Abstract
Among urban, primarily African American pregnant women, 74% were identified with Nugent-score BV. All BV-associated bacteria were more prevalent among women with Nugent-score BV. BVAB3 had the highest positive predictive value while Gardnerella vaginalis and Atopobium spp. had the highest sensitivity. Atopobium spp. levels had the most significant AUC.
Keywords: Nugent-score Bacterial vaginosis, Atopobium spp, BVAB3, Gardnerella vaginalis, pregnancy
Understanding the relationship between BV and reproductive health has increasingly become important, particularly with the advent of numerous methods to measure and quantify BV 1,2,3. BV is most commonly assessed using two diagnostic approaches: the Amsel criteria or the Nugent criteria4, 5. The Amsel criteria are based on reported symptomatology and the Nugent criteria are based on bacterial morphology. Molecular assays using quantitative polymerase chain reaction (qPCR) have also been developed to assess the vaginal level of various hard-to culture BV-associated bacteria among pregnant and non-pregnant women 1,2,6 Numerous reports have documented the ubiquity of BV among reproductive age women and have consistently concluded that the highest rates of BV occur among urban, primarily African-American women 7,8. Reports have linked BV to a two-fold increased risk of spontaneous preterm birth (SPTB) but many BV positive women deliver at term 9–13. Thus, the identification of the particular BV-associated bacteria most linked to BV could dramatically improve the efficacy of BV treatment, the development of prenatal screening methods to identify high risk pregnant women, and the assessment of more effective treatment options to reduce SPTB among high risk subgroups of pregnant women 14.
In this assessment, six hundred and eighty two pregnant women with singleton pregnancies less than 16 weeks gestation (mean/std: 10±6 weeks) and contributing multiple vaginal swabs were included. This study was conducted in accordance with Temple University’s Institutional Review Board. Woman self-collected two, dry epicenter vaginal swabs and self-collection has been shown to have excellent agreement to assess Nugent-score BV and to quantify BV-associated bacteria 15–16. Leptotrichia/Sneathia species, Megasphaera-like species, Gardnerella vaginalis, Mobiluncus spp., Atopobium spp. and Bacterial Vaginosis-Associated Bacterium (BVAB) 1, BVAB2 and BVAB3 were measured using rRNA gene quantitative PCR assay, as described elsewhere1,6. For the qPCR levels, a below the detectable threshold level was noted for values of 125, 250 or 500 gene copies per swab, depending on assay, and a value of 250 was assigned as non-detectable when assessing means. For the mean comparisons, the qPCR values were transformed to log base 10.
Vaginal fluid from the second swab was used for BV identification using the Nugent criteria 4. We compared women with Nugent-score BV, defined as a Nugent score ≥7, to women with intermediate flora (Nugent score of 4–6) and women with normal flora (Nugent score less than 4). Given the low prevalence of BV negative women, we grouped women with intermediate and normal flora.
Individual receiver operator characteristic (ROC) curves were created using the continuous log levels of Atopobium spp., BVAB3, and Gardnerella vaginalis to demonstrate the relationship between sensitivity and specificity over differing levels of Nugent-score BV. Cutoffs for Atopobium spp., BVAB3, and Gardnerella vaginalis were also generated by selecting the log level of each bacterium which resulted in high sensitivity and reasonable specificity to detect Nugent-score BV. Second ROC curves were developed using these dichotomized cutoff’s to understand the utility of the dichotomous grouping in identifying the Nugent-score BV. SPSS statistical package version 19.0 and SAS version 9.2 were used for the analysis and a 2-sided p value of <0.05 was considered statistically significant.
Seventy-four percent of women had Nugent-score BV, 17% had intermediate flora, and 9% had normal flora. As expected, women with Nugent-score BV compared to women with normal/intermediate flora were more likely to be African American (67.2% vs. 52.0%, p-value<0.001), report current alcohol use (26.6% vs. 17.9, p-value=0.02), report a new sexual partner during the pregnancy (3.0% vs. 0.6%. p-value=0.06), and report a higher mean number of male sexual partners in the past year (1.9 vs. 1.6, p-value=0.08). Mean perceived stress scores were not related to Nugent-score BV (5.3 vs. 5.3; p-value=0.96).
