Abstract
Introduction
Despite the advancements in the field, there is a lack of data when it comes to co-infections in poultry. Therefore, this study was designed to address this issue.
Material and Methods
Broiler birds were experimentally infected with E. coli (O78) and low pathogenic avian influenza (LPAI) strain, alone or in combination. The experimental groups were negative control.
Results
The infected birds showed most severe clinical signs in E. coli+LPAI group along with a significant decrease in weight and enhanced macroscopic and microscopic pathological lesions. The survival rate was 60%, 84%, and 100% in birds inoculated with E. coli+LPAI, E. coli, and LPAI virus alone, respectively. The results showed that experimental co-infection with E. coli and H9N2 strain of LPAI virus increased the severity of clinical signs, mortality rate, and gross lesions. The HI titre against LPAI virus infection in the co-infected group was significantly higher than the HI titre of LPAI group, which may indicate that E. coli may promote propagation of H9N2 LPAI virus by alteration of immune response.
Conclusion
The present study revealed that co-infection with E. coli and H9N2 LPAI virus caused more serious synergistic pathogenic effects and indicates the role of both pathogens as complicating factors in poultry infections.
Keywords: broilers, Escherichia coli, avian influenza virus, co-infection, pathology
Introduction
Respiratory diseases have a major impact on poultry health, not only directly influencing growth and viability, but possibly also leading to secondary infections. Low pathogenic avian influenza viruses (LPAIVs) are particularly important due to their widespread circulation in domestic poultry (13). Considerable information on the epidemiology of LPAIVs in chickens and wild birds has been reported over the last few years because of influenza surveillance schemes throughout the world (15, 19). LPAIV (H9N2) infections are emerging respiratory problems in poultry industry causing huge economic losses, especially in the presence of other co-infecting pathogens. H9 subtype of avian influenza virus (AIV) is one of the subtypes most frequently isolated from domestic chickens (17).
Infections with H9N2 viruses in domestic poultry are usually associated with decreased feed and water consumption and egg production, and are presented as mild respiratory diseases with low mortality (15). However, during the last decade, the outbreaks of LPAIV (H9N2) infections with severe clinical signs, high mortality (20%–65%) and reduced production (up to 75%) have been reported in commercial poultry farms (14). Similarly, an outbreak of H9N2 influenza virus infection in chickens in Hong Kong (A/chicken/Hong Kong/739/94) was associated with coughing and respiratory distress in 75% of the birds, with 10% mortality. Antibiotic treatment reduced the mortality rate, suggesting that bacteria may play a role in exhibition of the clinical signs (9, 12). Specific information about the pathological alterations during the course of co-infection with LPAIV (H9N2) and E. coli in poultry is not available. Hence the decision to study the effect of LPAIV infection on broiler chickens simultaneously challenged with E. coli. H9N2 AIV used in this study was isolated from live poultry markets of Pakistan. This study has been conducted as an experimental infection that addresses the query whether E. coli challenges pathogenesis and immune responses in avian influenza virus infections. The successful reproduction of clinical disease with this experimental model allowed us to investigate the role of E. coli in the pathogenesis of LPAIV (H9N2) infection and vice versa.
Material and Methods
Experimental birds and management
The study was carried out on three-week-old broiler chicks (n=100), procured from local hatchery. The chicks were shown to be negative for AIV infection using haemagglutination inhibition test and virus isolation for H5 and H9 subtypes (4). Standard management was provided with feed and water ad libitum. Different persons performed the feeding of the broiler chicken in order to prevent cross-contamination. All experimental groups were provided identical management protocol. The birds were given standard antibacterial free feed having an anti-coccidial agent (salinomycin).
Preparation of inoculum
E. coli strain (avian pathogenic strain) was kindly provided by Dr Muhammad Usman (Poultry Research Institute, Rawalpindi, Pakistan), originally isolated from chicken. Stock cultures of E. coli strain were stored in 40% glycerol broth at −80°C. E. coli stock culture was prepared by inoculating MacConkey agar with a loopful of reference E. coli strain culture and incubating at 37°C for 24 h. To prepare E. coli cultures for infecting birds by aerosol, 250 mL of Dulbecco's Modified Eagle's medium (DMEM, with Hepes 25 mM as neutralising medium) was inoculated with colonies from MacConkey agar plate and incubated in an orbital shaker at 37°C for 22 to 24 h. The estimated colony count was confirmed by plating 0.1 mL of 105 dilution of the final culture onto separate MacConkey agar plates (20).
