Abstract
Introduction
Infections with bovine foamy virus (BFV) were found in many countries but there is a lack of large-scale surveys on the prevalence of BFV among dairy cattle. The aim of this study was to develop and validate the recombinant Gag protein-based ELISA and to estimate the prevalence of antibodies against BFV.
Material and Methods
Gag coding region from BFV was cloned into expression vector pT7Arg-STOP, which expressed a high level of recombinant Gag protein from E.coli. The ELISA was standardised, and the cut-off value and sensitivity and specificity of the test were calculated using a receiver operating characteristic and Bayesian estimation.
Results
A total of 3,051 serum samples were tested by ELISA and 939 (30.8%) sera were recognised as positive. When Bayesian approach was used, the overall true BFV prevalence was 29.7% (95% CI: 25.9–33.4%).
Conclusion
Expressed Gag protein of BFV has been used successfully as an antigen for ELISA. Eventually, this study provides basic information about the epidemiological status of infection with BFV in dairy cattle in Poland, which can be used for further studies on dissemination and transmission of BFV infection.
Keywords: cattle, bovine foamy virus, seroprevalence, Bayesian estimation
Introduction
Bovine foamy virus (BFV) belongs to the least known subfamily of retroviruses called Spumaretrovirinae. BFV was first isolated from lymphocytes, lymph nodes, and milk sediment of cattle with lymphosarcoma (18). It was described as the syncytia-forming agent leading to a rapid lysis of bovine embryonic spleen culture. The organisation of BFV genome showed features typical for other known spumaviruses (24). The BFV genome consists of genes encoding structural and functional proteins, namely capsid protein (gag), glycoprotein (env), and Pol polyprotein (pol), being the precursor of viral reverse transcriptase, integrase, and protease. Moreover, two additional open reading frames were reported, which encode for regulatory proteins Borf-1 and Bet.
The natural transmission of BFV occurs similarly to other foamy viruses (FVs) by direct contact, probably through saliva (12) orvia colostrum or milk (25, 20). BFVs were also recovered from foetal tissues, placenta, and testes, as well as from fluids used to flush the uterus and oviducts of superovulated cows (2, 16). Interestingly, recent studies on naturally or experimentally infected animals have shown that BFV DNA is present in most tissues (lung, salivary glands, liver, spleen, bone marrow) (21) similarly to other FVs (22). Despite the fact that BFV is highly lytic for in vitro infection in various types of cells, infection in cattle is not associated with a defined disease or disease symptoms. Some reports confirmed mixed infection of cattle with BFV and bovine leukaemia virus (BLV) or bovine immunodeficiency virus (BIV) under natural conditions, and suggested possible role of BFV as a cofactor for other viruses (11).
Infection with BFV was found in a high percentage of cattle in different countries (1, 11, 12, 23, 25). In Poland, BFV was detected by virus isolation from blood of naturally infected animals (19) and one field isolate, called BFV100, was sequenced (9). However, the large-scale seroprevalence study of BFV infection has never been performed. The present study reports the development and validation of a recombinant Gag protein-based ELISA and the results of a survey for the presence of antibodies against BFV conducted in dairy cattle in Poland.
Material and Methods
Serum samples
Blood samples were collected from a total of 3,051 Holstein-Friesian cattle, 2–10 years old, which came from 86 farms. Blood was collected as part of the routine brucellosis-leukosis control programme, which involved 20, 40, and 26 farms located in Lower Silesian, Greater Poland, and Pomerania provinces, respectively. For serum collection, all blood samples were collected and allowed to coagulate and the tubes were centrifuged at 1,500 × g for 15 min. The sera were stored frozen at –20°C until used.
