Abstract
Background
The logopenic variant of primary progressive aphasia (lvPPA) manifests due to a breakdown of the language network with prominent hypometabolism of the left temporoparietal region. LvPPA is strongly associated with amyloid deposition, yet there is question as to whether it is a homogeneous clinical entity.
Objective
This study investigated whether differences in temporoparietal metabolic patterns on 18-F positron emission tomography (FDG-PET) could elucidate brain regions preferentially affected in lvPPA.
Method
We used differences in FDG-PET metabolic z-scores relative to controls for means of left lateral temporal, inferior parietal, and superior parietal regions to classify 53 amyloid-positive lvPPA patients into temporal, parietal, or temporoparietal predominate groups. Clinical features and FDG-PET regions of hypometabolism outside of the temporoparietal region were then compared across the three groups; the latter using statistical parametric mapping.
Results
Of the 53 lvPPA patients, 15 were classified as temporal, 14 as temporoparietal, and 22 as parietal predominate. There were no significant differences between the groups on demographic measures, language evaluation, or apolipoprotein E genotype. Compared to the other two groups, individuals with the parietal predominate pattern had extensive hypometabolism in left frontal lobe and the precuneus. Furthermore, this group had greater behavioral dyscontrol and deficits in executive function, visuospatial skills, visual memory retention, working memory, and cognitive flexibility (Bonferroni p < 0.05).
Conclusions
This study demonstrates that there is clinical heterogeneity within amyloid-positive lvPPA. Patients with lvPPA with predominant parietal hypometabolism, unlike those with temporal or temporoparietal predominant hypometabolism, demonstrated widespread cognitive and behavioral changes.
Keywords: primary progressive aphasia, fludeoxyglucose F18, Positron Emission tomography, Beta-amyloid, visuospatial deficit, executive function, working memory
Introduction
Logopenic primary progressive aphasia (lvPPA) is a neurodegenerative disorder distinguished by the initial preferential involvement and impairment of language processing [1–4]. More recent literature has identified amyloid-β deposition suggestive of Alzheimer’s disease (AD) in a majority of patients with lvPPA [2–6], and therefore lvPPA is also considered an atypical presentation of AD. However, phenotypic presentations in atypical AD with primary language dysfunction can vary significantly [7–9]. A majority of patients in a study characterized as “mixed” or “unclassified” PPA demonstrated underlying AD pathology and temporoparietal atrophy consistent with lvPPA [7–9]. While the core lvPPA criteria (i.e. impaired word retrieval in spontaneous speech and impaired repetition of long sentences) appear robust [1, 2], variability exists in clinical and neuroimaging findings, which questions whether lvPPA is a homogenous entity [1–7, 10–13].
We explored whether lvPPA is a homogenous entity based on the degree of neural involvement as measured by hypometabolism on FDG-PET. Prior lvPPA studies reveal a consistent pattern of hypometabolism on fluorodeoxyglucose 18-F Positron Emission Tomography (FDG-PET) involving left temporal and left inferior parietal regions [1–4]. Additional involvement of frontal areas and the superior parietal lobule has been demonstrated, [3, 11] but not specifically investigated.
In this study, we classified amyloid-positive lvPPA patients into three groups (temporal, parietal, or temporoparietal predominate) based on a quantitative analysis of predominant regions of hypometabolism on FDG-PET. We also investigated differences between groups in regions not involved in the diagnosis along with clinical and neuropsychological features of each classification.
Methods
We prospectively recruited patients who fulfilled the international diagnostic criteria for lvPPA[1, 2] between July 2010 and January 2015 from a larger neurodegenerative speech and language disorder study[2]. Inclusion criteria were native English speakers, 18 years or older, and accompanied by an informant, initial language complaint, and met criteria for lvPPA. We excluded patients meeting criteria for another degenerative disease (such as corticobasal syndrome). All participants underwent a detailed speech and language examination, neurological evaluation, neuroimaging, and neuropsychological testing [2, 10].
A speech-language pathologist (JRD or EAS) blinded to all imaging data (FDG-PET, MRI, and PiB) made the clinical diagnosis. Based on this criteria 59 patients with lvPPA were identified. We then excluded 6 amyloid-β negative patients to limit influence of confounding effects of differing underlying neuropathologies that have been associated with lvPPA. The Mayo Clinic Institutional Review Board approved this study and all participants consented for enrollment into the study.
Speech & Language Assessment
The speech and language assessment was conducted by a speech-language pathologist (JRD or EAS) which included the Western Aphasia Battery (WAB; [14]). The WAB Aphasia Quotient (AQ) was the primary measure of global language ability and aphasia severity which is a composite score derived from scores on Spontaneous Speech responses (including ratings of Information Content and a composite rating of fluency, grammaticality and paraphasias), and scores on measures of Auditory-Verbal Comprehension, Repetition, Naming, and Word Finding. In addition, a 15-item Boston Naming Test (BNT;[15]), a measure of verbal fluency, and the Pyramids and Palm Trees [16] test were administered. A score of greater than two standard deviations below the mean from the normative data was considered abnormal on all language tests with published or derived means and standard deviations.
Video recordings of speech and language assessments for all patients were reviewed by two speech-language pathologists (JRD and EAS) who rendered a consensus diagnosis based on the international lvPPA diagnostic criteria [1]. The criteria included: 1) slowed rate of verbal expression due to pauses for word retrieval or verbal formulation, 2) anomia, but target words typically recognized with cuing, 3) relatively spared single word comprehension with increased difficulty with complex sentence comprehension, 4) impaired sentence repetition or sentence comprehension, or phonemic paraphasias, 5) absence of agrammatic or telegraphic verbal output, 6) informant report of intact word comprehension. The raters were blinded to patients’ performance on the neurological, neuroimaging, and neurocognitive evaluation.
Neuroimaging
All patients underwent standardized neuroimaging that included FDG-PET and Pittsburgh Compound B PET (PiB-PET), as previously described [17, 18]. Presence of amyloid-β deposition was assessed using the PiB-PET scans, where patients were classified as PiB-positive using a cortical-to-cerebellar (SUVR) ratio cut-point of 1.5, as previously defined [19]. All patients also underwent a 3T volumetric MRI that included a 3D magnetization prepared rapid acquisition gradient echo (MPRAGE) sequence. Only patients with lvPPA and amyloid-β positive scans were included in this study.