Avian influenza A virus used in this study, namely A/chicken/Pakistan/10RS3039-284-48/2010 (H9N2), was a reasserting field virus isolated from diseased chicken and kindly provided by Dr Abdul Ghafar (National Agricultural Research Centre, Islamabad, Pakistan). Viral stocks were prepared and titrated in 10-day-old chicken embryonated eggs; the median embryo infectious dose (EID50) was calculated according to already reported procedures. The viral stocks were diluted in medium containing antimicrobial agents (brain-heart infusion (BHI) broth containing 200 U/mL of penicillin, 200 μg/mL of streptomycin, 100 U/mL of polymyxin B sulfate, and 250 μg/mL of gentamicin) to yield a final titre of 106 EID50/mL (4, 18).
Experimental design
The experimental birds were randomly allocated into four groups with 25 birds in each group. The experimental groups were identified as follows: negative control group, E. coli, LPAI, and E. coli+LPAI. All birds were confirmed serologically naive and influenza virus free by haemagglutination inhibition test. The birds in negative control group remained untreated while the birds in group E. coli and LPAI were inoculated with E. coli (106 cfu/mL) and LPAIV H9N2 (106 EID50/mL/bird) through intratracheal route (IT), respectively, whereas the birds in group E. coli+LPAI were co-infected with both pathogens on the same day at dosages of 103 cfu and 103 EID50 per bird, respectively.
Clinical examination
The birds were monitored for clinical signs twice a day up to 14 days post-infection (dpi). All birds were weighed at the start and end of experiment and observations were recorded in the form of general condition of birds, as well as clinical signs of disease and mortality rate. Attention was paid to any kind of pathology, but especially to disorders of the respiratory system (head swelling, nasal discharge, sneezing, tracheal rales, coughing, and difficult breathing). A scoring system was used to evaluate the severity of clinical signs. Each clinical sign was scored by the following scale: 0 – no sign; 1 – mild or slight; 2 – moderate; 3 – severe. The mean clinical score was based on the sum of clinical scores for each sign divided by the number of birds in each group at each observation time as previously described (5).
Macroscopic and microscopic examinations
Birds were euthanised using an intracephalic injection of' pentobarbital sodium (Anpro Pharmaceutical, Arcadia, USA) at 4, 8, and 12 dpi. Necropsy was performed immediately after the birds were euthanised. The pathological lesions were examined, with special attention paid to respiratory organs. Lesions of the trachea, bronchi, lungs, and air sacs were scored for gross severity. Briefly, respiratory organs were scored altogether on scale of 0 to 3, where 0 – no lesions, l – mild or slight lesions, 2 – moderate lesions, and 3 – severe lesions. The totals of scores of one experimental group were used for statistical comparison of the harshness of the lesions between the experimental birds. Trachea, lung, and air sac samples were taken from each necropsied bird and fixed in 10% formalin. After fixation, tissues were processed in paraffin, sectioned at 3 µm, and stained with haematoxylin and eosin. Histological lesions were graded as: (−) no lesion, (+) light, (++) moderate, or (+++) marked lesions as described previously (6). Histopathological analyses were carried out by two certified veterinary pathologists.
Statistical analysis
Data was expressed as means ±SEM (standard error of the mean) and analysed using GraphPad Prism 6 software (Graph Pad Software Inc., USA). The Shapiro-Wilk Normality Test was used to test normal distribution of the data. One-way analysis of variance (ANOVA) including Bonferroni correction was used for normally distributed data at different time points in different groups. Not normally distributed data were analysed with a Kruskal-Wallis one-way ANOVA. The body weights were also analysed by the Kruskal-Wallis test. The mean tracheal mucosa thickness was analysed using the Tukey-Kramer honest significant difference test. A chi-square test for equality of proportions was performed to measure the statistical significance of the mortality. Differences were assumed as statistically significant at P < 0.05.