Molecular cloning and development of recombinant Gag protein of BFV
A 1641 bp long fragment of whole gag gene was amplified by PCR with the following primers: Gag5N (5’-CATATGGCTCTTAATGACTTCGACC-3’) and Gag3P (5’–AAGCTT AGATGATTGC CCT TGATTTCC-3’), thereby introducing sites for Nde I (Gag5N) and Hind III (Gag3P). PCR reaction was performed in TGradient thermocycler (Biometra, Germany) under following conditions: 94ºC for 3 min and 35 cycles at 94ºC for 45 s, 55ºC for 45 s and 72ºC for 1 min, with final step at 72ºC for 10 min, using 2.5U of DyNazyme II polymerase (Finnzymes, Finland), 1 × PCR buffer, 1.5 mM of MgCl2, 0.1 mM of each dNTP, 0.2 μM of each primer, and 0.5 μg of genomic DNA of Cf2Th/BFV100. The PCR product was digested with restriction enzymes cleaving at the introduced sites, subcloned into pDRIVE vector (Qiagen), and finally fused in frame with His-tag included in the pT7Arg-STOP expression plasmid (kindly provided by Prof. Płucienniczak, IBA, Warsaw). E. coli Rosetta (DE3) pLys cells (kindly provided by Prof. Martin Löchelt, DKFZ, Heidelberg) were transformed with the pT7Arg-STOP/gag plasmid and positive clones were identified by enzyme digestion and sequencing. Selected clones were grown at 30°C in LB medium containing 100 μg/mL of ampicillin and the expression of Gag protein was induced by adding 0.25 mM of isopropyl-D-thio-galactoside (IPTG). The bacteria were harvested by centrifugation at 4,000 rpm and the pellet was resuspended in lysis buffer (QIAexpress Ni-NTA Fast Start, Qiagen, Germany) completed with protease inhibitor cocktail (Roche, Germany). Then, the cells were lysed by freezing-thawing and incubation with lysozyme (100 mg/mL) and RNase (5 g/mL). Next, the lysate was sonicated 10 times with 10 s intervals. Gag protein was purified by affinity chromatography using QIAexpress Ni-NTA Fast Start columns (Qiagen). Protein concentration was measured by Bradford method and the homogeneity of eluates was checked by SDS-PAGE. Protein was aliquoted and stored at –70ºC till use.
Western blotting
A total of 30 µg of purified Gag protein was electrophoresed on 12% polyacrylamide gel and transferred onto nitrocelulose filter (Hybond C, Amersham, UK). The blot was blocked with 2% BSA in TBST buffer, pH 7.5, and incubated with a pool of five BFV-positive and a pool of five BFV-negative bovine sera diluted 1:100 in 0.1% BSA in TBST, pH 7.5. Anti-bovine IgG-PO (SIGMA, USA) was used as secondary antibody. Blot was developed with 4-chloro-1-naphtol (SIGMA, USA).
ELISA
Ninety-six-well microtiter plates (Nunc Immoblizer Ni-chelate, Nunc, Denmark) were coated with His-tag Gag protein at a 1.5 µg/mL dilution of 0.01 M KCl for 2 h at 37°C. The coated plates were washed and incubated with bovine sera diluted 1:100 in PBST, pH 7.5, for 1 h at room temperature. After another washing the plates were incubated with anti-bovine IgG peroxidase conjugate (SIGMA, USA) diluted 1:5,000. Substrate reaction was performed for 10 min using ABTS prepared according to manufacturer’s protocol (SIGMA, USA). Quantification was performed using ELISA reader (Dynatech MR 5000) at 410 nm. All incubations were performed with a volume of 100 µL/well. The ELISA was standardised to determine the desirable signal-to-noise ratio by using BFV-positive and BFV-negative bovine control sera. All sera were tested in duplicate and the mean value for each specimen was calculated as a sample-to-positive ratio (S/P-ratio = (OD sample-OD negative control)/(OD positive control-OD negative control)). Two independent panels of bovine reference sera (panel I consisting of 82 sera – 33 positive and 49 negative, and panel II, consisting of 120 sera – 87 positive and 33 negative, as tested by WB) were used for validation of ELISA. To assess the cut-off value for positive sera, a receiver operating characteristic (ROC) analysis was performed, using STATISTCA software, version 8.0. Evaluation was performed by testing serum samples from panel I. Additionally, sensitivity (Se) and specificity (Sp) of the newly developed ELISA were estimated using Bayesian analysis and serum samples from panels I and II. The results from ELISA and Western blot were used for estimation of the prior distributions, which were specified by modelling to generate the posterior distribution probabilities of sensitivity and specificity (13). Prior estimation for Se and Sp of ELISA was based on approximation of β distribution and was expressed as median with CI (0.025–0.975 percentiles). To compare the performances of the two methods, the following tests were used: kappa value, proportions of positive and negative agreement, and McNemar’s chi-square values (4). Corresponding P values were calculated for both kappa and chi-squared tests. The “RSurveillance” and “epR” modules in the “R” software for CRAN project were used (https://r-forge.r-project.org).