Classification of groups
In order to classify subjects according to their patterns of hypometabolism, median metabolism was calculated for each patient in the left lateral temporal lobe (inferior, middle and superior temporal gyri), left inferior parietal lobe (inferior parietal lobe, angular gyrus and supramarginal gyrus) and left superior parietal lobe, using the automated anatomical labelling (AAL) atlas[20]. Briefly, the FDG-PET images were co-registered to the patients’ MPRAGE using 6 degrees-of-freedom registration. The AAL atlas was then transformed into the MPRAGE native anatomical space for each patient and multiplied by a grey matter mask created using SPM5. Median FDG-PET uptake in each grey matter region-of-interest was divided by median uptake in the pons. Regional FDG-PET uptake ratios were also calculated using the same method in 49 age and gender matched healthy controls, and the FDG-PET uptake ratios in the lvPPA patients were expressed as z-scores showing differences from the control cohort.
The z-scores for the left temporal, left inferior parietal, and left superior parietal regions were used to classify the patients with lvPPA into temporal, parietal, or temporoparietal predominate groups based on the region with greater hypometabolism (more negative z-score), which has been used previously[21]. Low Z-scores are indicative of reduced 18F-FDG uptake in the patients relative to the control mean. Patients were classified into a group based on the following parameters.
Temporal predominate group: if z-score for the temporal region was less than 0.5 of the z-score for both inferior parietal and superior parietal region.
Parietal predominate group: if z-score for either inferior or superior parietal group is less than 0.5 compared to the temporal z-score.
Temporoparietal group: if the z-score difference between temporal and parietal regions are less than or equal to 0.5.
Based on this classification, we investigated differences in z-scores for the three anatomical regions (left temporal, left inferior parietal, and left superior parietal). Consistent with the method of classification, the parietal predominate group showed greater hypometabolism compared to the temporal group in both the inferior (F=9.90, p <0.001) and superior parietal regions (F=16.33, p< 0.001). The parietal group showed greater hypometabolism in the inferior parietal region compared to the temporoparietal group (F=16.33, p=0.004). There were no differences between the groups in the temporal region (F= 1.09, p = 0.34). These scores reflect literature on lvPPA reporting hypometabolism in the temporoparietal regions [1]. Significant hypometabolism in the parietal region has been reported but not specifically investigated. The greater hypometabolism in the parietal predominate group in the inferior and superior parietal region, significantly distinguishes this group from the temporal and temporoparietal predominate groups.
Voxel-level comparisons
A voxel-level FDG-PET analysis was also performed to illustrate the patterns of hypometabolism for each lvPPA group using SPM5. This analysis was performed to provide a graphical representation of the group classification, and additionally to allow us to investigate areas of hypometabolism outside the temporoparietal regions that were used in the classification of groups. For each patient, all voxels in the FDG-PET image were divided by the median FDG uptake of the pons to form uptake ratio images, which were transformed into custom template space as previously described [2, 17, 18] and smoothed at 8mm full width at half maximum. Two-sided t-tests were used to assess voxel-wise patterns of FDG hypometabolism in each lvPPA group compared to the control cohort, corrected for multiple comparisons (family wise error correction at p<0.001). Since the direct comparisons between the three lvPPA groups did not survive correction for multiple comparisons, results were assessed uncorrected for multiple comparisons at p<0.001. A group-level PiB-PET analysis was also performed using identical methods, except that the PiB-PET images were normalized by the median uptake in the cerebellar grey matter.
Genetic testing
Apolipoprotein E (APOE) genotype testing was performed using previously described procedures [5].
Neuropsychological Assessment
The neuropsychological evaluation was performed separately from the comprehensive speech and language evaluation under the supervision of a trained neuropsychologist (MMM). The neurocognitive battery included domains of (1) memory [Wechsler Memory Scale-III (WMS-III; [22] Logical Memory I/II which assesses immediate and delayed recall of paragraph-length stories; Visual Reproduction I/II which assesses immediate and delayed recall of designs; the Rey Auditory Verbal Learning Test (AVLT; [23], a list learning test that includes five learning trials, an interference trial, immediate recall and delay recall trials, and recognition; (2) processing speed [Trail Making Test (TMT) Part A[24]], a test of scanning and visuomotor tracking]; (3) executive function [TMT Part B, which assesses divided attention and cognitive flexibility[24]], and Delis-Kaplan Executive Function (DKEFS) Card Sort[25], a conceptual task that evaluates problem-solving, verbal and nonverbal concept formation, and flexibility of thinking]; and, (4) visuospatial function [Rey-Osterreith Complex Figure Test[26]], a measure of visual perception and constructional praxis and Visual Object and Space Perception (VOSP) cube and incomplete letters subtests[27]].
Norms used in this study have been previously described [11]. For the participants who were younger than the MOANS normative sample, the lowest age grouping was used to derive standard scores. Participants were assigned a scaled score of 1 if they attempted but were unable to complete the task. Participants were assigned a scaled score of 0 if they did not comprehend task instructions [11].
Neurological evaluation
A neurologic examination was performed by a behavioral neurologist (KAJ). Patients completed the Mini-Mental Status Examination (MMSE; [28]), the Montreal Cognitive Assessment battery (MoCA;[29]), Frontal Behavioral Inventory (FBI; [30]), a brief questionnaire form of the Neuropsychiatric Inventory (NPI; [31]), the limb apraxia subscale of the WAB[14], the Movement Disorders Society-sponsored version of the Unified Parkinson’s Disease Rating Scale part III (MDS-UPDRS;[32]), modified CDR sum of boxes (MCDRSB; [33]) and Frontal Assessment Battery (FAB;[34]).
Statistical Analyses
Non-parametric Chi-Square and parametric univariate analysis of variance (ANOVA) were used to assess demographic and clinical features across groups, as appropriate (p <0.05). Post-hoc comparisons using Bonferroni correction at p <0.05 was used to correct for multiple comparisons for all ANOVAs conducted in this study (IBM SPSS version 21.0).
Results
From the remaining 53 patients with amyloid-positive lvPPA, 15 were classified as “temporal predominate”, 16 as “temporoparietal predominate,” and 22 as “parietal predominate” based on the rating process described above.
