Abstract
Background
To help general practitioners (GPs) in early identification of patients with palliative care (PC) needs, this pilot study aimed to determine the potential of the combined original surprise question (SQ1) (‘Would I be surprised if this patient died within the next 12 months?’) and the second surprise question (SQ2) (‘Would I be surprised if this patient was still alive after 12 months?’). We hypothesized that answering these SQs would trigger them to make a multidimensional care plan.
Methods
26 Slovenian GPs, randomized into 4 groups, were invited to write a care plan for each of the four patients described in case vignettes (2 oncologic, 1 organ failure and 1 frailty case). GPs in group 1 were only asked to write a care plan for each patient. GPs in group 2 answered SQ1 and GPs in groups 3 and 4 answered SQ1 and SQ2 before writing the care plan. The type and number of PC aspects mentioned in the respective care plans were quantified into a numeric RADboud ANTicipatory (RADIANT) score.
Results
Mean RADIANT scores in groups 1-4 were 2.2, 3.6, 2.5 and 3.1, respectively. When comparing the different vignettes, vignette B (terminal oncologic patient) scored best (3.6). Mean RADIANT scores in groups 3 and 4 were slightly higher for GPs who would be surprised compared to GPs who would not be surprised if the patient was still alive in 12 months.
Conclusion
The combined SQs were considered helpful in the early identification of patients in need of PC in Slovenian general practice.
Keywords: palliative care, surprise question, general practitioners, Slovenia, cancer, organ failure, frail elderly, dementia
Izvleček
Uvod
Namen te študije kot pomoč splošnim zdravnikom (SZ) pri zgodnjem prepoznavanju pacientov s potrebo po paliativni oskrbi (PO) je določanje potenciala kombiniranega izvirnega vprašanja presenečenja (VP1): »Ali bi me presenetilo, če bi pacient umrl v naslednjih 12 mesecih?« ter drugega vprašanja presenečenja (VP2): »Ali bi me presenetilo, če bi bil ta pacient živ čez 12 mesecev?« Naša hipoteza temelji na domnevi, da bi odgovarjanje na ti dve VP sprožilo pripravo večdimenzionalnega načrta oskrbe.
Metode
Šestindvajset slovenskih SZ, ki so bili naključno razvrščeni v štiri skupine, smo prosili, naj pripravijo načrt oskrbe za vsakega od štirih pacientov, ki so bili opisani v vinjetah s primeri (2 onkoloska primera, 1 odpoved organov in 1 primer krhkosti). SZ v 1. skupni so morali napisati poročilo o oskrbi za vsakega pacienta. SZ v 2. skupini so odgovorili na VP1, SZ v 3. in 4. skupini pa so odgovorili na VP1 in VP2, preden so pričeli pripravljati načrt oskrbe. Vrsta in število stališč PO, ki so bili omenjeni v načrtih oskrbe, so bili izmerjeni v numerični rezultat RADboud ANTicipatory (RADIANT).
Rezultati
Povprečni rezultati RADIANT od 1. do 4. skupine so bili 2,2, 3,6, 2,5 in 3,1. Pri primerjanju različnih vinjet je vinjeta B (umirajoči onkološki pacient) pridobila najboljši rezultat (3,6). Povprečni rezultati RADIANT v 3. in 4. skupini so bili rahlo višji pri SZ, ki bi bili presenečeni, v primerjavi s SZ, ki ne bili presenečeni, če bi bil pacient še vedno živ čez 12 mesecev.
Zaključek
Kombinirana VP pripomorejo k zgodnjemu prepoznavanju pacientov s potrebo po PO v splošni zdravstveni oskrbi v Sloveniji.
Ključne besede: paliativna oskrba, vprašanje presenečenja, splošni zdravniki, Slovenija, rak, odpoved organov, krhkost pri starostnikih, demenca
1. Introduction
During advanced stages of chronic life-limiting diseases, patients might benefit from palliative care (PC). Many patients in the Western world wish to remain at home during this palliative phase and to die there. Therefore, general practitioners (GPs) should play an important role in PC provision (1,2,3,4). In Slovenia, this is challenging since the average consultation time per patient is 7 minutes, and GPs do not receive extra payment for home visits (5). Other barriers in PC provision are the lack of knowledge, PC skills and experience, suboptimal communication with patients and with other healthcare professionals, and the uncertainty and unpredictability of illness trajectories, especially in non-cancer illnesses. (6,7,8,9,10). Therefore, PC is often restricted to physical symptom relief in the terminal phase, including emergency visits by the GP, transfers and unplanned hospital admissions (11). Moreover, 4% of the elderly Slovenian population have severe limitations, for which they do not receive any care (12). Without a universally accepted definition of ‘early’ palliative care, the dilemma arises of marking the right moment to start anticipatory PC alongside or instead of disease-oriented care in the advanced stages of chronic diseases (13,14). Physicians can approach this dilemma by (silently) asking themselves the surprise question (SQ1): ‘Would I be surprised if this patient died within the next 12 months?’ PC, including anticipating future problems, needs and wishes, would be indicated if the answer to this question was ‘no.’ The usefulness of this SQ has been validated in different populations (15,16,17). However, two recent reviews conclude that there is a wide range in accuracy and that further research is needed to develop more accurate tools (18, 19). Therefore, the second SQ (SQ2) was formulated: ‘Would I be surprised if this patient was still alive in 12 months?’ The aim of this pilot study was to determine the potential of using both SQ1 and SQ2 as tools to help GPs in the early identification of patients with a high chance to deteriorate or die.
