Abstract
Introduction
Dogs harbour zoonotic parasites that cause serious infections in humans, such as visceral larva migrans, ocular larva migrans, cystic echinococcosis, and alveolar echinococcosis. Studies on dogs’ gastrointestinal parasites in different geographical locations are required to increase knowledge of the risk of canine zoonoses in human populations.
Material and Methods
The presence of parasites was examined in 450 faecal samples collected from eight zones of Zanjan province, northwest Iran from June to November 2015. The samples were examined using the sedimentation concentration method and modified Ziehl-Neelsen staining.
Results
Gastrointestinal parasites were found in 86 (19.1%) faecal samples. Sarcocystis spp. (7.3%), Taenia/Echinococcus spp. (5.6%), Toxocara spp. (1.8%), and Cystoisospora spp. (1.6%) were the most common parasites observed. The other detected parasites consisted of Dicrocoelium dendriticum (0.7%), Eimeria spp. (0.7%), Cryptosporidium spp. (0.4%), Physaloptera spp. (0.4%), Giardia spp. (1.3%), and Spirocerca lupi (1.3%). The lowest parasite infection rates belonged to Trichuris vulpis and Acanthocephalans (0.2% each).
Conclusion
This study provides current information on the infection rates in dog populations in Zanjan Province. Furthermore, the study shows a high prevalence of gastrointestinal parasitic infections, including zoonotic ones and particularly Taenia/Echinococcus spp., potentially transmissible to humans and thus relevant to public health.
Keywords: dog, gastrointestinal parasites, zoonoses, public health, Iran
Introduction
The worldwide dog population has been estimated to be more than 500 million (29, 20). Although dogs bring many advantages to human life, including being guides for blind people, therapeutic agents, security guards, and hunters, they are associated with many potentially zoonotic organisms of parasitic origin (6). Gastrointestinal parasites are among the main enteropathogens and the major cause of mortality in dogs. Echinococcus granulosus, Toxocara canis, Cryptosporidium spp., and Dipylidium caninum are common parasites of dogs which can also affect humans (6, 8). There are two major transmission modes for dog gastrointestinal parasites, namely indirect and direct. The former includes consumption of foods and water contaminated with dogs’ secretions and excretions, particularly parasite eggs, cysts, and oocysts, shed through animal faeces into the environment. The latter includes direct contact with dogs since the majority of intestinal parasites have a faecal-oral transmission cycle. Free-roaming (i.e., stray) dogs often exist in urban and rural areas, and their behavioural characteristics such as unrestricted movement within a human environment can easily result in the contamination of the environment (15, 3). In fact, various parasitic forms such as eggs, larvae, cysts, and oocysts excreted through dog faeces can remain infective in the environment over a long time, depending on different conditions. They comprise a risk factor for animal and human infection, among whom pregnant women, children, and immunocompromised people are at higher risk of these infections and their consequences (37, 42). Therefore, understanding the epidemiology of the parasites in the canine population is required to reduce the risk of human infection (17). Several studies on the prevalence of gastrointestinal parasites in stray dogs from different areas of the world have been conducted (35, 40, 28, 41), including a number in Iran (7). However, there are no published data about the epidemiology of parasites existing in canine faeces in Zanjan Province, so the present research was designed to determine the prevalence of gastrointestinal parasites in dog faeces in the region. The study placed emphasis on zoonotic parasites with potential risk of transmission to the province’s human population.
