Abstract
Over the past 15 years, mesenchymal stem cells (MSCs) have been assessed for their capacity to suppress inflammation and promote tissue repair. Regardless of whether the cells are primed (exposed to instructive cues) before administration, their phenotype will respond to environmental signals present in the pathophysiological setting being treated. Since hypoxia and inflammation coexist in the settings of acute injury and chronic diseases, we sought to explore how the proteome and metabolome of MSCs changes when cells were exposed to 48 hours of 1% oxygen, interferon gamma (IFN-γ), or both cues together. We specifically focused on changes in cell metabolism, immune modulation, extracellular matrix secretion and modification, and survival capacity. IFN-γ promoted expression of anti-pathogenic proteins and induced MSCs to limit inflammation and fibrosis while promoting their own survival. Hypoxia instead led to cell adaption to low oxygen, including upregulation of proteins involved in anaerobic metabolism, autophagy, angiogenesis, and cell migration. While dual priming resulted in additive effects, we also found many instances of synergy. These data lend insight to how MSCs may behave after administration to a patient and suggest how priming cells beforehand could improve their therapeutic capacity.
Keywords: mesenchymal stem cell, priming, hypoxia, interferon-gamma, proteome, metabolome, extracellular matrix, immune, survival
INTRODUCTION
Mesenchymal stem cells (MSCs) are multipotent cells that are found in a wide variety of tissues.1,2 Indeed, it is reasonable to expect that most, if not all, tissues have resident MSC-like cells, which participate in local regeneration upon injury or disease. Because these endogenous cells exist in limited numbers, many investigators have explored administration of large numbers of exogenous MSCs to enhance their therapeutic effect.3 These cells are most often given by intravenous injection, with the expectation that they can home to affected sites and have long-ranging impact by secreting paracrine factors.4
The expression profile and phenotype of MSCs residing in healthy tissues or expanded under basal culture conditions are generally different from those expressed by therapeutic MSCs. Upon administration, MSCs enter the diseased microenvironment of the patient and react to the new cues that are present. Two of these cues, which are common to most ailments, are inflammation and hypoxia.5 Hypoxia specifically connotes an oxygen tension that is lower than physoxia, which is 3–9% oxygen in most tissues.6 It affects cell behavior because hypoxia inducible factors (HIF) become hydroxylated at higher oxygen levels and face proteasomal degradation, but at lower oxygen tensions, they are more stable (specifically the alpha subunit), and serve as transcription factors. While the signaling pathways for inflammation and hypoxia start off as being distinct, they have many opportunities for cross-talk, as has been reviewed elsewhere.5,7,8
In acute injury (such as heart infarction or stroke), a blood clot leads to a transient decrease in tissue perfusion, and inflammatory cells rush in to tackle foreign invaders and remove damaged tissue. In this situation, the hypoxia and inflammation may be short lived (days to a week), contingent upon successful resolution of the injury.9 However, in many chronic disease states these two factors co-exist for prolonged periods of time. For example, in rheumatoid arthritis the synovial joint space is characterized by regions of hypoxia and inflammation, which are thought to influence local cell survival, angiogenesis, and energy metabolism.9 Another autoimmune disease – inflammatory bowel disease – also exhibits chronic inflammation, with oxygen gradients at the intestinal mucosa that lead to regions of hypoxia. Curiously, these hypoxic regions are relatively protected compared with the rest of the intestinal tract, as the HIF pathway seems to promote epithelial survival while inducing neutrophil apoptosis.9 Lastly, in solid tumors, hypoxia and inflammation facilitate tumor vascularization and immune escape.7
In order to understand how MSCs serve as reparative and regenerative cells, we explored how they would respond to a generic pathological microenvironment in which hypoxic and inflammatory cues were provided in vitro. To simulate hypoxia, we chose an incubator setting of 1% O2, as this is a lower oxygen tension than the physoxic state of most tissues without being anoxic,10 and to simulate inflammation, we used interferon-gamma (IFN-γ). While many pro-inflammatory cytokines and TLR agonists have been shown to affect MSC behavior, the response of MSCs to IFN-γ is considered to be a standard benchmark of cell function by the ISCT, and IFN-γ is also natural to a generic inflammatory setting than polyI:C, for example.11–15 We then analyzed the proteome and metabolome of adipose-derived MSCs upon 48-hour exposure to control conditions, hypoxia (1% O2), inflammation (IFN-γ), or both cues together (a scenario we refer to as “dual priming”).
METHODS
MSC culture and priming
The protocol for MSC priming follows our previously published work.16 Briefly, frozen vials of adipose-derived MSCs from fully de-identified commercially obtained human lipoaspirates (LaCell, New Orleans, LA) were tested for tri-lineage differentiation as well as positive expression of in vitro MSC surface markers, as previously published.14 All experiments were done using passage 5 MSCs from 3 separate cryovials to generate biological triplicates. This passage number was chosen to represent a feasible level of cell expansion for a human clinical trial without over expanding cells to the point of senescence.17,18
Cells were cultured in MSC media (DMEM 11965 (Thermo Fisher, Bridgewater, NJ) with 10% FBS and 1% Penicillin/Streptomycin (Thermo Fisher) and plated in 6-well plates at an initial seeding density of 5,000 cells/cm2. Cells reached near confluence after approximately two population doublings, with a final cell density of ~22,000 cells/cm2. They were then exposed to 48 hours of either control conditions, 500 U/mL IFN-γ (Peprotech), hypoxia (1% O2; Eppendorf Galaxy 14S incubator), or both IFN-γ and hypoxia. Because priming occurred after cell expansion, its effect on cell division could not be determined.
MSCs were routinely tested as mycoplasma-free using the MycoAlert kit from Lonza (Allendale, NJ). The above steps were performed ahead of sample collection for mass spectrometry and then again for metabolomics and any confirmatory studies (See Supplemental Methods). Cell concentration and viability were determined using trypan blue exclusion and a Countess instrument (Life Technologies).
