Abstract
Neuroinflammation is an essential mechanism involved in the pathogenesis of subarachnoid hemorrhage (SAH)-induced brain injury. Recently, Netrin-1 (NTN-1) is well established to exert anti-inflammatory property in non-nervous system diseases through inhibiting infiltration of neutrophil. The present study was designed to investigate the effects of NTN-1 on neuroinflammation, and the potential mechanism in a rat model of SAH. Two hundred and ninety-four male Sprague Dawley rats (weight 280–330 g) were subjected to the endovascular perforation model of SAH. Recombinant human NTN-1 (rh-NTN-1) was administered intravenously. Small interfering RNA (siRNA) of NTN-1 and UNC5B, and a selective PPARγ antagonist bisphenol A diglycidyl ether (BADGE) were applied. Post-SAH evaluations included neurobehavioral function, brain water content, Western blot analysis, and immunohistochemistry. Our results showed that endogenous NTN-1 and its receptor UNC5B level were increased after SAH. Administration of rh-NTN-1 reduced brain edema, ameliorated neurological impairments, and suppressed microglia activation after SAH, which were concomitant with PPARγ activation, inhibition of NFκB, and decrease in TNF-α, IL-6, and ICAM-1, as well as myeloperoxidase (MPO). Knockdown of endogenous NTN-1 increased expression of pro-inflammatory mediators and MPO, and aggravated neuroinflammation and brain edema. Moreover, knockdown of UNC5B using specific siRNA and inhibition of PPARγ with BADGE blocked the protective effects of rh-NTN-1. In conclusion, our findings indicated that exogenous rh-NTN-1 treatment attenuated neuroinflammation and neurological impairments through inhibiting microglia activation after SAH in rats, which is possibly mediated by UNC5B/PPARγ/NFκB signaling pathway. Exogenous NTN-1 may be a novel therapeutic agent to ameliorating early brain injury via its anti-inflammation effect.
Keywords: rain edema, Early brain injury, Microglia, Netrin-1, Neuroinflammation, Subarachnoid hemorrhage
1. Introduction
Activation of neuroinflammatory response plays a crucial role in the progression and exacerbation of brain injury following subarachnoid hemorrhage (SAH) (Lucke-Wold et al., 2016). The innate immune response triggered by SAH is characterized by leukocyte infiltration, resident immune cells activation, and release of pro-inflammatory mediators within brain tissues, which result in blood-brain barrier (BBB) disruption and brain injury via various mechanisms (Frontera et al., 2012; Frontera et al., 2017). Therefore, inhibition of inflammatory response represent a potential treatment for attenuating brain injury following SAH (Chen et al., 2014; Provencio et al., 2016).
Netrin-1 (NTN-1), a laminin-related molecule, was initially discovered as a chemoattractive or chemorepulsive cue for directing axon outgrowth and neuron migrating during the development of the nervous system (Serafini et al., 1996). NTN-1 is also known to modulate the immune response via the inhibition of inflammatory cells migration and control inflammation in non-nervous system diseases, such as hypoxia, acute lung injury, liver injury, peritonitis, and inflammatory bowel disease (Aherne et al., 2013; Aherne et al., 2012; Ly et al., 2005; Mirakaj et al., 2011; Mirakaj et al., 2010; Rosenberger et al., 2009; Schlegel et al., 2016). Exogenous NTN-1 treatment has been shown to reduce the inflammation-mediated kidney injury by suppressing leukocytes infiltration and the production of inflammatory cytokine and chemokine through activating its receptor uncoordinated family member 5 B (UNC5B), which is abundantly expressed in leukocytes (Ranganathan et al., 2013a; Tadagavadi et al., 2010). A recent study reported that NTN-1 could prevent immune cells infiltration into brain parenchyma and ameliorate the severity of experimental autoimmune encephalomyelitis (EAE)-related inflammation, suggesting that NTN-1 exerts the potent anti-inflammatory property in autoimmune central nervous system diseases (Podjaski et al., 2015). Our previous study also showed that NTN-1 was upregulated in the brain and possessed neuroprotective functions of anti-apoptosis and preserving BBB integrity after SAH via distinct pathways (Xie et al., 2017b; Xie et al., 2017c). Nevertheless, the effect of NTN-1 on neuroinflammation after SAH has not been determined so far.
Peroxisome proliferator-activated receptor gamma (PPARγ), belonging to the nuclear hormone receptor superfamily, is a pivotal transcription factor that regulates inflammation, apoptosis, and oxidative stress (Glatz et al., 2010; Lehrke and Lazar, 2005; Zhao et al., 2015). PPARγ was identified as a downstream molecule of NTN-1 in response to inflammation during ischemia-reperfusion (IR) injury in kidney and heart (Mao et al., 2014; Ranganathan et al., 2013b). Moreover, activation of PPARγ effectively diminished inflammation through inhibiting NFκB pathway and reducing the production of pro-inflammatory cytokines after stroke (Culman et al., 2007; Zhao et al., 2015).
In the present study, we hypothesized that exogenous recombinant human NTN-1 (rh-NTN-1) binding UNC5B receptor could attenuate neuroinflammation and early brain injury (EBI) after SAH, and the anti-inflammation mechanism of rh-NTN-1 is mediated through PPARγ/NFκB-related signaling pathway.
2. Materials and Methods
2.1. Animals
All experimental protocols in this study were approved by the Institutional Animal Care and Use Committee at Loma Linda University. The study complied with the National Institutes of Health’s Guide for the Care and the Use of Laboratory Animals and the ARRIVE (Animal Research: Reporting In Vivo Experiments) guidelines. Adult male Sprague Dawley rats (weight 280–330 g; Indianapolis, IN) were housed in a controlled humidity and temperature room with a 12-h light/dark cycle and ad libitum access to water and food.
2.2. Experimental design
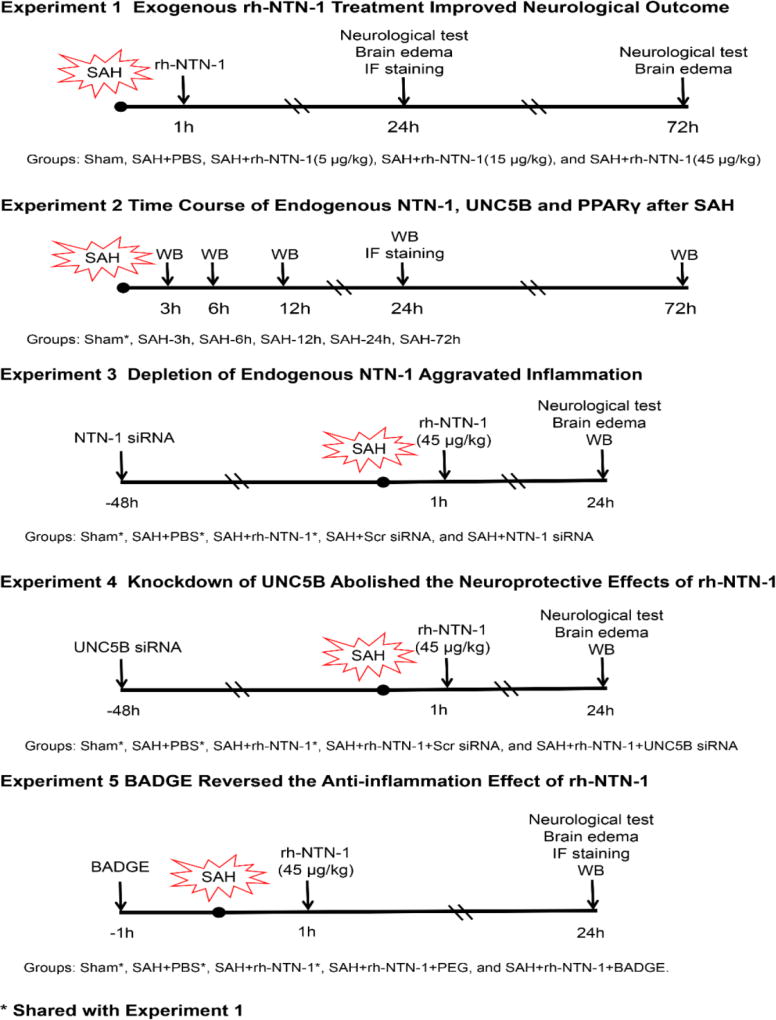
Five separate experiments were performed in a rat model of SAH, as shown in Figure 1. A total of 294 rats were used (Table 1).
Fig. 1.
Experimental design and animal groups. BADGE, bisphenol A diglycidyl ether; IF staining, immunofluorescence staining; NTN-1 siRNA, Netrin-1 siRNA; PEG, polyethyleneglycol; rh-NTN-1, recombinant human Netrin-1; SAH, Subarachnoid hemorrhage; Scr siRNA, Scrambled siRNA; siRNA, small interfering RNA; UNC5B siRNA, UNC5B siRNA; WB, Western blot.
Table 1.
