–8. doi: 10.1016/j.eururo.2010.10.044. [DOI] [PubMed] [Google Scholar]
- 17.Goh AC, Gill IS, Lee DJ, de Castro Abreu AL, Fairey AS, Leslie S, et al. Robotic intracorporeal orthotopic ileal neobladder: Replicating open surgical principles. Eur Urol. 2012;62:891–901. doi: 10.1016/j.eururo.2012.07.052. [DOI] [PubMed] [Google Scholar]
- 18.Smith AB, Raynor M, Amling CL, Busby JE, Castle E, Davis R, et al. Multi-institutional analysis of robotic radical cystectomy for bladder cancer: Perioperative outcomes and complications in 227 patients. J Laparoendosc Adv Surg Tech A. 2012;22:17–21. doi: 10.1089/lap.2011.0326. [DOI] [PubMed] [Google Scholar]
- 19.Yuh BE, Nazmy M, Ruel NH, Jankowski JT, Menchaca AR, Torrey RR, et al. Standardized analysis of frequency and severity of complications after robot-assisted radical cystectomy. Eur Urol. 2012;62:806–13. doi: 10.1016/j.eururo.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Collins JW, Sooriakumaran P, Sanchez-Salas R, Ahonen R, Nyberg T, Wiklund NP, et al. Robot-assisted radical cystectomy with intracorporeal neobladder diversion: The Karolinska experience. Indian J Urol. 2014;30:307–13. doi: 10.4103/0970-1591.134251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Azzouni FS, Din R, Rehman S, Khan A, Shi Y, Stegemann A, et al. The first 100 consecutive, robot-assisted, intracorporeal ileal conduits: Evolution of technique and 90-day outcomes. Eur Urol. 2013;63:637–43. doi: 10.1016/j.eururo.2012.11.055. [DOI] [PubMed] [Google Scholar]
- 22.Niegisch G, Albers P, Rabenalt R. Perioperative complications and oncological safety of robot-assisted (RARC) vs. Open radical cystectomy (ORC) Urol Oncol. 2014;32:966–74. doi: 10.1016/j.urolonc.2014.03.023. [DOI] [PubMed] [Google Scholar]
- 23.Raza SJ, Al-Daghmin A, Zhuo S, Mehboob Z, Wang K, Wilding G, et al. Oncologic outcomes following robot-assisted radical cystectomy with minimum 5-year follow-up: The Roswell park cancer institute experience. Eur Urol. 2014;66:920–8. doi: 10.1016/j.eururo.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 24.Yuh B, Torrey RR, Ruel NH, Wittig K, Tobis S, Linehan J, et al. Intermediate-term oncologic outcomes of robot-assisted radical cystectomy for urothelial carcinoma. J Endourol. 2014;28:939–45. doi: 10.1089/end.2014.0073. [DOI] [PubMed] [Google Scholar]
- 25.Sim A, Balbay MD, Todenhöfer T, Aufderklamm S, Halalsheh O, Mischinger J, et al. Robot-assisted radical cystectomy and intracorporeal urinary diversion – Safe and reproducible? Cent European J Urol. 2015;68:18–23. doi: 10.5173/ceju.2015.01.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuh B, Wilson T, Bochner B, Chan K, Palou J, Stenzl A, et al. Systematic review and cumulative analysis of oncologic and functional outcomes after Robot-assisted radical cystectomy. Eur Urol. 2015;67:402–22. doi: 10.1016/j.eururo.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Raza SJ, Wilson T, Peabody JO, Wiklund P, Scherr DS, Al-Daghmin A, et al. Long-term oncologic outcomes following robot-assisted radical cystectomy: Results from the international robotic cystectomy consortium. Eur Urol. 2015;68:721–8. doi: 10.1016/j.eururo.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 28.Asimakopoulos AD, Campagna A, Gakis G, Corona Montes VE, Piechaud T, Hoepffner JL, et al. Nerve sparing, Robot-assisted radical cystectomy with intracorporeal bladder substitution in the male. J Urol. 2016;196:1549–57. doi: 10.1016/j.juro.2016.04.114. [DOI] [PubMed] [Google Scholar]