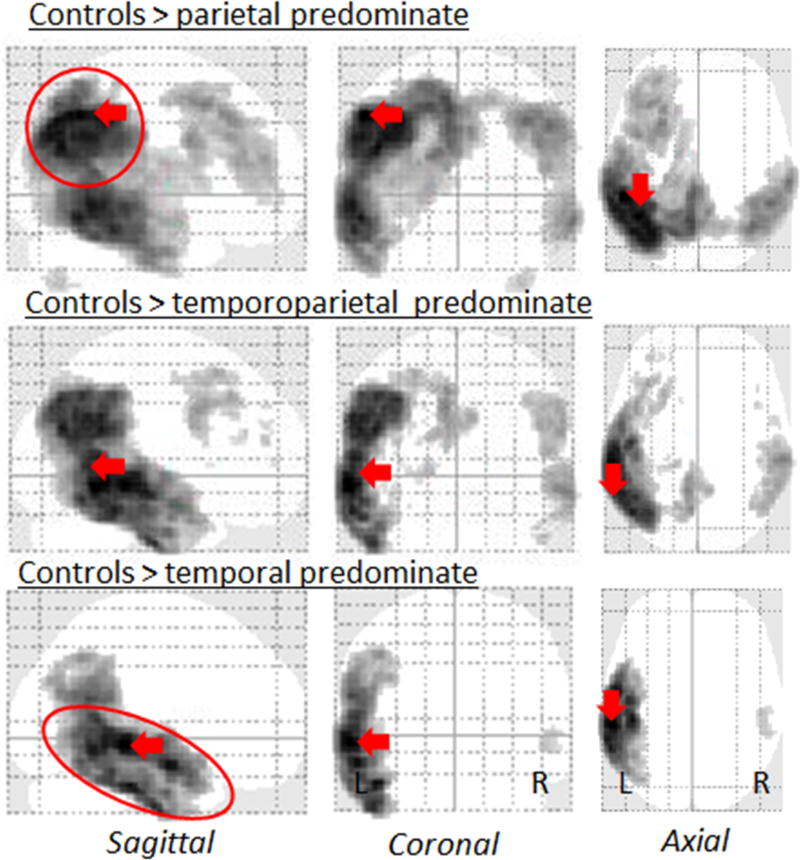
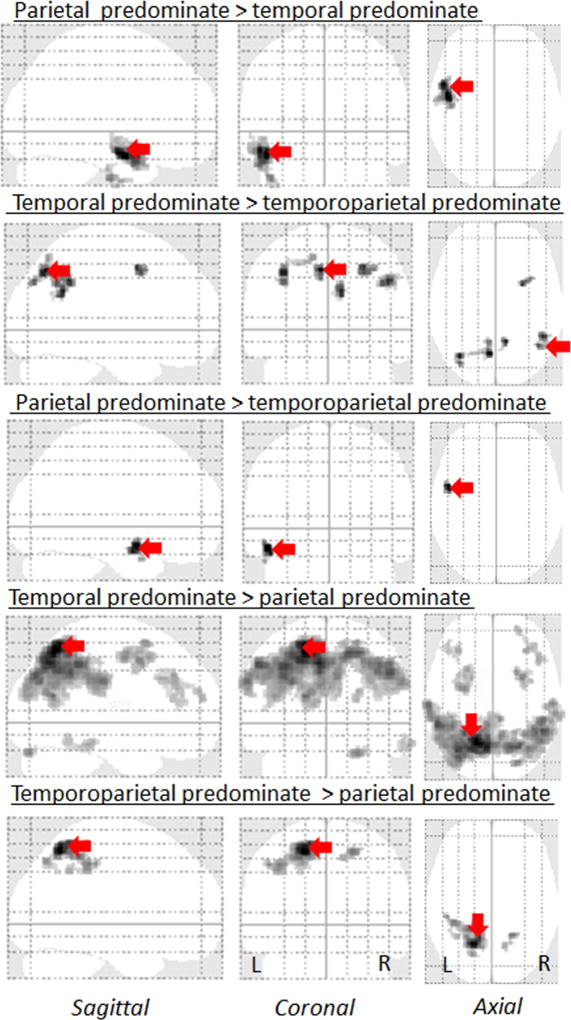
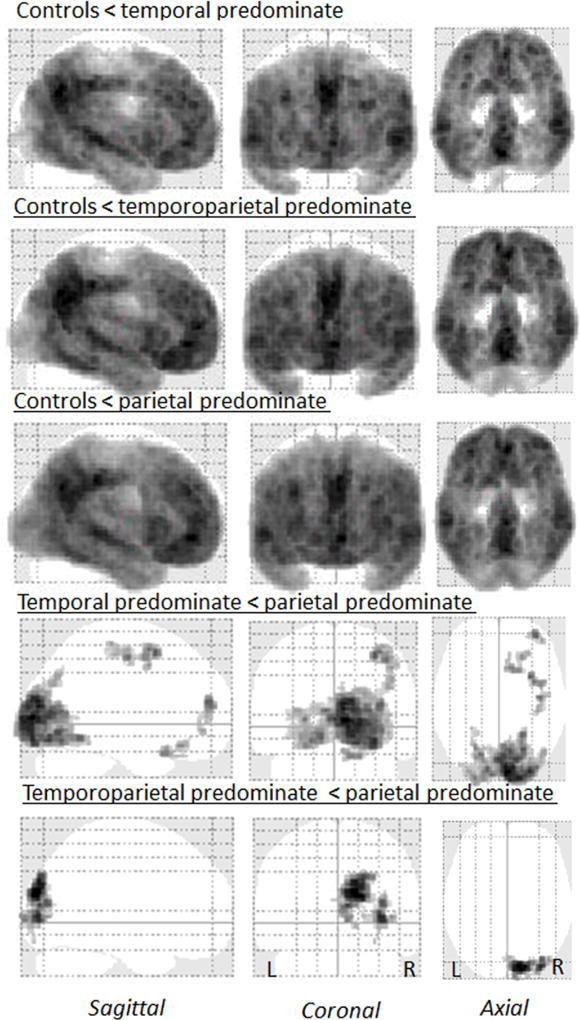
The different degrees of lateral temporal and parietal involvement that were used to classify the groups can be visualized in the voxel-level FDG-PET maps in Figure 1. Consistent with our classification, the voxels showing the greatest z-score were located in the parietal lobe in the parietal predominate group and in the lateral temporal lobe in the temporal predominate group. Furthermore, on direct comparison between the groups, the left lateral temporal lobe showed greater hypometabolism in both the temporal predominate and temporoparietal predominate groups compared to the parietal predominate groups (Figure 2). Conversely, bilateral regions in the lateral parietal lobe showed greater hypometabolism in both the parietal and temporoparietal predominate groups compared to the temporal predominate group (Figure 2). This substantiates the quantitative z-score values used to categorize the sample.
Figure 1. Patterns of FDG-PET hypometabolism in the three lvPPA groups compared to controls.
Glass brain renders showing patterns of FDG-PET hypometabolism in the parietal, temporoparietal, and temporal predominate lvPPA groups compared to controls. Results are shown after correction for multiple comparisons at p<0.001. Red arrows highlight the voxel with the peak t score for each comparison.
Figure 2. Patterns of FDG-PET hypometabolism in the three lvPPA groups compared to each other.
