Abstract
Introduction
The objective of the present research was to carry out a comparative assessment of copper, zinc, and selenium concentrations in the meat of edible land snails collected in Poland (Helix pomatia, Cornu aspersum maxima, and Cornu aspersum aspersum), as well as to determine the effect of preliminary processing of Roman snails (Helix pomatia) on the content of the aforementioned elements.
Material and Methods
In the first stage, determinations were made on unprocessed snail meat. In the second stage, the study focused on Roman snails and consisted in an additional evaluation of frozen meat after full processing. Zinc and copper contents were determined by flame atomic absorption spectrometry and the selenium content was established by graphite furnace atomic absorption spectrometry.
Results
The selenium content differed significantly among all three species. The copper content in Roman snails differed significantly from that in farmed snails. No significant difference in the zinc level was noted among the three snail species. The selenium content in raw and processed meat of Roman snails did not show any significant difference while the copper and zinc level was significantly higher in processed meat samples.
Conclusion
The present research on the meat of edible snails showed different levels of selenium, copper, and zinc, depending on the species, collection site, and subjection to processing.
Keywords: Helix pomatia, Cornu aspersum, selenium, copper, zinc
Introduction
In the last decade, increasing global consumption of snail meat has been observed along with a clear rise in consumer demand for both free-living and farmed snails. The most commonly consumed snail species from the Helicidae family are free-living Roman snails (Helix pomatia) and farmed common snails from the Cornu genus (Cornu aspersum maxima – CAM – and Cornu aspersum aspersum – CAA). In nomenclature use is still made of older scientific names for CAM – Helix aspersa maxima – and CAA – Helix aspersa aspersa. These species from the Cornu genus can also be known by their common names: “petit-gris” or brown garden snail for CAA and “gros-gris” or big brown snail for CAM. Snails harvested in Poland are exported to France and other European Union countries, but a small portion is also consumed in Poland. Snails are exported live, hibernated, or in the form of frozen meat (14).
The chemical composition of edible snail meat proves its high nutritional quality, which results primarily from the presence of complete protein and essential unsaturated fatty acids. Helix pomatia meat is characterised by higher protein and fat contents and a lower water level compared with the meat of farmed snails of the Cornu genus. Snail meat has a low carbohydrate content and energy value, but provides large amounts of calcium, potassium, magnesium, copper, zinc, and selenium, as well as B-group vitamins (14).
Trace elements, including selenium, copper, and zinc, are of fundamental importance to the human body. All three element concentrations show natural variation in the environment, and their ambient content affects the levels of these micronutrients in snail meat because of the high bioaccumulation potential of snails (8, 9, 18). Snails of the Helix pomatia and Helix aspersa species can accumulate copper above the environmental concentration, but zinc at or below its ambient level (10). Generally, the human diet should be rich in micronutrients, which are vital for maintaining health. Hence it is important to determine the micromineral content in snail meat.
The objective of the study was to carry out a comparative analysis of selenium, copper, and zinc levels in the meat of snails collected in Poland and to assess the effect of pre-consumption procedures on the contents of these elements in Roman snail meat.
Material and Methods
Material
The study was performed on three species of edible snails harvested in Poland: Roman snails (Helix pomatia) and two species of farmed snails from the Cornu genus (Cornu aspersum maxima and Cornu aspersum aspersum). The study material comprised a total of 160 snail meat samples, including 80 samples from Roman snails, 40 samples from brown garden snails, and 40 samples from big brown snails. The Roman snails were harvested in four provinces in Poland (Greater Poland, West Pomerania, Lower Silesia, and Lubuskie), and from each province 20 Roman snail meat samples were examined (10 samples immediately post-euthanasia and 10 after full processing). The Cornu species were gathered from heliculture situated in Greater Poland Province.