- 29.Gandaglia G, De Groote R, Geurts N, D’Hondt F, Montorsi F, Novara G, et al. Oncologic outcomes of Robot-assisted radical cystectomy: Results of a high-volume robotic center. J Endourol. 2016;30:75–82. doi: 10.1089/end.2015.0482. [DOI] [PubMed] [Google Scholar]
- 30.Pyun JH, Kim HK, Cho S, Kang SG, Cheon J, Lee JG, et al. Robot-assisted radical cystectomy with total intracorporeal urinary diversion: Comparative analysis with extracorporeal urinary diversion. J Laparoendosc Adv Surg Tech A. 2016;26:349–55. doi: 10.1089/lap.2015.0543. [DOI] [PubMed] [Google Scholar]
- 31.Simone G, Papalia R, Misuraca L, Tuderti G, Minisola F, Ferriero M, et al. Robotic intracorporeal padua ileal bladder: Surgical technique, perioperative, oncologic and functional outcomes. Eur Urol. 2016 doi: 10.1016/j.eururo.2016.10.018. pii: S0302-2838(16)30721-7. [DOI] [PubMed] [Google Scholar]
- 32.Kim TH, Sung HH, Jeon HG, Seo SI, Jeon SS, Lee HM, et al. Oncological outcomes in patients treated with radical cystectomy for bladder cancer: Comparison between open, laparoscopic, and robot-assisted approaches. J Endourol. 2016;30:783–91. doi: 10.1089/end.2015.0652. [DOI] [PubMed] [Google Scholar]
- 33.Bak DJ, Lee YJ, Woo MJ, Chung JW, Ha YS, Kim HT, et al. Complications and oncologic outcomes following robot-assisted radical cystectomy: What is the real benefit? Investig Clin Urol. 2016;57:260–7. doi: 10.4111/icu.2016.57.4.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan WS, Lamb BW, Tan MY, Ahmad I, Sridhar A, Nathan S, et al. In-depth critical analysis of complications following robot-assisted radical cystectomy with intracorporeal urinary diversion. Eur Urol Focus. 2017;3:273–9. doi: 10.1016/j.euf.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 35.DiLizia EM, Sadeghi F. Surgical and pathological outcomes of robotic-assisted radical cystectomy for bladder cancer in the community setting. J Robot Surg Aug. 2017 Aug 23; doi: 10.1007/s11701-017-0740-y. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 36.Li K, Lin T, Fan X, Xu K, Bi L, Duan Y, et al. Systematic review and meta-analysis of comparative studies reporting early outcomes after robot-assisted radical cystectomy versus open radical cystectomy. Cancer Treat Rev. 2013;39:551–60. doi: 10.1016/j.ctrv.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 37.Leow JJ, Reese S, Trinh QD, Bellmunt J, Chung BI, Kibel AS, et al. Impact of surgeon volume on the morbidity and costs of radical cystectomy in the USA: A contemporary population-based analysis. BJU Int. 2015;115:713–21. doi: 10.1111/bju.12749. [DOI] [PubMed] [Google Scholar]
- 38.Fonseka T, Ahmed K, Froghi S, Khan SA, Dasgupta P, Shamim Khan M, et al. Comparing robotic, laparoscopic and open cystectomy: A systematic review and meta-analysis. Arch Ital Urol Androl. 2015;87:41–8. doi: 10.4081/aiua.2015.1.41. [DOI] [PubMed] [Google Scholar]