Summary of experimental groups, sample sizes, and mortality rate in the study
| Experimental Groups | Neurological test Brain edema |
IF staining |
WB | Exclusion | Mortality | Subtotal |
|---|---|---|---|---|---|---|
| Sham | 12 | 6 | 0 | 0 | 18 | |
| SAH (3h, 6h, 12h, 24h, 72h) | 3 | 6×5 | 5 | 11 (22.45%) | 49 | |
| SAH+PBS | 12 | 4 | 6 | 4 | 8 (23.53%) | 34 |
| SAH+rh-NTN-1 (5 µg/kg) | 12 | 2 | 4 (22.22%) | 18 | ||
| SAH+rh-NTN-1 (15 µg/kg) | 12 | 0 | 3 (20%) | 15 | ||
| SAH+rh-NTN-1 (45 µg/kg) | 12 | 4 | 6 | 3 | 5 (16.67%) | 30 |
| SAH+Scr siRNA | 6 | 6 | 2 | 4 (22.22%) | 18 | |
| SAH+NTN-1 siRNA | 6 | 6 | 2 | 5 (26.32%) | 19 | |
| SAH+rh-NTN-1+Scr siRNA | 6 | 6 | 2 | 3 (17.65%) | 17 | |
| SAH+rh-NTN-1+UNC5B | 6 | 6 | 2 | 4 (22.22%) | 18 | |
| siRNA | ||||||
| SAH+rh-NTN-1+PEG | 6 | 6 | 1 | 3 (18.75%) | 16 | |
| SAH+rh-NTN-1+ BADGE | 6 | 4 | 6 | 2 | 5 (21.74%) | 23 |
| Naive +PBS | 3 | 0 | 0 | 3 | ||
| Naive +rh-NTN-1 | 3 | 0 | 0 | 3 | ||
| Naive +Scr siRNA | 3 | 0 | 0 | 3 | ||
| Naive +NTN-1 siRNA | 3 | 0 | 0 | 3 | ||
| Naive +UNC5B siRNA | 3 | 0 | 0 | 3 | ||
| SAH+UNC5B siRNA | 3 | 0 | 1 (25%) | 4 | ||
| Total | 25 | 56 (19.05%) | 294 |
BADGE, bisphenol A diglycidyl ether; IF staining, immunofluorescence staining; NTN-1 siRNA, Netrin-1 siRNA mixtures; PBS, phosphate-buffered saline; PEG, polyethyleneglycol; rh-NTN-1, recombinant human Netrin-1; SAH, Subarachnoid hemorrhage; Scr siRNA, Scrambled siRNA; siRNA, small interfering RNA; UNC5B siRNA, UNC5B siRNA mixtures; WB, Western blot.
Experiment 1
The role of rh-NTN-1 treatment in neuroinflammation was determined at 24 h and 72 h after SAH. Exogenous rh-NTN-1 (R&D Systems, USA) dissolved in phosphate-buffered saline (PBS) was administered through tail vein with a total volume of 200 µL at 1 h after SAH induction. Neurobehavioral function, and brain water content were examined at 24 h and 72 h post-SAH. Microglia activation was detected by immunofluorescence staining at 24 h after SAH. Rats were randomly divided into five groups: sham, SAH + PBS, SAH + rh-NTN-1 (5 µg/kg), SAH + rh-NTN-1 (15 µg/kg), and SAH + rh-NTN-1 (45 µg/kg).
Experiment 2
The time course of endogenous NTN-1, its receptor UNC5B, and PPARγ protein levels in the ipsilateral/left cerebral cortex at 3, 6, 12, 24, and 72h after SAH were measured by Western blot analysis. The cellular localization of NTN-1 and UNC5B were detected using double immunofluorescence staining.
Experiment 3
To evaluate the effect of in vivo knockdown of endogenous NTN-1 on neuroinflammation. NTN-1 small interfering RNA (NTN-1 siRNA) was administered by intracerebroventricular injection (i.c.v.) at 48 h before SAH induction. SAH grade, neurobehavioral function, brain water content, and Western blot were measured at 24 h after SAH. Rats were randomly divided into five groups: sham, SAH + PBS, SAH + rh-NTN-1 (45 µg/kg), SAH + scrambled siRNA (Scr siRNA), and SAH + NTN-1 siRNA.
Experiment 4
To assess the role of UNC5B receptor in the neuroprotective effects of exogenous rh-NTN-1. UNC5B siRNA was administered by i.c.v. at 48 h prior to SAH induction and then followed with rh-NTN-1 (45 µg/kg) treatment at 1 h after SAH. Neurobehavioral function and brain water content were evaluated, and inflammation-related molecules were detected by Western blot at 24 h after SAH. Rats were randomly divided into five groups: sham, SAH + PBS, SAH + rh-NTN-1, SAH + rh-NTN-1 + Scr siRNA, and SAH + rh-NTN-1 + UNC5B siRNA.
Experiment 5
To explore the role of PPARγ in the anti-inflammation effect of exogenous rh-NTN-1. The selective PPARγ antagonist bisphenol A diglycidyl ether (BADGE) (30 mg/kg, Sigma-Aldrich, MO, USA) [22] dissolved in polyethyleneglycol (PEG) was injected intraperitoneally (i.p.) with a total volume of 200 µL at 1 h before SAH induction, and then followed with rh-NTN-1 (45 µg/kg) treatment at 1 h after SAH. Control animals were injected PEG (2 ml/kg) at 1 h before SAH induction and then followed with rh-NTN-1 (45 µg/kg) treatment. Neurobehavioral function, brain water content, and Western blot were evaluated at 24 h post-SAH. Rats were randomly divided into five groups: sham, SAH + PBS, SAH + rh-NTN-1, SAH + rh-NTN-1 + PEG, and SAH + rh-NTN-1 + BADGE.
2.3. SAH model
The endovascular perforation model of SAH was performed as previously described (Enkhjargal et al., 2017). Briefly, rats were anesthetized with 3% isoflurane and mechanically ventilated throughout the operation. Body temperature was maintained at 37 °C ± 0.5 °C using a heating lamp. A sharpened, 4-0 monofilament nylon suture was inserted into the left internal carotid artery from the external carotid artery, and then advanced 3 mm further to perforate the bifurcation of the anterior and middle cerebral arteries. In the sham group, rats were subjected to the same procedures without perforation. After the surgical procedure was completed, the rats were allowed to recover on a heated blanket.
2.4. Intracerebroventricular injection
As described previously (Huang et al., 2015), rats were anesthetized with 3% isoflurane and placed on a stereotaxic frame. The needle of a 10-µL Hamilton syringe (Microliter 701; Hamilton Company, USA) was inserted through a burr hole into the right lateral ventricle according to the following coordinates relative to bregma: 1.5 mm posterior, 1.0 mm lateral, and 3.2mm below the horizontal plane of the bregma. Following the manufacturer’s instructions, a total volume of 5 µL (500 pmol) of rat NTN-1 siRNA (Thermo Fisher Scientific, USA), or UNC5B siRNA (Thermo Fisher Scientific, USA) dissolved in nuclease-free water was injected into the right ventricle by a pump at the rate of 0.5 µL/min at 48 h before SAH. The same volume of Scr siRNA (Thermo Fisher Scientific, USA) was used as a negative control. The needle was kept in place for an additional 5 minutes after injection to prevent possible leakage and was slowly withdrawn within 5 minutes. After the needle was removed, the burr hole was sealed with bone wax, the incision was closed with sutures and rats were allowed to recover.
2.5. Short-term neurological score evaluation
Neurobehavioral function were evaluated before euthanasia by an investigator (W.W.) blind to group information using the modified Garcia and Beam balance tests as previously described (Wu et al., 2016). The modified Garcia test assessed spontaneous activity, spontaneous movement of all limbs, forelimbs outstretching, climbing, touch of trunk, and vibrissae touch. Each part of the test has a score ranging from 0 to 3, with a maximum score of 18. Beam balance test evaluated rat’s walking distances on a wooden beam for 1 minute. The scores for this test ranged from 0 to 4. Higher scores represented better neurological function.
2.6. SAH grade
Assessment of the severity of SAH was performed in a blinded fashion with a grading system immediately after euthanasia as previously described (Sugawara et al., 2008). The base of the brain was divided into six parts, each part was scored (0–3) according to the amount of subarachnoid blood. The total score was calculated as the total SAH grade (maximum SAH grade = 18). SAH rats with a score < 8 at 24 hours were excluded from this study.
2.7. Brain water content
Brains were quickly removed at 24 h or 72 h after surgery, and separated into left hemisphere, right hemisphere, cerebellum, and brain stem. Each part was weighted immediately after removal (wet weight) and then dried in an oven at 105 °C for 72 h (dry weight). After that, the percentage of brain water content was calculated as [(wet weight-dry weight)/wet weight] × 100% (Chen et al., 2015).
2.8. Immunohistochemistry staining
Immunohistochemistry staining was performed as described previously (Guo et al., 2016). Briefly, at 24 h after operation, rats were transcardially perfused under deep anesthesia with ice-cold PBS (0.1M, pH 7.4), then perfused with 10% paraformaldehyde. Brains were removed and fixed in 10% paraformaldehyde at 4°C for 24 h, and then in 30% sucrose for 72 h. Frozen coronal slices (10 µm) were sectioned in cryostat (CM3050S; Leica Microsystems). Sections were blocked with 5% donkey serum for 1 hour and incubated overnight at 4°C with the following primary antibodies: rabbit anti-NTN-1 (1:500, Abcam, USA), rabbit anti-UNC5B (1:200, Abcam, USA), mouse anti-CD31 (1:100, Abcam, USA), goat anti-ionized calcium binding adaptor molecule 1 (Iba-1, 1:200, Abcam, USA), mouse anti-NeuN (1:500, Abcam, USA), and mouse anti-glial fibrillary acidic protein (GFAP, 1:500, Abcam, USA). Appropriate FITC-conjugated secondary antibodies (Jackson ImmunoResearch, West Grove, PA) were applied in the dark for 2 h at room temperature. Negative control staining was performed by omitting the primary antibody. The sections were visualized under a fluorescence microscope Leica DMi8 (Leica Microsystems, Germany). Microphotographs were analyzed with LASX software. The number of Iba-1 positive cells was counted in 3 different fields in the ipsilateral cortex from 5 random coronal sections per brain using a magnification of ×400 over a microscopic field of 0.01 mm2, and data were expressed as cells/field (Zhang et al., 2015).