Meat sample preparation
The snails were cold stored at 4°C–12°C for 10–12 days to purge the digestive tract, then washed under running water, salted, and euthanised with steam. The determinations were performed on the edible portions of snails, that is the foot with collar and a fragment of the mantle. The study consisted of two stages. In the first stage, determinations were made on snail meat obtained immediately post euthanasia without any further processing (raw meat). In the second stage, the study focused on Roman snails and consisted in an additional evaluation of frozen meat after full pre-consumption processing, i.e. scalding, evisceration, cooking, cooling, and freezing (Fig. 1). Prior to the examination, snail meat was put in a fridge and kept at 4°C for 24 h to thaw. A laboratory specimen comprised 20 randomly selected snail carcasses homogenised to obtain uniform research material using a handheld blender with stainless steel blades. The samples (0.5 mg) were taken from homogenate and mineralised with 3 mL of nitric acid (V) in a Mars Xpress microwave oven digester (CEM Corporation, USA). Three levels of mineralisation were applied. After mineralisation, the samples were transferred into 50 mL graduated flasks and diluted to the mark with distilled water. After that, the content of selected metals was determined. The 0.5 g samples were mineralised in a Mars Xpress microwave mineraliser (CEM Corporation, USA).
Fig. 1.
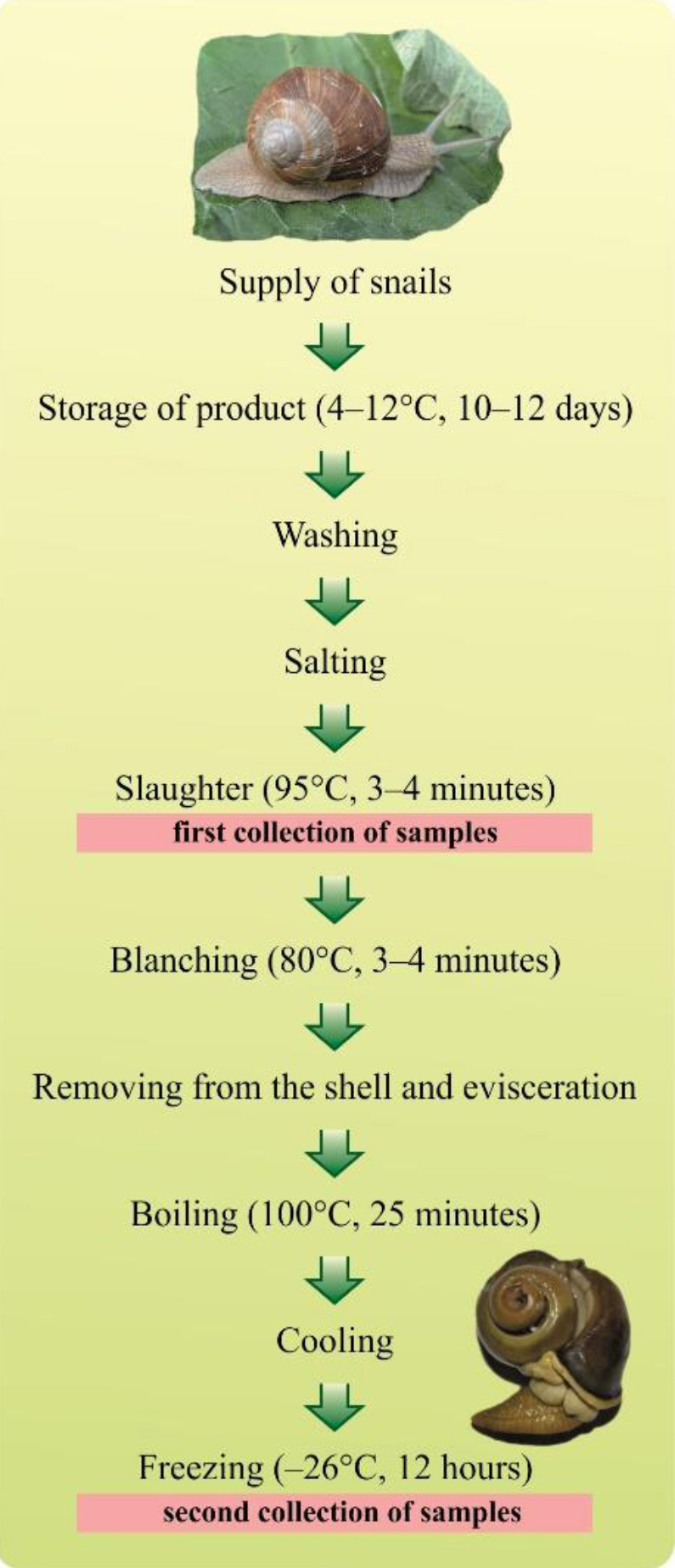
The pattern of snail meat processing stages
Chemical analysis
Zinc and copper contents were determined by flame atomic absorption spectrometry (FAAS) in a SpectrAA 280 FS fast sequential atomic absorption spectrometer (Varian, Australia) in an air-acetylene flame, using an auto diluter for standards and samples (SIPS). The selenium content was established by graphite furnace atomic absorption spectrometry (GFAAS) in a SpectrAA 280 Z atomic absorption spectrometer (Varian) with electrothermal atomisation.