2. Methods
2.1. Population
In June 2016, invitations to participate in this study were sent to 240 recipients of the Slovenian Family Medicine Journal in the Ljubljana area, all being registered GPs. Because of the lack of responses, another 39 GPs from all over Slovenia, of whom the email addresses were know by one of the authors (DRP), were invited one month later. All responses were gathered in July and August 2016.
2.2. Design
Participating GPs were randomized into four groups and were sent the matching questionnaire through the valid online software application CastorEDC. Each questionnaire contained the same four case vignettes based on real patients’ cases that were adapted to guarantee anonymity. The vignettes were written in English and described one organ failure patient (Vignette A), one terminal oncology patient (Vignette B), one frail elderly patient with dementia (Vignette C), and one incurable, but not yet terminal oncology patient (Vignette D) (Appendix 1). GPs in the first group were only asked whether they would plan any care for each of these patients. Those who decided to initiate care were asked to describe their care plan in detail. The GPs in group 2 were asked to answer SQ1. GPs in groups 3 and 4 were asked to answer SQ1 and SQ2 before answering the questions as described for the first group. GPs in group 4 were also shown the problem square (PS), a document designed to help structuring multidimensional care planning (Figure 1), before describing their care plan (20). Lastly, GPs were asked which aspects generally trigger them to start PC, and they were asked to give their opinion on the helpfulness and usefulness of the SQs and PS. The care plans and opinion section could be written in English or in Slovenian. Slovenian answers were translated to English by an independent, native Slovenian speaker.
Figure 1.
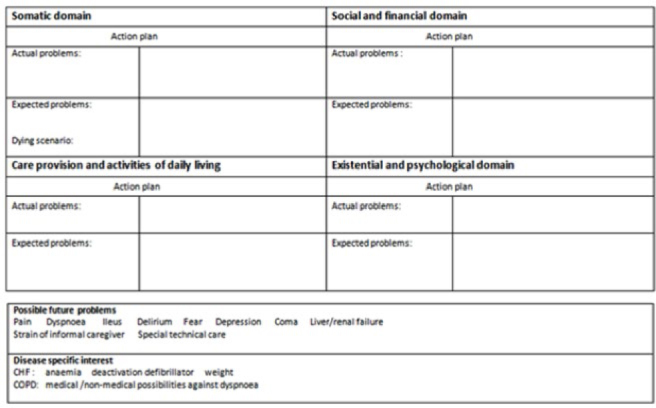
Problems square (Thoonsen et al. 2011 (18)).
Data Management
The open text content of each PC plan was quantified into a numeric score, the RADboud ANTicipatory (RADIANT) score, by one author (C.K.), using a score form (Figure 2). The form was developed by researchers from the Radboud university medical centre based in the Netherlands, on the WHO definition of PC and Dutch palliative care guidelines (21,22,23,24). The maximum score was 20 points.
Figure 2.
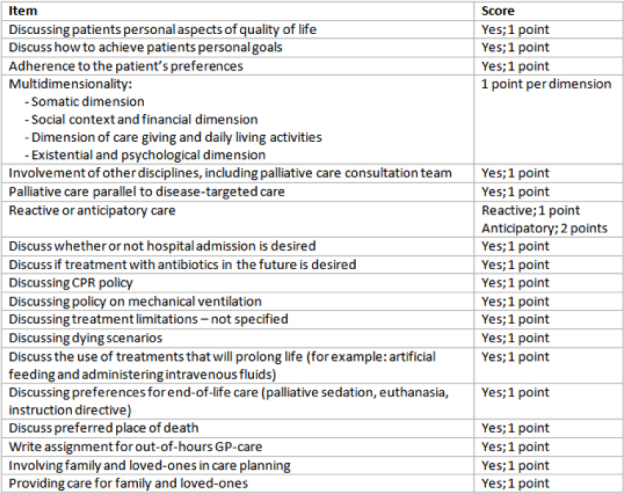
Score form.
The primary outcome measure was the answer combination given to SQ1 and SQ2 in relation to the RADIANT scores for each care plan. The secondary outcome measures were:
Differences in RADIANT scores between the four study groups and between the four vignettes.
Proportion of multidimensional care plans in each study group and each vignette.
Proportion of reactive and anticipatory care plans per vignette within each group.
Differences in mean RADIANT scores between care plans written in Slovenian and in English.
A qualitative review regarding the aspects that trigger GPs to start PC as well as their opinions on the usefulness of the SQs and PS.