Estimation of BFV seroprevalence
The apparent and true seroprevalences of BFV infection in naturally infected animals were estimated according to the overall population and each province. The apparent prevalence was calculated as the number of test-positive animals among the total number of animal tested. Confidence intervals for apparent prevalence use the Wilson binomial approximation (3, 6). The true prevalence was obtained from apparent prevalence by correction for misclassification of diagnostic test via methodology described by Gardner et al. (6). The posterior values were calculated using Epitools (Sergeant ESG: 2009, Epitools epidemiological calculators. AusVet Animal Health Services and Australian Biosecurity Cooperative Research Centre for Emerging Infectious Disease) (http://epitools.ausvet.com.au).
Results
Development of recombinant antigen
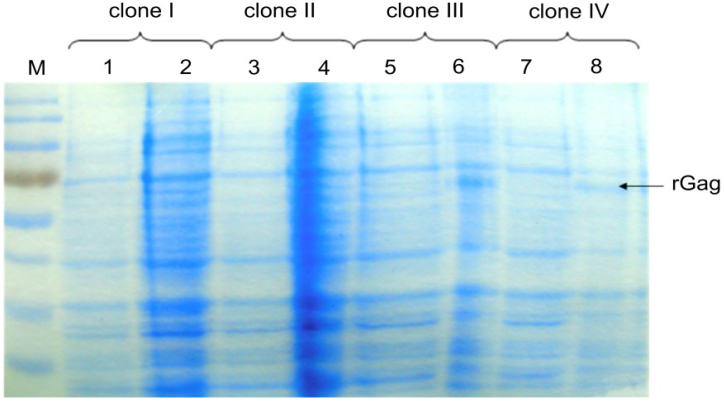
A fragment of 1,641 bp of gag gene was generated by PCR and cloned into the prokaryotic expression plasmid pT7Arg-STOP for the production of Gag protein. The pT7Arg-STOP/gag plasmid was sequenced near the fusion junction to ensure preservation of correct reading frame (data not shown). After electrophoresis and Coomassie blue staining, a distinct 68 kDa Gag-specific band was observed in bacterial lysates after IPTG induction (Fig. 1). The noted molecular mass was in agreement with the predicted molecular mass of Gag protein and thioredoxin and His-tag at NH2 terminus of expressed protein. The recombinant protein constituted approximately 20% of the total protein after IPTG induction and the final yield of the fusion protein was about 0.5 mg/mL. In order to analyse immunoreactivity of the expressed Gag protein, Western blot was applied. Gag protein showed a strong and specific reaction with positive control serum and the sera collected from naturally infected cows (Fig. 2). Negative control serum and BFV negative sera did not recognise the Gag protein.
Fig. 1.
PAGE-SDS of bacterial lysates of E. coli clones transformed with plasmid pT7Arg-STOP/Gag before induction with IPTG (lanes: 1, 3, 5, 7) and after 5 h of induction (lanes: 2, 4, 6, 8). Only lysates of clones III and IV showed the presence of additional band representing recombinant Gag protein
Fig. 2.
