Abstract
Overexpression of complementary DNA (cDNA) represents the most commonly used gain-of-function approach for interrogating gene functions and for manipulating biological traits. However, this approach is challenging and inefficient for multigene expression due to increased labor for cloning, limited vector capacity, requirement of multiple promoters and terminators, and variable transgene expression levels. Synthetic transcriptional activators provide a promising alternative strategy for gene activation by tethering an autonomous transcription activation domain (TAD) to an intended gene promoter at endogenous genomic locus through a programmable DNA-binding module. Among the known custom DNA-binding modules, the nuclease-dead Streptococcus pyogenes Cas9 (dCas9) protein, which recognizes a specific DNA target through base pairing between a synthetic guide RNA (sgRNA) and DNA, outperforms zinc finger proteins (ZFPs) and transcription activator-like effectors (TALEs), both of which target through protein-DNA interactions1. Recently, three potent dCas9-based transcriptional activation systems, namely VPR, SAM, and Suntag, have been developed for animal cells2–6. However, an efficient dCas9-based transcriptional activation platform is still lacking for plant cells7–9. Here, we developed a new potent dCas9-TAD named dCas9-TV through plant cell-based screens, which confers far stronger transcriptional activation of a single or multiple target genes than the routinely used dCas9-VP64 activator in both plant and mammalian cells.
Among synthetic gene activators, dCas9-TADs potentially offer unparalleled simplicity and multiplexability compared to ZFP-TADs and TALE-TADs because sgRNAs can be easily modified to achieve new targeting specificities and dCas9 guided by multiple sgRNAs can simultaneously bind to several different target loci10. However, a dCas9 fusion with VP64, a frequently used TAD11, only weakly activates target gene using a single sgRNA in plant and mammalian cells7–9,12–15. Using Arabidopsis protoplast-based promoter-luciferase (LUC) assays, we confirmed that dCas9-VP64 with a single sgRNA only weakly (maximally 2.4-fold) or ineffectively activated target genes (Supplementary Results; Supplementary Figs. 1 and 2). Interestingly, when the target sequence lacks a 5′ G, an extra G appended to the 5′ end of the sgRNA was found to enhance the promoter activation (Supplementary Fig. 1), presumably by promoting the transcription initiation of the sgRNA by the U6 promoter. Therefore, we routinely add a G to the 5′ end of sgRNAs when the target sequences start with a non-G nucleotide.
Although multiple sgRNAs tiling the proximal promoter of the target gene can synergistically boost the dCas9-VP64-mediated gene activation7–9,12–15, this strategy reduces the scalability of the system2 and may increase the risk of dCas9-mediated transcriptional perturbation at off-target non-promoter loci15–17. Therefore, we sought to devise and screen for an improved dCas9-TAD (Fig. 1a) that would allow potent transcriptional activation of target genes using only a single sgRNA, thus maximizing the system’s scalability. As the first step, we modified dCas9-VP64 by adding additional VP64 moieties (Supplementary Sequences). By using a WRKY30 promoter-LUC reporter and a pre-screened optimal sgRNA (WRKY30#2, Supplementary Fig. 2a), we observed that dCas9-VP128 outperformed dCas9-VP64 by increasing the LUC activation from 2-fold to more than 5-fold relative to the basal level (Fig. 1b). In contrast, dCas9-VP192 and dCas9-VP256 exhibited a sharp decrease of protein production and severe protein degradation, leading to an overall reduced LUC activation (Fig. 1b). The fact that VP192 and VP256 correspond to 12 and 16 repeats of the VP16 motif11, respectively, suggests that their expression and stability issues may be incurred by highly repetitive sequences.
Figure 1.