Glass brain renders showing a direct comparisons of the three lvPPA groups, uncorrected for multiple comparisons at p<0.001. Red arrows highlight the voxel with the peak t score for each comparison.
Differences across the lvPPA groups were also observed in regions that were not used to classify the groups, i.e. outside the lateral temporoparietal lobe. Both the parietal and temporoparietal groups showed medial parietal and frontal hypometabolism when compared to controls (Figure 1). In fact, the parietal group showed greater hypometabolism in middle and superior frontal gyri, and bilateral precuneus, compared to the temporal group and greater hypometabolism in the precuneus (left>right) compared to the temporoparietal group (Figure 2). The temporoparietal predominate group also showed greater hypometabolism in the bilateral precuneus and right posterior frontal lobule compared to the temporal group (Figure 2).
All three groups showed widespread amyloid-β deposition, although regional differences were observed (Figure 3). The parietal predominate group demonstrated greater PiB deposition in the right occipital lobe compared to the temporoparietal predominate group and greater PiB deposition in the right occipital lobe, prefrontal cortex, and sensorimotor cortex compared to the temporal group (Figure 3).
Figure 3. Amyloid-β deposition on PiB-PET in both lvPPA groups.
Glass brain renders showing amyloid-β deposition on PiB-PET in the parietal, temporoparietal, and temporal predominate lvPPA groups, corrected for multiple comparisons at p<0.001.
There were no group differences in demographic characteristics, the speech language evaluation, global PiB-PET SUVR, or presence of apolipoprotein E4 allele (Table 1 and 2).
Table 1.
Descriptive characteristics of the lvPPA groups classified based on FDG-PET pattern
| Temporal (n=15) Mean (SD) |
Temporoparietal (n=16) Mean (SD) |
Parietal (n=22) Mean (SD) |
p | |
|---|---|---|---|---|
| Age at onset | 63.67 (8.40) | 64.60 (10.77) | 60.91 (7.12) | 0.40 |
| Age at evaluation | 67.20 (7.84) | 67.44 (10.59) | 63.95 (8.18) | 0.40 |
| Illness duration | 6.97 (3.37) | 4.67 (3.74) | 4.77 (3.01) | 0.10 |
| Education | 16.00 (2.73) | 14.63 (2.28) | 15.23 (2.52) | 0.32 |
| WRAT-R SS | 90.47 (7.78) | 85.19 (18.06) | 87.55 (18.46) | 0.66 |
| MMSE | 24.27 (4.18) | 20.47 (6.61) | 21.77 (10.41) | 0.42 |
| PiB-PET SUVR Ratio | 2.09 (0.26) | 2.20 (0.24) | 2.17 (0.25) | 0.46 |
| Gender (M/F) | 5/10 | 8/8 | 12/10 | 0.43* |
| APOE4 allele (% present) | 61% | 64% | 47% | 0.79* |
Note:
= Chi-Sqaure, WRAT SS: Wide Range Achievement Test Scaled Score, MMSE: Mini mental status examination; PiB-PET SUVR: Pittsburgh Compound B PET cortical-to-cerebellar (SUVR) ratio; APOE: Apopoliprotein
Table 2.
Language evaluation for the lvPPA groups classified based on FDG-PET pattern
| Temporal (n=15) Mean (SD) |
Temporoparietal (n=16) Mean (SD) |
Parietal (n=22) Mean (SD) |
p | |
|---|---|---|---|---|
| WAB AQ (/100) | 84.42 (7.53) | 73.25 (17.78) | 78.35 (15.68) | 0.11 |
| WAB Repetition (/10) | 7.82 (1.04) | 6.98 (1.92) | 7.66 (1.77) | 0.32 |
| WAB Naming | 7.81 (1.51) | 6.76 (2.49) | 7.44 (2.18) | 0.37 |
| PPT (/52) | 48.87 (2.83) | 43.38 (8.57) | 45.67 (5.58) | 0.055 |
| BNT Total (/15) | 7.67 (4.72) | 6.25 (4.04) | 8.09 (4.67) | 0.45 |
| Action Fluency | 11.20 (4.13) | 7.88 (4.46) | 8.18 (4.75) | 0.08 |
| Letter Fluency | 22.20 (9.91) | 17.75 (10.14) | 14.00 (12.83) | 0.11 |
| Animal Fluency | 10.40 (4.55) | 8.25 (4.95) | 9.36 (6.18) | 0.54 |
Note: WAB AQ: Western Aphasia Battery Aphasia Quotient; PPT: Pyramids and palm trees; BNT= Boston Naming Test
On the neurological evaluation, the parietal predominate group demonstrated greater behavioral dysregulation on the Frontal Assessment Battery (p= 0.008) and the Frontal Behavioral Inventory (p=0.012). Participants in this group scored worse on the MoCA (p = 0.04) especially on the clock drawing (p <0.001) and calculation (p=0.002) compared to the temporal group. Furthermore, the Modified CDR-sum of boxes (p=0.041) showed greater global impairment in the parietal group compared to the temporal group. There were no significant differences between the temporal vs. temporoparietal groups or the temporoparietal vs. parietal groups (Table 3). The p values reflect significance after multiple comparison correction using the Bonferroni method.
Table 3.
Neurological data for lvPPA groups classified based on FDG-PET pattern
| Temporal (n=15) Mean (SD) |
Temporoparietal (n=16) Mean (SD) |
Parietal (n=22) Mean (SD) |
p | |
|---|---|---|---|---|
| FBI (/72) | 9.47 (6.08) | 14.73 (6.30) | 19.18 (12.69) | 0.01a |
| FAB (/18) | 13.60 (2.53) | 10.00 (4.95) | 10.07 (4.95) | 0.02b |
| Apraxia (/60) | 55.53 (4.58) | 49.73 (14.46) | 51.59 (8.86) | 0.27 |
| UPDRS III (132) | 5.07 (4.96) | 5.83 (6.36) | 5.59 (4.62) | 0.92 |
| MCDRSB (/24) | 3.87 (2.95) | 5.83 (2.66) | 7.19 (4.73) | 0.04a |
| NPI- Q (/36) | 2.80 (2.70) | 2.73 (2.02) | 3.64 (4.15) | 0.64 |
| MOCA | 18.40 (6.05) | 14.60 (7.39) | 12.73 (6.34) | 0.04b |
| MoCA – Clock (3pts) | 2.20 (0.86) | 1.44 (1.09) | 0.95 (0.65) | <0.001b |
| MoCA- Calculation (5pts) | 3.67 (1.99) | 2.50 (2.19) | 1.36 (1.50) | 0.002b |
Note: Bolded = Significant with Bonferroni correction at p< 0.05;
= parietal > temporal;
= temporal > parietal; FBI = frontal behavioral inventory, FAB= Frontal Assessment Battery, UPDRS III= Unified Parkinson Disease Rating Scale Part 3, MCDRSB=modified Clinical Dementia Rating Scale Sum of Boxes, NPI= Neuropsychiatric Inventory, MoCA = Montreal Cognitive Assessment
The parietal predominate group showed greater impairment on majority of the neurocognitive measures. Compared to the temporal predominate group, the parietal group performed worse on verbal learning (LM 1; p=0.02), verbal recall (LM II; p=0.016), visual learning (VR1; p=0.02), visual recall (VR II; p= 0.016) and visual retention (VR; p= 0.013), AVLT recognition (p= 0.003), Trails A (p=0.012), Trails B (p=0.001), ReyO (p=0.002) and VOSP cube (p = 0.002). The parietal group performed lower than the temporoparietal group on AVLT recognition (p = 0.018), Trails B (p= 0.041), and Rey-O (0.046). The p values reflect significance after multiple comparison correction using the Bonferroni method.