Immunoreactivity of recombinant BFV Gag protein determined by Western blot. BFV positive serum samples (strips: 8 – 13) and BFV negative serum samples (strips: 1 – 7). P – BFV positive control serum sample, N – BFV negative control serum samples
Development of ELISA
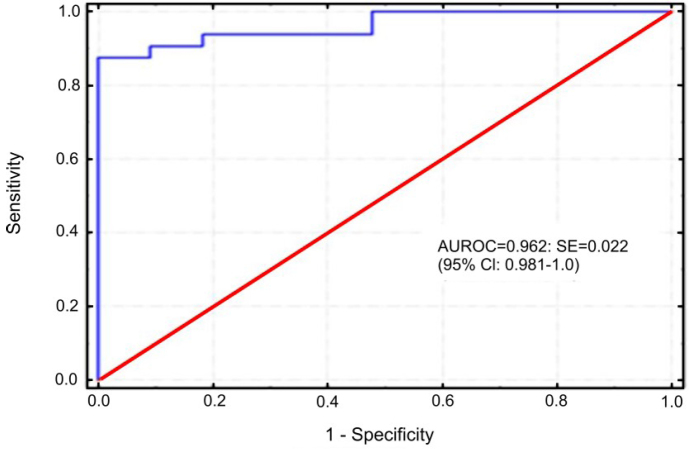
Series of titration experiments were set up to determine the optimal dilution of reagents using positive and negative control serum. The selected dilutions were those for which the highest disparities of OD values between BFV positive and BFV negative sera were noted. The most optimal results were obtained when concentration of 1.5 µg/mL of antigen was used and the sera and conjugate were diluted 1:100 and 1:5,000, respectively. In order to establish a positive cut-off value, 82 bovine sera with known BFV status were tested by ELISA, the S/P ratios were calculated and a single-graph ROC plot was generated (Fig. 3). Samples were considered positive when the S/P ration was equal or higher than 0.89. Under this condition, one negative serum was considered positive by ELISA while two positive sera were negative by ELISA. Using standard formula, sensitivity and specificity of ELISA were 93.93% and 97.95%, respectively. The value of the area under the curve (AUC) 0.962 indicated that the ELISA was also highly accurate. Additionally, statistical analysis using Bayesian method was performed on the data sets generated from examination of two independent panels of sera. Table 1 shows prior and posterior medians and lower and upper limits of the posterior equally tailed 95% credible intervals for the sensitivity and specificity for both diagnostic tests. The comparison of performances of the two tests revealed perfect concordance between them, as was estimated by kappa value K = 0.908 (0.849–0.967) and McNemar's chi-square statistic, (< 0.00001, P = 1.0). The same results were obtained when the proportions of positive and negative agreement were calculated. However, better agreement was noted for serum samples from infected (0.962) than from uninfected (0.946) animals, representing both panels.
Fig. 3.
Diagram presenting the ROC analysis
Table 1.
The prior and posterior medians and lower and upper limits of the posterior equally tailed 95% credible intervals for the sensitivity and specificity of ELISA and Western blot (WB)
| Parameters | Prior information | Posterior information | |
|---|---|---|---|
| Values of apparent parameters | Values of true parameters | ||
| Panel I | Panel II | (as median and the 95% CI)b | |
| ELISA Se | 0.939 (0.803–0.981) | 0.966 (0.904–0.988) | 0.969 (0.927–0.993) |
| ELISA Sp | 0.98 (0.896–0.985) | 0.909 (0.763–0.967) | 0.944 (0.880–0.983) |
| WB Se | 0.969 (0.842–0.993) | 0.966 (0.904–0.988) | 0.974 (0.935–0.995) |
| WB Sp | 0.96 (0.865–0.988) | 0.909 (0.763–0.967) | 0.944 (0.880–0.983) |
Cl – Wilson binomial confidence limits
CI – Bayesian credible interval
Estimation of true BFV prevalence in cattle
In total, 3,051 serum samples were tested by ELISA and specific BFV antibodies were detected in 939 (30.8%) samples, while 2,112 samples remained negative. The presence of true prevalence was then verified by the Bayesian estimation. Under this model, out of 3,051 cattle tested, 906 (29.7%; 95% CI: 25.9–33.4%) showed the presence of BFV antibodies. The percentage of cattle positive for BFV antibodies varied by the provinces and showed the highest value for Pomeranian Province (33.4%), while in Silesian and Greater Poland Provinces specific antibodies were detected in 30.9% and 28.2% animals, respectively. True prevalence noted in cattle from the provinces was slightly lower than AP values (Table 2).
Table 2.