Material and Methods
Study area
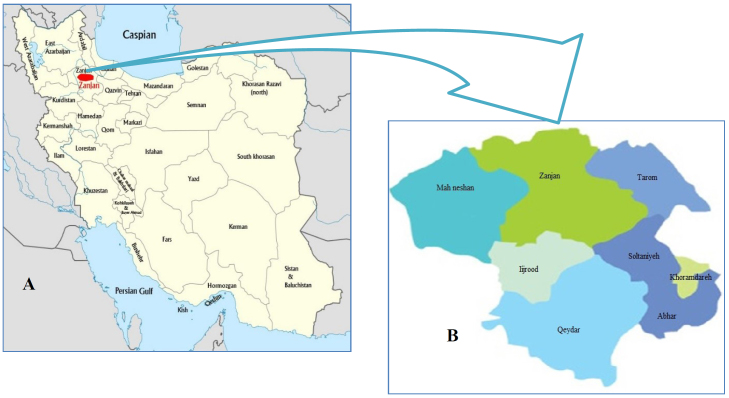
The province of Zanjan is situated in the northwest of Iran at latitude 36°40′24″ N and longitude 48°28′43″ E. It is divided into eight cities and has an area of 22,164 km2 occupying 1.34% of Iran’s total territory (Fig. 1-A). The population of the province is 1,015,734. The majority of the population (62.5%) lives in urban areas and the rest in rural areas. Zanjan is one of the coldest provinces of Iran, with an average minimum temperature of –19°C that drops to –27°C during the coldest winter days (Fig. 1-A) (23).
Fig. 1.
Geographic location. A – location of Zanjan province in Iran. B – map of Zanjan province and its cities, where dog faecal samples were collected
Sample collection
The study was conducted between June and November 2015. A total of 450 samples of fresh dog faeces were collected from streets in urban locations as well as from farms in rural areas of different parts of the province (Fig. 1-B). The faecal samples were collected in wide-mouth zip-top plastic faecal containers, which were properly labelled. The collected samples were transported to the Research Laboratory of the Department of Parasitology and Mycology, on the day of collection and underwent microscopic examinations. All samples were examined with the naked eye for adult helminths and proglottids of cestodes before concentration.
Parasitological procedures
Each faecal sample was suspended in 10% formalin and processed by the formalin-ethyl acetate sedimentation concentration method. The pellets were microscopically examined at 100× and 400× magnification. Identification of parasites was made by their morphological characteristics (43). In addition, the samples were screened for coccidian oocysts such as Cryptosporidium spp. by means of modified Ziehl-Neelsen staining (14). A thin smear was made of the pellet obtained from the sedimentation concentration method and stained by the modified Ziehl-Neelsen method. The smears were consequently examined at 400× and 1000× magnification for the detection of Cryptosporidium spp. and other coccidian oocysts. Samples containing at least one form of either helminthic or protozoan parasite were considered as positive.
Statistical analyses
Statistical analysis was performed by SPSS 16.0 (IBM, USA). The prevalence of each parasite was calculated as the number of positive samples divided by the total samples.
Results
The gastrointestinal parasite eggs, oocysts, and cysts found in the faecal samples are shown in Table 1. A total of 86 (19.1%) samples out of 450 examined contained at least one gastrointestinal parasite. They consisted of thirteen genera of gastrointestinal parasites (Figs 2 and 3), with helminth infections more prevalent than protozoan parasites. Sarcocystis spp. were the most common observed protozoan parasite with 7.3% frequency. Cystoisospora spp. were second and found in seven dogs (1.6%). Giardia spp. were detected in six dogs (1.3%), and Eimeria spp. were found in three (0.7%). Taenia/Echinococcus spp. were the most frequently detected gastrointestinal helminth parasites with 5.6% frequency, and Toxocara spp. followed. Spirocerca lupi was detected in six dogs (1.3%) and Toxascaris leonina was found in four (0.9%). Based on modified Ziehl-Neelsen staining, Cryptosporidium spp. were identified in two (0.4%) faecal samples. The lowest prevalence was shown by Acanthocephalans and Trichuris vulpis (0.2% each). Of the 450 faecal samples, 72 (16%) were infected with a single parasite species, and 14 (3.1%) samples had multiple infections, including 13 (2.9%) with 2 and 1 (0.2%) with 3 species of gastrointestinal parasite (Table 2).
Table 1.