Results
There were no clinical signs, gross lesions, or mortality in the uninfected control chickens. Birds in the E. coli group showed clinical signs of mild to moderate intensity sickness, including depression (25 birds), anorexia (25), sneezing (6), respiratory distress (8), ocular/nasal discharge (6), head swelling (8), and loose droppings (7). Moreover, clinical signs of mild severity, including anorexia (9), depression (8), sneezing (2), and respiratory distress (4), were noticed in birds of the LPAI group. On the other hand, clinical signs of severe respiratory disease such as sickness (25 birds), depression (25), anorexia (25), sneezing (12), respiratory distress (18), ocular/nasal discharge (11), head swelling (19), and loose droppings (12) were more pronounced and seen more frequently in E. coli+LPAI group than in the case of mono-infection with LPAI or E. coli (Table 1). Mean clinical scores of each group are presented in Fig. 1.
Table 1.
Number of infected chickens showing clinical disease signs
| Clinical signs | Negative control | E. co/z-challenged | LPAI-challenged | E. co/Z+LPAI-challenged |
|---|---|---|---|---|
| General sickness | – | ++ (25) | + (8) | +++ (25) |
| Depression | – | ++ (25) | + (8) | +++ (25) |
| Anorexia | – | ++ (25) | + (9) | ++ (25) |
| Sneezing | – | + (6) | + (2) | ++(12) |
| Respiratory distress | – | + (8) | + (4) | ++ (18) |
| Ocular/nasal discharge | – | + (6) | – | ++ (11) |
| Head swelling | – | + (8) | – | ++ (19) |
| Loose droppings | – | + (7) | – | + (12) |
| Mortality | – | (4) | – | (10)* |
| Mean body weight **± SEM | 1.889 ± 19.5a | 1.775 ± 55.4b | 1.848 ± 13.7a | 1.662 ± 17.6c |
– absence of apparent clinical disease signs, + mild, ++ moderate, and +++ severe signs
Mortality of five chickens at day 4 post virus challenge,
body weight was measured at the end of experiment (14 days post virus inoculation).
Mean values in the same row that do not share a common letter differ significantly (P < 0.05)
Fig. 1.
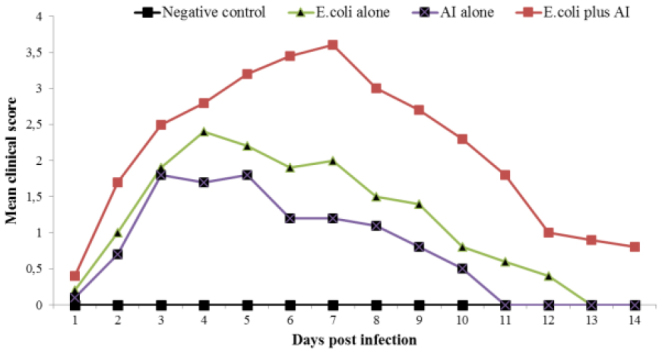
Clinical scores in different groups of broilers after inoculation with LPAIV or E. coli (O78) or a combination of both. Each clinical sign was scored by the following scale: 0 – no sign, 1 – mild or slight; 2 – moderate, 3 – severe
Gross and histopathological findings
No bird in the negative control group demonstrated body cavity lesions and all organs were normal in size, shape and consistency. In E. coli group, walls of thoracic air sacs were cloudy and thick. The amassing of serofibrinous exudates of varying amounts in thoracic /air sac, as well as fibrin accumulation in the liver and heart, and haemorrhages in the kidneys were also noticed in this group. On the other hand, only mild airsacculitis, tracheal congestion and nephritis were observed in birds of the LPAI group. In E. coli+LPAI group, large amounts of catarrhal exudates were grossly seen in the trachea. Mean gross lesions scores of respiratory organs are presented in Fig. 2. The tracheal mucosal membranes of these birds were erythematous and thick, having a layer of mucus over it (Table 2). Both the thoracic air sacs and the abdominal air sacs were recurrently filled by serofibrinous exudate. Severe gross lesions in the liver and heart increased in size, haemorrhages, oedema, and fibrin accumulation were more prominent in this group. The gross lesions such as tracheal congestion, airsacculitis, pneumonia, and perihepatitis were most prominent on 4 dpi in all infected groups while less prominent on 8 and 12 dpi. The comparison of pathological lesion scores in the respiratory system is shown in Table 3. Lesion scores in LPAI group did not show a statistically significant difference (P < 0.005) compared to the non-challenged control broiler birds, apart from a mild congestion of tracheal mucosa and kidneys. The pathological score as well as total lesion score of birds in E. coli group were higher (P < 0.005) than in the control birds. The pathological lesion score in chickens of E. coli+LPAI group had statistically significant increased values compared to E. coli group or control negative group (P < 0.005).