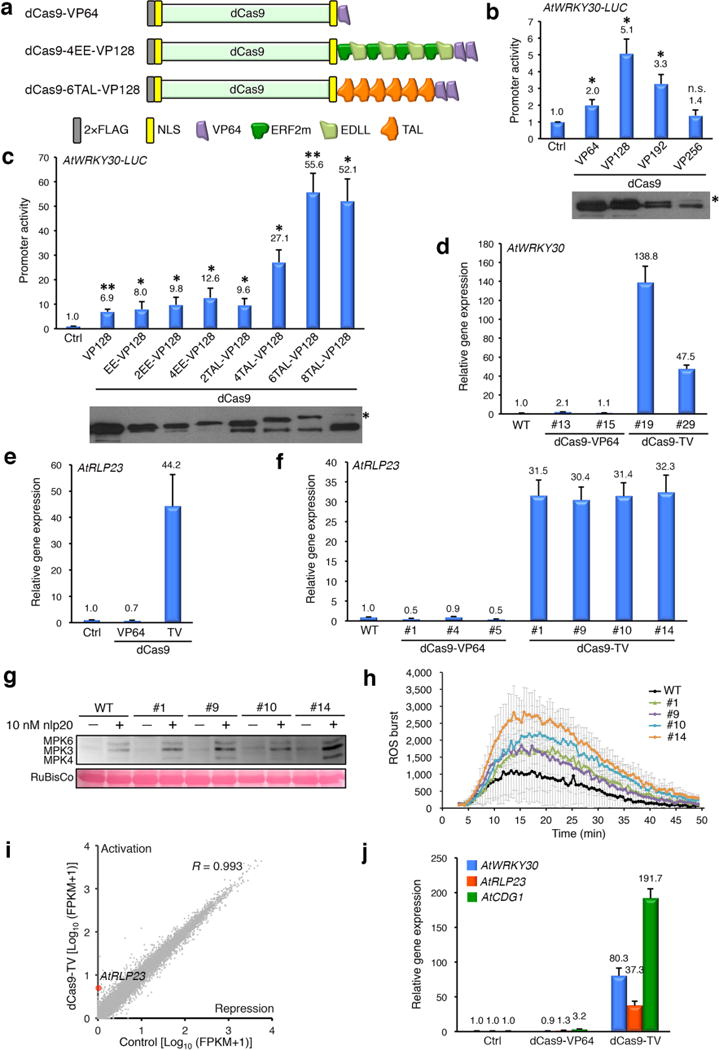
dCas9-TV-mediated gene activation in Arabidopsis. (a) Diagram of three representative dCas9 gene activators tested in this study. (b) dCas9-VP128 exhibits the highest activity among dCas9-(VP64)n (n=1-4) activators in Arabidopsis protoplasts. dCas9-VP256 is marked by an asterisk in the blot. (c) dCas9-6TAL-VP128 (dCas9-TV) exhibits the highest activity among all screened dCas9 activators in Arabidopsis protoplasts. dCas9-8TAL-VP128 is marked by an asterisk in the blot. (d) Representative transgenic Arabidopsis T1 plants co-expressing dCas9-TV and sgRNA-WRKY30 show strong induction of endogenous WRKY30. (e, f) Arabidopsis protoplasts (e) or representative transgenic T2 plants (f) co-expressing dCas9-TV and sgRNA-RLP23 show robust induction of endogenous RLP23. (g, h) Arabidopsis transgenic T2 plants with RLP23 induction accordingly exhibit enhanced MAPK activation (g) and ROS production (h) in response to the pathogen elicitor nlp20. RuBisCo in (g) indicates equal protein loading. (i) Evaluation of dCas9-TV specificity by RNA-seq. Arabidopsis protoplasts expressing or not expressing dCas9-TV and sgRNA-RLP23 exhibit very similar transcriptome profiles. R indicates Pearson’s correlation coefficient. RLP23 is marked by a red dot close to the y-axis as a highly activated gene. (j) Co-expression of dCas9-TV and 3 sgRNAs leads to simultaneous induction of WRKY30, RLP23 and CDG1 in Arabidopsis protoplasts. Empty vectors were used in Control (Ctrl) samples to replace constructs expressing dCas9 activator and sgRNAs. Gene activation was determined by promoter-LUC assays (b, c) or RT-qPCR (d-f, j), and data are shown as mean (indicated with a number on the top) ± sd (n=3). *P < 0.05, **P < 0.01 (student’s t-test). n.s., not significant.