Statistical analysis
The obtained results were analysed statistically with STATISTICA 9.1 (StatSoft, part of Tibco, USA) and expressed as the arithmetic means and standard deviation. The normal distribution in each group was checked with the Shapiro-Wilk test. The influence of each variability factor on the determined parameters was established using the one-way analysis of variance (ANOVA) for groups for which the assumption of homogenous variances was fulfilled. Rejecting the null hypothesis that all the means are equal, the post-hoc Tukey’s t-test with multiple confidence intervals was applied to compare the groups for statistically significant differences. Those differences between the means were determined at the P 0.05 level. In the case of Cu (Table 1) and Zn in processed meat (Table 2) when the assumption of homogenous variances was not fulfilled, a Kruskal-Wallis test was used. After null hypothesis rejection the Mann-Whitney U test for multiple comparisons was applied.
Table 1.
Selenium, copper, and zinc contents in the raw snail meat (mg/kg)
| Snail species | Se | Cu | Zn |
|---|---|---|---|
| Helix pomatia n = 40 | 0.09a ±0.05 | 14.8b ±10.1 | 19.81 ±5.14 |
| Cornu aspersa maxima n = 40 | 0.16b ±0.02 | 6.22a ±1.85 | 20.29 ±3.41 |
| Cornu aspersa aspersa n = 40 | 0.19c ±0.03 | 3.99a ±2.47 | 20.93 ±3.42 |
- the mean values marked with small letters differ statistically significantly at P ≤ 0.05 vertically
Table 2.
Selenium, copper, and zinc contents in the raw and processed meat of Helix pomatia harvested in different regions of Poland (mg/kg)
| Selenium | Copper | Zinc | ||||
|---|---|---|---|---|---|---|
| Province | Raw meat | Processed meat | Raw meat | Processed meat | Raw meat | Processed meat |
| Greater Poland n = 10 | 0.04a ± 0.04 | 0.04a ± 0.02 | 19.96Ab ± 5.27 | 28.91Bc ± 6.79 | 14.90Aa ± 1.13 | 37.06Bb ± 8.75 |
| West Pomeranian n = 10 | 0.11Abc ± 0.01 | 0.14Bc ± 0.01 | 4.82Aa ± 1.51 | 7.34Ba ± 2.76 | 25.42Ab ± 1.79 | 29.03Ba ± 4.63 |
| Lower Silesian n = 10 | 0.07ab ± 0.05 | 0.08b ± 0.05 | 27.17c ± 5.98 | 29.4c ± 2.57 | 16.78Aa ± 4.85 | 31.60B ± 1.56 |
| Lubuskie n = 10 | 0.12Ac ± 0.01 | 0.14Bc ± 0.01 | 7.25Aa ± 1.69 | 16.71Bb ± 3.57 | 22.15Ab ± 2.93 | 31.83B ± 6.25 |
| AVERAGE n = 40 | 0.09 ± 0.05 | 0.1 ± 0.05 | 14.8A ± 10.1 | 20.59B ± 10.17 | 19.81A ± 5.14 | 32.38B ± 6.4 |
– the mean values marked with capital letters differ statistically significantly at P ≤ 0.05 horizontally
– the mean values marked with small letters differ statistically significantly at P ≤ 0.05 vertically
Results
The results are presented in Tables 1 and 2. Table 1 compares the Se, Cu, and Zn contents in raw meat samples from the three snail species. The selenium concentration differed significantly among the three snail species. The Se level was the lowest in the raw meat of Roman snails (0.09 mg/kg), higher in the meat of large brown garden snails (0.16 mg/kg), and the highest in the meat of small brown garden snails (0.19 mg/kg).
The copper concentration in H. pomatia was 14.8 mg/kg and differed significantly from that in raw meat samples of farmed snails, which amounted to 6.22 mg/kg in Cornu aspersum maxima and 3.99 mg/kg in Cornu aspersum aspersum. No significant differences were found among the samples from farmed snails.
No significant differences in the Zn content were found among the meat samples. Its concentration ranged from 19.81 mg/kg in Roman snails to 20.93 mg/kg in small brown garden snails.