Fig. 2.
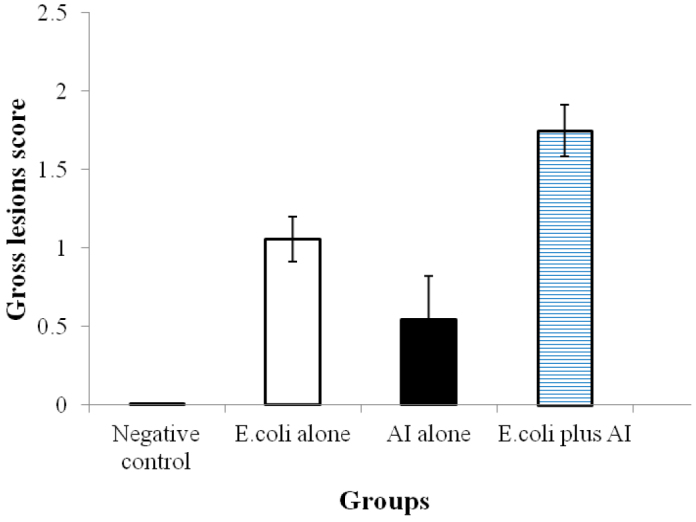
Macroscopic lesions score in the trachea, lungs, and airsacs of broiler chickens challenged with E. coli (O78) and LPAIV singly or in combination
Table 2.
Summary of post-challenge mortality and tracheal mucosal thickness analysis
| Post challenge mortality | Tracheal mucosal thickness | ||||
|---|---|---|---|---|---|
| Experimental group | Number of dead | Percentage of dead | Statistical | Mean thickness | Statistical |
| birds | birds | significance | (µm)A | significance | |
| Control negative | 0 | 0 | C | 71.25 ± 35.68 | C |
| E. coli-challenged | 4 | 16 | B | 106.5 ± 21.29 | B |
| LPAI-challenged | 0 | 0 | C | 78.17 ± 16.85 | C |
| E. coli +LPAI-challenged | 10 | 40 | A | 163.12 ± 18.25 | A |
Mean of mucosal thickness of 10 tracheas, measured at four equidistant points. Values with different capital letters are significantly different at P < 0.05
Table 3.
Summary of microscopic lesions and their intensity (+++ severe, ++ moderate, + mild, – absent or normal)
| Intensity of microscopic lesions | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Trachea/bronchi | Lungs/parabronchi | Air sacs | ||||||||
| Groups | Tracheitis /bronchitis | Necrosis and exfoliation of mucosal epithelium | Cellular infiltrates | Fibrino-leukocytic exudates | Pneumonia | Fibrino-leukocytic exudate | Cellular infiltrates | Airsacculitis | Fibrinous exudate | |
| Control negative | – | – | – | – | – | – | – | – | – | |
| E. coli | – | – | + | – | – | – | + | ++ | ++ | |
| LPAI | ++ | ++ | ++ | + | + | + | ++ | + | + | |
| E. coli+LPAI | +++ | +++ | +++ | +++ | +++ | ++ | +++ | +++ | ++ | |
In the control negative birds, all examined organs were histologically normal and there were no detectable lesions. The thickness of tracheal and primary bronchial respiratory epithelium in control group was 75.78 mm on an average. In LPAI group, the thickness of tracheal and primary bronchial respiratory epithelium did not show any statistical difference when compared to the control birds.