Mass spectrometry
Protein was collected from MSCs via a methanol-chloroform extraction protocol. MSC plates were washed × 3 with ice-cold PBS to thoroughly remove residual FBS and cytokines. 450 µL of lysis buffer consisting of TBS with 3% SDS and 50 µL protease inhibitors (Sigma # P8340) were applied to each MSC plate. Each lysate was collected in a 1.5 mL tube and incubated at 60 °C for 30 minutes. 400 µL of methanol and 100 µL of chloroform were added, and samples were vortexed and centrifuged at 16 000 × g for 1 min. The protein pellet was washed with methanol three times and allowed to air dry before final resuspension in 30 µL of solubilization buffer (100 mM ammonium bicarbonate, 8 M urea, and 0.1 M DTT in Optima water) and snap freezing with liquid N2 for storage at −80 °C. Immediately prior to analysis, samples were further reduced, alkylated, and trypsin digested, and yeast alcohol dehydrogenase was added as an internal control. Samples were collected from biological triplicates, where each replicate of a given priming condition came from a separately thawed cryovial of MSCs. Each individual sample was additionally run in technical duplicates. Mass spectrometry was performed at the Quantitative Proteomics and Metabolomics Center at Columbia University with an UltiMate 3000 RSLCNano ultrapressure liquid chromatograph coupled to a Q Exactive HF (Orbitrap) mass spectrometer (Thermo Fisher Scientific, Bremen, Germany). More detailed methods can be found in the supplemental information (SI).
iPathway Guide (Advaita, 2017) and String v10.5 (string-db.org/) were used to understand the nature of the differentially expressed (DE) proteins, which were defined by a log2 fold change (log2FC)| > 0.6 and p < 0.05. All proteins are listed by their related gene names, as these are the names used in the iPathway Guide program.
Metabolomic Analysis
MSC plates (6-well) were washed three times with ice-cold PBS to thoroughly remove residual FBS and cytokines. 500 µL of chilled 80% methanol containing internal standard (17 µM 4-chlorophenylacetic acid) were then added to each well, and the plates were placed on a shaker for 30 minutes in a cold room. Samples were subsequently collected into 15 mL Falcon tubes, with lysate from two full 6-well plates used for each individual sample. As with the proteomics set up, there were three biological replicates for each priming condition. Samples were stored at −80 °C before analysis at the Quantitative Proteomics and Metabolomics Center at Columbia University, where the metabolomics analysis was performed (detailed methods in SI). Because, in some instances, no metabolite was detected in control MSCs, the relative expression is presented instead of. the log2FC.
RESULTS
Overview of Data
MSCs used for proteomic and metabolomic analyses had a viability of >94%, based on trypan blue exclusion, with no significant differences based on exposure to hypoxia or IFN-γ. While we did not observe a change in morphology indicative of undesired differentiation, exposure to IFN-γ did increase the average cell diameter (Figure S1), as has been observed by others.19 Our prior studies have also shown minimal changes to MSC surface marker expression, with the exception that IFN-γ leads to expression of HLA-DR, which is not expressed at baseline.16
A total of 3,465 proteins were detected, with 2,435 proteins identified by more than one peptide (conservative set). Many proteins identified by one peptide are accurate results and have been validated by others; however, only the conservative set will be presented in figures, and tables will indicate the proteins outside of this set. Our own validation studies can be seen in Figure S2, which also serves to validate some of the smaller magnitude (yet significant) findings.
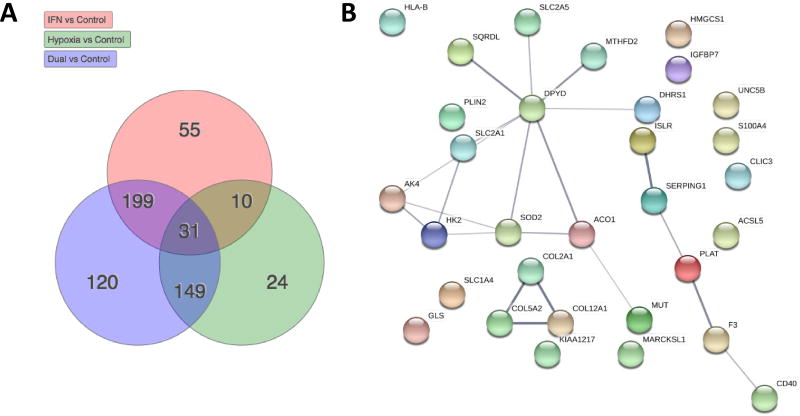
Figure 1A shows the overlap in differential expression (DE) of proteins amongst priming conditions, with the 31 proteins DE in all priming conditions shown in Figure 1B. The central node is dihydropyrimidine dehydrogenase (DPYD), the rate-limiting-step in pyrimidine metabolism.
Figure 1. Overview of data.
A) The total number, and overlap amongst priming conditions, of proteins that show significant differential expression compared with control MSCs B) STRING network of the 31 proteins that are differentially expressed from all priming conditions.
Table 1 documents the percentages of the 2,435 proteins that are DE for each priming condition. IFN-γ priming led to a significant change in expression in ~12% of the proteins, hypoxia led to a change in ~9% while dual IFN-γ/hypoxia priming led to a change ~21%. The very neat addition of these percentages (9+12=21), suggests that IFN-γ and hypoxia alter distinct pathways without much overlap (only 41 proteins as per Figure 1A). In further support of this, most proteins with DE following hypoxia were mitochondrial, whereas IFN-γ predominantly affected non-mitochondrial proteins. The complete protein data set is provided in Supp. File 1.
Table 1.
Overview of the impact of IFN-y and/or hypoxia on the MSC proteome.
| IFN-γ | Hypoxia | Dual | |
|---|---|---|---|
| % of proteins that are DE | 12.10% | 8.80% | 20.50% |
| % of DE proteins that are in the mitochondria | 12.20% | 62.60% | 29.30% |
Metabolomic analysis identified forty-four intracellular metabolites with strong confidence. The relative abundances of nine metabolites are highlighted in Table 2 (please see Supp. File 2 for complete list). Priming with IFN-γ resulted in higher increases in β-alanine levels than priming with hypoxia, but both cues acted together to produce the highest amounts of this metabolite in dual-primed cells. Taurine and hypotaurine showed similar trends to β-alanine. While a relative abundance of 1E+99 implies there was no detectable metabolite in the control MSCs, the relative abundance of taurine in IFN-γ primed vs. hypoxia primed cells was 6.06-fold higher (Supp. File 2). There was no significant difference in relative abundance of kynurenine or tyrosine in IFN-γ primed vs. dual primed cells.
Table 2.
Relative metabolite abundance in IFN-γ and hypoxia primed MSCs normalized to the abundance in control MSCs.
| IFN-γ | Hypoxia | Dual | |
|---|---|---|---|
| Beta- alanine | 6.56 | 1.63 | 10.60 |
| hypotaurine | 2.27 | 1.51 | 3.45 |
| L-kynurenine | 1E+99 | 0.00 | 1E+99 |
| L-tyrosine | 1E+99 | 0.00 | 1E+99 |
| D-glucose | 1.20 | 1.45 | 2.88 |
| O-Phosphoethanolamine | 1.15 | 2.92 | 2.45 |
| pyruvic acid | 0.72 | 0.44 | 0.49 |
| L-(+) lactic acid | 0.55 | 1.54 | 1.83 |
| taurine | 1E+99 | 1E+99 | 1E+99 |
Given the above changes in the expression of proteins and metabolites, we analyzed their relevance to four biological settings: metabolism, immune modulation, extracellular matrix (ECM), and cell survival. Of note, these are only tentative boundaries, as one pathway often has direct influence in more than one setting.