The highest Se amount was found in Roman snails from Lubuskie and West Pomeranian provinces, whereas the lowest was in those from Greater Poland province (Table 2). Cu was the most concentrated in snails from Lower Silesian province, and the least in those from West Pomeranian province. Zinc had the best bioaccumulation in H. pomatia from West Pomeranian province, and the worst in those from Lower Silesian province.
The comparative study of Se contents in the raw and processed meat of Roman snails originating from all the regions investigated did not reveal any significant differences. The raw meat samples contained 0.09 mg/kg of Se, whereas the processed ones yielded 0.1 mg/kg. In West Pomeranian and Lubuskie provinces, higher selenium content was determined in processed meat. Taking into account the mean values of all four regions, an increase in the Cu content was observed from 14.8 mg/kg in raw meat to 20.59 mg/kg in processed meat. Notably, the increase in copper concentration in processed meat was not statistically significant only for Lower Silesian province. All samples from all provinces showed significantly higher Zn contents in processed meat. A comparison of the mean Zn contents in Roman snails revealed that the concentration of this mineral amounted to 19.81 mg/kg in raw meat, whereas in processed meat it increased to as much as 32.38 mg/kg.
Discussion
The analysis of edible snail tissues showed significant differences in selenium concentration between free-living Helix pomatia and farmed snails of the Cornu genus, which may be attributed to the selenium-poor habitats of Roman snails. The Se concentration in the environment affects its content in snail meat because of the snail’s great ability to bioaccumulate this element (10). Debski et al. (6) showed that over 70% of the total area of Poland is Se-deficient. On the other hand, soil on which snails of the Cornu genus are farmed may have a higher natural Se level or may be Se supplemented. Selenium content in the environment has decisive impact on its level in snail meat as Se has been shown to bioaccumulate easily in snails (8, 18). Among all the Roman snail samples investigated, those originating from the Greater Poland province had the lowest Se content (0.04 mg/kg). The studies on Se concentration in soil from this province, which is considered the Se-deficient area, showed this element at 0.19 mg/kg content on average (2). Arable land on the Cornu genus snail-producing farms here may naturally have a higher Se content or be supplemented with this micronutrient. Higher Se concentration was noted in the soils of southern Poland, a region known to be under strong human pressure, where the range 0.060–0.818 mg/kg was measured (1). The meat of snails originating from Moldavia, Ukraine, and Russia had Se content between 130 and 423 μg/kg, depending on the geochemical characteristics of the habitat, with the highest Se content determined in snails from Moldavia (18). Generally, it is vital to choose an appropriate soil environment for heliciculture. The supplementation of soil with Se may be an effective method of increasing the content of this micronutrient in snail meat. The concentration of this essential mineral in the meat of the snails under study, especially in farmed snails, was higher than in pork (0.078 mg/kg), beef (0.064 mg/kg), and horsemeat (0.039 mg/kg) from Poland (15, 17). Therefore, snails may be an excellent source of Se in the human diet.
The present study revealed a significantly higher Cu content in Roman snails compared with farmed snails. Given that snails from the Helicidae family are known to readily accumulate the element, the difference in the Cu content between the free-living helix genus and the farmed cornu genus may have been due to differences in its content in local soils (8). Subject to soil type Cu and Zn contents can markedly vary. It is generally recognised that an elevated concentration of trace metals in soil can serve as an indicator of human impact on the environment (16). The highest Cu concentration (27.17 mg/kg) was found in the H. pomatia snails originating from the Lower Silesian province where, in the Legnica – Gogów copper belt, Cu deposits and smelters are located. Mining of Cu ores, their processing and metallurgical engineering are the major sources of soil contamination with Cu, especially in close proximity to smelters (11).
Compared with those observed in the present study, higher Cu and Zn concentrations were noted in the meat of three snail species (Helix pomatia, Helix aspersa, and Arion rufus) harvested in northern Italy. Markedly higher Zn levels were determined in the hepatopancreas, whereas the Cu content in this organ and in the foot did not substantially exceed the content determined in this work (13). Coughtrey and Martin (3) collectedHelix aspersa from three sites in Great Britain and showed a higher Cu concentration and a lower Zn content in the foot of these snails compared with the farmed snails used in the present research. Besides, it was found that most Zn was accumulated in the hepatopancreas, whereas higher Cu amounts occurred in the foot. Zinc is deposited in many organs, but its highest level, of up to 70%, is found in the hepatopancreas. On the other hand, the distribution of Cu in snail organs is relatively even, without a clear preference for the hepatopancreas (5).