Discussion
This study reveals clinical heterogeneity within a sample of patients with amyloid-positive lvPPA an average of 5.37 years from symptom onset. Classification of the groups into lateral temporal, parietal, or temporoparietal predominate regions of hypometabolism was based on data from each individual’s FDG-PET scan. The three amyloid-β positive lvPPA groups showed different patterns of neuroanatomical involvement outside the temporoparietal region and were associated with unique neurological and neurocognitive profiles.
All participants in this study have underlying amyloid-β deposition and hence would meet pathological classification for AD [35–38]. Consistent with the literature, 90% of patients with lvPPA (53/59) had significant amyloid-β deposition, which substantiates lvPPA as an atypical presentation of AD [2–6]. However, this study sample differs from typical AD in clinical and neuroimaging features. All lvPPA participants presented with language-related complaints as the first and most debilitating symptom which differs from the initial amnestic presentation which is a hallmark for a typical AD diagnosis. Furthermore, all patients were reliably classified as lvPPA based on an in-depth language evaluation by two speech language pathologists blinded to the imaging and other clinical data. The three groups did not differ based on the language evaluation (Table 2), which corroborates the lvPPA diagnosis.
The FDG-PET hypometabolism patterns in this sample differ from typical AD. All participants with lvPPA demonstrated an asymmetric hypometabolism with greater involvement of the left hemisphere, which is unlike the symmetric pattern of involvement in typical AD [3, 4, 6]. Furthermore, while the parietal predominate group demonstrates greater PiB deposition in the occipital lobe compared to the other two groups, the overall PiB SUVR ratios do not differ between the three identified groups, suggesting that PiB distribution does not likely account for the FDG-PET differences reported here.
The voxel-level FDG-PET analyses supported the classification of the three lvPPA groups, but also highlighted differences across the groups that would argue against one group being a more severe manifestation of the other. For example, the parietal predominate group showed the most widespread patterns of hypometabolism, with the greatest involvement of the frontal cortices, yet, despite this, the lateral temporal cortex was involved to a greater degree in the temporal predominate group compared to the parietal predominate group. Hence, it is unlikely that the parietal predominate group represents a more severe manifestation of the temporal predominate group, and more likely that they represent different patterns of disease progression. The same argument applies to the parietal predominate group compared to the temporoparietal group. The similar illness duration and aphasia severity across groups also argues against one classification presenting as a more severe manifestation of another. This finding contrasts prior studies demonstrating an association between additional involvement and disease progression or underlying pathology in lvPPA [3, 4, 6]. In our study, the sample was rather large compared to other lvPPA cohorts and was restricted to amyloid-positive lvPPA which may partly explain the contrary results.
A neurocognitive evaluation in patients with language disorders is challenging due to the high verbal demand on most neuropsychological tests. Despite this caveat, the three variants of PPA present with distinct neurocognitive profiles [10, 12]. Cognitive profiles of patients with lvPPA generally show deficits in working memory, verbal memory, fluency, digit span, mental flexibility and visuospatial skills [10, 11, 39], which is observed in this study across the identified lvPPA groups.
There are also distinct group differences in the neurocognitive profiles in this study. Interestingly, the cognitive results are consistent with the neuroanatomical findings. Cognitive deficits were associated with the area of predominant hypometabolism and with the regions of additional neural involvement in each group. For example, there is greater hypometabolism in the precuneus in the parietal and temporoparietal groups. The precuneus plays a role in visuospatial tasks and episodic memory retrieval [40] which are areas of cognitive deficits in parietal and temporoparietal group but not in the temporal predominate group which did not demonstrate precuneus involvement.
The role of the superior parietal lobule in working memory has been demonstrated using functional imaging, resting-state imaging, repetitive transcranial stimulation research, and lesion studies[41–44]. The parietal predominate group, which has significant superior parietal involvement, illuminates the association between the neuroanatomy (superior parietal) and working memory deficits associated with this group compared to the temporal and temporoparietal predominate groups which do not show significant superior parietal involvement. The greater cognitive deficits in the parietal predominate group may be explained by the dual role played by the superior parietal lobule in the language and working memory resting-state networks in lvPPA [41, 44]. It appears that our temporal predominate group is associated with predominant dysfunction in the language network, whereas the parietal predominate group shows dysfunction in the language network with striking additional dysfunction of the working memory network. The temporoparietal group exhibits similar trends as the parietal group, but to a lesser extent, presumably due to comparatively decreased involvement of the parietal lobes in the mixed temporoparietal group. It must be emphasized that the cognitive deficits described here survived stringent multiple comparison correction which suggests these findings are not merely coincidental.
The parietal predominate group further distinguishes itself with greater behavioral dysregulation likely due to increased frontal lobe involvement. This group differs from the recently described behavioral/dysexecutive variant of AD [45] as the former involves asymmetric left temporoparietal hypometabolism compared to the symmetric presentation in the latter [45]. Furthermore, dysexecutive symptoms were not the chief complaint or endorsed as functionally debilitating by the patients or their informant even 5 years from illness onset. However, it is possible that the parietal predominate group demonstrates a constellation of symptoms common to other atypical forms of AD, such as the frontal variant [36, 45] or posterior cortical atrophy (PCA) [38].