Prevalence of the gastrointestinal parasites in Zanjan Province
| Parasite species | Number of parasite species in infected dogs | Prevalence (%) |
|---|---|---|
| Sarcocystis spp. | 33 | 7.3% |
| Taenia/Echinococcus spp. | 25 | 5.6% |
| Toxocara spp. | 8 | 1.8% |
| Cystoisospora spp. | 7 | 1.6% |
| Giardia spp. | 6 | 1.3% |
| Spirocerca lupi | 6 | 1.3% |
| Toxascaris leonina | 4 | 0.9% |
| Dicrocoelium dendriticum | 3 | 0.7% |
| Eimeria spp. | 3 | 0.7% |
| Cryptosporidium spp. | 2 | 0.4% |
| Physaloptera spp. | 2 | 0.4% |
| Trichuris vulpis | 1 | 0.2% |
| Acanthocephalans | 1 | 0.2% |
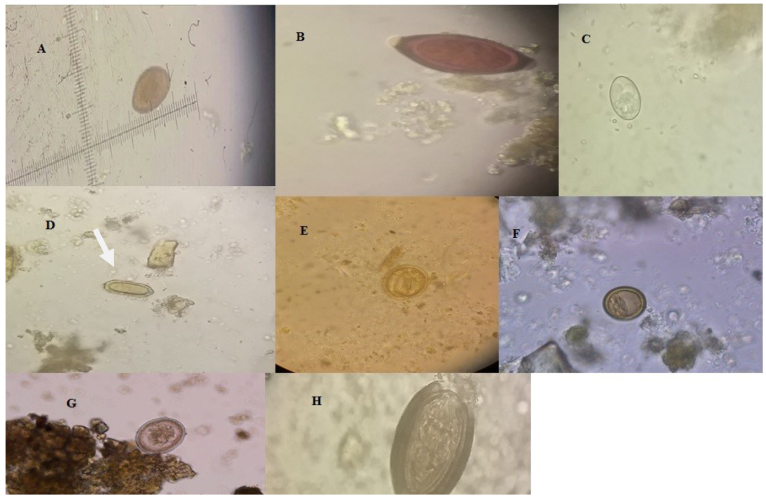
Fig. 2.
Helminthic parasites found in dogs’ faeces from Zanjan. A – Dicrocoelium dendriticum, B – Trichuris vulpis , C – Physaloptera spp., D –Spirocerca lupi , E – Toxocara spp., F – Taenia/Echinococcus spp., G – Toxascaris leonina, H – Acanthocephalan
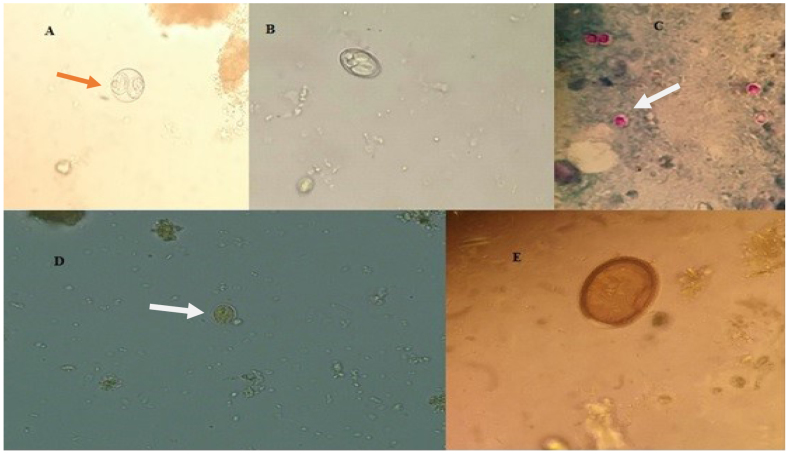
Fig. 3.
Protozoan parasites found in dogs’ faeces from Zanjan. A – Cystoisospora spp., B – Sarcocystis spp., C – Cryptosporidium spp., D – Giardia spp., E – Eimeria spp.
Table 2.