2.9. Western blot analysis
After rats were perfused with ice-cold PBS (0.1M, pH 7.4) at 24 h post-operation, the ipsilateral cortex were collected and stored in −80 °C freezer until use. Western blot was performed as described previously (Tong et al., 2017). After protein samples preparation, equal amounts of protein (50 µg) were separated by SDS-PAGE gel electrophoresis, and then transferred onto nitrocellulose membranes. Membranes were blocked and incubated with the following primary antibodies overnight at 4 °C: rabbit anti-NTN-1 (1:800, Abcam, USA), rabbit anti-UNC5B (1:1000, Abcam, USA), rabbit anti-PPARγ (1:500, Abcam, USA), rabbit anti-NFκB P65 (1:1000, Abcam, USA), mouse anti-IL-6 (1:1000, Abcam, USA), goat anti-TNF-α (1:1000, Abcam, USA), rabbit anti-ICAM-1 (1:1000, Santa Cruz Biotechnology, USA), and rabbit anti-myeloperoxidase (MPO, 1:1000, Santa Cruz Biotechnology, USA). β-actin was used as the internal loading control. Then, membranes were incubated with horseradish-peroxidase conjugated secondary antibodies (Santa Cruz Biotechnology, USA) for 1 h at room temperature, The immunoblots were probed with an ECL Plus chemiluminescence reagent kit (Amersham Biosciences, USA). The relative density of protein was analyzed by ImageJ software (ImageJ 1.5, NIH, USA).
2.10. Statistics analysis
All Data were presented as a mean ± SD. All analyses were performed using SigmaPlot 11.0 and GraphPad Prism 6 (GraphPad software). Data normality was first confirmed using the Shapiro-Wilk normality test. For the data that passed the normality test, the statistical differences among groups were further analyzed using one-way ANOVA followed by Tukey multiple comparison post hoc analysis. For the data that failed the normality test, Kruskal-Wallis one-way ANOVA on Ranks was used, followed by Tukey multiple comparison post hoc analysis. P value of less than 0.05 was considered statistically significant.
3. Results
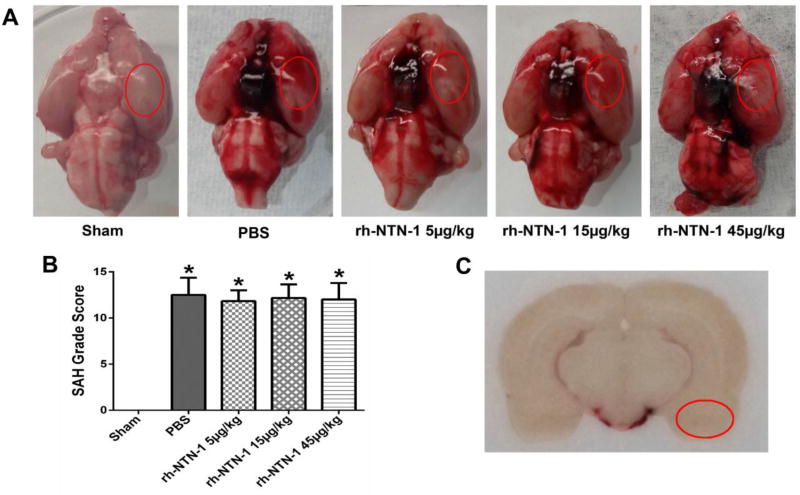
3.1. Mortality rates and SAH grade score
The overall mortality of SAH was 19.05% (56/294). There was no significant difference in mortality rates among all SAH groups (P > 0.05). No rats died in the sham group. Representative brain samples in each group are presented in Fig. 2A. At 24 h after SAH, subarachnoid blood clots were obviously present around the Circle of Willis (Fig. 2A). The SAH grade scores were not significantly different among all SAH groups (P > 0.05, Fig. 2B). According to the SAH grade score, 25 rats with mild SAH were excluded from this study (Table 1). The basal cortex of the left hemisphere was the region of interest for Immunochemistry staining and Western blot (Fig. 2C).
Fig. 2.
Representative pictures of brains from each group and SAH grade score at 24 h after SAH. (A) Representative brains from sham, PBS, rh-NTN-1 (5 µg/kg), rh-NTN-1 (15 µg/kg), and rh-NTN-1 (45 µg/kg). (B) Quantitative analyses of SAH grade score. n=6/group. *P<0.05 vs sham. (C) The schematic diagram shows the optimal region of brain section for Immunochemistry staining and Western blot (red circle).
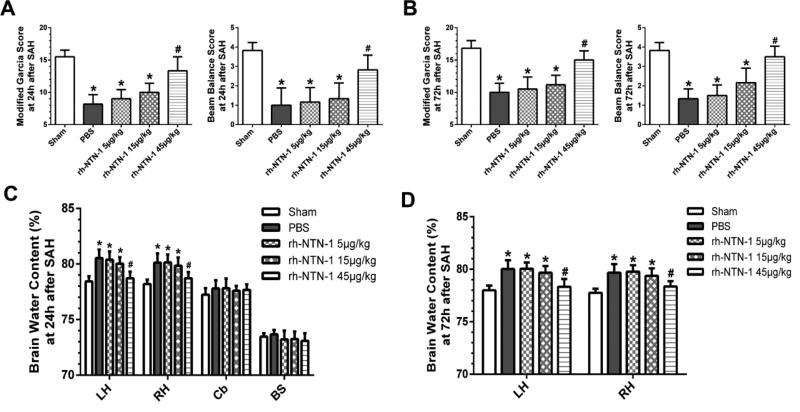
3.2. Exogenous rh-NTN-1 treatment attenuated neurobehavioral deficits and brain edema after SAH
At 24 h post-SAH, rats in the PBS and rh-NTN-1 (5 and 15 µg/kg) groups showed severe neurological deficits (P < 0.05, Fig. 3A) and higher brain water content in the left and right hemispheres (P < 0.05, Fig. 3C), when compared with sham group. However, administration of rh-NTN-1 at a dose of 45 µg/kg evidently improved neurological performance (P < 0.05, Fig. 3A, B) and diminished brain water content in both hemispheres (P < 0.05, Fig. 3C, D) both at 24 h and 72 h after SAH, when compared with PBS, rh-NTN-1 groups at dose of 5 and 15 µg/kg. Given that the high dose of rh-NTN-1 (45 µg/kg) was most effective on neurobehavioral score and brain water content, we decided to use this high dose for the following studies.
Fig. 3.
The effects of exogenous recombinant human Netrin-1 (rh-NTN-1) on neurological function and brain edema after SAH. Administration of exogenous rh-NTN-1 at a dose of 45 µg/kg markedly improved neurological impairments (A, B) and reduced brain water content in the left and right hemispheres (C, D) both at 24 h and 72 h post-SAH. n=6/group. *P<0.05 vs sham; #P<0.05 vs PBS, rh-NTN-1 (5 µg/kg), and rh-NTN-1 (15 µg/kg). BS, brain stem; Cb, cerebellum; LH, left hemisphere; and RH, right hemisphere.
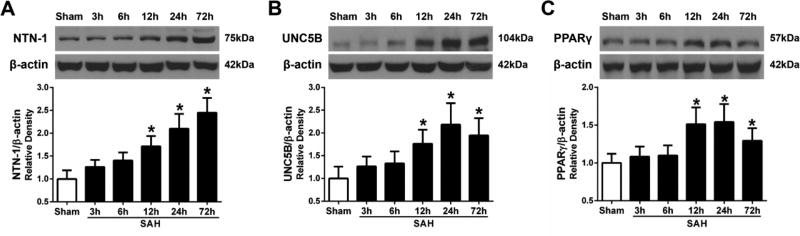
3.3. Expression of endogenous NTN-1, UNC5B receptor and PPARγ after SAH
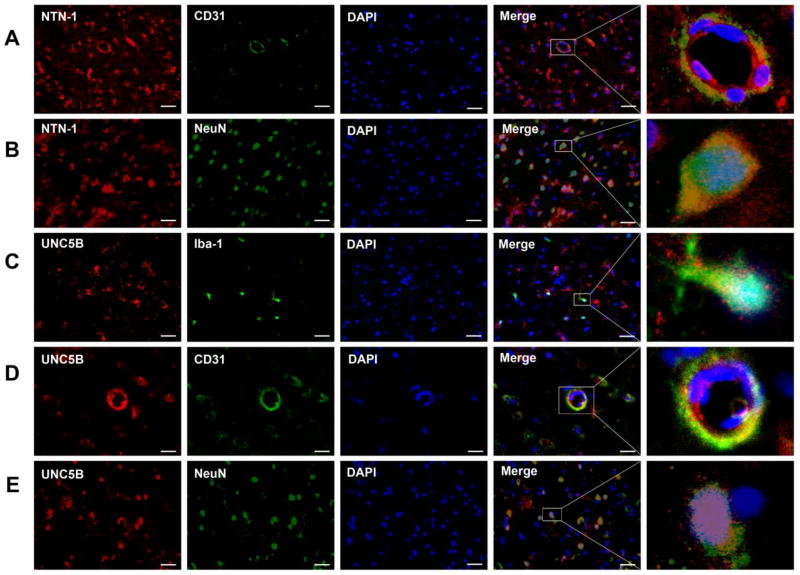
Western blot analysis showed there was significant increase in endogenous NTN-1 level in the ipsilateral cortex at 12, 24, and 72 h after SAH (P < 0.05, Fig. 4A). UNC5B level was slowly increased from 12 h, and reached its peak at 24 h, but declined at 72 h after SAH (P < 0.05, Fig. 4B). PPARγ was significantly increased from 12 h and peaked at 24 h, but decreased at 72 h after SAH (P < 0.05, Fig. 4C). Double immunofluorescence staining revealed that NTN-1 was predominantly expressed in endothelial cells (Fig. 5A), and neurons (Fig. 5B) in cerebral cortex at 24 hours after SAH. UNC5B was expressed in microglia (Fig. 5C), endothelial cells (Fig. 5D), and neurons (Fig. 5E) at 24 h after SAH.