However, E. coli group had significantly thicker respiratory epithelium (P < 0.05) as compared to control and LPAI group. Furthermore, the respiratory epithelium of the trachea and primary bronchi in E. coli+LPAI group was statistically significantly thicker than those of control birds (P < 0.05), LPAI group (P < 0.05), and E. coli group (P < 0.05).
The respiratory epithelium of control birds was topped by ciliated columnar epithelium and mucus filled goblet cells. No lymphocyte and histiocyte infiltration was recorded. In the LPAI group, in some places the respiratory epithelium was discontinued and absence of cilia and desquamation were observed in the epithelial cells in these places. The histological lesion score was statistically higher (P < 0.05) than that of the control group. In E. coli group, the epithelial cells of various parts of the trachea and primary bronchi did not have cilia, which were degenerated, and had fewer mucosal glands, located haphazardly in the oedematous and lymphocyte and histiocyte infiltrated respiratory epithelium. The histological score was statistically higher (P < 0.05) as compared to LPAI group. The respiratory epithelium of co-infected birds showed epithelial cell degeneration. A large number of these cells showed the loss of cilia (thrown off in various places). Mucosal glands were reduced and were found infiltrated with lymphocytes and histiocytes. These lesions were found on the entire respiratory epithelium. Lesion score of this group was statistically higher (P < 0.05) when compared to birds in LPAI and E. coli group.
Histological examination of the lung revealed non-ciliated epithelial flat cells in the secondary bronchi and parabronchi of control birds. No lymphocyte infiltration was observed in the inter-atrial and inter-parabronchial septa or around the blood vessels. Few small-sized germinal centres were seen. The lung of the birds in the group was similar to the control birds. The lungs of E. coli group had degeneration and hyperplasia of non-ciliated epithelial cell layer in the secondary bronchi and parabronchi. Furthermore, lymphocyte infiltration was observed in the inter-atrial and inter-parabronchial septa. Germinal centres were increased in size around secondary bronchi and blood vessels. In co-challenged birds, thick inter-atrial and inter-parabronchial septa were seen in the lungs. The infiltration of dispersed lymphocytes around the secondary bronchi and blood vessels was combined with germinal centres. All these lesions depict interstitial pneumonia. The assembling of cellular debris, serous exudates, and neutrophil infiltration in the atrium, infundibulum, para-bronchi, and secondary bronchi (catarrhal pneumonia) was observed less commonly in E. coli and E. coli+LPAI challenged group.
Seroconversion
The sera of birds from control negative group and E. coli group were negative in the haemagglutination-inhibition test while those of the birds from group LPAI and group E. coli+LPAI were positive for the inoculated virus using HI test on the 4th, 8th, and 12th day of infection (Table 4). The HI titre in birds of LPAI group was significantly lower (P < 0.05) than HI titre of the group E. coli+LPAI. Jointly, the geometric mean titre (GMT) was higher (P < 0.05) in birds of the group E. coli+LPAI than the group LPAI at 4, 8 and 12 days post virus infection. In our study, the geometric mean titres of LPAI and E. coli+LPAI groups were log2 4.8 on 4 dpi to log2 6.1 on 12 dpi, and log27.4 on 4 dpi to log2 8.4 on 12 dpi, respectively.
Table 4.
HI titres in sera from virus inoculated chickens in different groups
| Groups | Pre-infection HI titre log2 HI titre | Number of chickens positive for HI/total chickens log2 HI titre range (GMT; mean ± SEM) | ||
|---|---|---|---|---|
| 4 dpi | 8 dpi | 12 dpi | ||
| LPAI-challenged | <2 | 10/10 (4.8 ±0.3)a | 10/10 (5.2 ±0.7)a | 6/6 (6.1 ±0.5)a |
| E. coli+LPAI-challenged | <2 | 10/10 (7.4 ±0.5)b | 10/10 (8.6 ±0.6)b | 6/6 (8.4 ±0.3)b |
GMT − geometric mean titre, dpi − days post virus infection. HI titres 8 or lower were considered negative for seroconversion.