Each of the eight BV-associated bacteria were significantly more prevalent among pregnant women with Nugent-score BV and the mean levels of each of the BV-associated bacteria were significantly higher among women with Nugent-score BV. In the final multivariate model predicting Nugent-score BV, which included all eight BV-associated microbiota, race, alcohol use and new sexual partner, the presence of BVAB3 (aOR=8.17, 95% CI: 3.82–17.48) or the presence of Atopobium spp. (aOR=2.63, 95%CI: 1.40–4.90) remained significantly predictive of Nugent-score BV. A significant interaction predicting Nugent-score BV was found among women positive for both Gardnerella vaginalis and Atopobium spp (aOR=11.32, 95% CI: 7.18–17.84).
The sensitivity and specificity of the BV-associated bacteria over differing levels of Nugent-score BV were similar to the findings among non-pregnant women6. BVAB3 had the highest PPV (0.97, 95% CI: 0.94–0.98) and high specificity (0.94, 95% CI: 0.90–0.97) for Nugent-score BV. The presence of Gardnerella vaginalis or the presence of Atopobium spp. had the highest sensitivity for Nugent-score BV (0.98, 95% CI: 0.96–0.99 and 0.93, 95% CI: 0.90–0.95; respectively). In this sample, in contrast to findings by Menard et al, the presence of both Atopobium spp. and Gardnerella vaginalis did not dramatically improve the accuracy of identifying Nugent-score BV (sensitivity: 0.93, specificity 0.48, PPV: 0.83 and NPV: 0.70)17.
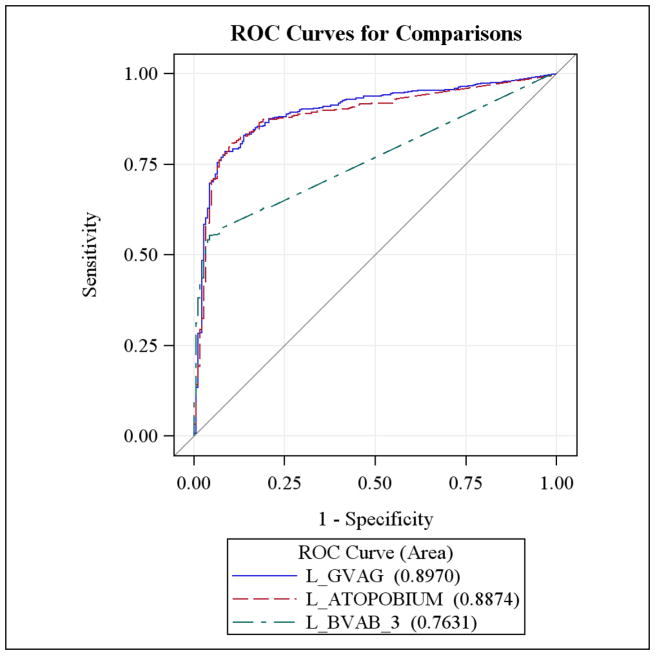
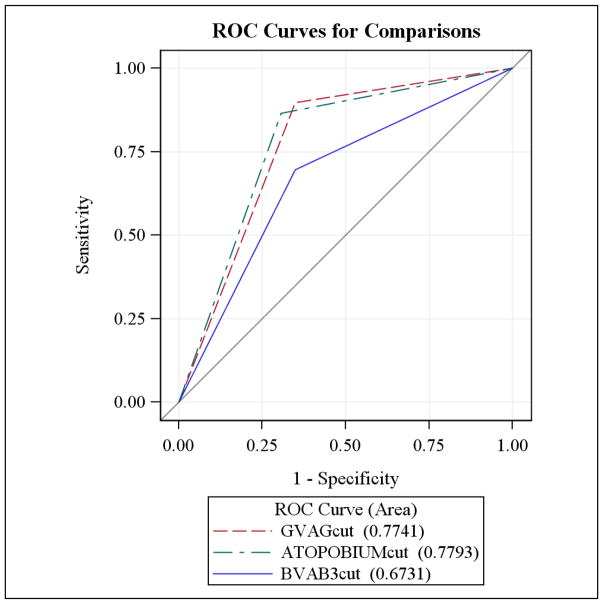
The continuous level of Atopobium spp. and Gardnerella vaginalis had the highest area under the curve (AUC) for Nugent-score BV (AUC=0.89, 95% CI: 0.86–0.91 and AUC = 0.89, 95% CI: 0.87–0.92, respectively). The continuous level of BVAB3 had a moderate AUC (AUC = 0.76, 95% CI: 0.73–0.79). Grouping Atopobium spp. at or above the cutoff value continued to yield the highest sensitivity for Nugent-score BV (AUC = 0.78, 95% CI: 0.75–0.81) (Figure 1). Using the cutoffs for Gardnerella vaginalis and BVAB3, the AUC were 0.77 (95% CI: 0.74–0.81) and 0.67 (95% CI: 0.64 – 0.71), respectively.