Fig. 4.
Time course of endogenous Netrin-1 (NTN-1), UNC5B receptor, and PPARγ expression after SAH. Representative Western blot bands and quantitative analyses of NTN-1 (A), UNC5B (B), and PPARγ expression (C) in the ipsilateral cortex after SAH. Relative densities of each protein have been normalized against the sham group. n=6/group. *P<0.05 vs sham.
Fig. 5.
The cellular localization of Netrin-1 (NTN-1) and UNC5B receptor in the ipsilateral cortex. Representative microphotographs of double immunofluorescence staining showed that NTN-1 was expressed in endothelial cells (A) and neurons (B) in cerebral cortex at 24 h after SAH. UNC5B was co-localized with ionized calcium binding adaptor molecule 1 (Iba-1) positive microglia (C), also expressed in endothelial cells (D) and neurons (E) in cerebral cortex at 24 h after SAH. n=3/group. Scale bar=50 µm.
3.4. Depletion of endogenous NTN-1 exacerbated neurological impairments, brain edema and inflammatory response after SAH
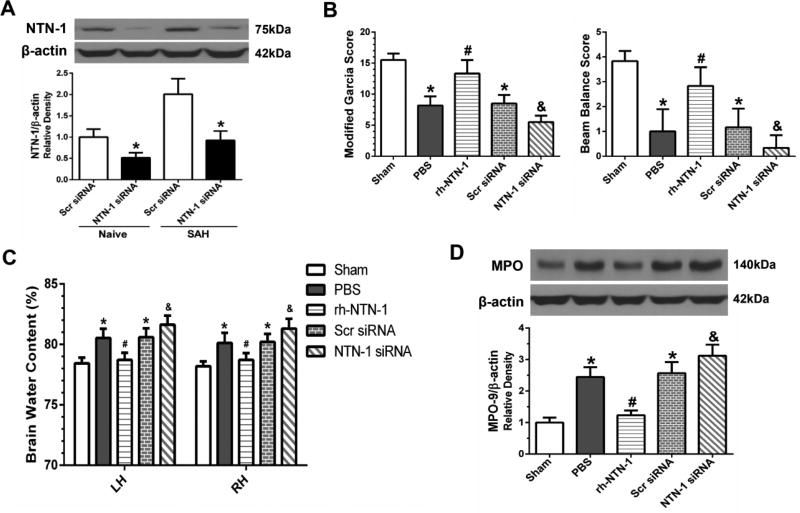
The depletion efficiency of NTN-1 siRNA was confirmed by Western blot. The result showed that NTN-1 expression in the ipsilateral cortex was inhibited by NTN-1 siRNA (P < 0.05, Fig. 6A). Depletion of endogenous NTN-1 exacerbated neurological impairments (P < 0.05, Fig. 6B), and increased brain water content in both hemispheres (P < 0.05, Fig. 6C) at 24 h after SAH. We explored the effects of NTN-1 siRNA on MPO expression in the ipsilateral cortex by Western blot. As shown in Figure 6D, administration of exogenous rh-NTN-1 decreased MPO expression in the ipsilateral cortex at 24 h after SAH (P < 0.05, Fig. 6D). However, knockdown of endogenous NTN-1 using specific siRNA increased MPO expression (P < 0.05, Fig. 6D).
Fig. 6.
The effects of depletion of endogenous Netrin-1 (NTN-1) on neurological scores, brain edema, and myeloperoxidase (MPO) expression after SAH. (A) NTN-1 expression in the ipsilateral cortex was significantly inhibited by NTN-1 siRNA. n=3/group. *P<0.05 vs Scr siRNA. Depletion of endogenous NTN-1 significantly exacerbated neurological deficits (B), and increased brain water content (C) in both hemispheres at 24 h after SAH. (D) Representative Western blot band and quantitative analyses of MPO expression indicated that exogenous rh-NTN-1 treatment decreased MPO expression in the ipsilateral cortex, and NTN-1 siRNA pretreatment increased MPO expression. Relative density of MPO protein has been normalized against the sham group. n=6/group. *P<0.05 vs sham; #P<0.05 vs PBS; and &P<0.05 vs PBS, rh-NTN-1 and Scr siRNA. NTN-1 siRNA, Netrin-1 siRNA; Scr siRNA, scrambled siRNA. LH, left hemisphere; RH, right hemisphere.
3.5. Effect of exogenous rh-NTN-1 on expression of inflammatory molecules after SAH
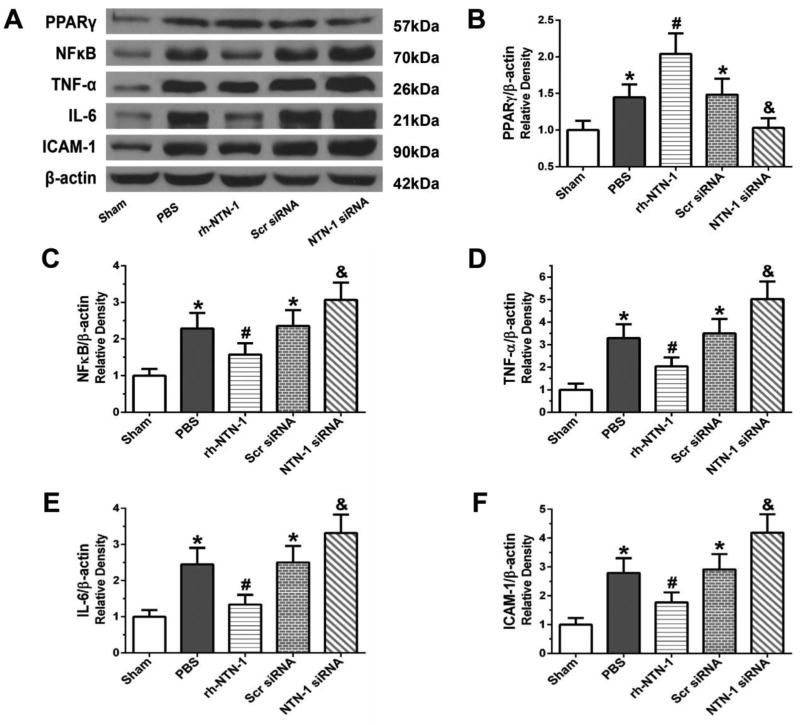
Administration of exogenous rh-NTN-1 further augmented PPARγ expression in the ipsilateral cortex at 24 h post-SAH, which suppressed the expression of downstream inflammation-related proteins including NFκB, TNF-α, IL-6, and ICAM-1 (P < 0.05, Fig. 7A–F), compared with PBS group. In contrast, depletion of endogenous NTN-1 by NTN-1 siRNA reduced PPARγ level and enhanced the expression of inflammation-related molecules (P < 0.05, Fig. 7A–F).
Fig. 7.
The effects of recombinant human Netrin-1 (rh-NTN-1) and knockdown of endogenous NTN-1 on expression of inflammatory molecules at 24 h after SAH. Exogenous rh-NTN-1 treatment further augmented PPARγ expression, and inhibited the expression of NFκB, TNF-α, IL-6, and ICAM-1 in the ipsilateral cortex (A–F). In contrast, when depleted the endogenous NTN-1 with specific siRNA, PPARγ expression was decreased, but the expression of NFκB, TNF-α, IL-6, and ICAM-1 were increased (A–F). Relative densities of each protein have been normalized against the sham group. n=6/group. *P<0.05 vs sham; #P<0.05 vs PBS; and &P<0.05 vs PBS, rh-NTN-1 and Scr siRNA. NTN-1 siRNA, Netrin-1 siRNA; Scr siRNA, scrambled siRNA.