- 39.Xia L, Wang X, Xu T, Zhang X, Zhu Z, Qin L, et al. Robotic versus open radical cystectomy: An updated systematic review and meta-analysis. PLoS One. 2015;10:e0121032. doi: 10.1371/journal.pone.0121032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Novara G, Catto JW, Wilson T, Annerstedt M, Chan K, Murphy DG, et al. Systematic review and cumulative analysis of perioperative outcomes and complications after robot-assisted radical cystectomy. Eur Urol. 2015;67:376–401. doi: 10.1016/j.eururo.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 41.Hu JC, Chughtai B, O’Malley P, Halpern JA, Mao J, Scherr DS, et al. Perioperative outcomes, health care costs, and survival after robotic-assisted versus open radical cystectomy: A National comparative effectiveness study. Eur Urol. 2016;70:195–202. doi: 10.1016/j.eururo.2016.03.028. [DOI] [PubMed] [Google Scholar]
- 42.Matulewicz RS, DeLancey JO, Manjunath A, Tse J, Kundu SD, Meeks JJ, et al. National comparison of oncologic quality indicators between open and robotic-assisted radical cystectomy. Urol Oncol. 2016;34:431.e9–431.e15. doi: 10.1016/j.urolonc.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 43.Shen Z, Sun Z. Systematic review and meta-analysis of randomised trials of perioperative outcomes comparing robot-assisted versus open radical cystectomy. BMC Urol. 2016;16:59. doi: 10.1186/s12894-016-0177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tan WS, Khetrapal P, Tan WP, Rodney S, Chau M, Kelly JD, et al. Robotic assisted radical cystectomy with extracorporeal urinary diversion does not show a benefit over open radical cystectomy: A Systematic review and meta-analysis of randomised controlled trials. PLoS One. 2016;11:e0166221. doi: 10.1371/journal.pone.0166221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Son SK, Lee NR, Kang SH, Lee SH. Safety and effectiveness of robot-assisted versus open radical cystectomy for bladder cancer: A Systematic review and meta-analysis. J Laparoendosc Adv Surg Tech A. 2017;27:1109–20. doi: 10.1089/lap.2016.0437. [DOI] [PubMed] [Google Scholar]
- 46.Pak JS, Lee JJ, Bilal K, Finkelstein M, Palese MA. Utilization trends and short-term outcomes of robotic versus open radical cystectomy for bladder cancer. Urology. 2017;103:117–23. doi: 10.1016/j.urology.2016.10.067. [DOI] [PubMed] [Google Scholar]
- 47.Wang YL, Jiang B, Yin FF, Shi HQ, Xu XD, Zheng SS, et al. Perioperative blood transfusion promotes worse outcomes of bladder cancer after radical cystectomy: A Systematic review and meta-analysis. PLoS One. 2015;10:e0130122. doi: 10.1371/journal.pone.0130122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abel EJ, Linder BJ, Bauman TM, Bauer RM, Thompson RH, Thapa P, et al. Perioperative blood transfusion and radical cystectomy: Does timing of transfusion affect bladder cancer mortality? Eur Urol. 2014;66:1139–47. doi: 10.1016/j.eururo.2014.08.051. [DOI] [PubMed] [Google Scholar]
- 49.Buchner A, Grimm T, Schneevoigt BS, Wittmann G, Kretschmer A, Jokisch F, et al. Dramatic impact of blood transfusion on cancer-specific survival after radical cystectomy irrespective of tumor stage. Scand J Urol. 2017;51:130–6. doi: 10.1080/21681805.2017.1295399. [DOI] [PubMed] [Google Scholar]
- 50.Siemens DR, Jaeger MT, Wei X, Vera-Badillo F, Booth CM. Peri-operative allogeneic blood transfusion and outcomes after radical cystectomy: A population-based study. World J Urol. 2017;35:1435–42. doi: 10.1007/s00345-017-2009-5. [DOI] [PubMed] [Google Scholar]