Effects of IFN-γ and hypoxia on cellular metabolism
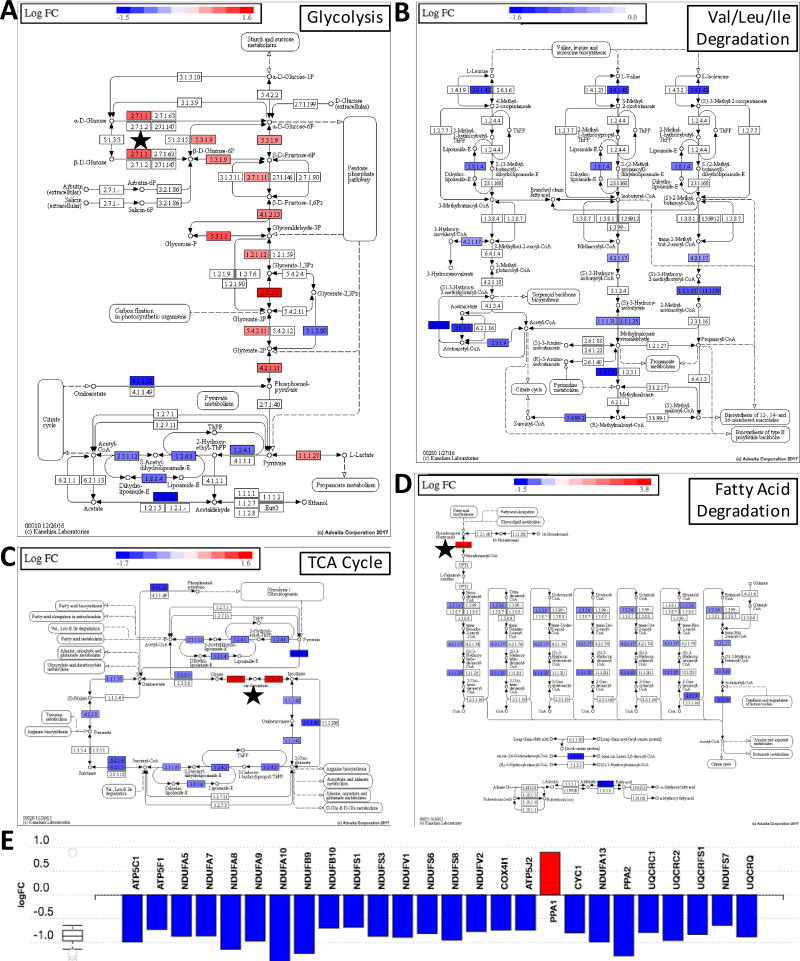
Exposure to hypoxia resulted in significant and comprehensive changes in carbon metabolism. MSCs had broad upregulation of proteins involved in glycolysis and a greater capacity for uptake of glucose (GLUT1/SLC2A1) and fructose (GLUT5/SLC2A5), as well as for export of lactate (MCT4/SLC16A3) and hydrogen ions (CA12). By contrast, citric acid (TCA) cycle proteins were mostly downregulated, as were the enzymes involved in fat degradation and transport, amino acid catabolism and transport, and the electron transport chain (ETC). Consistent with the fewer mitochondrial proteins, mitochondrial ribosomal proteins (MRP family) were downregulated. Hypoxia also influenced glycogen turnover, as both glycogen synthase and glycogen phosphorylase L were upregulated.
In contrast, IFN-γ had a selective impact on metabolism. While generally not altering any pathway at every step, IFN-γ led to an upregulation of GLUT5 (log2FC = 5.32 vs. only 1.55 from hypoxia), cytoplasmic aconitase (ACO1/IRP1), hexokinase II (HKII), and long chain acyl-CoA synthetase (ACSL5). Curiously, these proteins were also upregulated by hypoxia (Fig. 1B). Thus, when MSCs were exposed to both hypoxia and IFN-γ (dual priming), they aligned closer to the metabolic changes observed with hypoxia, but with an even greater induction of the above four proteins. These trends can be seen in Figure 2A–E, which demonstrates the combined changes in metabolism for dual primed MSCs. Only dual primed cells significantly upregulated proteins involved in gluconeogenesis (Figure S3).
Figure 2. The effect of dual IFN-γ/hypoxia priming.
A) on glycolysis, B) neutral amino acid degradation C) the TCA cycle D) fat degradation and E) the electron transport chain. Note that the trends are similar for hypoxia primed cells, however, co-exposure to IFN-γ reinforced the upregulation of HKII, ACO1, and ACSL5, which are indicated with a black star (★) in panels A, C, and D, respectively. Beyond upregulating these proteins, IFN-γ had an unremarkable impact on the above pathways.
Effects on immune modulation
The immune regulatory function of MSCs is thought to be one of their most important qualities and highly responsive to microenvironmental cues. Curiosly, hypoxia did not lead to DE of proteins implicated in traditional immune pathways. By contrast, IFN-γ had a large impact. For example, IFN-γ induced expression of proteins associated with antigen presentation (Figure S4), the defense against viral and microbial pathogens (IFIT, TRIM, NOD, and CXCL families; Supp. File 1), and chemotaxis (CXCL9-CXCL11, CD47, CD97).
IFN-γ exposure also resulted in upregulation of factors that promote the complement cascade (C1QBP, C1R, C1S). While these factors could put MSCs at greater risk for complement-related cell death and clearance, it is notable that SERPING1 and complement factor H (CFH) were highly induced (Table 3), which could provide protection. Other factors that could protect MSCs from immune mediated clearance were upregulated, such as CD47, HLA-E, HLA-F, and PD-L1, as well as the tryptophan catabolizing enzyme indoleamine-2,3-dioxygenase (IDO1). TGF-β1 had the highest induction in dual primed cells but only at a low level (log2FC = 0.58). However, a related protein, LRRC32, was significantly induced in dual primed cells. Several proteins associated with MSC immune modulation - HLA-G, HGF, LIF, NOS2/iNOS, and TSG-6 - were not detected in any MSC groups.4
Table 3.