The groups described in this study are not distinct variants but rather represent a continuum of features in patients diagnosed with lvPPA. The greatest polarity in imaging and clinical characteristics exists between the parietal predominate and the temporal predominate categories. Forty-two percent (22/53) of the study participants demonstrated predominant parietal involvement on FDG-PET, which is not rare. The parietal predominate group demonstrates greater behavioral dysregulation and worse outcome on neurocognitive tasks measuring visuospatial skills, working memory, and mental flexibility. Overall, there is substantial and expected neuroanatomical overlap between the groups, given that these patients with lvPPA have analogous language dysfunction presumably due to involvement of similar neural regions. However, there are distinctly different regions involved in each group that map onto corresponding behavioral and cognitive deficits.
All participants in this study presented with primary language complaints as the most salient initial symptom. We carefully selected patients who strictly met lvPPA diagnostic criteria [1, 2] as determined by consensus by two speech language pathologists. This study demonstrates that patients with amyloid-positive lvPPA develop differing neurological and neurocognitive symptoms over a comparable illness duration (i.e., 5.37 years), influenced by region of neuroanatomical involvement. This corroborates prior findings that lvPPA may not be a discrete entity [4, 7]. Prior lvPPA studies report cognitive impairments in domains of visuospatial functioning, attention, and memory in addition to deficits in language related cognitive function[46, 47].
This is the largest cohort of lvPPA patients reported in the literature, which allowed for a nuanced investigation into the varying FDG-PET patterns of hypometabolism in lvPPA. Future studies are needed to substantiate these findings.
In later stages of neurodegenerative disorders, when multiple systems may be impacted, medical providers vary in preference on whether to diagnose patients based on current presentation or on symptom progression. For example, case studies of patients who meet diagnostic criteria for lvPPA at time 1, developed symptoms consistent with dementia with Lewy bodies 2 and 6 years after initial symptom onset [48, 49]. This raises the issue of whether the patient should be diagnosed as lvPPA, DLB secondary to lvPPA, or just DLB at follow-up. None of the patients in this study reported early cognitive deficits. We have previously noted a preference to modify existing lvPPA criteria to add specifiers that separate patients with lvPPA and focal aphasia from patients with lvPPA with more generalized cognitive decline [50, 51]. These specifiers may assist patients and their families in understanding the patients’ difficulties (i.e. a patient with lvPPA may have co-occurring dysexecutive symptoms and visuospatial difficulties over time or focal aphasia with minor cognitive deficits). This clarification, instead of a diagnosis of mixed PPA or atypical AD, may also be more meaningful to patients. Furthermore, the suggested classification may be useful for research purposes to reduce variance associated with heterogenous presentations.
In conclusion, we demonstrate that lvPPA is not a distinct entity. There exist varying patterns of hypometabolism in patients with lvPPA with predominant involvement of different areas with the temporoparietal region. This study extends prior findings to demonstrate a link between neuroanatomical involvement shown on FDG-PET and the corresponding neurological and neurocognitive profiles [4, 12]. Atypical presentations of AD such as PCA, lvPPA, behavioral/dysexecutive variant, or early-onset AD can present with temporoparietal atrophy[38, 39, 45], which provides further support to the hypothesis that atypical variants of AD demonstrate a fluid boundary in the clinical manifestations of the disease[38, 39, 45].
Table 4.
Neurocognitive evaluation for lvPPA groups classified based on FDG-PET pattern
| Temporal (n=15) Mean (SD) |
Temporoparietal (n=16) Mean (SD) |
Parietal (n=22) Mean (SD) |
p | |
|---|---|---|---|---|
| WMS- III LM 1 ss | 5.00 (3.91) | 2.81 (2.10) | 2.45 (2.15) | 0.02b |
| WMS III LM II ss | 7.40 (4.21) | 4.50 (2.92) | 3.64 (2.63) | 0.004b,c |
| WMS III LM % retention ss | 11.47 (4.37) | 8.27 (4.85) | 7.82 (5.49) | 0.09 |
| WMS III VR 1 ss | 7.40 (4.31) | 4.13 (3.59) | 3.91 (3.29) | 0.015b,c |
| WMS III VR II ss | 9.47 (4.41) | 6.60 (2.44) | 5.55 (2.54) | 0.002b, c |
| WMS III VR % retention ss | 10.13 (4.42) | 7.20 (3.32) | 6.36 (3.58) | 0.014b |
| AVLT LOT MOANS | 8.64 (4.48) | 7.13 (3.91) | 6.29 (3.72) | 0.24 |
| AVLT delayed Recall MOANS | 7.50 (4.50) | 5.06 (2.86) | 4.73 (3.19) | 0.06 |
| AVLT recognition MOANS | 8.71 (2.92) | 5.69 (2.94) | 5.23 (2.83) | 0.003b,c |
| Trails A MOANS | 8.00 (4.00) | 6.13 (3.26) | 4.18 (3.92) | 0.02b |
| Trails B MOANS | 5.87 (4.34) | 2.94 (3.26) | 1.64 (2.01) | 0.001b,c |
| DKEFS Scaled Score | 7.27 (3.84) | 4.56 (3.46) | 4.64 (3.62) | 0.07 |
| ReyO MOANS | 8.87 (4.56) | 5.19 (3.17) | 3.68 (2.98) | <0.001b,c |
| VOSP Letters Raw | 17.86 (4.91) | 16.79 (5.16) | 17.30 (3.11) | 0.81 |
| VOSP Cube Raw | 8.86 (2.11) | 6.79 (3.89) | 5.30 (3.40) | 0.01b |
Notes: Bolded = Significant with Bonferroni correction at p< 0.05;
= parietal score > temporal;
= temporal score > parietal;
= temporal > temporoparietal, ss = scaled score (mean 10, standard deviation 2); MOANS = Mayo Older American Normative Studies (mean 10, standard deviation 2); WMS-III = Wechsler Memory Scale, Third Edition; LM = Logical Memory I (Immediate recall) and II (Delayed recall); VR = Visual Reproduction I (Immediate recall) and II (Delayed recall); LOT = Learning Over Trials, TMT = Trail Making Test; DKEFS = Delis-Kaplan Executive Function System; Rey-O = Rey-Osterreith Complex Figure; VOSP = Visual Object and Space Perception
Acknowledgments
Study Funding
This study was funded by National Institute of Health Grant R01 DC010367 (PI Josephs) and Alzheimer’s Association Grant New Investigator Research Grant-12-242215 (PI Whitwell).
Drs. Josephs, Machulda, and Whitwell receive funding from the NIH (NIA and NIDCD). Drs. Duffy and Strand receives funding from the NIH/NIDCD. Dr. Jack serves on the scientific advisory board for Eli Lilly & Company; receives research support from the NIH/NIA, the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Foundation; and holds stock in Johnson & Johnson. Dr. Lowe serves on scientific advisory boards for Bayer Schering Pharma, Piramal Life Sciences and receives research support from GE Healthcare, Siemens Molecular Imaging, AVID Radiopharmaceuticals and the NIH (NIA, NCI).