As a second step to enhance the activity of dCas9-VP128, we incorporated other sequence-unrelated portable TADs, which included plant-specific EDLL18 and ERF2m19 (modified ERF2) motifs as well as a TAD from Xanthamonas TALEs20. To minimize potential interference between different TADs, flexible GGSGG linkers were used as spacers between TADs (Supplementary Sequences). We observed that the combination of VP128 with up to four copies of tandem ERF2m-EDLL motifs (thereafter simplified as EE) activated LUC expression by 12.6-fold relative to the basal level (Fig. 1c), and the combination of VP128 with up to six copies of the TALE TAD motif (thereafter simplified as TAL) exhibited a maximal activation of LUC expression by more than 55-fold (Fig. 1c). Of note, further insertion of TAL motifs triggered severe protein degradation presumably due to the high sequence repetition, resulting in an overall decreased LUC induction (Fig. 1c). Therefore, the cell-based screens identified dCas9-6TAL-VP128 as a potentially strong transcription activator, which was renamed dCas9-TV for simplicity. In addition, when we used an Arabidopsis U6-26 rather than U6-1 promoter to express the sgRNA-WRKY30#2, we detected a further boost of LUC expression to 201-fold relative to the basal level (Supplementary Fig. 3). Therefore, the U6-26 promoter was used for sgRNA expression in the following experiments in Arabidopsis. To validate the data from cell-based assays, we generated transgenic Arabidopsis plants to co-express the sgRNA-WRKY30#2 along with dCas9-TV or dCas9-VP64. As quantified by RT-qPCR, the expression of endogenous WRKY30 was strongly induced by dCas9-TV by 48- and 139-fold, respectively, in two independent T1 transgenic lines (Fig. 1d), whereas negligible WRKY30 activation by dCas9-VP64 was observed in representative transgenic lines (Fig. 1d).
No Cpf1-derived gene activator has so far been explored in plant or mammalian cells. Therefore, we also evaluated the transcriptional activation activity of a nuclease-dead Acidaminococcus sp. BV3L6 Cpf1 (dCpf1)-TV fusion (Supplementary Results), since this dCpf1 has been recently reported to outperform its homologs in targeted gene suppression21. When dCpf1-TV and dCas9-TV were designed to target overlapping or proximal sequences within the WRKY30 promoter, we only detected minimal activation (maximally 4.7-fold) of the promoter by dCpf1-TV in contrast to a strong activation by dCas9-TV (about 215-fold, Supplementary Fig. 4). Although we cannot exclude the possibility that other dCpf1 homolog may be suitable for targeted transcriptional activation, we focused the following study only on dCas9-TV.
To generalize our finding about the potency of dCas9-TV, we pre-screened a single sgRNA to target each promoter of six more Arabidopsis genes (Supplementary Fig. 5), and then compared the transcriptional activation efficiencies of dCas9-TV and dCas9-VP64 side by side using cell-based promoter-LUC assays. Induction of LUC expression by dCas9-TV was detected for all six genes from 1.6- to 92-fold, whereas dCas9-VP64 only conferred marginal transcriptional upregulation or even suppression of target gene expression (Supplementary Fig. 6). Interestingly, the magnitudes of gene activation by dCas9-TV were roughly negatively correlated with the basal expression levels of these genes, as genes with weak basal expression (Supplementary Fig. 7), such as RLP23, WRKY30 and CDG1, tended to be better induced than those already under vigorous transcription, such as FLS2, EFR, AVP1 and HDC1. Similar trends have also been documented for other strong dCas9 activators (e.g., VPR and SAM) in mammalian cells2,5,6, indicating the generality of this phenomenon. We further validated the contrasting transcriptional activation efficiencies between dCas9-TV and dCas9-VP64 by RT-qPCR on the endogenous expression of RLP23. While dCas9-TV activated RLP23 expression by 44-fold in protoplasts (Fig. 1e), dCas9-VP64 slightly suppressed RLP23 expression (Fig. 1e). Moreover, we generated transgenic Arabidopsis plants co-expressing sgRNA-RLP23 and dCas9-TV or dCas9-VP64. As quantified by RT-qPCR, multiple T2 transgenic lines co-expressing the sgRNA and dCas9-TV exhibited robust induction of endogenous RLP23 by over 30-fold (Fig. 1f). In contrast, several T2 transgenic lines co-expressing the sgRNA and dCas9-VP64 showed compromised RLP23 expression (Fig. 1f), consistent with what has been observed in protoplasts (Fig. 1e).