Differential expression (log2 FC relative to control MSCs) of proteins related to the complement cascade, immune tolerance, and leukocyte migration.
| IFN -γ | H yp o x | Dual | Function | |
|---|---|---|---|---|
| C1QBP | −0.12 | −1.2 | −1.13 | inhibits complement factor |
| C1R | 2.55 | −0.51 | 2.66 | forms complement factor 1 |
| C1S* | 2.01 | −0.07 | 1.94 | forms complement factor 1 |
| CD47 | 1.24 | 0.01 | 1.19 | protection from phagocytosis; transendothelial migration |
| CD97 | 1.39 | 0.17 | 1.23 | chemotaxis |
| CFH* | 6.8 | 0.49 | 7.38 | helps direct complement to pathogens vs. host cells |
| CSF1 | 3.41 | −0.28 | 3.18 | monocyte maturation |
| CXCL9 | 6.51 | 0.03 | 6.8 | chemotaxis; antimicrobial |
| CXCL10* | 2.04 | 0.12 | 1.96 | chemotaxis; antimicrobial |
| CXCL11* | 2.44 | −0.13 | 2.38 | chemotaxis; antimicrobial |
| HLA-E | 3.01 | −0.03 | 3.47 | protects cells from NK cells |
| HLA-F* | 7.16 | 0.16 | 6.97 | possibly immune tolerance |
| IDO1 | 6.64 | 0.19 | 6.51 | immune tolerance |
| LGALS3BP | 1.54 | −0.22 | 1.32 | galectin 3 binding protein |
| LGALS9B* | 4.76 | −0.88 | 4.11 | analog of galectin 9 |
| LRRC32 | 0.3 | 0.35 | 0.89 | promotes surface expression of TGF-β on T-regs |
| SERPING1 | 3.61 | 0.81 | 3.7 | inhibits complement factor |
| PDL1* | 6.38 | −0.2 | 6.27 | immune tolerance |
| WARS | 3.88 | −0.08 | 3.78 | trytophan production |
identified by only 1 peptide.
Effects on extracellular matrix
ECM remodeling is a fundamental part of both healthy and pathological wound repair, as is cell migration. Both IFN-γ and hypoxia had large impact on the ECM (Table 4). IFN-γ predominantly affected the production of collagen components, with significant downregulation of collagens 1–3, 5, 12, 15 and 16, but upregulation of collagen 4. IFN-γ also increased laminin alpha chains and agrin, while downregulating elastin, fibulin, and connective tissue growth factor. Hypoxia upregulated collagen 6 and had many effects similar to IFN-γ. Consequently, the DE patterns of dual primed cells were often suggestive of synergistic influences of the two cues, with the most dramatic example being COL4A2, where IFN-γ or hypoxia conditioning resulted in log2FC = 0.5, while dual priming resulted in log2FC = 4.18.
Table 4.
Differential expression (relative to control MSCs) of proteins related to the ECM.
| IFN-γ | Hypoxia | Dual | |
|---|---|---|---|
| AGRN* | 4.35 | 1.47 | 6.16 |
| COL1A1 | −1.37 | −0.57 | −2.22 |
| COL1A2 | −1.03 | −0.48 | −1.61 |
| COL2A1 | −1.42 | −0.64 | −2.67 |
| COL3A1 | −1.05 | 0.13 | 1.16 |
| COL4A1 | 0.65 | 0.46 | 1.21 |
| COL4A2 | 0.51 | 0.54 | 4.18 |
| COL5A1 | −0.75 | −0.14 | −0.92 |
| COL5A2 | −0.9 | −0.74 | 2.27 |
| COL6A2 | 0.18 | 0.61 | 0.89 |
| COL6A3 | 0.1 | 0.63 | 0.76 |
| COL12A1 | −0.97 | −0.96 | −2.59 |
| COL15A1 | −1.06 | −0.56 | −1.77 |
| COL16A1 | −0.71 | −0.2 | −0.67 |
| CTGF | −2.25 | 0.46 | −1.94 |
| ELN | −1.27 | −0.26 | −1.36 |
| FBLN1 | −0.73 | −0.45 | 0.17 |
| FBN1 | −0.02 | 0.26 | 0.91 |
| LAMA2* | 0.67 | −0.15 | 1.34 |
| LAMA4 | 1.67 | 0.27 | 1.83 |
| LAMB1 | −1.03 | −0.37 | 1.11 |
| LAMC1 | −0.67 | 0.05 | −0.38 |
| LOX | −0.43 | 1.05 | 0.96 |
| LOXL1* | 0.22 | 0.72 | 2.11 |
| LOXL2 | −0.79 | 0.99 | 0.27 |
| LOXL3* | 0.65 | 2.72 | 4.21 |
| PLOD1 | 0.08 | 0.68 | 0.49 |
| PLOD2 | 0.27 | 1.74 | 1.9 |
| SPARC | −1.56 | 0.21 | −1.13 |
| THBS1 | −1.25 | 0.13 | 0.76 |
| THBS2 | −1.62 | 0.16 | −1.04 |
| TIMP1 | 0.38 | 0.91 | 0.89 |
| TIMP3 | −0.56 | 0.37 | −0.86 |
| TNC | 0.03 | 0.01 | 0.62 |
log2 FC is shown.
proteins identified by only 1 peptide.
These cues also affected regulators of ECM metabolism and angiogenesis. Hypoxia had a large effect on collagen crosslinking, as it upregulated several lysyl oxidases (LOX, LOXL1-3) and lysyl hydroxylases (PLOD1&2). When MSCs were dual primed, induction of LOXL1 and LOXL3 was bolstered even further, suggesting another area of synergy. Dual priming also affected expression of TIMP1 and TIMP3, which were upregulated and downregulated, respectively, and led to unique induction of tenascin-C. Lastly, IFN-γ and dual primed cells downregulated thrombospondins 1&2, which act to inhibit angiogenesis.
Effects on cell survival
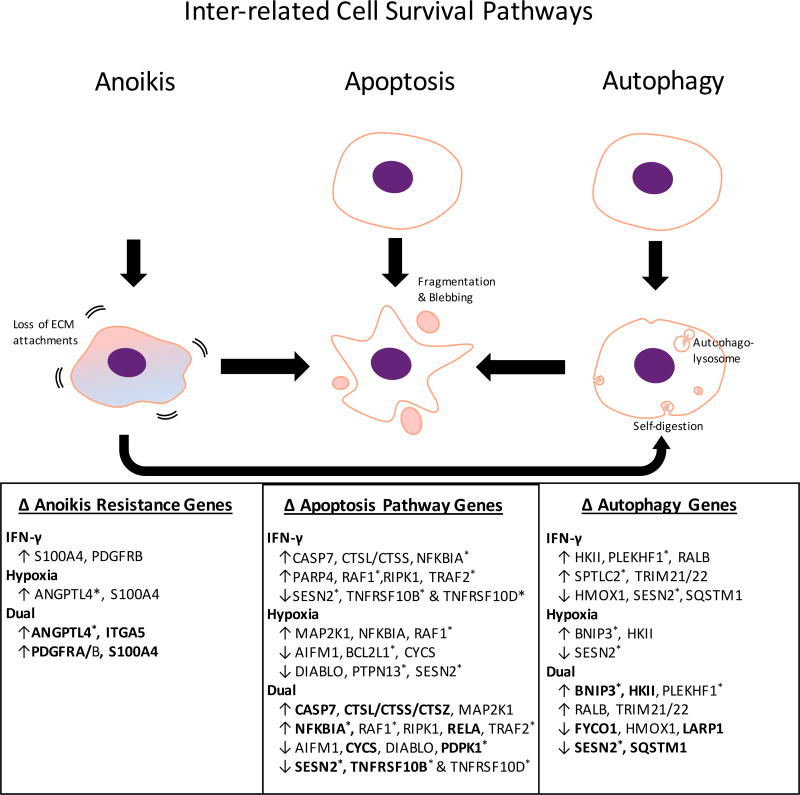
When cultured in monolayers at basal cuture conditions, MSCs experience cues that maintain cell survival. Once injected into patients, however, the MSCs lose their attachment sites and experience stressful signals such as hypoxia and inflammation. We therefore investigated how these cues affect the pathways directly related to cell survival: anoikis, apoptosis, and autophagy.