- 51.Moschini M, Dell’ Oglio P, Capogrosso P, Cucchiara V, Luzzago S, Gandaglia G, et al. Effect of allogeneic intraoperative blood transfusion on survival in patients treated with radical cystectomy for nonmetastatic bladder cancer: Results from a single high-volume institution. Clin Genitourin Cancer. 2015;13:562–7. doi: 10.1016/j.clgc.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 52.Bochner BH, Dalbagni G, Sjoberg DD, Silberstein J, Keren Paz GE, Donat SM, et al. Comparing open radical cystectomy and robot-assisted laparoscopic radical cystectomy: A Randomized clinical trial. Eur Urol. 2015;67:1042–50. doi: 10.1016/j.eururo.2014.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith ND, Castle EP, Gonzalgo ML, Svatek RS, Weizer AZ, Montgomery JS, et al. The RAZOR (randomized open vs.robotic cystectomy) trial: Study design and trial update. BJU Int. 2015;115:198–205. doi: 10.1111/bju.12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hautmann RE, de Petriconi RC, Volkmer BG. 25 years of experience with 1,000 neobladders: Long-term complications. J Urol. 2011;185:2207–12. doi: 10.1016/j.juro.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 55.Hautmann RE, Volkmer BG, Schumacher MC, Gschwend JE, Studer UE. Long-term results of standard procedures in urology: The ileal neobladder. World J Urol. 2006;24:305–14. doi: 10.1007/s00345-006-0105-z. [DOI] [PubMed] [Google Scholar]
- 56.Herr H, Lee C, Chang S, Lerner S Bladder Cancer Collaborative Group. Standardization of radical cystectomy and pelvic lymph node dissection for bladder cancer: A collaborative group report. J Urol. 2004;171:1823–8. doi: 10.1097/01.ju.0000120289.78049.0e. [DOI] [PubMed] [Google Scholar]
- 57.Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, et al. Radical cystectomy in the treatment of invasive bladder cancer: Long-term results in 1,054 patients. J Clin Oncol. 2001;19:666–75. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 58.Madersbacher S, Hochreiter W, Burkhard F, Thalmann GN, Danuser H, Markwalder R, et al. Radical cystectomy for bladder cancer today – A homogeneous series without neoadjuvant therapy. J Clin Oncol. 2003;21:690–6. doi: 10.1200/JCO.2003.05.101. [DOI] [PubMed] [Google Scholar]
- 59.Ghoneim MA, Abdel-Latif M, el-Mekresh M, Abol-Enein H, Mosbah A, Ashamallah A, et al. Radical cystectomy for carcinoma of the bladder: 2,720 consecutive cases 5 years later. J Urol. 2008;180:121–7. doi: 10.1016/j.juro.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 60.Yafi FA, Aprikian AG, Chin JL, Fradet Y, Izawa J, Estey E, et al. Contemporary outcomes of 2287 patients with bladder cancer who were treated with radical cystectomy: A Canadian multicentre experience. BJU Int. 2011;108:539–45. doi: 10.1111/j.1464-410X.2010.09912.x. [DOI] [PubMed] [Google Scholar]
- 61.Dotan ZA, Kavanagh K, Yossepowitch O, Kaag M, Olgac S, Donat M, et al. Positive surgical margins in soft tissue following radical cystectomy for bladder cancer and cancer specific survival. J Urol. 2007;178:2308–12. doi: 10.1016/j.juro.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 62.Konety BR, Joslyn SA, O’Donnell MA. Extent of pelvic lymphadenectomy and its impact on outcome in patients diagnosed with bladder cancer: Analysis of data from the surveillance, epidemiology and end results program data base. J Urol. 2003;169:946–50. doi: 10.1097/01.ju.0000052721.61645.a3. [DOI] [PubMed] [Google Scholar]