3.6. Knockdown of UNC5B abolished the neuroprotective effects of rh-NTN-1 after SAH
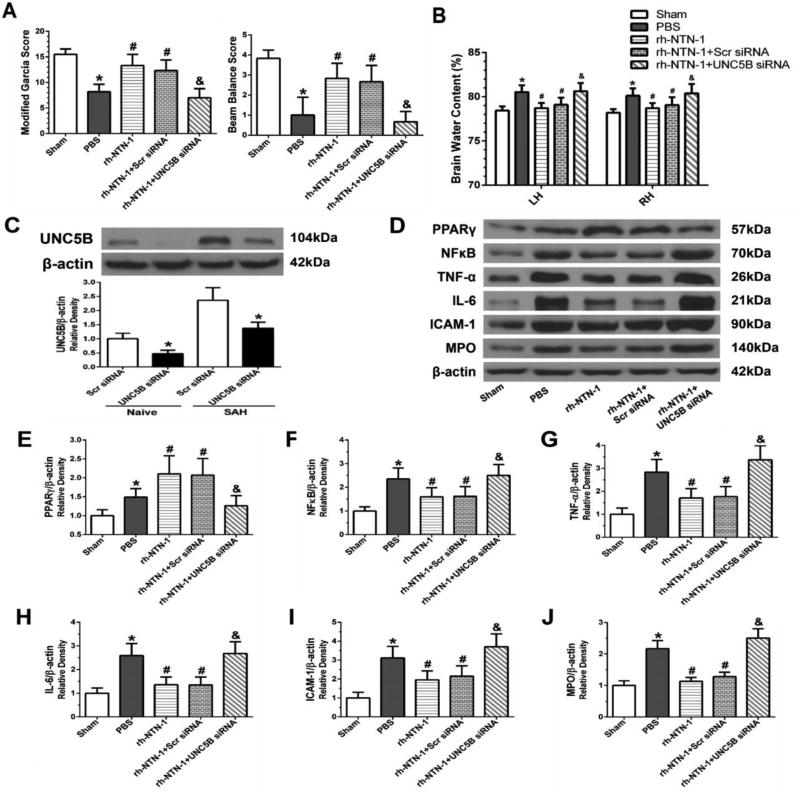
Knockdown of UNC5B by specific siRNA significantly abolished the neuroprotective effects of rh-NTN-1 against neurological impairments (P < 0.05, Fig. 8A) and brain edema (P < 0.05, Fig. 8B) at 24 h post-SAH. Western blot result showed UNC5B siRNA inhibited UNC5B expression in ipsilateral cortex at 72 h after siRNA injection (P < 0.05, Fig. 8C). Knockdown of UNC5B significantly decreased PPARγ expression, which was accompanied by increasing the expression of NFκB, TNF-α, IL-6, and ICAM-1, as well as MPO in the ipsilateral cortex at 24 h after SAH (P < 0.05, Fig. 8D–J).
Fig. 8.
The effects of UNC5B siRNA on the neuroprotection associated with recombinant human Netrin-1 (rh-NTN-1) at 24 h after SAH. Knockdown of UNC5B significantly abolished the neuroprotective effects of rh-NTN-1 against neurological impairments (A) and brain edema (B) at 24 h post-SAH. (C) UNC5B siRNA efficiently knocked down UNC5B expression in the ipsilateral cortex. n=3/group. *P<0.05 vs Scr siRNA. Knockdown of UNC5B abolished the effects of rh-NTN- 1 on the promotion of PPARγ expression, and inhibition of the expression of NFκB, TNF-α, IL-6, and ICAM-1, as well as MPO in the ipsilateral cortex (D–J). Relative densities of each protein have been normalized against the sham group. n=6/group. *P<0.05 vs sham; #P<0.05 vs PBS; and &P<0.05 vs rh-NTN-1 and rh-NTN-1+Scr siRNA. UNC5B siRNA, UNC5B siRNA; Scr siRNA, scrambled siRNA.
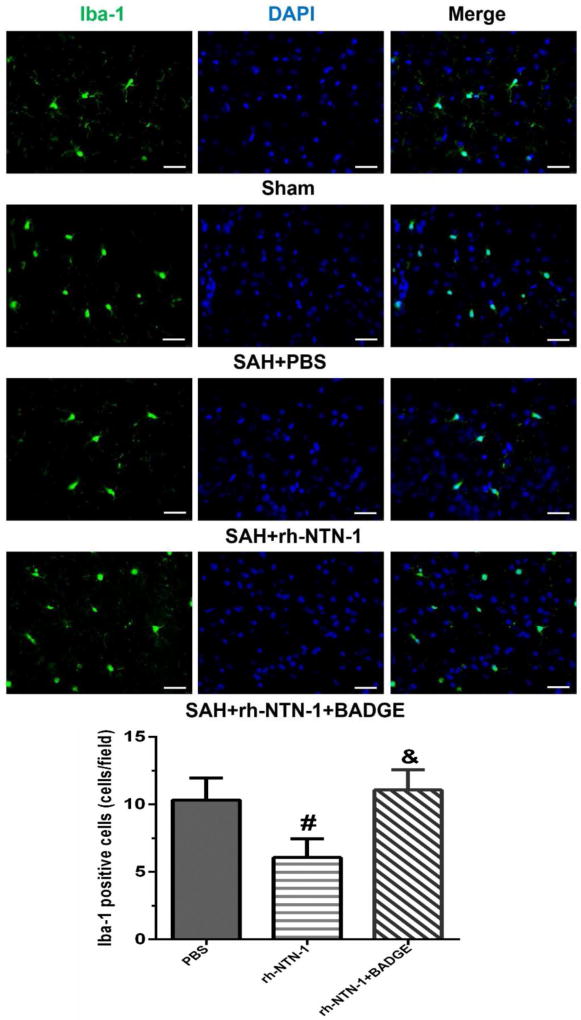
3.7. Exogenous rh-NTN-1 treatment suppressed microglia activation after SAH
We examined the effects of rh-NTN-1 and BADGE on Iba-1 positive cells. As shown in Figure 9, the numbers of Iba-1positive cells were significantly increased in the ipsilateral cortex in PBS group at 24 h after SAH, and activated microglia showed shorter processes (Fig. 9A). Administration of exogenous rh-NTN-1 significantly reduced the number of Iba-1-positive cells (P < 0.05, Fig. 9A, B). However, BADGE reversed the effect of exogenous rh-NTN-1 (P < 0.05, Fig. 9A, B).
Fig. 9.
Microglia activation in the ipsilateral cortex at 24 h after SAH. Representative immunofluorescence microphotographs and quantitative analysis of ionized calcium binding adaptor molecule 1 (Iba-1) positive cells showed that administration of exogenous recombinant human Netrin-1 (rh-NTN-1) significantly decreased the number of Iba-1 positive cells. However, BADGE abolished the effect of exogenous rh-NTN-1. n=4/group, 15 fields/rat. #P<0.05 vs PBS; and &P<0.05 vs rh-NTN-1. BADGE, bisphenol A diglycidyl ether. Scale bar=100 µm.
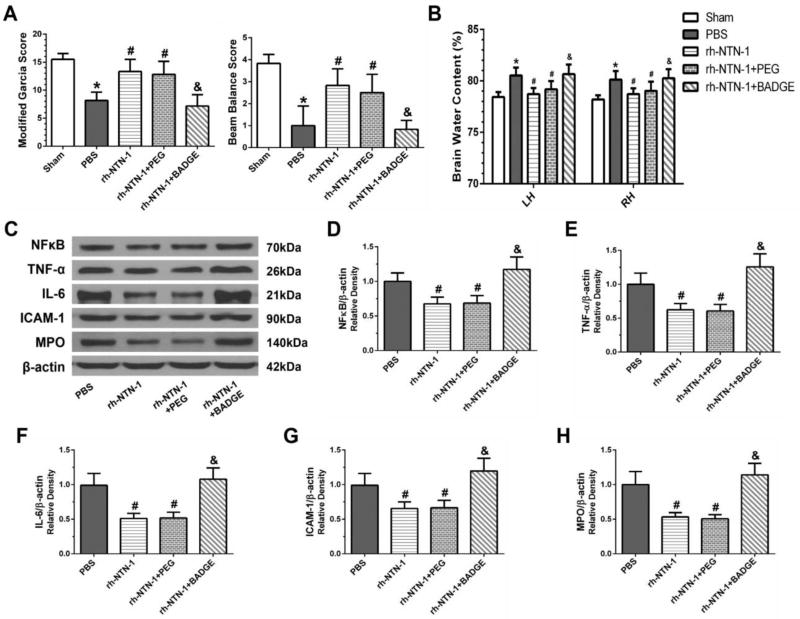
3.8. Inhibition of PPARγ reversed the anti-inflammation function of rh-NTN-1 after SAH
PPARγ antagonist BADGE significantly aggravated neurological impairments (P < 0.05, Fig. 10A) and increased brain water content (P < 0.05, Fig. 10B) at 24 h after SAH. Moreover, BADGE upregulated the expression of NFκB, TNF-α, IL-6, ICAM-1, and MPO in the ipsilateral cortex at 24 h post-SAH (P < 0.05, Fig. 10C–H).
Fig. 10.
Inhibition of PPARγ by BADGE reversed the anti-inflammation effect of recombinant human Netrin-1 (rh-NTN-1) at 24 h after SAH. BADGE significantly aggravated neurological deficits (A) and increased brain edema (B). BADGE reversed the effects of rh-NTN-1 on the inhibition of the expression of NFκB, TNF-α, IL-6, and ICAM-1, as well as MPO in the ipsilateral cortex (C–H). Relative densities of each protein have been normalized against the PBS group. n=6/group. *P<0.05 vs sham; #P<0.05 vs PBS; and &P<0.05 vs rh-NTN-1 and rh-NTN-1+PEG. BADGE, bisphenol A diglycidyl ether; PEG, polyethyleneglycol; LH, left hemisphere; RH, right hemisphere.