Footnotes
Disclosures of conflict of interest
The other authors report no disclosures.
References
- 1.Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Ogar JM, Rohrer JD, Black S, Boeve BF, Manes F, Dronkers NF, Vandenberghe R, Rascovsky K, Patterson K, Miller BL, Knopman DS, Hodges JR, Mesulam MM, Grossman M. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Botha H, Duffy JR, Whitwell JL, Strand EA, Machulda MM, Schwarz CG, Reid RI, Spychalla AJ, Senjem ML, Jones DT, Lowe V, Jack CR, Josephs KA. Classification and clinicoradiologic features of primary progressive aphasia (PPA) and apraxia of speech. Cortex; a journal devoted to the study of the nervous system and behavior. 2015;69:220–236. doi: 10.1016/j.cortex.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teichmann M, Kas A, Boutet C, Ferrieux S, Nogues M, Samri D, Rogan C, Dormont D, Dubois B, Migliaccio R. Deciphering logopenic primary progressive aphasia: a clinical, imaging and biomarker investigation. Brain : a journal of neurology. 2013;136:3474–3488. doi: 10.1093/brain/awt266. [DOI] [PubMed] [Google Scholar]
- 4.Mesulam MM, Weintraub S, Rogalski EJ, Wieneke C, Geula C, Bigio EH. Asymmetry and heterogeneity of Alzheimer's and frontotemporal pathology in primary progressive aphasia. Brain : a journal of neurology. 2014;137:1176–1192. doi: 10.1093/brain/awu024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Lowe VJ, Jack CR, Jr, Whitwell JL. APOE epsilon4 influences beta-amyloid deposition in primary progressive aphasia and speech apraxia. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2014;10:630–636. doi: 10.1016/j.jalz.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leyton CE, Villemagne VL, Savage S, Pike KE, Ballard KJ, Piguet O, Burrell JR, Rowe CC, Hodges JR. Subtypes of progressive aphasia: application of the International Consensus Criteria and validation using beta-amyloid imaging. Brain : a journal of neurology. 2011;134:3030–3043. doi: 10.1093/brain/awr216. [DOI] [PubMed] [Google Scholar]
- 7.Sajjadi SA, Patterson K, Nestor PJ. Logopenic, mixed, or Alzheimer-related aphasia? Neurology. 2014;82:1127–1131. doi: 10.1212/WNL.0000000000000271. [DOI] [PubMed] [Google Scholar]
- 8.Rohrer JD, Rossor MN, Warren JD. Alzheimer's pathology in primary progressive aphasia. Neurobiology of Aging. 2012;33:744–752. doi: 10.1016/j.neurobiolaging.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gil-Navarro S, Llado A, Rami L, Castellvi M, Bosch B, Bargallo N, Lomena F, Rene R, Montagut N, Antonell A, Molinuevo JL, Sanchez-Valle R. Neuroimaging and biochemical markers in the three variants of primary progressive aphasia. Dementia and geriatric cognitive disorders. 2013;35:106–117. doi: 10.1159/000346289. [DOI] [PubMed] [Google Scholar]
- 10.Butts AM, Machulda MM, Duffy JR, Strand EA, Whitwell JL, Josephs KA. Neuropsychological Profiles Differ among the Three Variants of Primary Progressive Aphasia. Journal of the International Neuropsychological Society : JINS. 2015:1–7. doi: 10.1017/S1355617715000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Machulda MM, Whitwell JL, Duffy JR, Strand EA, Dean PM, Senjem ML, Jack CR, Jr, Josephs KA. Identification of an atypical variant of logopenic progressive aphasia. Brain and language. 2013;127:139–144. doi: 10.1016/j.bandl.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rohrer JD, Ridgway GR, Crutch SJ, Hailstone J, Goll JC, Clarkson MJ, Mead S, Beck J, Mummery C, Ourselin S, Warrington EK, Rossor MN, Warren JD. Progressive logopenic/phonological aphasia: erosion of the language network. Neuroimage. 2010;49:984–993. doi: 10.1016/j.neuroimage.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whitwell JL, Duffy JR, Strand EA, Machulda MM, Senjem ML, Schwarz CG, Reid R, Baker MC, Perkerson RB, Lowe VJ, Rademakers R, Jack CR, Jr, Josephs KA. Clinical and neuroimaging biomarkers of amyloid-negative logopenic primary progressive aphasia. Brain and language. 2015;142:45–53. doi: 10.1016/j.bandl.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kertesz A. Western Aphasia Battery Revised. Psych Corp; San Antonio: 2007. [Google Scholar]
- 15.Lansing AE, Ivnik RJ, Cullum CM, Randolph C. An empirically derived short form of the Boston naming test. Archives of clinical neuropsychology : the official journal of the National Academy of Neuropsychologists. 1999;14:481–487. [PubMed] [Google Scholar]
- 16.Howard D, Patterson KE. The Pyramids and Palm Trees Test: A test of semantic access from words and pictures. Thames Valley Test Company; 1992. [Google Scholar]
- 17.Jack CR, Jr, Lowe VJ, Senjem ML, Weigand SD, Kemp BJ, Shiung MM, Knopman DS, Boeve BF, Klunk WE, Mathis CA, Petersen RC. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer's disease and amnestic mild cognitive impairment. Brain : a journal of neurology. 2008;131:665–680. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Master AV, Lowe VJ, Jack CR, Jr, Whitwell JL. Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain : a journal of neurology. 2012;135:1522–1536. doi: 10.1093/brain/aws032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Josephs KA, Duffy JR, Strand EA, Machulda MM, Vemuri P, Senjem ML, Perkerson RB, Baker MC, Lowe V, Jack CR, Jr, Rademakers R, Whitwell JL. Progranulin-associated PiB-negative logopenic primary progressive aphasia. Journal of neurology. 2014;261:604–614. doi: 10.1007/s00415-014-7243-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 21.Seeley WW, Bauer AM, Miller BL, Gorno-Tempini ML, Kramer JH, Weiner M, Rosen HJ. The natural history of temporal variant frontotemporal dementia. Neurology. 2005;64:1384–1390. doi: 10.1212/01.WNL.0000158425.46019.5C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wechsler D. Wechsler Memory Scale-III. Psychological Corporation; New York: 1997. [Google Scholar]
- 23.Rey A. L'examen clinique en psychologie. Presses Universitaires de France; Paris: 1964. [Google Scholar]
- 24.Reitan R. Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual & Motor Skills. 1958;9:271–276. [Google Scholar]
- 25.Delis D, Kaplan E, Kramer J. Delis-Kaplan Executive Function System (DKEFS): Examiner's manual. The Psychological Corporation; San Antonio: 2001. [Google Scholar]
- 26.Osterrieth P. Le test de copie d'une figure complex: Coontribution a l'etude de la perception et de la memoire. 1944:286–356. [Google Scholar]