Figure 1.
ROC curves using the continuous levels of BVAB3, Gardnerella vaginalis and Atopobium spp.
In this assessment, we documented the high level of Nugent-score BV among urban, primarily African American pregnant women and, similar to other research, we reported both a high prevalence of Atopobium spp. among pregnant women and confirmed that high Atopobium spp. levels were linked to Nugent-score BV. Menard and others have also found a high prevalence of Atopobium spp. among primarily Caucasian European pregnant women and have identified Atopobium spp. as highly sensitive and specific to Nugent-score BV17,18 In addition, a recent assessment found excellent sensitivity (0.95), specificity (0.99) and high PPV and NPV values for BV detection (0.95 and 0.99, respectively) among pregnant women with high levels of both Atopobium spp. (≥ 108 copies/mL) and Gardnerella vaginalis (≥ 109 copies/mL)17. We found high agreement between Nugent-score BV and high levels of Atopobium spp. but we did not find an improvement in the prediction of BV among pregnant women who were positive for both Atopobium spp. and Gardnerella vaginalis. Menard et al also reported that pregnant women presenting with signs and symptoms of preterm labor (PTL) and having elevated levels of both Atopobium vaginae and Gardnerella vaginalis were at a higher risk of SPTB19. Future research should determine the importance of high levels of Atopobium vaginae and the combination of high levels of Atopobium vaginae and Gardnerella vaginalis and subsequent risk of SPTB among African American women and pregnant women with and without PTL.
When interpreting these data, it is important to recognize several limitations. First, this is a sample of urban, primarily African American pregnant women which may not be generalizable to all pregnant women. In fact, some of the differences between this work and Menard et al may be due to the higher prevalence of Nugent-score BV in our sample of urban women or differences in the microbial diversity of BV-associated bacteria found among African American women. Second, we used the Nugent criteria to identify BV. As noted by Martin, not all women with high Nugent scores are deficient in Lactobacilli and Ravel et at recently reported that women with vaginal samples dominated by L iners and L gasserii were commonly identified as having intermediate or high Nugent scores, again indicating that some Lactobacillus species are associated with high Nugent scores20,21.
Given the high prevalence of intermediate flora among this group of pregnant women, it is important to understand the importance of identifying women with intermediate flora. Some have suggested that women with intermediate flora are in transition between normal flora and BV, and others suggest that a subset of women with intermediate flora exhibit vaginal microbiota closely related to women with normal flora and another subset of women with intermediate flora resemble Nugent-score BV 22–24. In addition, Donders et al has proposed that women with abnormal, intermediate flora represent a select group of women with aerobic vaginitis reflecting high levels of group B streptococci and E. coli 25. It is currently unclear if the group of pregnant women with intermediate flora are more or less likely to become BV positive as pregnancy progresses and the relative importance of intermediate vaginal flora on the risk of SPTB. We previously reported an increase risk of miscarriage among women with Nugent-score intermediate flora which followed a dose-dependent increase 26. Future research should focus on the large group of pregnant women with Nugent-score intermediate flora to determine the importance of this classification in predicting adverse pregnancy outcomes and to examine the short and long-term microbial diversity among women with intermediate flora.