Several anoikis-resistance factors were upregulated following hypoxia and IFN-γ exposure (Figure 3). S100A4 was upregulated by both IFN-γ and hypoxia (log2FC = 0.75 and 0.64, respectively), while the greatest induction came from dual priming (log2FC = 1.59). Dual primed cells also showed the greatest upregulation of ANGPTL4 (log2FC = 2.56 vs. 2.05 from hypoxia alone), induction of both PDGFRA and PDGFRB, and unique upregulation of integrin alpha chain V (ITGA5).
Figure 3. Effect of IFN-γ and/or hypoxia on the interrelated survival pathways of anoikis, apoptosis, and autophagy.
* proteins that were identified by a single peptide. Bold type indicates significant effects of dual priming when compared to single priming. Proteins listed for apoptosis and autophagy were identified using pathways on iPathway Guide, and those related to survival from anoikis were determined from literature.20–24
The changes to apoptosis-related genes were suggestive of both promotion and inhibition of this process. The promotion of apoptosis was evidenced by the increases in expression of cathepsins, caspase 7 and NFKBIA, and decreases in expression of BCL2L1 and PDPK1. The inhibition of apoptosis was evidenced by increased expression of MAP2K1, RAF1 and RELA, and decreased expression of AIFM1, CYCS, DIABLO, SESN2, and TNFR10B & D (TRAIL receptors). Other proteins listed in Figure 3 have variable, context-specific effects. Overall, IFN-γ and hypoxia seemed to have mixed effects on apoptosis, which was also seen with dual priming.
All proteins listed under autophagy served to facilitate that pathway (Figure 3). As with apoptosis, there were changes that both promote and inhibit this process. IFN-γ upregulated TRIM21 and TRIM22, whereas hypoxia highly induced BNIP3 (log2FC = 3.47), and both factors induced HKII. BNIP3 and HKII had an even greater induction from dual priming, while SESN2 was synergistically downregulated (the most downregulated protein in the whole data set). Since SESN2 is also pro-apoptotic, its downregulation may have consequences for both autophagy and apoptosis.
Beyond the pathways listed above, some additional metabolomics changes described in Table 2 are worth highlighting. For example, taurine has many anti-oxidant and cytoprotective properties,25–27 and this metabolite was found in all primed cells but was undetected in control MSCs. β-alanine was also upregulated in all priming conditions. This amino acid is the precursor to the anti-oxidant carnosine, which has shown cytoprotective properties in similar situations to taurine.28,29 β-alanine is made from pyrimidine metabolism, and its accumulation in primed cells is consistent with their upregulation of DPYD – the first enzyme in this pathway.
DISCUSSION
Several meritorious studies have been dedicated to identifying the protein expression profile of MSCs derived from different tissue sources.30,31 However, if basic culture conditions are used, none of these profiles represented how MSCs would behave in a clinical setting, because MSCs are highly responsive to microenvironmental cues, which change in the settings of injury and disease. Since inflammation and hypoxia are common to many pathological environments, we sought to better understand the biology of therapeutic MSCs via simulating these signals with 1% O2 and IFN-γ. We used a 48-hour conditioning regimen, as MSCs are not thought to survive more than a few days in vivo, making this exposure time realistic for the clinical setting.32 While we have validated the significance of the metabolic and immune modulatory changes from this priming regimen in our previous work,16 our goal here was to reveal the global proteomic and metabolomic changes of MSCs co-exposed to these cues, and to parse out the relative contribution of each factor and identify synergies. The major themes we uncovered are that IFN-γ induces MSCs to contain an injury by limiting damage from infection, inflammation and fibrosis, while hypoxia promotes adaptation to low oxygen via increasing proteins involved in anaerobic metabolism, angiogenesis, autophagy, and cell migration. Combining these cues roughly combines their individual effects, with additional synergies.
As expected, IFN-γ had a strong impact on MSC immune modulation. Our study confirmed some well-known downstream effects of interferons, such as upregulation of HLA proteins, anti-microbial and anti-viral factors, and the immunosuppressive proteins IDO and PD-L1.14 However, we also uncovered some less described proteins and metabolites that could confer MSC protection. For example, IDO is known to inhibit T-cells via metabolizing tryptophan to kynurenine (supported by our metabolomics data). This tryptophan depletion could also be harmful to MSCs. Thus, it is interesting that IFN-γ upregulated tryptophanyl tRNA-synthetase (a.k.a. WARS), which could serve as a MSC-protective method by replenishing intracellular tryptophan stores.33 Our proteomics and flow cytometry validation study also show that MSCs have high baseline expression of CD47, but this undergoes two-fold upregulation in response to IFN-γ. This protein, which has thus far received little attention in the MSC literature, binds to the SIRPα receptor on macrophages and has been described as a “don’t eat me” signal (blocks phagocytosis) that may also promote peripheral tolerance.34 This pathway has been implicated as a mechanism for tumor immune escape, and could be an interesting lead to pursue for MSC immunomodulation.35
We also noted strong induction of the complement inhibitory factor – CFH. MSCs, particularly the cryopreserved products used in industry-sponsored clinical trials, have been shown to activate the complement cascade, expediting MSC lysis and clearance.36 Since the therapeutic utility of MSCs would be better if they had a longer half-life, one group coated the cell surface with CFH to prevent this type of clearance.37 Our data indicate that a similar effect may result from simply exposing MSCs to IFN-γ. Lastly, we observed induction of the cell-protective metabolites, taurine and β-alanine, the latter being protective upon conversion to carnosine.26–29
Beyond immune modulation, IFN-γ induces an anti-fibrotic MSC phenotype. Except for collagen IV and agrin, IFN-γ downregulates all other structural ECM proteins as well as CTGF, which is thought to be highly involved in fibrosis.38 Agrin is an ECM proteoglycan from the basement membrane, so its upregulation with collagen IV is consistent with preserving basement membrane formation. However, agrin has also been implicated as a part of the immune synapse, and the upregulation of agrin may thus be related to that function.39
The otherwise widespread decrease in structural proteins is significant, because tissue fibrosis from excessive deposition of ECM is a common consequence of unchecked inflammation and is detrimental to having functional tissues.40 Thus, IFN-γ priming of MSCs may help prevent pathological fibrosis by both inhibiting inflammation and decreasing ECM secretion. This anti-fibrotic effect has clinical relevance. In one study, patients treated with recombinant IFN-γ significantly reduced the development of liver fibrosis in the setting of chronic hepatitis B.41
While IFN-γ instructs MSCs to resolve tissue injury, hypoxia leads to many survival adaptations. First, hypoxia shifts cell metabolism towards non-oxygen dependent strategies – glycolysis and glycogenolysis. To facilitate the reliance on these pathways, cells increase lactate dehydrogenase, lactate transporters, glucose and fructose transporters, and carbonic anhydrase (to assist with pH balance)42. Degradation and transport of amino acids and fatty acids, which generally feed into the TCA cycle and ETC (oxygen dependent), are downregulated, as is the mitochondrial protein expression in general.