Arabidopsis RLP23 encodes a cell-surface immune receptor that perceives nlp20, a conserved eliciting peptide across bacteria, fungi, and oomycetes22. Overexpression of RLP23 could sensitize plant cells to a low concentration of nlp20 (Supplementary Fig. 8), which otherwise cannot be efficiently detected due to the low basal expression level of RLP23 (Supplementary Fig. 7). We found that those T2 transgenic plants with dCas9-TV-mediated RLP23 activation all exhibited enhanced immune responses to nlp20, as exemplified by the elevated MAPK activation (Fig. 1g) and reactive oxygen species (ROS) burst (Fig. 1h). These results highlighted the competence of the dCas9-TV/single sgRNA system as a tool to rewire cellular responses.
A critical concern for a targeted gene activation system is its specificity. Because RLP23 encodes an Arabidopsis immune receptor that is only activated upon binding to its ligand nlp20, we reasoned that dCas9-TV-mediated transcriptional upregulation of RLP23 in cells without nlp20 stimulation should provoke minimal secondary transcriptional perturbation to confound the specificity analysis. Therefore, we assessed the genome-wide transcriptional activation specificity of RLP23 by dCas9-TV in protoplasts using RNA-sequencing (RNA-seq). Very similar transcriptome profiles were detected between samples expressing or not expressing dCas9-TV and sgRNA-RLP23 (Pearson’s correlation coefficient 0.993; Fig. 1i), suggesting that gene expression at the whole genome level is not broadly influenced by dCas9-TV. In addition, none of the six Arabidopsis genes with potential off-target binding sites (Supplementary Table 1) showed altered gene expression. However, as revealed by RNA-seq (Supplementary Database), whereas RLP23 was induced by dCas9-TV/sgRNA-RLP23 by 129-fold as expected, five other genes also exhibited increased expression (> 6-fold), which might be due to the secondary effect of RLP23 upregulation since no predictable binding sites of sgRNA-RLP23 with up to five mismatches could be identified within the 2 kb promoters of these genes (data not shown).
We also evaluated the multiplexability of our transcriptional activation system in cell-based assays by co-expressing dCas9-TV with three sgRNAs targeting to WRKY30, RLP23 and CDG1. As quantified by RT-qPCR, the endogenous gene expression was induced by 80-fold for WRKY30, 37-fold for RLP23, and 192-fold for CDG1 (Fig. 1j). These results demonstrated the robust multiplexability of the dCas9-TV system.
To further strengthen the dCas9-TV-based transcriptional activation, we adopted a modified sgRNA (thereafter referred to as the SAM sgRNA) that is able to recruit a chimeric TAD (Fig. 2a) consisting of the MS2 phage coat protein (MCP) and 4EE-VP128 or TV through internally embedded MCP-binding aptamers2. When using the SAM sgRNA-WRKY30#2 to target the WRKY30 promoter, dCas9-VP64 paired with either MCP-4EE-VP128 or MCP-TV resulted in much stronger induction of WRKY30-LUC than the combination of dCas9-VP64 and a normal sgRNA (Supplementary Fig. 9), suggesting that MCP-4EE-VP128 and MCP-TV are both active transcription activators upon association with the target promoter. Notably, the combination of dCas9-TV and a normal sgRNA was still more active than that of dCas9-VP64, SAM sgRNA and MCP-TV (Supplementary Fig. 9). We reasoned that the combination of dCas9-TV with the SAM sgRNA and MCP-4EE-VP128 or MCP-TV may lead to additive or synergistic activation of the target gene. However, this three-component activation system failed to enhance the WRKY30-LUC induction and even led to decreased RLP23-LUC induction compared to the two-component system of dCas9-TV and normal sgRNAs (Fig. 2b; Supplementary Fig. 10). These results suggested that MCP-TADs piggybacked on the SAM sgRNA are unable to strengthen the dCas9-TV-mediated transcriptional activation in plant cells, presumably due to the saturation of local transcriptional machineries or steric incompatibility between MCP-TAD and dCas9-TV. Interestingly, a recent similar endeavor to upgrade the transcriptional activation of target gene by combining the robust dCas9-VPR activator with the SAM-based transcriptional activation strategy was also unsuccessful in mammalian cells6.