In conclusion, we confirmed in this sample of young, urban primarily African American women that Atopobium spp. was related to Nugent-score BV. Continuous levels and high values of Atopobium spp. were also highly sensitive and specific over differing levels of Nugent-score BV. Future studies should validate these findings, confirm the role of high levels of Atopobium spp. among pregnant women, and assess the impact of BV-associated bacteria on adverse pregnancy outcomes.
Figure 2.
ROC curves using the derived cutoffs for BVAB3, Gardnerella vaginalis and Atopobium spp.
Summary.
High levels of Atopobium spp. were related to Nugent-score BV among urban, pregnant women. Levels of Atopobium spp. early in pregnancy were highly sensitive and specific for BV.
Acknowledgments
This work was supported by funding from the National Institute of Child Health and Human Development (R01HD038856 to Nelson DB)
We gratefully acknowledge and thank Jill Wadlin for laboratory technical assistance, the nurses and nurse managers at Temple University hospital, and the Philadelphia Department of Public Health.
Footnotes
No conflict of interest
References
- 1.Fredricks DN, Diedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. NEJM. 2005;353:1899–1911. doi: 10.1056/NEJMoa043802. [DOI] [PubMed] [Google Scholar]
- 2.Cartwright CP, Lembke BD, Ramachandran K, Body BA, Nye MB, Rivers CA, Schwebke JR. Development and Validation of a Semiquantitative, Multitarget PCR Assay for Diagnosis of Bacterial Vaginosis. JCM. 2012;50(7):2321–2329. doi: 10.1128/JCM.00506-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rauh VA, Culhane JF, Hogan VK. Bacterial vaginosis: a public health problem for women. JAMWA. 2000;55:220–2. [PubMed] [Google Scholar]
- 4.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis: Diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74(1):14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- 6.Fredricks DN, Fiedler TL, Thomas KK, Oakley BB, Marrazzo JM. Targeted PCR for Detection of Vaginal Bacteria Associated with Bacterial Vaginosis. Journal of Clinical Microbiology. 2007;45(10):3270–3276. doi: 10.1128/JCM.01272-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ness RB, Hillier S, Richter HE, Soper DE, Stamm C, Bass DC, et al. Can known risk factors explain racial differences in the occurrence of bacterial vaginosis. J Natl Med Assoc. 2003;95(3):201–2. [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson DB, Hanlon A, Hassan S, Britto J, Geifman-Holtzman O, Haggery C, Fredricks DN. Preterm labor and bacterial vaginosis-associated bacteria among urban women. J Perinat Med Assoc. 2009;37:130–134. doi: 10.1515/JPM.2009.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hillier SL, Nugent RP, Eschenbach DA, Krohn MA, Gibbs RS, Martin DH, et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. N Engl J Med. 1995;333:1737–1742. doi: 10.1056/NEJM199512283332604. [DOI] [PubMed] [Google Scholar]
- 10.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;333:1737–1742. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 11.Hay PE, Lamont RF, Taylor-Robinson D, Morgan DJ, Ison C, Pearson J. Abnormal bacterial colonization of the genital tract and subsequent preterm delivery and late miscarriage. Br Med J. 1994;308(6924):295–298. doi: 10.1136/bmj.308.6924.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meis PJ, Goldenberg RL, Mercer B, Moawad A, Das A, McNellis D, et al. The preterm prediction study: Significance of vaginal infections. Am J Obstet Gynecol. 1995;173(4):1231–1235. doi: 10.1016/0002-9378(95)91360-2. [DOI] [PubMed] [Google Scholar]
- 13.Hillier SL, Nugent RP, Eschenbach DA, Krohn MA, Gibbs RS, Martin DH, Cotch MF, Edelman R, Pastorek JG, Rao AV, et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The Vaginal infections and prematurity study group. N Engl J Med. 1995;333(26):1737–42. doi: 10.1056/NEJM199512283332604. [DOI] [PubMed] [Google Scholar]