Single and multiple infections of dogs with gastrointestinal parasites in Zanjan Province
| Parasite species | Number of positive dogs | |
|---|---|---|
| Single infections | ||
| Sarcocystis spp. | 24 | |
| Taenia spp./Echinococcus spp. | 20 | |
| Toxocara spp. | 6 | |
| Cystoisospora spp. | 4 | |
| Single Giardia spp. | 4 | |
| Spirocerca lupi | 3 | |
| infections Toxascaris leonina | 2 | |
| Dicrocoelium dendriticum | 2 | |
| Eimeria spp. | 3 | |
| Cryptosporidium spp. | 2 | |
| Physaloptera spp. | 0 | |
| Trichuris vulpis | 1 | |
| Acanthocephalans | 1 | |
| Cystoisospora spp.+ Giardia spp. | 2 | |
| Multiple infections | ||
| Toxocara spp.+ Physaloptera spp. | 1 | |
| Sarcocystis spp.+ Spirocerca lupi | 3 | |
| Sarcocystis spp.+ Toxascaris leonina | 2 | |
| Sarcocystis spp.+ Cystoisospora spp. | 1 | |
| Sarcocystis spp.+ Taenia./Echinococcus spp. | 3 | |
| Taenia/Echinococcus spp.+ Physaloptera spp. | 1 | |
| Taenia./Echinococcus spp.+ Dicrocoelium dendriticum + Toxocara spp. | 1 |
Discussion
This study showed a relatively high prevalence of gastrointestinal parasitic infections (19.1%) in dogs in Zanjan Province. More than twelve parasite species were found based on the detection of protozoan cysts or oocysts and helminthic eggs in collected faecal samples, including several zoonotic species. The most prevalent infection belonged to Sarcocystis spp., followed by Taenia spp./Echinococcus spp. with infection rates of 7.3% and 5.6% respectively. The prevalence rates for other parasites ranged between 0.2% and 1.6% of the examined samples.
Canine gastrointestinal parasites are of great concern to public health, due to the potential risk of transmission of zoonotic species to humans in both rural and urban areas. Parasite transmission to humans primarily takes place through contact with the faeces of infected animals. The parasitic elements (eggs, larvae, cysts, and oocysts) excreted with the faeces of dogs as befoulment can survive for a long time, depending on the environmental conditions (38). In many countries, there are unrestrained populations of stray dogs, where overcrowding, a low level of hygiene, and lack of awareness increase the risk of zoonotic parasite transmission (37). The results of the present study showed a high prevalence (7.3%) of Sarcocystis species compared to other studies (Table 3). Sarcocystosis is a protozoan infection with worldwide distribution in many animal species. It is a two-host parasite, generally with a herbivore as the intermediate host and a carnivore as the definitive host. There are more than 200 species of the genus Sarcocystis; however, complete life cycles are known for only 26 species (10). Dogs are the definitive hosts for some species such as S. cruzi (S. bovicanis), S. miescheriana (S. suicanis), and S. tenella (S. ovicanis) (13). Not all species of the genus Sarcocystis cause disease in their host species, but the species using canids as definitive hosts are more pathogenic than the others (25). The species that infect herbivores such as cattle and sheep have economic importance because they cause illness and may lead to weight loss or death of animals. Dogs harbour some zoonotic helminths, several examples of which are in the Taenidae family. The eggs of Taenia /Echinococcus spp. were found in 5.6% of the examined samples in the study area, which is a relatively higher percentage than that of other studies in some countries. It was 1.0% in Czech Republic (11), 1.7% in Denmark (2), and 0.34% in Portugal (28) and Iran (5). Although concentration methods are capable of detecting taeniid eggs, distinguishing these zoonotic parasites needs either bioassay or molecular techniques such as PCR. Echinococcosis, one of the most important zoonotic infections, is caused by Echinococcus spp. belonging to the Taenidae family. Echinococcosis is endemic in various parts of Iran (39).
Table 3.