Figure 2.
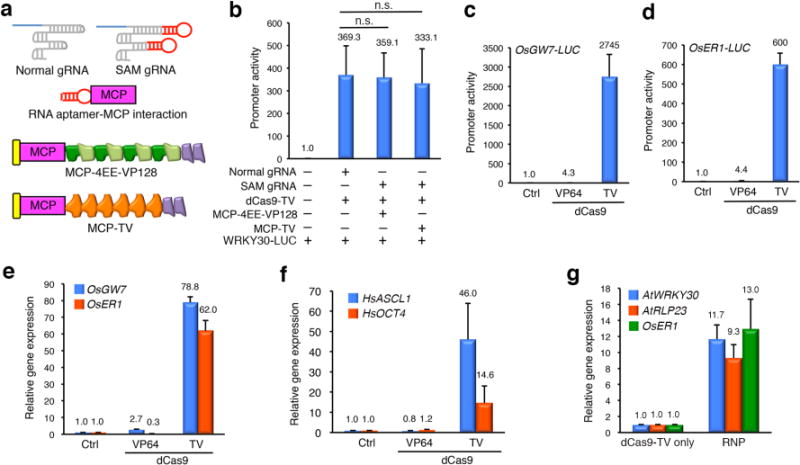
Modified strategies of dCas9-TV-mediated gene activation in Arabidopsis and other cells. (a) Diagram of the SAM sgRNA, MCP activators and their interactions. (b) SAM sgRNA-associated MCP activators fail to strengthen dCas9-TV-mediated WRKY30 activation in Arabidopsis protoplasts. (c, d) Rice protoplasts co-expressing dCas9-TV and sgRNAs targeting to GW7 (c) or ER1 (d) exhibit strong target gene induction. (e) Co-expression of dCas9-TV and 2 sgRNAs leads to simultaneous induction of GW7 and ER1 in rice protoplasts. (f) HEK 293T cells co-expressing dCas9-TV and sgRNAs targeting to ASCL1 or OCT4 exhibit clear induction of these genes. (g) dCas9-TV ribonucleoprotein (RNP)-mediated activation of WRKY30 and RLP23 in Arabidopsis protoplasts and ER1 in rice protoplasts. Empty vectors were used in Control (Ctrl) samples to replace constructs expressing dCas9 activator and sgRNAs. Gene activation was determined by promoter-LUC assays (b-d) or RT-qPCR (e-g), and data are shown as mean (indicated by a number on the top) ± sd (n=3). n.s., not significant.
In addition to Arabidopsis cells, which represent cells of dicotyledon species, we also investigated the transcriptional activation induced by dCas9-TV in cells of monocot species, such as rice. We targeted the proximal GW7 or ER1 promoter in rice cells using a single pre-screened sgRNA expressed by the rice U6a promoter (Supplementary Fig. 11), and found that dCas9-TV induced the target promoter activity two orders of magnitude stronger than dCas9-VP64 (Figs. 2c and 2d). Moreover, concurrent expression of both sgRNAs along with dCas9-TV in rice cells led to simultaneous induction of endogenous GW7 by 79-fold and ER1 by 62-fold (Fig. 2e), as quantified by RT-qPCR. By contrast, dCas9-VP64 only modestly activated GW7 by 2.7-fold and suppressed ER1 expression (Fig. 2e). These results suggested that dCas9-TV is broadly effective for targeted gene activation in various types of plant cells. Furthermore, since TAL is known as an active TAD in yeast20, we speculated that the dCas9-TV-mediated transcriptional activation may also work for mammalian cells, which are distantly related to Arabidopsis and rice cells. Indeed, when we targeted ASCL1 or OCT4 in human embryonic kidney (HEK) 293T cells with a single sgRNA2,23, we detected induction of endogenous ASCL1 by 46-fold and OCT4 by 14.6-fold, whereas dCas9-VP64 failed to enhance the expression of either gene (Fig. 2f).