A second approach to cell survival is through conservation of energy stores via autophagy. We observed significant induction of the pro-autophagic BNIP3, which is consistent with studies by others.22 Lastly, MSCs can attempt to restore a physoxic environment by promoting angiogenesis or migrating away from the low oxygen region. Consistent with previous reports, we found that hypoxia upregulates the entire family of lysyl oxidase (LOX, LOXL1-3) and lysyl hydroxylase (PLOD1&2) enzymes, which serve to cross-link collagen and elastin. These proteins are associated with promoting angiogenesis43 and cell migration.44,45 The additional upregulation of anoikis-resistance proteins like ANGPTL4 and S100A4 may facilitate survival during this process.10,21,22
The collective adaptation to hypoxia may inadvertently make MSCs a better cell therapy. Several animal studies have shown that hypoxic preconditioning of MSCs before administration enables them to better survive in the clinical setting of ischemia. Upregulation of autophagy has been directly shown to contribute. In one study, preconditioning MSCs with hypoxia improved their ability to repair infarcted hearts; however, this difference was partially abolished by an autophagy inhibitor. The changes in metabolism could also influence the immune modulatory function of MSCs. As has been described in the tumor literature, when cells prioritize glycolysis for their own survival, they inhibit inflammatory immune cells in the nearby environment through glucose competition and lactate signaling.46–48 This would explain why hypoxia preconditioned cells have a better ability to suppress T-cells in vitro without superior upregulation of immunosuppressive proteins like IDO.49
Clearly the response of MSC to IFN-γ and hypoxia are very different, and it makes sense that combining these cues results in additive effects. Indeed, dual primed cells demonstrate the metabolic changes of hypoxia, the immunomodulatory changes of IFN-γ, and the ECM and survival changes of both cues. Nevertheless, there were many points of synergy.
While hypoxia alone did not upregulate any immunosuppressive factors, dual IFN-γ/hypoxia exposure led to synergistic upregulation of LRRC32 (a.k.a. GARP), which is known to tether TGF-β1 (also most abundant in dual primed cells) to regulatory T-cells and megakaryocytes and play a role in the immunosuppressive function of MSCs.50,51 Dual priming also downregulates structural ECM proteins and upregulates basement membrane proteins beyond IFN-γ alone, and upregulates LOXL enzymes more than hypoxia alone. The LOXL family has been shown to cross-link ECM proteins to attract anti-inflammatory macrophages, promote angiogenesis, and mobilize cells to migrate to distal organs.44,45,52,53 Survival in this “metastatis-like” scenario is consistent with dual primed cells having the highest expression of ACSL5, ANGPTL4, BNIP3, HKII, PDGFRA, S100A4, as well as taurine and β-alanine (precursor to carnosine).
While we have attempted to profile how MSCs respond to a diseased microenvironment, several limitations should be considered. Clearly, hypoxia and IFN-γ are not the only cues present in vivo, as there are many other paracrine factors and immune cells that will affect MSC behavior. Our analysis is meant to isolate the effect of these two particular factors, individually and in combination, to help understand how they contribute to the whole. In doing so, we also gained insights into the many ways by which priming MSCs with these cues beforehand could enhance their therapeutic capacity (via immune modulation and pro-survival factors).54,55
A second limitation of our study is that our samples were healthy adherent cells after ~10 days in cell culture. MSCs used in industry sponsored clinical trials are cryopreserved, thawed, and injected into the patient within a few hours. Loss of attachment can be expected to affect cell viability, and cryopreservation is known to affect MSC metabolism, viability, and responsiveness to IFN-γ.56–58 Our data thus represent the best-case-scenario, as the proteome and metabolome of recently thawed cells may be less malleable.
Finally, there are several outstanding questions that are based on the results of this study:. 1) What is the role of ACO1/IRP1 upregulation from IFN-γ and hypoxia? 2) What is the function of GLUT5 upregulation when blood fructose levels are low? Given that IFN-γ upregulates ASCL5 and SLC27A3, does IFN-γ have a meaningful effect on long chain lipid metabolism? We expect that future studies will attempt to address these questions, to further our understanding of the effects of IFN-γ and hypoxia on the therapeutic capacity of MSCs and the clinical use of these cells.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the funding support of NIH (grants EB002520 and EB025765 to GVN), the New York State Stem Cell Science Board (NYSTEM, contract #C029159 to LMB) and the Columbia-Coulter Biomedical Accelerator Program (to HMW and GVN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Work was performed at Columbia University in the City of New York, NY, USA
DISCLOSURES
HMW and GVN are founders of a start-up company Immplacate Inc that uses the technology described in this paper. RDS is a scientific advisor for Immplacate.
AUTHOR CONTRIBUTIONS
The raw data required to reproduce these findings will be made available to interested i
DATA AVAILABILITY
All experimental data necessary to reoroduce the findings from this study will be made available to interested investigators.