Prevalence of gastrointestinal parasites in dogs from Zanjan Province compiled with prevalence data from other studies
| Parasite species | Present study Number (%) | Other studies | |
|---|---|---|---|
| Percentage and location | References | ||
| Sarcocystis spp. | 33 (7.3%) | 2.5%, Spain 2.7%, Brazil |
(27) (21) |
| Taenia / Echinococcus spp. | 25 (5.6%) | 1.0%, Czech Republic 1.7%, Denmark 0.34%, Portugal |
(11) (2) (28) |
| Toxocara spp. | 8 (1.8%) | 21.9 %, Slovak Republic 27.8%, Turkey |
(40) (18) |
| Cystoisospora spp. | 7 (1.6%) | 7.5%, Italy 0.21%, Milan, Italy |
(36) (44) |
| Giardia spp. | 6 (1.3 %) | 4.3%, Greece 1.6 %, Slovak Republic |
(33) (40) |
| Spirocerca lupi | 6 (1.3 %) | 0.2%, Venezuela 1.9%, Sao Paolo, Brazil |
(35) (32) |
| Toxascaris leonina | 4 (0.9 %) | 1.7%, Italy 0.6%, Denmark |
(36) (2) |
| Dicrocoelium dendriticum | 3 (0.7 %) | 14%, Iran | (5) |
| Eimeria spp. | 3 (0.7 %) | 19%, Iran 8.7%, Hungary |
(5) (16) |
| Cryptosporidium spp. | 2 (0.4 %) | 2.8%, Greece 3.04%, Portugal |
(33) (28) |
| Physaloptera sp. | 2 (0.4 %) | 1%, Cuba 3%, Iran |
(34) (5) |
| Trichuris vulpis | 1 (0.2 %) | 3.67%, Milan, Italy 6%, Iran |
(44) (5) |
| Acanthocephalans | 1 (0.2 %) | 4.82%, Iran | (7) |
The prevalence rate of cystic echinococcosis has previously been reported in human population (22) and livestock (12). However, canids as the definitive hosts of the parasite have not been surveyed, and the present study is the first one conducted on dog faeces. There are many rural areas in Zanjan Province, and livestock is important to rural residents. Almost all cattle owners and most rural inhabitants keep at least one dog to protect their possessions from wild predators and thieves. The free and unrestrained movement of dogs and excretion of their faeces everywhere spread parasitic agents (Echinococcus eggs) in the environment. Our previous study showed that 75.7% of patients were from rural areas (22), where life cycle components (i.e. sheep, cattle, dogs, etc.) are all present (22, 9). Cystic echinococcosis annually gives rise to major monetary losses in developing countries. In Iran, approximately 1% of admissions to surgical wards are related to the disease (39). According to our previous and present studies it is obvious that humans are at a high risk of cystic echinococcosis especially in rural areas of this province.
In the present study, the roundworms Toxocara spp. and Toxascaris leonina were found in 1.8% and 0.9% of canine faeces samples respectively. Dogs as the definitive host are an important source for human toxocarosis. Ingestion of the embryonated eggs of the parasite by humans can cause serious diseases, such as visceral larva migrans. Our study showed low prevalence of Toxocara spp. (1.9%) compared to other studies (Table 3). According to the study of Abdi et al. (1), the overall prevalence of toxocarosis in Iran was reported to be 21.6 %. However, a study that investigated the seroprevalence of toxocarosis in children referred to the hospitals of Zanjan Province (30) showed a 2% prevalence rate, which is the lowest prevalence of all areas investigated in Iran. Toxocara spp. eggs are senstive to low temperature. Okoshi et al. (31) showed that Toxocara spp. eggs died after five day exposure to –15°C. Zanjan is a cold region in Iran and this could be one of the reasons for the low prevalence of Toxocara infection in this region. The oocysts of Cystoisospora spp. were observed in 1.6% of the dogs’ samples. Cystoisospora spp. is strictly host specific; however, to reduce the risk of acquiring the infection, treatment should include all susceptible dogs (26). Giardia spp. cysts were observed in 1.6% of dogs’ samples. Differing from this, some studies conducted in Argentina (24), Canada (19), and Germany (4) showed high prevalence rates. Since Giardia cysts are excreted intermittently, sensitive methods such as antigen detection could help to find the actual infection with Giardia spp. The prevalence of other parasites such as Dicrocoelium dendriticum, Eimeria spp., Cryptosporidium spp., Physaloptera spp., Spirocerca lupi, Acanthocephalans, and Trichuris vulpis was lower than the prevalence rates reported in other studies.
In conclusion, a relatively high prevalence of gastrointestinal parasitic infections, including 13 different helminths and parotozoon species, was found in faecal samples collected from dogs in Zanjan Province. Several zoonotic species, with Taenia spp. particularly attention-worthy among them due to its high prevalence rate, were among the detected parasites which pose a potential risk of human infection and are of high public health relevance.