Lastly, we explored the possibility of targeted gene activation in plant cells by dCas9-TV ribonucleoprotein (RNP) complexes. To this end, purified E. coli-expressed dCas9-TV and in vitro transcribed sgRNA (Supplementary Fig. 12) targeting to Arabidopsis WRKY30 or RLP23 or rice ER1 were pre-assembled into RNP complexes and then delivered into Arabidopsis or rice protoplasts through polyethylene glycol (PEG)-mediated transfection. As quantified by RT-qPCR, induction of WRKY30 by 11.7-fold, RLP23 by 9.3-fold and ER1 by 13-fold was detected at 5 h after transfection (Fig. 2g). The proof-of-concept of dCas9-TV RNP-mediated transcriptional activation promises a possible DNA-free strategy to achieve short bursts of gene activation with minimal cloning efforts, and leverages the easy availability of recombinant dCas9-TV proteins, synthetic sgRNAs and plant protoplasts24.
In summary, dCas9-TV exhibits potent transcriptional activation activity in plant and mammalian cells, which can be utilized to efficiently and specifically activate single or multiple genes when these genes have modest basal expression levels. We envision that dCas9-TV will be particularly useful in basic and applied plant research, including but not limited to: (i) when combined with a genome-scale sgRNA library, dCas9-TV can be used in protoplast-based gain-of-function screens for regulatory genes in a signaling pathway of interest using a promoter-LUC/GFP reporter as a signaling readout; (ii) it is useful for generating a synthetic plant transcriptome25 to study the functions of transcription regulators; (iii) it can be applied in metabolic engineering by multiplex activation of lowly expressed enzymes throughout a metabolic pathway to increase the production of valuable metabolites; (iv) it can also be applied to upregulate crop genes conferring beneficial traits such as biotic and abiotic resistances. Future efforts will be needed to improve the rational design of sgRNAs, as different sgRNAs targeting the same promoter can lead to variable transcriptional regulation by the same dCas9 activators2,7,9,12–17 (Supplementary Figs. 2, 5 and 11), and a straightforward correlation between the parameters of sgRNAs (e.g., GC content or target site location, Supplementary Table 2) and their gene activation efficiencies has not been established. Meanwhile, since dCas9-TV is prone to protein degradation due to its high sequence repetition, future studies will address the possibility whether genetic fusion of XTEN26, 30Kc19α27 or other protein-stabilizing polypeptides to the N- or C-terminus of dCas9-TV may mitigate its degradation issue and further potentiate the dCas9-TV-mediated transcriptional activation.
METHODS
Methods and any associated references are available in the Supplementary Information in the online version of the paper.
Supplementary Material
Acknowledgments
We thank Frederick Ausubel and Zhenyu Cheng for critical reading of this manuscript. This work was supported by the National Natural Science Foundation of China grant 31522006 and start-up funds from China’s Thousand Young Talents Program to J.F.L. and the NIH grant R01GM70567 to J.S. This work was partially supported by the Guangzhou Science and Technology Project grant 201605030012.
Footnotes
Note: Any Supplementary Information and Source Data files are available in the online version of the paper.