- 27.Warrington EaMJ. The Visual Object and Space Perception Battery. Thames Valley Test Company; Bury St. Edmunds, Suffolk: 1991. [Google Scholar]
- 28.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 29.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 30.Kertesz A, Davidson W, Fox H. Frontal behavioral inventory: diagnostic criteria for frontal lobe dementia. The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques. 1997;24:29–36. doi: 10.1017/s0317167100021053. [DOI] [PubMed] [Google Scholar]
- 31.Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, Lopez OL, DeKosky ST. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. The Journal of neuropsychiatry and clinical neurosciences. 2000;12:233–239. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- 32.Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, van Hilten JJ, LaPelle N. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Movement disorders : official journal of the Movement Disorder Society. 2008;23:2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 33.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. The British journal of psychiatry : the journal of mental science. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 34.Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a Frontal Assessment Battery at bedside. Neurology. 2000;55:1621–1626. doi: 10.1212/wnl.55.11.1621. [DOI] [PubMed] [Google Scholar]
- 35.Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease. Neurobiology of aging. 1997;18:S1–2. [PubMed] [Google Scholar]
- 36.Bigio EH, Mishra M, Hatanpaa KJ, White CL, 3rd, Johnson N, Rademaker A, Weitner BB, Deng HX, Dubner SD, Weintraub S, Mesulam M. TDP-43 pathology in primary progressive aphasia and frontotemporal dementia with pathologic Alzheimer disease. Acta neuropathologica. 2010;120:43–54. doi: 10.1007/s00401-010-0681-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Josephs KA, Whitwell JL, Dickson DW, Boeve BF, Knopman DS, Petersen RC, Parisi JE, Jack CR., Jr Voxel-based morphometry in autopsy proven PSP and CBD. Neurobiology of aging. 2008;29:280–289. doi: 10.1016/j.neurobiolaging.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Migliaccio R, Agosta F, Rascovsky K, Karydas A, Bonasera S, Rabinovici GD, Miller BL, Gorno-Tempini ML. Clinical syndromes associated with posterior atrophy: early age at onset AD spectrum. Neurology. 2009;73:1571–1578. doi: 10.1212/WNL.0b013e3181c0d427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leyton CE, Hodges JR, McLean CA, Kril JJ, Piguet O, Ballard KJ. Is the logopenic-variant of primary progressive aphasia a unitary disorder? Cortex; a journal devoted to the study of the nervous system and behavior. 2015;67:122–133. doi: 10.1016/j.cortex.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 40.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain : a journal of neurology. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 41.Jones DT, Vemuri P, Murphy MC, Gunter JL, Senjem ML, Machulda MM, Przybelski SA, Gregg BE, Kantarci K, Knopman DS, Boeve BF, Petersen RC, Jack CR., Jr Non-stationarity in the "resting brain's" modular architecture. PloS one. 2012;7:e39731. doi: 10.1371/journal.pone.0039731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koenigs M, Barbey AK, Postle BR, Grafman J. Superior parietal cortex is critical for the manipulation of information in working memory. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:14980–14986. doi: 10.1523/JNEUROSCI.3706-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turken AU, Dronkers NF. The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analyses. Frontiers in systems neuroscience. 2011;5:1. doi: 10.3389/fnsys.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whitwell JL, Jones DT, Duffy JR, Strand EA, Machulda MM, Przybelski SA, Vemuri P, Gregg BE, Gunter JL, Senjem ML, Petersen RC, Jack CR, Jr, Josephs KA. Working memory and language network dysfunctions in logopenic aphasia: a task-free fMRI comparison with Alzheimer's dementia. Neurobiology of aging. 2015;36:1245–1252. doi: 10.1016/j.neurobiolaging.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ossenkoppele R, Pijnenburg YA, Perry DC, Cohn-Sheehy BI, Scheltens NM, Vogel JW, Kramer JH, van der Vlies AE, Joie RL, Rosen HJ, van der Flier WM, Grinberg LT, Rozemuller AJ, Huang EJ, van Berckel BN, Miller BL, Barkhof F, Jagust WJ, Scheltens P, Seeley WW, Rabinovici GD. The behavioural/dysexecutive variant of Alzheimer's disease: clinical, neuroimaging and pathological features. Brain : a journal of neurology. 2015;138:2732–2749. doi: 10.1093/brain/awv191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leyton CE, Hsieh S, Mioshi E, Hodges JR. Cognitive decline in logopenic aphasia: more than losing words. Neurology. 2013;80:897–903. doi: 10.1212/WNL.0b013e318285c15b. [DOI] [PubMed] [Google Scholar]
- 47.Zilli EM, Heilman KM. Spatial neglect in a patient with logopenic progressive aphasia. Neurocase. 2016;22:30–39. doi: 10.1080/13554794.2015.1031254. [DOI] [PubMed] [Google Scholar]
- 48.Teichmann M, Migliaccio R, Kas A, Dubois B. Logopenic progressive aphasia beyond Alzheimer's--an evolution towards dementia with Lewy bodies. Journal of neurology, neurosurgery, and psychiatry. 2013;84:113–114. doi: 10.1136/jnnp-2012-302638. [DOI] [PubMed] [Google Scholar]
- 49.Caselli RJ, Beach TG, Sue LI, Connor DJ, Sabbagh MN. Progressive aphasia with Lewy bodies. Dementia and geriatric cognitive disorders. 2002;14:55–58. doi: 10.1159/000064925. [DOI] [PubMed] [Google Scholar]
- 50.Whitwell JL, Lowe VJ, Duffy JR, Strand EA, Machulda MM, Kantarci K, Wille SM, Senjem ML, Murphy MC, Gunter JL, Jack CR, Jr, Josephs KA. Elevated occipital beta-amyloid deposition is associated with widespread cognitive impairment in logopenic progressive aphasia. Journal of neurology, neurosurgery, and psychiatry. 2013;84:1357–1364. doi: 10.1136/jnnp-2013-305628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wicklund MR, Duffy JR, Strand EA, Machulda MM, Whitwell JL, Josephs KA. Quantitative application of the primary progressive aphasia consensus criteria. Neurology. 2014;82:1119–1126. doi: 10.1212/WNL.0000000000000261. [DOI] [PMC free article] [PubMed] [Google Scholar]