Acknowledgements
This research is a part of the first author’s PhD thesis. The authors would like to express their gratitude and appreciation to Dr. Erfani and Mr. Azadvar, and other staff of the Veterinary Organisation of Zanjan Province. We would also like to extend our gratitude to Dr. Roberto Papini and Dr. Jaroslav Vadlejch for their helpful comments in parasite identification.
Footnotes
Conflict of Interests Statement: The authors declare that there is no conflict of interests regarding the publication of this article.
Financial Disclosure Statement: This research was supported by Zanjan University of Medical Sciences (grant no. A-12-153-8).
Animal Rights Statement: None required.
References
- 1.Abdi J., Darabi M., Sayehmiri K.. Epidemiological situation of toxocariasis in Iran: meta-analysis and systematic review. Pak J Biol Sci. 2012;15:1052–1055. doi: 10.3923/pjbs.2012.1052.1055. [DOI] [PubMed] [Google Scholar]
- 2.Al-Sabi M.N., Kapel C.M., Johansson A., Espersen M.C., Koch J., Willesen J.L.. A coprological investigation of gastrointestinal and cardiopulmonary parasites in hunting dogs in Denmark. Vet Parasitol. 2013;196:366–372. doi: 10.1016/j.vetpar.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 3.Baxter D., Leck I.. The deleterious effects of dogs on human health. 2. Canine zoonoses. J Public Health. 1984;6:185–197. doi: 10.1093/oxfordjournals.pubmed.a043711. [DOI] [PubMed] [Google Scholar]
- 4.Becker A.C., Rohen M., Epe C., Schnieder T.. Prevalence of endoparasites in stray and fostered dogs and cats in Northern Germany. Parasitol Res. 2012;111:849–857. doi: 10.1007/s00436-012-2909-7. [DOI] [PubMed] [Google Scholar]
- 5.Beiromvand M., Akhlaghi L., Massom S.H.F., Meamar A.R., Motevalian A., Oormazdi H., Razmjou E.. Prevalence of zoonotic intestinal parasites in domestic and stray dogs in a rural area of Iran. Prev Vet Med. 2013;109:162–167. doi: 10.1016/j.prevetmed.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Chomel B.B.. Emerging and re-emerging zoonoses of dogs and cats. Animals. 2014;4:434–445. doi: 10.3390/ani4030434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalimi A., Sattari A., Motamedi G.. A study on intestinal helminthes of dogs, foxes and jackals in the western part of Iran. Vet Parasitol. 2006;142:129–133. doi: 10.1016/j.vetpar.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 8.Dantas-Torres F., Otranto D.. Dogs, cats, parasites, and humans in Brazil: opening the black box. Parasit Vectors. 2014;7:1–25. doi: 10.1186/1756-3305-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deplazes P., van Knapen F., Schweiger A., Overgaauw P.A.. Role of pet dogs and cats in the transmission of helminthic zoonoses in Europe, with a focus on echinococcosis and toxocarosis. Vet Parasitol. 2011;182:41–53. doi: 10.1016/j.vetpar.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Dubey J.P.. Foodborne and waterborne zoonotic sarcocystosis. Zoonoses Public Health. 2008;55:406–413. [Google Scholar]
- 11.Dubná S., Langrová I., Nápravník J., Jankovská I., Vadlejch J., Pekár S., Fechtner J.. The prevalence of intestinal parasites in dogs from Prague, rural areas, and shelters of the Czech Republic. Vet Parasitol. 2007;145:120–128. doi: 10.1016/j.vetpar.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Farhadi M., Fazaeli A., Haniloo A.. Genetic characterization of livestock and human hydatid cyst isolates from northwest Iran, using the mitochondrial cox1 gene sequence. Parasitol Res. 2015;114:4363–4370. doi: 10.1007/s00436-015-4673-y. [DOI] [PubMed] [Google Scholar]