Mean values in the same column that do not share a common letter differ significantly (P < 0.05)
Discussion
It is a well-known fact that upper respiratory tract viral infections in poultry are often complicated by more serious bacterial diseases. Influenza virus is most commonly recognised in this context, and may also predispose to secondary infections. The co-infection with E. coli and influenza viruses in poultry has been observed under field conditions. Natural AIV/bacterial problems usually occur simultaneously and have been reported in poultry (9, 10), but the adverse effects of such co-infections on the health of broilers are not well-known. Co-infections of poultry present a complicated clinical picture confusing the identification and diagnosis, and unfortunately little is known about the interactions between co-infecting pathogens (3, 9).
In the present study, classical clinical signs of E. coli and LPAIV (H9N2) infection were observed in all infected groups, as described previously (4). However, clinical signs were more severe and prominent in co-infected birds. Moreover, mortality was observed in E. coli (16%) and E. coli+LPAI group (40%). The clinical signs and gross lesions in LPAIV (H9N2) infected chicks were less severe than the lesions reported previously in chickens naturally infected with H9N2 (7). In this study, no mortality occurred in the experimental group which was infected by H9N2 LPAIV. Nili and Asasi (8) reported that experimental infection of broilers with AIV H9N2 caused severe necrotising tracheitis and 19% mortality, but it was not shown that the inocula were free from other pathogens. In this study, the inocula used for experimental challenge were negative for bacterial and fungal contamination, as well as Newcastle disease virus and Mycoplasma gallisepticum. Lesions in the airsacs of both E. coli+LPAI group were more pronounced and of longer duration than in the E. coli group. In the airsacs and lungs, the numbers of macrophages remained high in the E. coli+LPAI group, whereas the number of macrophages in the E. coli group decreased. Higher population of both T cells and macrophages might be responsible for the enhanced disease severity and lesions, possibly due to overproduction of inflammatory cytokines, as demonstrated in a study with turkeys (11).
The present study revealed that pathogenicity of H9N2 virus is affected by the co-infection with E. coli. It is not known how E. coli bacteria enhance the replication of H9N2 virus in chickens. Post-translational proteolytic activation of the precursor of HA molecule into HA1 and HA2 subunits by host proteases is essential for infectivity and for the spread of the virus. Thus, virus activation by the host proteases plays a vital role in the spread of infection, tissue tropism, and pathogenicity of LPAIV (15). E. coli co-infection may have provided the protease enzymes and enhanced H9N2 pathogenicity in this experiment. It was demonstrated that the protease of S. aureus activated the HA of the influenza virus, allowing multiple cycles of virus replication in the lungs of mice (16). Co-infection with E. coli may confer a similar effect on H9N2 virus infection in chickens. An alternative explanation for the exacerbation of the pathogenicity of H9N2 influenza virus infection is that the stress of bacterial infection affects the immune system of chickens. This result implies that the intratracheal AI challenge by itself is not enough to produce severe disease and mortality in broilers and that the E. coli challenge was required to induce the mortality supporting findings of Nili and Asasi (8).
It is well known that E. coli, through various virulence factors such as hydrogen peroxide, nitrous oxide, and various proteases can cause significant necrosis of the host cells, excessive release of pro-inflammatory cytokines, inhibit phagocytosis, and affect the normal functions of B and T lymphocytes, which may increase replication of viruses. Therefore, it can be assumed that the presence of E. coli may predispose the host to several other viral and bacterial infections. Avian influenza HI titre in the group co-infected with LPAIV and E. coli was significantly higher than in the group infected with LPAIV alone on 4, 8, and 12 dpi (P < 0.05). This finding may show that E. coli could enhance the propagation of the virus and consequently an increase in its HI titre. It has been demonstrated that stimulation of host cells to produce or secrete more protease and the destruction of endogenous cell protease inhibitors may increase trypsin-like protease activity and enhance influenza virus pathogenicity (1). Our field observation indicates that flocks which are positive to H9N2 have shown increase in mortality in recent years probably due to increased pathogenicity of the virus or due to other undetected field infections. It was speculated that severe clinical signs linked to H9N2 infections in the field were due to stress and co-infections, most likely due to E. coli involvement (2). Moreover, a significant increase in the thickness of the trachea indicates that these pathogens have strong affinity for tracheal mucosa leading to loss of cilia, oedema, degeneration, and metaplasia of epithelial cells in E. coli+LPAI group.