AUTHOR CONTRIBUTIONS
J.F.L. and J.S. conceived the study. J.F.L. designed the experiments and supervised the study. D.Z. conducted the protoplast-based screens of dCas9 activators. Z.L. conducted other dCas9-TV experiments in Arabidopsis protoplasts and transgenic plants. X.X. and Z.L. conducted the dCas9-TV experiments in rice protoplasts. B.Y. conducted the dCas9-TV experiments in human HEK293T cells. Z.L., X.X. and W.X. performed the RNP-mediated gene activation. J.F.L. wrote the manuscript with input from J.S. and all other authors.
COMPETING FINANCIAL INTERESTS
The authors have filed a patent application based on some results reported in this paper.
References
- 1.Qi LS, et al. Cell. 2013;152:1173–1183. doi: 10.1016/j.cell.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Konermann S, et al. Nature. 2015;517:583–588. doi: 10.1038/nature14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanenbaum ME, et al. Cell. 2014;159:635–646. doi: 10.1016/j.cell.2014.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilbert LA, et al. Cell. 2014;159:647–661. doi: 10.1016/j.cell.2014.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chavez A, et al. Nat Methods. 2015;12:2–6. [Google Scholar]
- 6.Chavez A, et al. Nat Methods. 2016;13:563–567. doi: 10.1038/nmeth.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piatek A, et al. Plant Biotechnol J. 2015;13:578–589. doi: 10.1111/pbi.12284. [DOI] [PubMed] [Google Scholar]
- 8.Lowder LG, et al. Plant Physiol. 2015;169:971–985. doi: 10.1104/pp.15.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vazquez-Vilar M, et al. Plant Methods. 2016;12:10. doi: 10.1186/s13007-016-0101-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Didovyk A, Borek B, Tsimring L, Hasty J. Curr Opin Biotechnol. 2016;40:177–184. doi: 10.1016/j.copbio.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beerli RR, Segal DJ, Dreier B, Barbas CF., III Proc Natl Acad Sci USA. 1998;95:14628–14633. doi: 10.1073/pnas.95.25.14628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mali P, et al. Nat Biotechnol. 2013;31:833–838. doi: 10.1038/nbt.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perez-Pinera P, et al. Nat Methods. 2013;10:973–976. doi: 10.1038/nmeth.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maeder ML, et al. Nat Methods. 2013;10:977–979. doi: 10.1038/nmeth.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng AW, et al. Cell Res. 2013;23:1163–1171. doi: 10.1038/cr.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farzadfard F, Perli SD, Lu TK. ACS Synth Biol. 2013;2:604–613. doi: 10.1021/sb400081r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braun CJ, et al. Proc Natl Acad Sci USA. 2016;113:e3892–3900. doi: 10.1073/pnas.1600582113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tiwari SB, et al. Plant J. 2012;70:855–865. doi: 10.1111/j.1365-313X.2012.04935.x. [DOI] [PubMed] [Google Scholar]
- 19.Li J, et al. Plant Biotechnol J. 2013;11:671–680. doi: 10.1111/pbi.12057. [DOI] [PubMed] [Google Scholar]
- 20.Zhu W, et al. Plant Cell. 1999;11:1665–1674. doi: 10.1105/tpc.11.9.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang X, et al. Nat Plants. 2017;3:17018. doi: 10.1038/nplants.2017.18. [DOI] [PubMed] [Google Scholar]
- 22.Zipfel C, Oldroyd GED. Nature. 2017;543:328–336. doi: 10.1038/nature22009. [DOI] [PubMed] [Google Scholar]
- 23.Hu J, et al. Nucleic Acids Res. 2014;42:4375–4390. doi: 10.1093/nar/gku109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li JF, Zhang D, Sheen J. Nat Protoc. 2015;9:939–949. doi: 10.1038/nprot.2014.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puchta H. Plant J. 2016;87:5–15. doi: 10.1111/tpj.13100. [DOI] [PubMed] [Google Scholar]
- 26.Schellenberger V, et al. Nat Biotechnol. 2009;27:1186–1190. doi: 10.1038/nbt.1588. [DOI] [PubMed] [Google Scholar]
- 27.Ryu J, et al. Biotechnol J. 2016;11:1443–1451. doi: 10.1002/biot.201600040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