Gomot and Pihan (8) highlighted some differences in the affinity for Cu and Zn between H. aspersa aspersa and H. aspersa maxima. It was demonstrated that the small brown garden snail has greater bioaccumulation ability than the large garden brown snail. These findings are not consistent with the results of the present research in which the contents of both metals in the meat of the two farmed snail species were similar.
According to the present research results, the processing procedures for snail meat result in higher Cu and Zn contents, but do not change the Se content significantly. Elevated Cu and Zn concentrations in the processed meat can result from relative dry mass increase caused by water loss during the thermal protein denaturation process. During cooking, proteins denature and lose water binding capacity. An analysis of the effect of culinary treatment on Zn concentration in veal also showed a significantly higher content of this element in cooked meat (5.39 mg/100 g) than in raw meat (3.44 mg/100 g) which likely results from water loss during thermal processing (12). Similar findings concerning Cu and Se contents were reported in fish meat studied in Portugal. The cooked meat of fish had more Cu (0.25 mg/kg) than raw meat did (0.19 mg/kg) with no significant difference in the Se concentration (4). Slightly different relationships between cooked and raw meat were noted in rainbow trout meat. The research underlined a decline in Zn concentration from 9.68 mg/kg in raw meat to 3.20 mg/kg in cooked. Similarly, the Cu content was lower in cooked meat (0.08 mg/kg) than in raw (0.33 mg/kg) (7).
The content of the studied micronutrients in snail meat greatly correlates to their concentration in the soil. copper, zinc, and selenium concentrations in the environment are closely associated with their naturally varied distribution patterns in soil as well as environmental contamination as the effect of human activities in agriculture and industrial processes. It is important to select the appropriate land for snail farming, hence Se enrichment of soil can be a good way to supplement its level in farmed snail meat. What should be emphasised is the risk associated with Cu and Zn excess in the snail-gathering areas and, therefore, the need for action to prevent these micronutrients from entering the human body in undue amounts. It is advisable to perform detailed studies on Cu and Zn contents in the H. pomatia harvest sites.
In conclusion, the present research on the meat of edible snails showed different levels of Se, Cu, and Zn depending on species, collection site, and technological processing. Roman snails harvested in the natural habitat accumulate significantly more Cu and less Se compared with farmed snails. According to literature data, these snails are very efficient Se bioaccumulators, which implies that they were obtained from a low-Se environment. Cu concentration in the Roman snail meat was two-fold higher as compared to farmed snail meat, which is likely to arise from H. pomatia’s occurrence in Cu-contaminated areas. There were no significant differences in Zn and Cu contents between Cornu aspersum maxima and Cornu aspersum aspersum, but the latter species had a significantly higher Se content, and that implies its higher bioaccumulation capacity for this micronutrient. It was also shown that processing, especially cooking followed by freezing, resulted in significantly increased Cu and Zn concentrations in Roman snail meat, without affecting the Se concentration.
Footnotes
Conflict of Interests Statement: The authors declare that there is no conflict of interests regarding the publication of this article.
Financial Disclosure Statement: The manuscript was supported by Grant for Young Scientists no. WKH/MN/2 of the University of Life Sciences in Lublin, Poland.
Animal Rights Statement: None required.