- 13.Fayer R., Esposito D.H., Dubey J.P.. Human infections with sarcocystis species. Clin Microbiol Rev. 2015;28:295–311. doi: 10.1128/CMR.00113-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fayer R., Xiao L. CRC press; United States: 2007. Cryptosporidium and cryptosporidiosis. [Google Scholar]
- 15.Feldmann B.M., Carding T.H.. Free-roaming urban pets. Health Serv Rep. 1973;88:956–962. [PMC free article] [PubMed] [Google Scholar]
- 16.Fok E., Szatmari V., Busak K., Rozgonyi F.. Epidemiology: prevalence of intestinal parasites in dogs in some urban and rural areas of Hungary. Vet Q. 2001;23:96–98. doi: 10.1080/01652176.2001.9695091. [DOI] [PubMed] [Google Scholar]
- 17.Garedaghi Y., Karimi B.. Prevalence of intestinal protozoan parasites in stray dogs of Tabriz city, Iran. Ind J Fund Appl Life Sci. 2014;4:20–24. [Google Scholar]
- 18.Gürler A.T., Bölükbaş C., Pekmezci G.Z., Umur S., Açıcı M.. Nematode and cestode eggs scattered with cats-dogs feces and significance of public health in Samsun, Turkey. Üniv Vet Fak Derg. 2015;62:23–26. [Google Scholar]
- 19.Jacobs S.R., Forrester C., Yang J.. A survey of the prevalence of Giardia in dogs presented to Canadian veterinary practices. Can Vet J. 2001;42:45–46. [PMC free article] [PubMed] [Google Scholar]
- 20.Kantere M., Athanasiou L., Chatzopoulos D., Spyrou V., Valiakos G., Kontos V., Billinis C.. Enteric pathogens of dogs and cats with public health implications. Am J Anim Vet Sci. 2014;9:84–94. [Google Scholar]
- 21.Katagiri S., Oliveira-Sequeira T.. Prevalence of dog intestinal parasites and risk perception of zoonotic infection by dog owners in Sao Paulo State, Brazil. Zoonoses Public Health. 2008;55:406–413. doi: 10.1111/j.1863-2378.2008.01163.x. [DOI] [PubMed] [Google Scholar]
- 22.Kohansal M.H., Nourian A., Bafandeh S.. Human cystic echinococcosis in Zanjan area, Northwest Iran: A retrospective hospital based survey between 2007 and 2013. Iran J Public Health. 2015;44:1277–1282. [PMC free article] [PubMed] [Google Scholar]
- 23.Kohansal M.H., Nourian A., Haniloo A., Fazaeli A.. Molecular detection of Taenia spp. in dogs’ feces in Zanjan Province, Northwest of Iran. Vet World. 2017;10:445–449. doi: 10.14202/vetworld.2017.445-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lavallén C., Dopchiz M., Lobianco E., Hollmann P., Denegri G.. Intestinal parasites of zoonotic importance in dogs from the District of General Pueyrredón (Buenos Aires, Argentina) Rev Vet. 2011;22:19–24. [Google Scholar]
- 25.Lindsay D.S., Blagburn B.L., Braund K.G.. Sarcocystis spp. and sarcocystosis. Br Med J. 1995;5:249–254. [Google Scholar]
- 26.Lindsay D.S., Dubey J.P., Blagburn B.L.. Biology of Isospora spp. from humans, nonhuman primates, and domestic animals. Clin Microbiol Rev. 1997;10:19–34. doi: 10.1128/cmr.10.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinez-Moreno F., Hernández S., López-Cobos E., Becerra C., Acosta I., Martínez-Moreno A.. Estimation of canine intestinal parasites in Cordoba (Spain) and their risk to public health. Vet Parasitol. 2007;143:7–13. doi: 10.1016/j.vetpar.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 28.Mateus T.L., Castro A., Ribeiro J.N., Vieira-Pinto M.. Multiple zoonotic parasites identified in dog feces collected in Ponte de Lima, Portugal-a potential threat to human health. Environ Res Public Health. 2014;11:9050–9067. doi: 10.3390/ijerph110909050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morey D.F.. The early evolution of the domestic dog. Am Sci. 1994;82:336–347. [Google Scholar]