The results of testing of bovine sera using ELISA
| Province | Number of tested samples | Number of BFV-positive samples | Apparent seroprevalence of BFV (with 95% Cl)a | True seroprevalence of BFV (with 95% CI)b |
|---|---|---|---|---|
| Silesian | 1,055 | 326 | 0.309 (0.282–0.338) | 0.298 (0.253–0.343) |
| Greater Poland | 1,030 | 290 | 0.282 (0.255–0.310) | 0.268 (0.223–0.313) |
| Pomeranian | 966 | 323 | 0.334 (0.305–0.365) | 0.326 (0.280–0.371) |
| Total | 3,051 | 939 | 0.308 (0.292–0.324) | 0.297 (0.259–0.334) |
Cl – Wilson binomial confidence limits
CI – Bayesian credible interval
Discussion
The first serological study on BFV infection was conducted in cattle in Canada in 1979 (7). By now, a variety of serological analyses have been described for BFV; however, there were no large-scale surveys on the prevalence and distribution of BFV among dairy cattle. The lack of extensive surveys for BFV was partially due to the difficulties in obtaining sufficient amounts of antigen for diagnostic test. The first attempt in this direction was made using GST-capture ELISA with recombinant Gag, Bet, and Env proteins of BFV (25). However, only Gag protein was acknowledged as an antigen of choice for serological detection of BFV infection. Gag protein is known to be highly immunogenic and contains the sequences which are conserved among different isolates of BFV (9). It has also been demonstrated that Gag protein elicited a strong and long-lasting immune response in calves and sheep experimentally inoculated with BFV (21). Specific antibodies could be detected as early as two weeks after BFV inoculation and persisted at high and stable level for at least three years. Several other reports dedicate Gag protein to detection of antibodies against other foamy viruses (8, 10, 14, 26).
In this study ELISA was developed using the recombinant Gag protein as an antigen for detection of BFV antibody. Recombinant BFV Gag protein offers some advantages over preparation of the virus from tissue culture. Additionally, an advantage of chosen approach is the high concentration of obtained protein and application of His-tag instead of GST. As opposed to GST-tag which is highly immunogenic (17), especially in cows, His-tag shows relatively lower immunogenicity and allows avoiding additional steps in ELISA procedure, such as preincubation of GST-tag with tested sera during assay performance. Furthermore, the use of His-tagged protein and its affinity to nickel-chelate plates, allowed us to avoid the blocking step, currently included in standard protocol of ELISA. Considering all advantages of established ELISA with Gag recombinant protein as antigen, we provided a simple and fast assay to detection of BFV specific antibodies.
In this study, newly developed ELISA was validated according to the results obtained by Western blot. When ROC analysis was performed, a cut-off of S/P 0.89 gave the optimum sensitivity of 93.93% and specificity of 97.95%. A previous study addressed the efficiency of anti-FFV and BFV antibody detection using either biotinylated recombinant protein from nucleocapside FFV or GST-capture ELISA with Gag recombinant protein (26, 25). Both tests showed perfect specificity when the cut-off value was calculated using negative reference sera tested by Western blot. However, Western blot is not a suitable gold standard for estimation of true infection status, since this method itself fails to properly identify all infected and uninfected animals. Therefore, the Bayesian estimation was applied since this technique is especially useful in the absence of gold standard method, and allows for estimation of stable test parameters, as well as easily interpretable intervals. Our data showed that posterior parametres, calculated by Bayesian estimation, were 96.9% and 94.4% for sensitivity and specificity, respectively, and these values were slightly different from those calculated by ROC analysis (apparent parametres), but the differences were not statistically significant, as was showed by kappa value, proportions of positive and negative agreement, and McNemar's chi-square values. Both methods of validation showed the relatively high overall specificity of the newly developed ELISA. This is not surprising, since the use of a purified recombinant antigen is expected to significantly decrease false-positive reactions to other proteins in the antigen preparation, as was shown for detection of PRRS antibodies in swine (5).
When the developed ELISA was used for the detection of BFV-specific antibodies among dairy cattle, the seropositivity was noted in approximately 30.8% of the tested sera and was similar across all provinces. The true prevalence values were somewhat lower, which may suggest the tendency for the overestimation of apparent data that can be associated with either true seropositive animals or false-positive results (6, 13). Serological surveys for BFV infections were performed in some countries, showing different percentages of BFV seropositive cattle ranging from 50% and 40% in Canada (7, 11), 39% in Great Britain (1) and Australia (12) to 7% in Germany (25). However, it is difficult to refer these results to those from our study due to the different number of tested samples and variety of serological methods used. For example the data from Canada were generated by immunodiffusion test, which is known to be less sensitive than ELISA, and the results were not adjusted for sensitivity and specificity (11). This relatively high seroprevalence of BFV noted in this study can be explained by the fact that BFV can be easily transmitted by direct contact between animals. It was found that shedding of BFV can occur via saliva, and possibly through sneezing or licking. This could lead to the infection of calves, negative at birth, through being kept together with infected adults (12). In this context, faeces could also be considered a possible source of infection for cattle, as was suggested for simian foamy virus in chimpanzees (15). Additionally, perinatal modes of transmission via colostrum or milk have also been postulated (20, 25). In conclusion, this study provides basic information about the epidemiology of infection with BFV in dairy cattle in Poland and the obtained data can be further used to investigate the transmission and dissemination of BFV infection.