- 14.Marrazzo JM, Martin DH, Watts DH, Schulte J, Sobel JD, Hillier SL, Deal D, Fredricsk DN. Bacterial Vaginosis: Identifying Research Gaps Proceedings of a Workshop Sponsored by DHHS/NIH/NIAID. Sex Transmit Dis. 2010;37(12):732–744. doi: 10.1097/OLQ.0b013e3181fbbc95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menard J-P, Fenollar F, Raoult D, Boubli L, Bretelle F. Self-collected vaginal swabs for the quantitative real-time polymerase chain reaction assay of Atopobium vaginae and Gardnerella vaginalis and the diagnosis of bacterial vaginosis. Eur J Clin Microbiol Infect Dis. 2012;31:513–518. doi: 10.1007/s10096-011-1341-8. [DOI] [PubMed] [Google Scholar]
- 16.Nelson DB, Bellamy S, Odibo AO, Nachamkin I, Ness RB, Allen-Taylor L. Vaginal Symptoms and Bacterial Vaginosis(BV): How Useful is Self-Report? Development of a Scoring System for Prediction BV Status. Epidemiology and Infection. 2007;135(8):1369–1375. doi: 10.1017/S095026880700787X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menard JP, Fenollar F, Henry M, Bretelle F, Raoult D. Molecular quantification of Gardnerella vaginalis and Atopobium vaginae loads to predict bacterial vaginosis. Clin Infect Dis. 2008;47(1):33–43. doi: 10.1086/588661. [DOI] [PubMed] [Google Scholar]
- 18.Menard J-P, Mazouni C, Fenollar F, Raoult D, Boubli L, Bretelle F. Diagnostic accuracy of quantitative real-time PCR assay versus clinical and Gram stain identification of bacterial vaginosis. Eur J Clin Microbiol Infect Dis. 2010;29:1547–1552. doi: 10.1007/s10096-010-1039-3. [DOI] [PubMed] [Google Scholar]
- 19.Menard J-P, Mazouni C, Salem-Cherif I, Fenollar F, Raoult D, Boubli L, Gamerre M, Bretelle F. High Vaginal Concentrations of Atopobium vaginea and Gardnerella vaginalis in Women Undergoing Preterm Labor. Obstetrics and Gynecology. 2010;115(1):134–140. doi: 10.1097/AOG.0b013e3181c391d7. [DOI] [PubMed] [Google Scholar]
- 20.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SSK, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM, Davi CC, Ault K, Peralta L, Forney LJ. Vaginal microbiome of reproductive-age women. PNAS. 2011;108(1):4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin DH. The Microbiota of the Vagina and Its Influence on Women’s Health and Disease. The American Journal of the Medical Sciences. 2012;343(1):2–9. doi: 10.1097/MAJ.0b013e31823ea228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verhelst R, Verstaelen H, Claeys G, et al. Comparison between Gram stain and culture for the characterization of vaginal microflora: definition of a distinct grade that resemble grade I microflora and revised categorization of grade I microflora. BMC Microbiol. 2005;5:61. doi: 10.1186/1471-2180-5-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larson PG, Carlsson B, Fahraeus L, Jakobsson T, Forsum U. Diagnosis of bacterial vaginosis: need for validation of microscopic image area used for scoring bacterial morphotypes. Sex Transmit Infect. 2004;80:63–7. doi: 10.1136/sti.2003.006106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thoma ME, Gray RH, Kiwanuka N. The short-term variability of bacterial vaginosis diagnosed by Nugent Gram Stain Criteria Among Sexually Active Women in Rakai, Uganda. Sex Trans Dis. 2011;38:111–6. doi: 10.1097/OLQ.0b013e3181f0bdd0. [DOI] [PubMed] [Google Scholar]
- 25.Donders GGG, Vereecken A, Bosmans E, Dekeersmaecker A, Salembier G, Spitz B. Definition of a type of abnormal vaginal flora that is distinct from bacterial vaginosis: aerobic vaginitis. BJOG. 2002;109:34–43. doi: 10.1111/j.1471-0528.2002.00432.x. [DOI] [PubMed] [Google Scholar]
- 26.Nelson DB, Bellamy S, Nachamkin I, Ness RB, Allen-Taylor L. First Trimester Bacterial Vaginosis, Individual Microorganism Levels and Risk of Second Trimester Pregnancy Loss Among Urban Women. Fertility and Sterility. 2007;88(5):1396–1403. doi: 10.1016/j.fertnstert.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]