- 63.Lowrance WT, Rumohr JA, Chang SS, Clark PE, Smith JA, Jr, Cookson MS, et al. Contemporary open radical cystectomy: Analysis of perioperative outcomes. J Urol. 2008;179:1313–8. doi: 10.1016/j.juro.2007.11.084. [DOI] [PubMed] [Google Scholar]
- 64.Wang GJ, Barocas DA, Raman JD, Scherr DS. Robotic vs.open radical cystectomy: Prospective comparison of perioperative outcomes and pathological measures of early oncological efficacy. BJU Int. 2008;101:89–93. doi: 10.1111/j.1464-410X.2007.07212.x. [DOI] [PubMed] [Google Scholar]
- 65.Nix J, Smith A, Kurpad R, Nielsen ME, Wallen EM, Pruthi RS, et al. Prospective randomized controlled trial of robotic versus open radical cystectomy for bladder cancer: Perioperative and pathologic results. Eur Urol. 2010;57:196–201. doi: 10.1016/j.eururo.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 66.Ng CK, Kauffman EC, Lee MM, Otto BJ, Portnoff A, Ehrlich JR, et al. Acomparison of postoperative complications in open versus robotic cystectomy. Eur Urol. 2010;57:274–81. doi: 10.1016/j.eururo.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 67.Gondo T, Yoshioka K, Nakagami Y, Okubo H, Hashimoto T, Satake N, et al. Robotic versus open radical cystectomy: Prospective comparison of perioperative and pathologic outcomes in Japan. Jpn J Clin Oncol. 2012;42:625–31. doi: 10.1093/jjco/hys062. [DOI] [PubMed] [Google Scholar]
- 68.Parekh DJ, Messer J, Fitzgerald J, Ercole B, Svatek R. Perioperative outcomes and oncologic efficacy from a pilot prospective randomized clinical trial of open versus robotic assisted radical cystectomy. J Urol. 2013;189:474–9. doi: 10.1016/j.juro.2012.09.077. [DOI] [PubMed] [Google Scholar]
- 69.Khan MS, Challacombe B, Elhage O, Rimington P, Coker B, Murphy D, et al. A dual-centre, cohort comparison of open, laparoscopic and robotic-assisted radical cystectomy. Int J Clin Pract. 2012;66:656–62. doi: 10.1111/j.1742-1241.2011.02888.x. [DOI] [PubMed] [Google Scholar]
- 70.Sung HH, Ahn JS, Seo SI, Jeon SS, Choi HY, Lee HM, et al. A comparison of early complications between open and robot-assisted radical cystectomy. J Endourol. 2012;26:670–5. doi: 10.1089/end.2011.0372. [DOI] [PubMed] [Google Scholar]
- 71.Styn NR, Montgomery JS, Wood DP, Hafez KS, Lee CT, Tallman C, et al. Matched comparison of robotic-assisted and open radical cystectomy. Urology. 2012;79:1303–8. doi: 10.1016/j.urology.2012.01.055. [DOI] [PubMed] [Google Scholar]
- 72.Richards KA, Kader AK, Otto R, Pettus JA, Smith JJ 3rd, Hemal AK, et al. Is robot-assisted radical cystectomy justified in the elderly. A comparison of robotic versus open radical cystectomy for bladder cancer in elderly ≥75 years old? J Endourol. 2012;26:1301–6. doi: 10.1089/end.2012.0035. [DOI] [PubMed] [Google Scholar]
- 73.Knox ML, El-Galley R, Busby JE. Robotic versus open radical cystectomy: Identification of patients who benefit from the robotic approach. J Endourol. 2013;27:40–4. doi: 10.1089/end.2012.0168. [DOI] [PubMed] [Google Scholar]
- 74.Kader AK, Richards KA, Krane LS, Pettus JA, Smith JJ, Hemal AK, et al. Robot-assisted laparoscopic vs.open radical cystectomy: Comparison of complications and perioperative oncological outcomes in 200 patients. BJU Int. 2013;112:E290–4. doi: 10.1111/bju.12167. [DOI] [PubMed] [Google Scholar]