Acknowledgements
The authors would like to thank Prof. Andrzej Płucienniczak for providing pT7Arg-STOP expression plasmid and Prof. Martin Löchelt for providing E. coli Rosetta (DE3) pLys cells
Footnotes
Conflict of Interests Statement: The authors declare that there is no conflict of interests regarding the publication of this article.
Financial Disclosure Statement: The research was supported by a grant no. 2 PO6K 043 27 from the Polish Ministry of Science and Higher Education.
Animal Rights Statement: None required.
References
- 1.Appleby R.C.. Antibodies to bovine syncytial virus in dairy cattle. Vet Rec. 1979;105:80–81. doi: 10.1136/vr.105.4.80. [DOI] [PubMed] [Google Scholar]
- 2.Bouillant A.M., Ruckerbauer G.M.. Isolation of bovine syncytial virus from lymphocytes recovered from fluids used to flush uterus and oviducts of superovulated cattle. Can J Comp Med. 1984;48:332–334. [PMC free article] [PubMed] [Google Scholar]
- 3.Brown L.D., Cat T.T., Das Gupta A.. Interval Estimation for a proportion. Statistical Science. 2001;16:101–133. [Google Scholar]
- 4.Cicchetti D.V., Feinstein A.R.. High agreement but low kappa: II. Resolving the paradoxes. J Clin Epidemiol. 1990;43:551–558. doi: 10.1016/0895-4356(90)90159-m. [DOI] [PubMed] [Google Scholar]
- 5.Ferrin N., Fang Y., Johnson C., Murtaugh M., Polson D., Torremorell M., Gramer M., Nelson E.. Validation of a blocking enzyme-linked immunosorbent assay for detection of antibodies against porcine reproductive and respiratory syndrome virus. Clin Diag Lab Immunol. 2004;11:503–514. doi: 10.1128/CDLI.11.3.503-514.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gardner I.A., Stryhn H., Lind P., Collins M.T.. Conditional dependence between tests affects the diagnosis and surveillance of animal diseases. Prev Vet Med. 2000;45:107–122. doi: 10.1016/s0167-5877(00)00119-7. [DOI] [PubMed] [Google Scholar]
- 7.Greig A.S.. A syncytium regression test to detect antibodies to bovine syncytia virus. Can J Comp Med. 1979;43:112–114. [PMC free article] [PubMed] [Google Scholar]
- 8.Hahn H., Baunach G., Bräutigam S., Mergia A., Neumann-Haeflin D., Daniel M.D., McClure M.O., Rethwilm A.. Reactivity of primate sera to foamy virus Gag and Bet proteins. J Gen Virol. 1994;75:2635–2644. doi: 10.1099/0022-1317-75-10-2635. [DOI] [PubMed] [Google Scholar]
- 9.Hechler T., Materniak M., Kehl T., Kuzmak J., Löchelt M.. Complete genome sequences of two novel European clade bovine foamy viruses from Germany and Poland. J Virol. 2012;86:10905–10906. doi: 10.1128/JVI.01875-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hussain A.I., Shanmugam V., Bhullar V.B., Beer B.E., Vallet D., Gauier-Hion A., Wolfe N.D., Karesh W.B., Kilbourn A.M., Tooze Z., Heneine W., Switzer W.M.. Screening for simian foamy virus infection by using a combined antigen Western blot assay evidence for a wide distribution among Old World primates and identification of four new divergent viruses. Virology. 2003;309:248–257. doi: 10.1016/s0042-6822(03)00070-9. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs R.M., Pollari F.L., McNab W.B., Jefferson B.. A serological survey of bovine syncytial virus in Ontario: associations with bovine leukemia and immunodeficiency-like viruses, production records, and management practices. Can J Vet Res. 1995;59:271–278. [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson R.H., Rosa R. de la, Abher I., Kertayadanya I.G., Entwistle K.W., Fordyce G., Holroyd R.G.. Epidemiological studies of bovine spumavirus. Vet Microbiol. 1988;16:25–33. doi: 10.1016/0378-1135(88)90124-1. [DOI] [PubMed] [Google Scholar]