4. Discussion
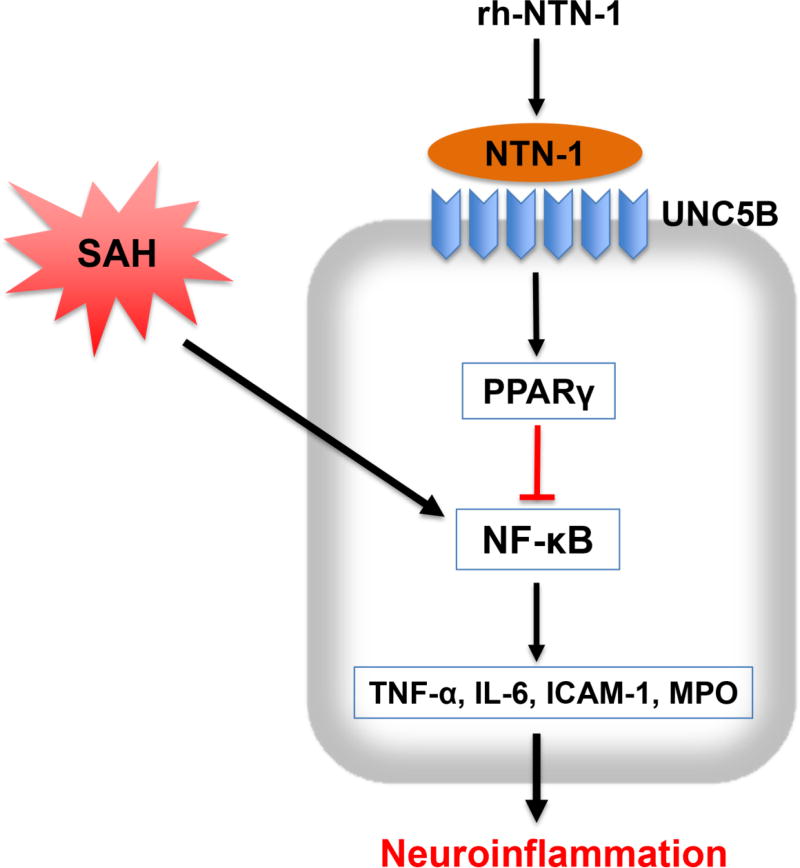
The novel findings in the current study were: (1) endogenous NTN-1 and UNC5B receptor levels were significantly increased in the ipsilateral cortex during the early stage of SAH; (2) administration of exogenous rh-NTN-1 at a dose of 45 µg/kg significantly ameliorated brain edema and neurological impairments after SAH; (3) exogenous rh-NTN-1 treatment suppressed microglia activation, and inhibited the expression of MPO, TNF-α, IL-6, and ICAM-1 in the ipsilateral cortex, thereby attenuated brain injury after SAH; (4) knockdown of endogenous NTN-1 by specific siRNA significantly increased the expression of inflammatory molecules, aggravated neurological impairments at 24 h post-SAH; (5) UNC5B siRNA reversed the beneficial effect of rh-NTN-1 on neuroinflammation at 24 h following SAH; (6) PPARγ antagonist BADGE also abolished the anti-inflammatory function of exogenous rh-NTN-1 after SAH. Taken together, our findings suggested that exogenous rh-NTN-1 binding UNC5B attenuated neuroinflammation and EBI through inhibiting microglia activation after SAH, which is at least in part mediated by PPARγ/NFκB signaling cascade (Fig. 11).
Fig. 11.
The schematic diagram of potential molecular mechanisms of anti-inflammation effects of recombinant human Netrin-1 (rh-NTN-1) through UNC5B/PPARγ/NFκB signaling pathway after SAH.
Mounting evidence from both clinical and preclinical studies suggests that early inflammation is an important factor to contribute to brain injury after SAH (Frontera et al., 2012; Lucke-Wold et al., 2016). The presence of subarachnoid blood contributes to microglial activation, and initiates vigorous inflammatory cascades after SAH. Microglia activation and leukocytes infiltration into brain parenchyma after stroke represent the hallmark of immune response, release inflammatory mediators such as TNF-α and IL-6, and trigger deleterious inflammation response that leads to neuroinflammation and brain injury (Chamorro et al., 2012; Xu et al., 2017). In turn, excessive production of pro-inflammatory cytokine and chemokine released from immune cells further amplify the inflammatory response, including further microglia activation and leukocyte infiltration into brain, subsequently trap in a vicious circle to exacerbate brain injury after stroke (Keep et al., 2012; Xie et al., 2017a). Therefore, suppressing microglia activation and neutrophils infiltration is beneficial to prevent excessive inflammation and ameliorate inflammation-induced brain injury after SAH.
NTN-1 has been reported to regulate the inflammation by inhibiting immune cells infiltration into tissues in different pathological settings (Aherne et al., 2013; Aherne et al., 2012; Ly et al., 2005; Mirakaj et al., 2011; Mirakaj et al., 2010; Rosenberger et al., 2009; Schlegel et al., 2016). During the acute kidney injury, administration of exogenous NTN-1 suppressed leukocytes infiltration into ischemic kidney and the production of cytokine and chemokine, and alleviated renal ischemia-reperfusion (IR) injury through decreasing the production of cyclooxygenase 2-mediated prostaglandin E2 and thromboxaneA2 (Ranganathan et al., 2013a; Tadagavadi et al., 2010). During the liver IR injury, NTN-1 decreased neutrophil migration and the expression of pro-inflammatory mediators, controlled the resolution of inflammation, and thereby promoted hepatic repair and regeneration (Schlegel et al., 2016). Up to date, there is only one study focused on the role of NTN-1 in regulating the inflammation in the central nervous system, which demonstrated that exogenous NTN-1 treatment could decrease pro-inflammatory cytokine secretion from human brain-derived endothelial cells in vitro, and inhibit immune cells invasion into brain, thereby mitigate the severity of inflammatory lesions after EAE in vivo (Podjaski et al., 2015). Consistent with the findings in EAE model, our results showed that administration of exogenous rh-NTN-1 resulted in a significant inhibition of microglia activation, and downregulation of MPO, TNF-α, IL-6, and ICAM-1, and ameliorated brain edema and neurological impairments after SAH. Whereas, silencing of endogenous NTN-1 by specific siRNA mainly suppressed the expressions of NTN-1 in neurons and endothelial cells, thus induced inflammatory response by increasing the expression of MPO, pro-inflammatory mediators and adhesion molecules, as well as exacerbated neurological dysfunction and brain edema, which were also associate with neuronal apoptosis and BBB breakdown induced by knockdown of endogenous NTN-1 with NTN-1 siRNA. These observations indicated that exogenous NTN-1 could suppress neuroinflammation by inhibiting the expressions of inflammatory molecules following SAH.
NTN-1, acting through UNC5B receptor, powerfully inhibited leukocyte infiltration into tissues and inflammation in vitro and in a peritonitis model (Ly et al., 2005). Likewise, previous studies also provided solid evidence that NTN-1 binding UNC5B receptor, attenuated inflammation by suppressing leukocytes infiltration and the production of inflammatory mediators. While neutralization of UNC5B receptor with blocking antibody abolished the beneficial effects of NTN-1 on inflammation in the kidney injury (Ranganathan et al., 2013a; Tadagavadi et al., 2010). In the current study, we observed that UNC5B receptor was expressed in microglia, endothelial cells, and neurons. Moreover, knockdown of UNC5B using specific siRNA significantly abolished the anti-inflammation property of exogenous rh-NTN-1, which suppressed microglia activation, and diminished the pro-inflammatory TNF-α and IL-6 levels as well as adhesion molecules ICAM-1 expressions. Therefore, it is reasonable to speculate that UNC5B receptor mediates NTN-1-induced anti-inflammation effects after SAH.
PPARγ has been shown to play an essential role in regulating inflammatory response (Chawla, 2010; Croasdell et al., 2015; Jiang et al., 1998). After stroke, activation of PPARγ with its agonists significantly reduced the expression of pro-inflammatory mediators, and suppressed migration of macrophages and neutrophils into brain, as well as promoted phagocytosis-mediated hematoma resolution by promoting the M2 phagocytic phenotype of microglial cells, suggesting that PPARγ, a cytoprotective protein, exerted anti-inflammation property (Culman et al., 2007; Gliem et al., 2015; Gu et al., 2015; Kumari et al., 2010; Zhao et al., 2007; Zhao et al., 2015). In addition, PPARγ was also proven to prevent against the development of intracranial aneurysmal rupture (Shimada et al., 2015). Recent studies have revealed that PPARγ is a downstream mediator of NTN-1-mediated anti-inflammation during the IR injury in kidney and heart. Ranganathan et al. showed that PPARγ signaling pathway was activated by NTN-1-induced macrophage M2 polarization and ameliorated inflammation in the renal IR injury, while inhibition of PPARγ activation offset the protective effects of NTN-1 on the kidney injury (Ranganathan et al., 2013b). Another study also documented that NTN-1 treatment promoted PPARγ activation in cardiac allograft and decreased infiltration of neutrophils and macrophages into allograft, and then mitigated myocardial IR injury, while the beneficial effects of NTN-1 was significantly abrogated by PPARγ antagonist (Mao et al., 2014). Furthermore, numerous studies have demonstrated that PPARγ serves as a negative factor to modulate NFκB signaling pathway (Feinstein et al., 2005; Wan et al., 2008; Zhao et al., 2015). NFκB is well-known to be involved in inflammation and immune processes through upregulating multiple proinflammatory cytokines, chemokines, proteases, and adhesion molecules (Behrouz, 2016; Harari and Liao, 2010). In this study, we observed that PPARγ protein level was increased after SAH, which was consistent with the previous findings in a stroke model (Victor et al., 2006; Xiong et al., 2016). However, the extent of endogenous PPARγ upregulation was not sufficient to inhibit NFκB elevation after SAH. After administration of exogenous rh-NTN-1, PPARγ protein level was further augmented, resulted in an effective inhibition of NFκB activation, and subsequent inflammatory molecules expression after SAH. In contrast, inhibition of PPARγ with BADGE reversed the anti-inflammatory effect of exogenous rh-NTN-1, promoted the NFκB activation, and up-regulated expression of TNF-α, IL-6, ICAM-1, and MPO, thereby worsened neurological deficits and brain edema. Thus, our findings supported the hypothesis that anti-inflammation effect of exogenous rh-NTN-1 is mediated at least in part through PPARγ/NFκB signaling pathway after SAH. Additionally, our previous study showed that NTN-1 possessed neuroprotective functions through inhibiting neuronal apoptosis and preserving BBB integrity after SAH via distinct signaling pathways (Xie et al., 2017b; Xie et al., 2017c). Thus, it cannot be ruled out that the anti-inflammation property of NTN-1 may be also related with NTN-1-mediated preservation of BBB integrity.