In summary, we have verified that co-infection of broilers with E. coli and LPAI H9N2 may result in adverse respiratory diseases leading to significant economic losses because of poor weight gain, dropped egg production, and higher mortality rate. New strategies are needed to fight against such co-infections. Continuous surveillance of AI infection and co-infections studies in experimental poultry models is warranted to find new strategies to control their circulation in domestic and wild poultry. The timing of co-infection would also require further systematic experimental studies to understand the role of prior/post/simultaneous inoculation in disease outcome, pathogenesis, and virus shedding pattern.
Acknowledgements
We are thankful to Dr Muhammad Usman (Poultry Research Institute, Rawalpindi, Pakistan) and Dr Abdul Ghafar (National Agricultural Research Centre, Islamabad, Pakistan) for kindly providing strains of E. coli and avian influenza virus for inoculation. We are also thankful to the laboratory staff for sampling and technical help during the study.
Footnotes
Conflict of Interests Statement: The authors declare that there is no conflict of interests regarding the publication of this article.
Financial Disclosure Statement: This study was not funded by any funding agency and was conducted at the expense of the research group to combat the rising problem in the poultry industry of the country.
Animal Rights Statement: The experiments and procedures involving broiler chickens were approved by the Animal Care and Research Committee of the College of Veterinary and Animal Sciences (CVAS), Jhang, Pakistan, and were conducted according to the guidelines of the committee.
References
- 1.Azizpour A., Goodarzi H., Charkhkar S., Momayez R., Hablolvarid M.H.. Study on clinical aspects of SPF chickens infected with Ornithobacterium rhinotracheale followed by H9N2 avian influenza. Eur J Exp Biol. 2013;3:186–189. [Google Scholar]
- 2.Bano S., Naeem K., Malik S.A.. Evaluation of pathogenic potential of avian influenza virus serotype H9N2 in chickens. Avian Dis. 2003;47:817–822. doi: 10.1637/0005-2086-47.s3.817. [DOI] [PubMed] [Google Scholar]
- 3.Costa-Hurtado M., Afonso C.L., Miller P.J., Spackman E., Kapczynski D.R., Swayne D.E., Shepherd E., Smith D., Zsak A., Pantin-Jackwood M.. Virus interference between H7N2 low pathogenic avian influenza virus and lentogenic Newcastle disease virus in experimental co-infections in chickens and turkeys. Vet Res. 2014;45:1–11. doi: 10.1186/1297-9716-45-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fatima Z., Khan MA, Ahmad MUD, Muhammad K., Khwaja KN., Khan A., Anwar Z., Ahad A., Mahmood A.. Cross sectional survey of live bird markets, and zoo birds for circulating influenza subtypes in Pakistan. Pak Vet J. 2017;37:185–189. [Google Scholar]
- 5.Jirjis F.F., Noll S.L., Halvorson D.A., Nagaraja K.V., Martin F., Shaw D.P.. Effects of bacterial coinfection on the pathogenesis of avian pneumovirus infection in turkeys. Avian Dis. 2004;48:34–49. doi: 10.1637/7017. [DOI] [PubMed] [Google Scholar]
- 6.Mahmood MS., Amir HW., Abbas RZ., Rafique A., Aslam B.. Evaluation of antiviral activity of Azadirachta indica (Neem) bark extract against Newcastle disease virus. Pak Vet J. 2017;37:1–4. [Google Scholar]