References
- 1.Biernacka E., Mauszyński M.J.. The content of cadmium, lead, and selenium in soils from selected sites in Poland. Pol J Environ Stud. 2006;15:7–9. [Google Scholar]
- 2.Bakowska M., Balicka-Ramisz A., Hendzel D., Pilarczyk B., Semeniuk M., Tomza-Marciniak A., Tylkowska A., Udaa J.. Selenium concentration in soil and selected tissues of roe deer (Capreolus capreolus) from Wielkopolska region. Acta Sci Pol Zootech. 2010;9:251–260. [Google Scholar]
- 3.Coughtrey P.J., Martin M.H.. The distribution of Pb, Zn, Cd, and Cu within the pulmonate mollusc Helix aspersa Müller. Oecologia. 1976;23:315–322. doi: 10.1007/BF00345960. [DOI] [PubMed] [Google Scholar]
- 4.Costa S., Afonso C., Bandarra N.M., Gueifão S., Castanheira I., Carvalho M.L., Cardoso C., Nunes M.L.. The emerging farmed fish species meagre (Argyrosomus regius): How culinary treatment affects nutrients and contaminants concentration and associated benefit-risk balance. Food Chem Toxicol. 2013;60:277–285. doi: 10.1016/j.fct.2013.07.050. [DOI] [PubMed] [Google Scholar]
- 5.Dallinger R., Wieser W.. Patterns of acumulation, distribution and liberation of Zn, Cu, Cd, and Pb in different organs of the land snail Helix pomatia L. Comp Biochem Phys C. 1984;79:117–124. doi: 10.1016/0742-8413(84)90173-7. [DOI] [PubMed] [Google Scholar]
- 6.Debski B., Zachara B., Wasowicz W.. Próby oceny poziomu selenu w Polsce oraz jego wpyw na zdrowotność ludzi i zwierzat. Folia Univ Agric Stetin Zootechnica. 2001;224:31–38. [Google Scholar]
- 7.Gokoglu N., Yerlikaya P., Cengiz E.. Effects of cooking methods on the proximate composition and mineral contents of rainbow trout (Oncorhynchus mykiss) Food Chem. 2004;84:19–22. [Google Scholar]
- 8.Gomot A., Pihan F.. Comparison of the bioaccumulation capacities of copper and zinc in two snail subspecies (Helix) Ecotox Environ Safe. 1996;38:85–94. doi: 10.1006/eesa.1997.1566. [DOI] [PubMed] [Google Scholar]
- 9.Gomot A., Pihan F.. Growing snails used as sentinels to evaluate terrestrial environment contamination by trace elements. Chemosphere. 2000;40:275–284. doi: 10.1016/s0045-6535(99)00246-5. [DOI] [PubMed] [Google Scholar]
- 10.Laskowski R., Hopkin S.P.. Accumulation of Zn, Cu, Pb, and Cd in the garden snail (Helix aspersa): implications for predators. Environ Pollut. 1996;91:289–297. doi: 10.1016/0269-7491(95)00070-4. [DOI] [PubMed] [Google Scholar]
- 11.Lis J., Pasieczna A.. Anthropogenic soils pollution within the Legnica–Gogów copper district. Pol Geol Inst Sp Papers. 2005;17:42–48. [Google Scholar]
- 12.Lopes A.F., Alfaia C.M.M., Partidário A.M.C.P.C., Lemos J.P.C., Prates J.A.M.. Influence of household cooking methods on amino acids and minerals of Barrosã-PDO veal. Meat Sci. 2015;99:38–43. doi: 10.1016/j.meatsci.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Menta C., Parisi V.. Metal concentrations in Helix pomatiaHelix aspersa, and Arion rufus: a comparative study. Environ Pollut. 2001;115:205–208. doi: 10.1016/s0269-7491(01)00110-5. [DOI] [PubMed] [Google Scholar]
- 14.Paszkiewicz W., Ziomek M., Szkucik K., Maćkowiak-Dryka M.. Production and quality of snail meat. Med Weter. 2014;70:673–679. [Google Scholar]
- 15.Pilarczyk B., Tomza-Marciniak A., Mituniewicz-Małek A., Wieczorek-Dabrowska M., Pilarczyk R., Wójcik J., Balicka-Ramisz A., Bakowska M., Dmytrów I.. Selenium content in selected products of animal origin and estimation of the degree of cover daily Se requirement in Poland. Int J Food Sci Technol. 2010;45:186–191. [Google Scholar]
- 16.Qishlaqi A., Moore F.. Statistical analysis of accumulation and sources of heavy metal occurrence in agricultural soils of Khoshk River Banks, Shiraz, Iran. Am Eurasian J Agric Environ Sci. 2007;2:565–573. [Google Scholar]
- 17.Szkucik K., Gondek M., Bekot Z., Kursa K.. Content of selenium in muscles and internal organs of slaughter horses depending on their age and sex. Zywn-Nauk Technol Jakosc. 2014;96:63–71. [Google Scholar]
- 18.Toader-Williams A., Golubkina N.. Investigation upon the edible snail’s potential as source of selenium for human health and nutrition observing its food chemical contaminant risk factor with heavy metals. Bulletin UASVM Agriculture. 2009;66:495–499. [Google Scholar]