- 13.Joseph L., Gyorkos T.W., Coupal L.. Bayesian estimation of disease prevalence and the parameters of diagnostic test in the absence of a gold standard. Am J Epidemiol. 1995;141:263–272. doi: 10.1093/oxfordjournals.aje.a117428. [DOI] [PubMed] [Google Scholar]
- 14.Khan A.S., Sears J.F., Muller J., Galvin T.A., Shahabuddin M.. Sensitive assays for isolation and detection of simian foamy retroviruses. J Clinic Μicrobiol. 1999;37:2678–2686. doi: 10.1128/jcm.37.8.2678-2686.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu W., Worobey M., Li Y., Keele B.F., Bibollet-Ruche F., Guo Y., Goepfert P.A., Santiago M.L., Ndjango J.B., Neel C., Clifford S.L., Sanz C., Kamenya S., Wilson M.L., Pusey A.E., Gross-Camp N., Boesch C., Smith V., Zamma K., Huffman M.A., Mitani J.C., Watts D.P., Peeters M., Shaw G.M., Switzer W.M., Sharp P.M., Hahn B.H.. Molecular ecology and natural history of simian foamy virus infection in wild-living chimpanzees. PloS Pathog. 2008;4:1–21. doi: 10.1371/journal.ppat.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luther P.D., Nuttall P.A., Gibbons R.A.. Isolation of viruses from cultures of bovine endometrial cells. J Infect Dis. 1978;138:660–663. doi: 10.1093/infdis/138.5.660. [DOI] [PubMed] [Google Scholar]
- 17.Mahnke C., Löchelt M., Bannert H., Flugel R.M.. Specific enzyme-linked immunosorbent assay for the detection of antibodies to the human spumavirus. J Virol Methods. 1990;29:13–22. doi: 10.1016/0166-0934(90)90003-x. [DOI] [PubMed] [Google Scholar]
- 18.Malmquist W.A., Maaten M.J., Van der Boothe A.D.. Isolation, immunodiffusion, immunofluorescence, and electron microscopy of a syncytial virus of lymphosarcomatous and apparently normal cattle. Cancer Res. 1969;29:188–200. [PubMed] [Google Scholar]
- 19.Materniak M., Bicka L., Kuźmak J.. Isolation and partial characterization of bovine foamy virus from Polish cattle. Polish J Vet Sci. 2006;9:207–211. [PubMed] [Google Scholar]
- 20.Materniak M., Sieradzki Z., Kuźmak J.. Detection of bovine foamy virus in milk and saliva of BFV seropositive cattle. Bull Vet Inst Pulawy. 2010;54:461–465. [Google Scholar]
- 21.Materniak M., Hechler T., Lochelt M., Kuzmak J.. Similar patterns of infection with bovine foamy virus in experimentally inoculated calves and sheep. J Virol. 2013;87:3516–3525. doi: 10.1128/JVI.02447-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray S.M., Picker L.J, Axthelm M.K, Linial M.L.. Expanded tissue targets for foamy virus replication with simian immunodeficiency virus-induced immunosuppression. J Virol. 2006;80:663–670. doi: 10.1128/JVI.80.2.663-670.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pamba R., Jeronimo C., Archambault D.. Detection of bovine retrospumavirus by the polymerase chain reaction. J Virol Methods. 1999;78:199–208. doi: 10.1016/s0166-0934(98)00179-7. [DOI] [PubMed] [Google Scholar]
- 24.Renshaw R.W., Casey J.W.. Analysis of the 5’long terminal repeat of bovine syncytia virus. Gene. 1994;141:221–224. doi: 10.1016/0378-1119(94)90575-4. [DOI] [PubMed] [Google Scholar]
- 25.Romen F., Backes P., Materniak M., Sting R., Vahlenkamp T.W., Riebe R., Pawlita M., Kuzmak J., Löchelt M.. Serological detection systems for identification of cows shedding bovine foamy virus via milk. Virology. 2007;364:123–131. doi: 10.1016/j.virol.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Winkler I.G., Löchelt M., Levesque J-P., Bodem J., Flügel R.M., Flower R.L.P.. A rapid streptavidin-capture ELISA specific for the detection of antibodies to feline foamy virus. J Immunol Methods. 1997;207:69–77. doi: 10.1016/s0022-1759(97)00109-9. [DOI] [PubMed] [Google Scholar]