There are several limitations in our study. Previous studies reported that NTN-1 possesses other protective functions, such as promoting angiogenesis and white matter repairing (He et al., 2013; Wilson et al., 2006). In this study, we only investigated the anti-inflammation effect of NTN-1 after SAH. Therefore, we cannot exclude the possibility that NTN-1 also exert other neuroprotective roles in EBI after SAH. Further studies are needed to elucidate other effects of NTN-1 after SAH and its underlying signaling mechanisms. We did not have any groups using BADGE or PEG only without rh-NTN-1 treatment. It is possible that BADG or PEG alone has some specific effects in addition to rh-NTN-1 effects. In addition, long-term neurological benefits of NTN-1 in the setting of SAH require further to be studied in the future.
5. Conclusions
Exogenous rh-NTN-1 binding UNC5B could attenuate neuroinflammation and improve neurological functions through inhibiting microglia activation via PPARγ/NFκB signaling pathway after SAH. Therefore, exogenous NTN-1 may serve as a promising therapeutic agent against brain injury for SAH patients.
Supplementary Material
Highlights.
Exogenous Netrin-1 (NTN-1) significantly attenuated neurological impairments and brain edema after SAH.
NTN-1 ameliated neuroinflammatory responses through inhibiting microglia activation after SAH.
NTN-1 binding UNC5B activated PPARγ and inhibited NFκB signaling pathway.
NTN-1 may serve as a promising therapeutic agent against neuroinflammation in early brain injury after SAH.
Acknowledgments
This study was partially supported by grants from the National Institutes of Health (NS081740 and NS082184), Chongqing Natural Science Foundation Project (CSTC2013jcyjA10054), and Medical Research Projects of Chongqing Municipal Health Bureau (2013-1-018).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare no competing financial interests.
References
- Aherne CM, Collins CB, Eltzschig HK. Netrin-1 guides inflammatory cell migration to control mucosal immune responses during intestinal inflammation. Tissue barriers. 2013;1:e24957. doi: 10.4161/tisb.24957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aherne CM, Collins CB, Masterson JC, Tizzano M, Boyle TA, Westrich JA, Parnes JA, Furuta GT, Rivera-Nieves J, Eltzschig HK. Neuronal guidance molecule netrin-1 attenuates inflammatory cell trafficking during acute experimental colitis. Gut. 2012;61:695–705. doi: 10.1136/gutjnl-2011-300012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrouz R. Re-exploring Tumor Necrosis Factor Alpha as a Target for Therapy in Intracerebral Hemorrhage. Translational stroke research. 2016;7:93–96. doi: 10.1007/s12975-016-0446-x. [DOI] [PubMed] [Google Scholar]
- Chamorro A, Meisel A, Planas AM, Urra X, van de Beek D, Veltkamp R. The immunology of acute stroke. Nature reviews. Neurology. 2012;8:401–410. doi: 10.1038/nrneurol.2012.98. [DOI] [PubMed] [Google Scholar]
- Chawla A. Control of macrophage activation and function by PPARs. Circulation research. 2010;106:1559–1569. doi: 10.1161/CIRCRESAHA.110.216523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Feng H, Sherchan P, Klebe D, Zhao G, Sun X, Zhang J, Tang J, Zhang JH. Controversies and evolving new mechanisms in subarachnoid hemorrhage. Progress in neurobiology. 2014;115:64–91. doi: 10.1016/j.pneurobio.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Zhang Y, Tang J, Liu F, Hu Q, Luo C, Tang J, Feng H, Zhang JH. Norrin protected blood-brain barrier via frizzled-4/beta-catenin pathway after subarachnoid hemorrhage in rats. Stroke; a journal of cerebral circulation. 2015;46:529–536. doi: 10.1161/STROKEAHA.114.007265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croasdell A, Duffney PF, Kim N, Lacy SH, Sime PJ, Phipps RP. PPARgamma and the Innate Immune System Mediate the Resolution of Inflammation. PPAR research. 2015;2015:549691. doi: 10.1155/2015/549691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culman J, Zhao Y, Gohlke P, Herdegen T. PPAR-gamma: therapeutic target for ischemic stroke. Trends in pharmacological sciences. 2007;28:244–249. doi: 10.1016/j.tips.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Enkhjargal B, McBride DW, Manaenko A, Reis C, Sakai Y, Tang J, Zhang JH. Intranasal administration of vitamin D attenuates blood-brain barrier disruption through endogenous upregulation of osteopontin and activation of CD44/P-gp glycosylation signaling after subarachnoid hemorrhage in rats. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2017;37:2555–2566. doi: 10.1177/0271678X16671147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein DL, Spagnolo A, Akar C, Weinberg G, Murphy P, Gavrilyuk V, Dello Russo C. Receptor-independent actions of PPAR thiazolidinedione agonists: is mitochondrial function the key? Biochemical pharmacology. 2005;70:177–188. doi: 10.1016/j.bcp.2005.03.033. [DOI] [PubMed] [Google Scholar]
- Frontera JA, Aledort L, Gordon E, Egorova N, Moyle H, Patel A, Bederson JB, Sehba F. Early platelet activation, inflammation and acute brain injury after a subarachnoid hemorrhage: a pilot study. Journal of thrombosis and haemostasis : JTH. 2012;10:711–713. doi: 10.1111/j.1538-7836.2012.04651.x. [DOI] [PubMed] [Google Scholar]
- Frontera JA, Provencio JJ, Sehba FA, McIntyre TM, Nowacki AS, Gordon E, Weimer JM, Aledort L. The Role of Platelet Activation and Inflammation in Early Brain Injury Following Subarachnoid Hemorrhage. Neurocritical care. 2017;26:48–57. doi: 10.1007/s12028-016-0292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatz T, Stock I, Nguyen-Ngoc M, Gohlke P, Herdegen T, Culman J, Zhao Y. Peroxisome-proliferator-activated receptors gamma and peroxisome-proliferator-activated receptors beta/delta and the regulation of interleukin 1 receptor antagonist expression by pioglitazone in ischaemic brain. Journal of hypertension. 2010;28:1488–1497. doi: 10.1097/HJH.0b013e3283396e4e. [DOI] [PubMed] [Google Scholar]
- Gliem M, Klotz L, van Rooijen N, Hartung HP, Jander S. Hyperglycemia and PPARgamma Antagonistically Influence Macrophage Polarization and Infarct Healing After Ischemic Stroke. Stroke; a journal of cerebral circulation. 2015;46:2935–2942. doi: 10.1161/STROKEAHA.115.010557. [DOI] [PubMed] [Google Scholar]
- Gu C, Wang Y, Li J, Chen J, Yan F, Wu C, Chen G. Rosiglitazone attenuates early brain injury after experimental subarachnoid hemorrhage in rats. Brain research. 2015;1624:199–207. doi: 10.1016/j.brainres.2015.07.025. [DOI] [PubMed] [Google Scholar]
- Guo Z, Hu Q, Xu L, Guo ZN, Ou Y, He Y, Yin C, Sun X, Tang J, Zhang JH. Lipoxin A4 Reduces Inflammation Through Formyl Peptide Receptor 2/p38 MAPK Signaling Pathway in Subarachnoid Hemorrhage Rats. Stroke; a journal of cerebral circulation. 2016;47:490–497. doi: 10.1161/STROKEAHA.115.011223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harari OA, Liao JK. NF-kappaB and innate immunity in ischemic stroke. Annals of the New York Academy of Sciences. 2010;1207:32–40. doi: 10.1111/j.1749-6632.2010.05735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Li Y, Lu H, Zhang Z, Wang Y, Yang GY. Netrin-1 overexpression promotes white matter repairing and remodeling after focal cerebral ischemia in mice. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2013;33:1921–1927. doi: 10.1038/jcbfm.2013.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Sherchan P, Wang Y, Reis C, Applegate RL, 2nd, Tang J, Zhang JH. Phosphoinositide 3-Kinase Gamma Contributes to Neuroinflammation in a Rat Model of Surgical Brain Injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:10390–10401. doi: 10.1523/JNEUROSCI.0546-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. The Lancet. Neurology. 2012;11:720–731. doi: 10.1016/S1474-4422(12)70104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari R, Willing LB, Patel SD, Krady JK, Zavadoski WJ, Gibbs EM, Vannucci SJ, Simpson IA. The PPAR-gamma agonist, darglitazone, restores acute inflammatory responses to cerebral hypoxia-ischemia in the diabetic ob/ob mouse. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2010;30:352–360. doi: 10.1038/jcbfm.2009.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrke M, Lazar MA. The many faces of PPARgamma. Cell. 2005;123:993–999. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- Lucke-Wold BP, Logsdon AF, Manoranjan B, Turner RC, McConnell E, Vates GE, Huber JD, Rosen CL, Simard JM. Aneurysmal Subarachnoid Hemorrhage and Neuroinflammation: A Comprehensive Review. International journal of molecular sciences. 2016;17:497. doi: 10.3390/ijms17040497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly NP, Komatsuzaki K, Fraser IP, Tseng AA, Prodhan P, Moore KJ, Kinane TB. Netrin-1 inhibits leukocyte migration in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:14729–14734. doi: 10.1073/pnas.0506233102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X, Xing H, Mao A, Jiang H, Cheng L, Liu Y, Quan X, Li L. Netrin-1 attenuates cardiac ischemia reperfusion injury and generates alternatively activated macrophages. Inflammation. 2014;37:573–580. doi: 10.1007/s10753-013-9771-3. [DOI] [PubMed] [Google Scholar]