- 7.Nili H., Asasi K.. Natural cases and an experimental study of H9N2 avian influenza in commercial broiler chickens of Iran. Avian Pathol. 2002;31:247–252. doi: 10.1080/03079450220136567. [DOI] [PubMed] [Google Scholar]
- 8.Nili H., Asasi K.. Avian influenza (H9N2) outbreak in Iran. Avian Dis. 2003;47:828–831. doi: 10.1637/0005-2086-47.s3.828. [DOI] [PubMed] [Google Scholar]
- 9.Pan Q., Liu A., Zhang F., Ling Y., Ou C., Hou N., He C.. Co-infection of broilers with Ornithobacterium rhinotracheale and H9N2 avian influenza virus. BMC Vet Res. 2012;8:104–110. doi: 10.1186/1746-6148-8-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pu J., Yu L.F., Zhe W., Bo M., Earl G.B., Jin H.L.. Pathogenicity of H3N8 influenza viruses isolated from domestic ducks in chickens with or without Escherichia coli coinfection. Avian Dis. 2012:597–600. doi: 10.1637/9984-110911-ResNote.1. [DOI] [PubMed] [Google Scholar]
- 11.Rautenschlein S., Miller R.L., Sharma J.M.. 1998. Interferon induction in turkeys by oral administration of the imidazoquinolinamine S-28828 and modulation of the pathogenesis of Escherichia coli. Vet Immunol Immunopathol. 1998;66:127–141. doi: 10.1016/s0165-2427(98)00197-4. [DOI] [PubMed] [Google Scholar]
- 12.Rashid F., Abbas MA, Siddique N., Rafique S., Ahmed S., Mehmood F., Shah A., Suleman M., Roomi S., Naeem K.. Induction of immunosuppression in broiler chicken upon co-infection of avian adenovirus-4 with low pathogenic avian influenza H9N2. Pak Vet J. 2017;37(3):311–315. [Google Scholar]
- 13.Stipkovits L., Laszlo E., Vilmos P., Andrea B., Ervin P., Maria S., Susan S., Bela D.. Effect of low-pathogenicity influenza virus H3N8 infection on Mycoplasma gallisepticum infection of chickens. Avian Pathol. 2012;41:51–57. doi: 10.1080/03079457.2011.635635. [DOI] [PubMed] [Google Scholar]
- 14.Suarez D.L. Swayne D.E. Avian Influenza. Blackwell Publishing; Ames: 2008. Influenza A virus; pp. 3–22. [Google Scholar]
- 15.Swayne D.E., Halvorson D.A. Saif Y.M., Glisson J.R., McDougald L.R., Nolan L.K., Swayne D.E. Diseases of Poultry. Blackwell Publishing; Ames: 2008. Influenza; pp. 153–184. [Google Scholar]
- 16.Tashiro M., Ciborowski P., Klenk H.D., Pulverer G., Rott R.. Role of staphylococcus protease in the development of influenza pneumonia. Nature. 1987;325:536–537. doi: 10.1038/325536a0. [DOI] [PubMed] [Google Scholar]
- 17.Umar S., Sarfraz S., Mushtaq A., Attique M.. Emerging threat of H9N2 viruses in poultry of Pakistan and vaccination strategy. World Poult Sci J. 2016;3:1–10. [Google Scholar]
- 18.Umar S., Younus M., Rehman M.U., Aslam A., Shah M.A.A., Munir M.T., Hussain S., Iqbal F., Fiaz M., Ullah S.. Role of aflatoxin toxicity on transmissibility and pathogenicity of H9N2 avian influenza virus in turkeys. Avian Pathol. 2015;44:305–310. doi: 10.1080/03079457.2015.1046813. [DOI] [PubMed] [Google Scholar]
- 19.Wibowo M.H., Anggoro D., Amanu S., Wahyuni A., Untari T., Artanto S., Asmara W.. Receptor binding and antigenic site analysis of hemagglutinin gene fragments of avian influenza virus serotype H5N1 isolated from Indonesia. Pak Vet J. 2017;37:123–128. [Google Scholar]
- 20.Xu X., Chen X., Gao S., Zhao L.. Pathogenicity of FtsK mutant of avian pathogenic Escherichia coli. J Vet Res. 2016;60:13–18. [Google Scholar]