References
- 1.Subramani B, et al. Generation and characterization of human cardiac resident and non-resident mesenchymal stem cell. Cytotechnology. 2016;68:2061–2073. doi: 10.1007/s10616-016-9946-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh A, Singh A, Sen D. Mesenchymal stem cells in cardiac regeneration: a detailed progress report of the last 6 years (2010–2015) Stem Cell Res. Ther. 2016;7:82. doi: 10.1186/s13287-016-0341-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Squillaro T, Peluso G, Galderisi U. Review Clinical Trials With Mesenchymal Stem Cells : An Update. 2016;25:829–848. doi: 10.3727/096368915X689622. [DOI] [PubMed] [Google Scholar]
- 4.Wobma HM, Liu D, Vunjak-Novakovic G. Paracrine Effects of Mesenchymal Stromal Cells Cultured in Three-Dimensional Settings on Tissue Repair. ACS Biomater. Sci. Eng. 2017 doi: 10.1021/acsbiomaterials.7b00005. acsbiomaterials.7b00005. [DOI] [PubMed] [Google Scholar]
- 5.Taylor CT, Colgan SP. Regulation of immunity and inflammation by hypoxia in immunological niches. Nat. Rev. Immunol. 2017;17:774–785. doi: 10.1038/nri.2017.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michiels C. Physiological and Pathological Responses to Hypoxia. Am. J. Pathol. 2004;164:1875–1882. doi: 10.1016/S0002-9440(10)63747-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eltzschig HK, Carmeliet P. Hypoxia and Inflammation. N. Engl. J. Med. 2011;364:656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Ignazio L, Bandarra D, Rocha S. NF-κB and HIF crosstalk in Immune Responses. FEBS J. 2015:n/a–n/a. doi: 10.1111/febs.13578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biddlestone J, Bandarra D, Rocha S. The role of hypoxia in inflammatory disease (Review) Int. J. Mol. Med. 2015;35:859–869. doi: 10.3892/ijmm.2015.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moya A, et al. Human Mesenchymal Stem Cell Failure to Adapt to Glucose Shortage and Rapidly use Intracellular Energy Reserves through Glycolysis Explains Poor Cell Survival After Implantation. Stem Cells. 2017 doi: 10.1002/stem.2763. [DOI] [PubMed] [Google Scholar]
- 11.Polchert D, et al. IFN-γ activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur. J. Immunol. 2008;38:1745–1755. doi: 10.1002/eji.200738129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cell (MSC) paradigm: Polarization into a pro-inflammatory MSC1 or an immunosuppressive MSC2 phenotype. PLoS One. 2010;5 doi: 10.1371/journal.pone.0010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohammadpour H, Akbar A, Nikougoftar M, Taher M. International Immunopharmacology TNF- α modulates the immunosuppressive effects of MSCs on dendritic cells and T cells. Int. Immunopharmacol. 2015;28:1009–1017. doi: 10.1016/j.intimp.2015.07.045. [DOI] [PubMed] [Google Scholar]
- 14.Krampera M, Galipeau J, Shi Y, Tarte K, Sensebe L. Immunological characterization of multipotent mesenchymal stromal cells-The international society for cellular therapy (ISCT) working proposal. Cytotherapy. 2013;15:1054–1061. doi: 10.1016/j.jcyt.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 15.DelaRosa O, et al. Requirement of IFN-gamma-mediated indoleamine 2,3-dioxygenase expression in the modulation of lymphocyte proliferation by human adipose-derived stem cells. Tissue Eng. Part A. 2009;15:2795–2806. doi: 10.1089/ten.TEA.2008.0630. [DOI] [PubMed] [Google Scholar]
- 16.Wobma HM, et al. Dual IFN-γ/hypoxia priming enhances immunosuppression of mesenchymal stromal cells through regulatory proteins and metabolic mechanisms. J Immunol Regen Med. doi: 10.1016/j.regen.2018.01.001. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mendicino M, Bailey AM, Wonnacott K, Puri RK, Bauer SR. MSC-based product characterization for clinical trials: An FDA perspective. Cell Stem Cell. 2014;14:141–145. doi: 10.1016/j.stem.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 18.Chinnadurai R, et al. Immune dysfunctionality of replicative senescent mesenchymal stromal cells is corrected by IFNγ priming. Blood Adv. 2017;1:628–643. doi: 10.1182/bloodadvances.2017006205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klinker MW, Marklein RA, Lo Surdo JL, Wei C-H, Bauer SR. Morphological features of IFN-γ-stimulated mesenchymal stromal cells predict overall immunosuppressive capacity. Proc. Natl. Acad. Sci. U. S. A. 2017;114:E2598–E2607. doi: 10.1073/pnas.1617933114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mashima T, et al. Acyl-CoA synthetase as a cancer survival factor: Its inhibition enhances the efficacy of etoposide. Cancer Sci. 2009;100:1556–1562. doi: 10.1111/j.1349-7006.2009.01203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen W, et al. S100A4 protects gastric cancer cells from anoikis through regulation of αv and α5 integrin. Cancer Sci. 2011;102:1014–1018. doi: 10.1111/j.1349-7006.2011.01915.x. [DOI] [PubMed] [Google Scholar]
- 22.Paoli P, Giannoni E, Chiarugi P. Anoikis molecular pathways and its role in cancer progression. Biochim. Biophys. Acta - Mol. Cell Res. 2013;1833:3481–3498. doi: 10.1016/j.bbamcr.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 23.Guadamillas MC, Cerezo A, del Pozo MA. Overcoming anoikis - pathways to anchorage-independent growth in cancer. J. Cell Sci. 2011;124:3189–3197. doi: 10.1242/jcs.072165. [DOI] [PubMed] [Google Scholar]
- 24.Liao Y-H, et al. Epidermal growth factor-induced ANGPTL4 enhances anoikis resistance and tumour metastasis in head and neck squamous cell carcinoma. Oncogene. 2017;36:2228–2242. doi: 10.1038/onc.2016.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang B, Yang X, Gao X. Taurine protects against bilirubin-induced neurotoxicity in vitro. Brain Res. 2010;1320:159–167. doi: 10.1016/j.brainres.2010.01.036. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z, et al. Taurine supplementation reduces oxidative stress and protects the liver in an iron-overload murine model. Mol. Med. Rep. 2014;10:2255–2262. doi: 10.3892/mmr.2014.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chowdhury S, Sinha K, Banerjee S, Sil PC. Taurine protects cisplatin induced cardiotoxicity by modulating inflammatory and endoplasmic reticulum stress responses. BioFactors. 2016;42:647–664. doi: 10.1002/biof.1301. [DOI] [PubMed] [Google Scholar]
- 28.Caruso G, et al. Carnosine modulates nitric oxide in stimulated murine RAW 264.7 macrophages. Mol. Cell. Biochem. 2017;431:197–210. doi: 10.1007/s11010-017-2991-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naghshvar F, Abianeh SM, Ahmadashrafi S, Hosseinimehr SJ. Chemoprotective effects of carnosine against genotoxicity induced by cyclophosphamide in mice bone marrow cells. Cell Biochem. Funct. 2012;30:569–573. doi: 10.1002/cbf.2834. [DOI] [PubMed] [Google Scholar]