- Mirakaj V, Gatidou D, Potzsch C, Konig K, Rosenberger P. Netrin-1 signaling dampens inflammatory peritonitis. Journal of immunology. 2011;186:549–555. doi: 10.4049/jimmunol.1002671. [DOI] [PubMed] [Google Scholar]
- Mirakaj V, Thix CA, Laucher S, Mielke C, Morote-Garcia JC, Schmit MA, Henes J, Unertl KE, Kohler D, Rosenberger P. Netrin-1 dampens pulmonary inflammation during acute lung injury. American journal of respiratory and critical care medicine. 2010;181:815–824. doi: 10.1164/rccm.200905-0717OC. [DOI] [PubMed] [Google Scholar]
- Podjaski C, Alvarez JI, Bourbonniere L, Larouche S, Terouz S, Bin JM, Lecuyer MA, Saint-Laurent O, Larochelle C, Darlington PJ, Arbour N, Antel JP, Kennedy TE, Prat A. Netrin 1 regulates blood-brain barrier function and neuroinflammation. Brain : a journal of neurology. 2015;138:1598–1612. doi: 10.1093/brain/awv092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencio JJ, Swank V, Lu H, Brunet S, Baltan S, Khapre RV, Seerapu H, Kokiko-Cochran ON, Lamb BT, Ransohoff RM. Neutrophil depletion after subarachnoid hemorrhage improves memory via NMDA receptors. Brain, behavior, and immunity. 2016;54:233–242. doi: 10.1016/j.bbi.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan PV, Jayakumar C, Mohamed R, Dong Z, Ramesh G. Netrin-1 regulates the inflammatory response of neutrophils and macrophages, and suppresses ischemic acute kidney injury by inhibiting COX-2-mediated PGE2 production. Kidney international. 2013a;83:1087–1098. doi: 10.1038/ki.2012.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan PV, Jayakumar C, Ramesh G. Netrin-1-treated macrophages protect the kidney against ischemia-reperfusion injury and suppress inflammation by inducing M2 polarization. American journal of physiology. Renal physiology. 2013b;304:F948–957. doi: 10.1152/ajprenal.00580.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberger P, Schwab JM, Mirakaj V, Masekowsky E, Mager A, Morote-Garcia JC, Unertl K, Eltzschig HK. Hypoxia-inducible factor-dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nature immunology. 2009;10:195–202. doi: 10.1038/ni.1683. [DOI] [PubMed] [Google Scholar]
- Schlegel M, Kohler D, Korner A, Granja T, Straub A, Giera M, Mirakaj V. The neuroimmune guidance cue netrin-1 controls resolution programs and promotes liver regeneration. Hepatology. 2016;63:1689–1705. doi: 10.1002/hep.28347. [DOI] [PubMed] [Google Scholar]
- Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, Skarnes WC, Tessier-Lavigne M. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87:1001–1014. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- Shimada K, Furukawa H, Wada K, Korai M, Wei Y, Tada Y, Kuwabara A, Shikata F, Kitazato KT, Nagahiro S, Lawton MT, Hashimoto T. Protective Role of Peroxisome Proliferator-Activated Receptor-gamma in the Development of Intracranial Aneurysm Rupture. Stroke; a journal of cerebral circulation. 2015;46:1664–1672. doi: 10.1161/STROKEAHA.114.007722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara T, Ayer R, Jadhav V, Zhang JH. A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. Journal of neuroscience methods. 2008;167:327–334. doi: 10.1016/j.jneumeth.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadagavadi RK, Wang W, Ramesh G. Netrin-1 regulates Th1/Th2/Th17 cytokine production and inflammation through UNC5B receptor and protects kidney against ischemia-reperfusion injury. Journal of immunology. 2010;185:3750–3758. doi: 10.4049/jimmunol.1000435. [DOI] [PubMed] [Google Scholar]
- Tong LS, Shao AW, Ou YB, Guo ZN, Manaenko A, Dixon BJ, Tang J, Lou M, Zhang JH. Recombinant Gas6 augments Axl and facilitates immune restoration in an intracerebral hemorrhage mouse model. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2017;37:1971–1981. doi: 10.1177/0271678X16658490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor NA, Wanderi EW, Gamboa J, Zhao X, Aronowski J, Deininger K, Lust WD, Landreth GE, Sundararajan S. Altered PPARgamma expression and activation after transient focal ischemia in rats. The European journal of neuroscience. 2006;24:1653–1663. doi: 10.1111/j.1460-9568.2006.05037.x. [DOI] [PubMed] [Google Scholar]
- Wan H, Yuan Y, Qian A, Sun Y, Qiao M. Pioglitazone, a PPARgamma ligand, suppresses NFkappaB activation through inhibition of IkappaB kinase activation in cerulein-treated AR42J cells. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2008;62:466–472. doi: 10.1016/j.biopha.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Wilson BD, Ii M, Park KW, Suli A, Sorensen LK, Larrieu-Lahargue F, Urness LD, Suh W, Asai J, Kock GA, Thorne T, Silver M, Thomas KR, Chien CB, Losordo DW, Li DY. Netrins promote developmental and therapeutic angiogenesis. Science. 2006;313:640–644. doi: 10.1126/science.1124704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Zhang Y, Yang P, Enkhjargal B, Manaenko A, Tang J, Pearce WJ, Hartman R, Obenaus A, Chen G, Zhang JH. Recombinant Osteopontin Stabilizes Smooth Muscle Cell Phenotype via Integrin Receptor/Integrin-Linked Kinase/Rac-1 Pathway After Subarachnoid Hemorrhage in Rats. Stroke; a journal of cerebral circulation. 2016;47:1319–1327. doi: 10.1161/STROKEAHA.115.011552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Guo H, Wang L, Xu L, Zhang X, Yu L, Liu Q, Li Y, Zhao N, Zhao N, Ye R, Liu X. Human albumin attenuates excessive innate immunity via inhibition of microglial Mincle/Syk signaling in subarachnoid hemorrhage. Brain, behavior, and immunity. 2017a;60:346–360. doi: 10.1016/j.bbi.2016.11.004. [DOI] [PubMed] [Google Scholar]
- Xie Z, Enkhjargal B, Reis C, Huang L, Wan W, Tang J, Cheng Y, Zhang JH. Netrin-1 Preserves Blood-Brain Barrier Integrity Through Deleted in Colorectal Cancer/Focal Adhesion Kinase/RhoA Signaling Pathway Following Subarachnoid Hemorrhage in Rats. Journal of the American Heart Association. 2017b:6. doi: 10.1161/JAHA.116.005198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Huang L, Enkhjargal B, Reis C, Wan W, Tang J, Cheng Y, Zhang JH. Intranasal administration of recombinant Netrin-1 attenuates neuronal apoptosis by activating DCC/APPL-1/AKT signaling pathway after subarachnoid hemorrhage in rats. Neuropharmacology. 2017c;119:123–133. doi: 10.1016/j.neuropharm.2017.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong D, Deng Y, Huang B, Yin C, Liu B, Shi J, Gong Q. Icariin attenuates cerebral ischemia-reperfusion injury through inhibition of inflammatory response mediated by NF-kappaB, PPARalpha and PPARgamma in rats. International immunopharmacology. 2016;30:157–162. doi: 10.1016/j.intimp.2015.11.035. [DOI] [PubMed] [Google Scholar]
- Xu H, Li J, Wang Z, Feng M, Shen Y, Cao S, Li T, Peng Y, Fan L, Chen J, Gu C, Yan F, Wang L, Chen G. Methylene blue attenuates neuroinflammation after subarachnoid hemorrhage in rats through the Akt/GSK-3beta/MEF2D signaling pathway. Brain, behavior, and immunity. 2017;65:125–139. doi: 10.1016/j.bbi.2017.04.020. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen Y, Wu J, Manaenko A, Yang P, Tang J, Fu W, Zhang JH. Activation of Dopamine D2 Receptor Suppresses Neuroinflammation Through alphaB-Crystalline by Inhibition of NF-kappaB Nuclear Translocation in Experimental ICH Mice Model. Stroke; a journal of cerebral circulation. 2015;46:2637–2646. doi: 10.1161/STROKEAHA.115.009792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Sun G, Zhang J, Strong R, Song W, Gonzales N, Grotta JC, Aronowski J. Hematoma resolution as a target for intracerebral hemorrhage treatment: role for peroxisome proliferator-activated receptor gamma in microglia/macrophages. Annals of neurology. 2007;61:352–362. doi: 10.1002/ana.21097. [DOI] [PubMed] [Google Scholar]
- Zhao XR, Gonzales N, Aronowski J. Pleiotropic role of PPARgamma in intracerebral hemorrhage: an intricate system involving Nrf2, RXR, and NF-kappaB. CNS neuroscience & therapeutics. 2015;21:357–366. doi: 10.1111/cns.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.