- 75.Maes AA, Brunkhorst LW, Gavin PW, Todd SP, Maatman TJ. Comparison of robotic-assisted and open radical cystectomy in a community-based, non-tertiary health care setting. J Robot Surg. 2013;7:359–63. doi: 10.1007/s11701-013-0401-8. [DOI] [PubMed] [Google Scholar]
- 76.Musch M, Janowski M, Steves A, Roggenbuck U, Boergers A, Davoudi Y, et al. Comparison of early postoperative morbidity after robot-assisted and open radical cystectomy: Results of a prospective observational study. BJU Int. 2014;113:458–67. doi: 10.1111/bju.12374. [DOI] [PubMed] [Google Scholar]
- 77.Nepple KG, Strope SA, Grubb RL 3rd, Kibel AS. Early oncologic outcomes of robotic vs.open radical cystectomy for urothelial cancer. Urol Oncol. 2013;31:894–8. doi: 10.1016/j.urolonc.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Khan MS, Gan C, Ahmed K, Ismail AF, Watkins J, Summers JA, et al. A single-centre early phase randomised controlled three-arm trial of open, robotic, and laparoscopic radical cystectomy (CORAL) Eur Urol. 2016;69:613–21. doi: 10.1016/j.eururo.2015.07.038. [DOI] [PubMed] [Google Scholar]
- 79.Hong X, Li T, Ling F, Yang D, Hou L, Li F, et al. Impact of surgical margin status on the outcome of bladder cancer treated by radical cystectomy: A meta-analysis. Oncotarget. 2017;8:17258–69. doi: 10.18632/oncotarget.12907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Snow-Lisy DC, Campbell SC, Gill IS, Hernandez AV, Fergany A, Kaouk J, et al. Robotic and laparoscopic radical cystectomy for bladder cancer: Long-term oncologic outcomes. Eur Urol. 2014;65:193–200. doi: 10.1016/j.eururo.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 81.Collins JW, Tyritzis S, Nyberg T, Schumacher M, Laurin O, Khazaeli D, et al. Robot-assisted radical cystectomy: Description of an evolved approach to radical cystectomy. Eur Urol. 2013;64:654–63. doi: 10.1016/j.eururo.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 82.Tan WS, Sridhar A, Ellis G, Lamb B, Goldstraw M, Nathan S, et al. Analysis of open and intracorporeal robotic assisted radical cystectomy shows no significant difference in recurrence patterns and oncological outcomes. Urol Oncol. 2016;34:257.e1–9. doi: 10.1016/j.urolonc.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 83.Nguyen DP, Al Hussein Al Awamlh B, O’Malley P, Khan F, Lewicki PJ, Golombos DM, et al. Factors impacting the occurrence of local, distant and atypical recurrences after robot-assisted radical cystectomy: A Detailed analysis of 310 patients. J Urol. 2016;196:1390–6. doi: 10.1016/j.juro.2016.05.101. [DOI] [PubMed] [Google Scholar]
- 84.Nguyen DP, Al Hussein Al Awamlh B, Wu X, O’Malley P, Inoyatov IM, Ayangbesan A, et al. Recurrence patterns after open and robot-assisted radical cystectomy for bladder cancer. Eur Urol. 2015;68:399–405. doi: 10.1016/j.eururo.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Collins JW, Hosseini A, Adding C, Nyberg T, Koupparis A, Rowe E, et al. Early recurrence patterns following totally intracorporeal robot-assisted radical cystectomy: Results from the EAU robotic urology section (ERUS) scientific working group. Eur Urol. 2017;71:723–6. doi: 10.1016/j.eururo.2016.10.030. [DOI] [PubMed] [Google Scholar]
- 86.Tyritzis SI, Hosseini A, Collins J, Nyberg T, Jonsson MN, Laurin O, et al. Oncologic, functional, and complications outcomes of robot-assisted radical cystectomy with totally intracorporeal neobladder diversion. Eur Urol. 2013;64:734–41. doi: 10.1016/j.eururo.2013.05.050. [DOI] [PubMed] [Google Scholar]