- 30.Nourian A., Amiri M., Ataeian A., Haniloo A., Mosavinasab S., Badali H.. Seroepidemiological study for toxocariasis among children in Zanjan-northwest of Iran. Pak J Biol Sci. 2008;11:1844–1847. doi: 10.3923/pjbs.2008.1844.1847. [DOI] [PubMed] [Google Scholar]
- 31.Okoshi S., Usui M.. Experimental studies on Toxascaris leonina. IV. Development of eggs of three ascarids, T. leonina, Toxocara canis and Toxocara cati, in dogs and cats. Jpn J Vet Sci. 1968;30:29–38. doi: 10.1292/jvms1939.30.29. [DOI] [PubMed] [Google Scholar]
- 32.Oliveira-Sequeira T., Amarante A., Ferrari T., Nunes L.. Prevalence of intestinal parasites in dogs from São Paulo State, Brazil. Vet Parasitol. 2002;103:19–27. doi: 10.1016/s0304-4017(01)00575-1. [DOI] [PubMed] [Google Scholar]
- 33.Papazahariadou M., Founta A., Papadopoulos E., Chliounakis S., Antoniadou-Sotiriadou K., Theodorides Y.. Gastrointestinal parasites of shepherd and hunting dogs in the Serres Prefecture, Northern Greece. Vet Parasitol. 2007;148:170–173. doi: 10.1016/j.vetpar.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 34.Puebla L.E.J., Nunez F.A., Rivero L.R., Hernandez Y.R., Garcia I.S., Millan I.A.. Prevalence of intestinal parasites in dogs from municipality La Lisa, Havana, Cuba. J Vet Sci Technol. 2015;6:1–3. [Google Scholar]
- 35.Ramírez-Barrios R.A., Barboza-Mena G., Muñoz J., Angulo-Cubillán F., Hernández E., González F., Escalona F.. Prevalence of intestinal parasites in dogs under veterinary care in Maracaibo, Venezuela. Vet Parasitol. 2004;121:11–20. doi: 10.1016/j.vetpar.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 36.Riggio F., Mannella R., Ariti G., Perrucci S.. Intestinal and lung parasites in owned dogs and cats from central Italy. Vet Parasitol. 2013;193:78–84. doi: 10.1016/j.vetpar.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 37.Robertson I.D., Irwin P., Lymbery A., Thompson R.. The role of companion animals in the emergence of parasitic zoonoses. Int J Parasitol. 2000;30:1369–1377. doi: 10.1016/s0020-7519(00)00134-x. [DOI] [PubMed] [Google Scholar]
- 38.Robertson I.D., Thompson R.. Enteric parasitic zoonoses of domesticated dogs and cats. Vet Parasitol. 2007;143:7–13. doi: 10.1016/s1286-4579(02)01607-6. [DOI] [PubMed] [Google Scholar]
- 39.Rokni M.. Echinococcosis/hydatidosis in Iran. Iran J Parasitol. 2009;4:1–16. [Google Scholar]
- 40.Szabová E., Juriš P., Miterpáková M., Antolová D., Papajová I., Šefčíková H.. Prevalence of important zoonotic parasites in dog populations from the Slovak Republic. Helminthologia. 2007;44:170–176. [Google Scholar]
- 41.Traub R.J., Pednekar R.P., Cuttell L., Porter R.B., Rani P.A.A.M., Gatne M.L.. The prevalence and distribution of gastrointestinal parasites of stray and refuge dogs in four locations in India. Vet Parasitol. 2014;205:233–238. doi: 10.1016/j.vetpar.2014.06.037. [DOI] [PubMed] [Google Scholar]
- 42.Traversa D., Frangipane di Regalbono A., Di Cesare A., La Torre F., Drake J., Pietrobelli M.. Environmental contamination by canine geohelminths. Parasit Vectors. 2014;7:1–9. doi: 10.1186/1756-3305-7-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zajac A.M., Conboy G.A. John Wiley & Sons;; United States: 2012. Veterinary clinical parasitology. [Google Scholar]
- 44.Zanzani S.A., Di Cerbo A.R., Gazzonis A.L., Genchi M., Rinaldi L., Musella V., Cringoli G., Manfredi M.T.. Canine fecal contamination in a Metropolitan Area (Milan, North-Western Italy): prevalence of intestinal parasites and evaluation of health risks. Sci World J. 2014;2014:1–6. doi: 10.1155/2014/132361. [DOI] [PMC free article] [PubMed] [Google Scholar]