- 30.Tachida Y, Sakurai H, Okutsu J. Proteomic Comparison of the Secreted Factors of Mesenchymal Stem Cells from Bone Marrow, Adipose Tissue and Dental Pulp. J. Proteomics Bioinform. 2015;8:266–273. [Google Scholar]
- 31.Rolandsson Enes S, et al. Quantitative proteomic characterization of lung-MSC and bone marrow-MSC using DIA-mass spectrometry. Sci. Rep. 2017;7:9316. doi: 10.1038/s41598-017-09127-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eggenhofer E, Luk F, Dahlke MH, Hoogduijn MJ. The life and fate of mesenchymal stem cells. Front. Immunol. 2014;5:1–6. doi: 10.3389/fimmu.2014.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mcgaha TL, et al. Amino acid catabolism: A pivotal regulator of innate and adaptive immunity. Immunol. Rev. 2012;249:135–157. doi: 10.1111/j.1600-065X.2012.01149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toledano N, Gur-Wahnon D, Ben-Yehuda A, Rachmilewitz J. Novel CD47: SIRPa Dependent Mechanism for the Activation of STAT3 in Antigen-Presenting Cell. PLoS One. 2013;8 doi: 10.1371/journal.pone.0075595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu X, Kwon H, Li Z, Fu Y. Is CD47 an innate immune checkpoint for tumor evasion? J. Hematol. Oncol. 2017;10:12. doi: 10.1186/s13045-016-0381-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moll G, et al. Do Cryopreserved Mesenchymal Stromal Cells Display Impaired Immunomodulatory and Therapeutic Properties? Stem Cells. 2014;32:2430–2442. doi: 10.1002/stem.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Qiu W, Zhang L, Fung J, Lin F. Painting factor H onto mesenchymal stem cells protects the cells from complement- and neutrophil-mediated damage. Biomaterials. 2016;102:209–219. doi: 10.1016/j.biomaterials.2016.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lipson KE, Wong C, Teng Y, Spong S. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis Tissue Repair. 2012;5:S24. doi: 10.1186/1755-1536-5-S1-S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kabouridis PS, et al. Distinct localization of T cell Agrin during antigen presentation - Evidence for the expression of Agrin receptor(s) in antigen-presenting cells. FEBS J. 2012;279:2368–2380. doi: 10.1111/j.1742-4658.2012.08615.x. [DOI] [PubMed] [Google Scholar]
- 40.Wynn Ta, Yugandhar VG, Clark Ma. Cellular and molecular mechanisms of fibrosis. J Pathol. 2013;46:26–32. [Google Scholar]
- 41.Mertens PR, Wei–min Cai t, Dooley S. Effect of Interferon-Gamma on Hepatic Fibrosis in Chronic Hepatitis B Virus Infection: A Randomized Controlled Study. 2005;3565:819–828. doi: 10.1016/s1542-3565(05)00404-0. [DOI] [PubMed] [Google Scholar]
- 42.Chiche J, et al. Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res. 2009;69:358–368. doi: 10.1158/0008-5472.CAN-08-2470. [DOI] [PubMed] [Google Scholar]
- 43.Baker A, et al. Lysyl oxidase plays a critical role in endothelial cell stimulation to drive tumor angiogenesis. 2013;73:583–594. doi: 10.1158/0008-5472.CAN-12-2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.El-Haibi CP, et al. Critical role for lysyl oxidase in mesenchymal stem cell-driven breast cancer malignancy. Proc. Natl. Acad. Sci. 2012;109:17460–17465. doi: 10.1073/pnas.1206653109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xie Q, et al. Hypoxia triggers angiogenesis by increasing expression of LOX genes in 3-D culture of ASCs and ECs. Exp. Cell Res. 2017;352:157–163. doi: 10.1016/j.yexcr.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 46.Romero-Garcia S, Moreno-Altamirano MMB, Prado-Garcia H, Sánchez-García FJ. Lactate Contribution to the Tumor Microenvironment: Mechanisms, Effects on Immune Cells and Therapeutic Relevance. Front. Immunol. 2016;7:52. doi: 10.3389/fimmu.2016.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mockler MB, Conroy MJ, Lysaght J. Targeting T cell immunometabolism for cancer immunotherapy; understanding the impact of the tumor microenvironment. Front. Oncol. 2014;4:107. doi: 10.3389/fonc.2014.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chang CH, et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell. 2015;162:1229–1241. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roemeling-Van Rhijn M, et al. Effects of hypoxia on the immunomodulatory properties of adipose tissue-derived mesenchymal stem cells. Front. Immunol. 2013;4:1–8. doi: 10.3389/fimmu.2013.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tran DQ, et al. GARP (LRRC32) is essential for the surface expression of latent TGF- on platelets and activated FOXP3+ regulatory T cells. Proc. Natl. Acad. Sci. 2009;106:13445–13450. doi: 10.1073/pnas.0901944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carrillo-Galvez aB, et al. Mesenchymal stromal cells express GARP / LRRC32 on their surface: Effects on their biology and immunomodulatory capacity. Stem Cells. 2015;33:183–95. doi: 10.1002/stem.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel) 2014;6:1670–1690. doi: 10.3390/cancers6031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumar V, Gabrilovich DI. Hypoxia-inducible factors in regulation of immune responses in tumour microenvironment. Immunology. 2014;143:512–519. doi: 10.1111/imm.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baer PC, Overath JM, Urbschat A, Schubert R, Geiger H. Preconditioning of Human Adipose-derived Stromal/Stem Cells: Evaluation of Short-term Preincubation Regimens to Enhance their Regenerative Potential. J. Stem Cell Res. Ther. 2016;6 [Google Scholar]
- 55.Huang YC, Parolini O, Deng L, Yu BS. Should hypoxia preconditioning become the standardized procedure for bone marrow MSCs preparation for clinical use? Stem Cells. 2016;34:1992–1993. doi: 10.1002/stem.2389. [DOI] [PubMed] [Google Scholar]
- 56.Chinnadurai R, et al. Cryopreserved Mesenchymal Stromal Cells Are Susceptible to T-Cell Mediated Apoptosis Which Is Partly Rescued by IFNγ Licensing. Stem Cells. 2016;34:2429–2442. doi: 10.1002/stem.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Francois M, et al. Cryopreserved mesenchymal stromal cells display impaired immunosuppressive properties as a result of heat-shock response and impaired interferon-γ licensing. Cytotherapy. 2012;14:147–152. doi: 10.3109/14653249.2011.623691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Killer MC, et al. Immunosuppressive capacity of mesenchymal stem cells correlates with metabolic activity and can be enhanced by valproic acid. Stem Cell Res. Ther. 2017;8:100. doi: 10.1186/s13287-017-0553-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oswald ES, Brown LM, Bulinski JC, Hung CT. Label-free protein profiling of adipose-derived human stem cells under hyperosmotic treatment. J. Proteome Res. 2011;10:3050–3059. doi: 10.1021/pr200030v. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.