- 87.Akbulut Z, Canda AE, Ozcan MF, Atmaca AF, Ozdemir AT, Balbay MD, et al. Robot-assisted laparoscopic nerve-sparing radical cystoprostatectomy with bilateral extended lymph node dissection and intracorporeal studer pouch construction: Outcomes of first 12 cases. J Endourol. 2011;25:1469–79. doi: 10.1089/end.2010.0632. [DOI] [PubMed] [Google Scholar]
- 88.Canda AE, Atmaca AF, Altinova S, Akbulut Z, Balbay MD. Robot-assisted nerve-sparing radical cystectomy with bilateral extended pelvic lymph node dissection (PLND) and intracorporeal urinary diversion for bladder cancer: Initial experience in 27 cases. BJU Int. 2012;110:434–44. doi: 10.1111/j.1464-410X.2011.10794.x. [DOI] [PubMed] [Google Scholar]
- 89.Tan WS, Sridhar A, Goldstraw M, Zacharakis E, Nathan S, Hines J, et al. Robot-assisted intracorporeal pyramid neobladder. BJU Int. 2015;116:771–9. doi: 10.1111/bju.13189. [DOI] [PubMed] [Google Scholar]
- 90.Satkunasivam R, Santomauro M, Chopra S, Plotner E, Cai J, Miranda G, et al. Robotic intracorporeal orthotopic neobladder: Urodynamic outcomes, urinary function, and health-related quality of life. Eur Urol. 2016;69:247–53. doi: 10.1016/j.eururo.2015.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee R, Ng CK, Shariat SF, Borkina A, Guimento R, Brumit KF, et al. The economics of robotic cystectomy: Cost comparison of open versus robotic cystectomy. BJU Int. 2011;108:1886–92. doi: 10.1111/j.1464-410X.2011.10114.x. [DOI] [PubMed] [Google Scholar]
- 92.Smith A, Kurpad R, Lal A, Nielsen M, Wallen EM, Pruthi RS, et al. Cost analysis of robotic versus open radical cystectomy for bladder cancer. J Urol. 2010;183:505–9. doi: 10.1016/j.juro.2009.09.081. [DOI] [PubMed] [Google Scholar]
- 93.Martin AD, Nunez RN, Castle EP. Robot-assisted radical cystectomy versus open radical cystectomy: A complete cost analysis. Urology. 2011;77:621–5. doi: 10.1016/j.urology.2010.07.502. [DOI] [PubMed] [Google Scholar]
- 94.Yu HY, Hevelone ND, Lipsitz SR, Kowalczyk KJ, Nguyen PL, Choueiri TK, et al. Comparative analysis of outcomes and costs following open radical cystectomy versus robot-assisted laparoscopic radical cystectomy: Results from the US nationwide inpatient sample. Eur Urol. 2012;61:1239–44. doi: 10.1016/j.eururo.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 95.Mmeje CO, Martin AD, Nunez-Nateras R, Parker AS, Thiel DD, Castle EP, et al. Cost analysis of open radical cystectomy versus robot-assisted radical cystectomy. Curr Urol Rep. 2013;14:26–31. doi: 10.1007/s11934-012-0292-7. [DOI] [PubMed] [Google Scholar]
- 96.Bansal SS, Dogra T, Smith PW, Amran M, Auluck I, Bhambra M, et al. Cost analysis of open radical cystectomy versus robot-assisted radical cystectomy. BJU Int. 2017 Oct 6; doi: 10.1111/bju.14044. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 97.Yeung C, Dinh T, Lee J. The health economics of bladder cancer: An updated review of the published literature. Pharmacoeconomics. 2014;32:1093–104. doi: 10.1007/s40273-014-0194-2. [DOI] [PubMed] [Google Scholar]
- 98.Parekh D, Gables C. A prospective, multicentre, randomized trial of open versus robotic radical cystectomy (RAZOR) J Urol. 2017;197((4) Suppl):e918. [Google Scholar]