All calculations were made using IBM SPSS software version 22. Significance testing was not performed because of the explorative nature of this study with a limited number of subjects.
3. Results
3.1. Population
297 GPs were invited to participate in this study. 35 (11.8%) agreed to participate, and 26 (8.8% of total, 74% of those who agreed) actually completed the survey (Figure 3). The participants’ characteristics are shown in Table 1.
Figure 3.
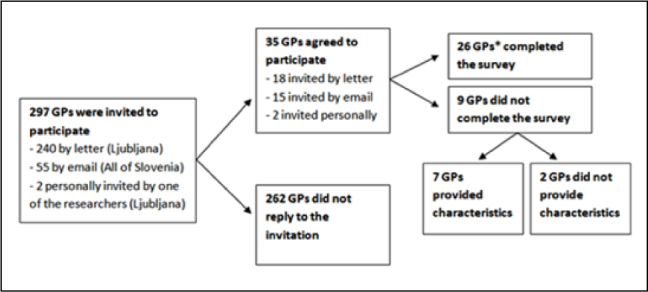
The process of inclusion of general practitioners.
Table 1.
Characteristics of participants.
| Age (years±SD) | 48±10.4 |
| Gender: male | 36% |
| Vocational training | 96% |
| Function | |
| • Employee in Healthcare Center | 56% |
| • Independent contract holder | 20% |
| • Employee in practice of independent contract holder | 20% |
| • Other | 4% |
| Type of practice | |
| • Practice in healthcare center | 56% |
| • Solo practice | 32% |
| • Nursing home | 8% |
| • Other | 4% |
| Teaching practice | 64% |
| Workload (hours per week ±SD) | 37±11.0 |
| After hours work (hours per month ±SD) | 20±13.7 |
| Consultation time (minutes per patient ±SD) | 9.5±4.9 |
| Home visits (no. per week ±SD) | 0.9±1.0 |
| Interest in palliative care (scale 1-10 ±SD) | 8.4±1.1 |
| Palliative care skills ( scale 1-10 ±SD) | 6.3±1.6 |
| Plans to improve PC skills | 93% |
3.2. Primary Outcome
In group 2, all participants (n=8) answered SQ1 with ‘no’ for each vignette (Table 2). In groups 3 (SQ1 and SQ2) and 4, (SQ1, SQ2 and PS) the patient B (terminal oncology patient) was the only patient for whom all GPs gave the answer combination no + yes (they would not be surprised if the patient died and would be surprised if the patient was still alive in 12 months). For the patient C (frail elderly patient with dementia), none of the GPs in group 3, and only 1 GP in group 4, gave this answer combination. In groups 3 and 4, the mean RADIANT scores were slightly higher for GPs who would be surprised if the patient was still alive in 12 months, compared to the GPs who would not be surprised if the patient was still alive in 12 months.
Table 2.
Answer combinations to SQ1+SQ2 and mean RADIANT scores per case vignette.
| Group 2 (n=8) | Group 3 (n=5) | Group 4 (n=5) | ||||
|---|---|---|---|---|---|---|
| Answer SQ1 | RADIANT score | Q1+SQ2 | RADIANT score | Answers SQ1+SQ2 | RADIANT score | |
| Vignette A | No (n=8) | 3.63 | No+Yes (n=2) | 3.5 | No+Yes (n=1) | 4.0 |
| No+No (n=2) | 1.0 | No+No (n=3) | 3.7 | |||
| Yes+No (n=1) | 0 | Yes+No (n=1) | 2.0 | |||
| Vignette B | No (n=8) | 4.0 | No+Yes (n=5) | 3.0 | No+Yes (n=5) | 4.0 |
| No+No (n=0) | - | No+No (n=0) | - | |||
| Yes+No (n=0) | - | Yes+No (n=0) | - | |||
| Vignette C | No (n=8) | 3.3 | No+Yes (n=0) | - | No+Yes (n=1) | 3.0 |
| No+No (n=4) | 2.0 | No+No (n=3) | 2.7 | |||
| Yes+No (n=1) | 3.0 | Yes+No (n=1) | 2.0 | |||
| Vignette D | No (n=8) | 3.4 | No+Yes (n=2) | 3.0 | No+Yes (n=1) | 5.0 |
| No+No (n=1) | 2.0 | No+No (n=4) | 1.8 | |||
| Yes+No (n=2) | 3.0 | Yes+No (n=0) | - | |||
Abbreviations: GP: general practitioner, SQ1: first surprise question, SQ2: second surprise question, RADIANT score: RADboud ANTicipatory score, a scoring method to quantify the open text content of palliative care plans made by GPs.
3.3. Secondary Outcome Measures
RADIANT scores were highest in group 2 (SQ1) and lowest in group 1 (no SQs). When comparing the RADIANT scores for the different vignettes, vignette B (terminal oncology case) scored higher than the other vignettes. Overall, the highest RADIANT score was found for vignette B in groups 2 (SQ1) and 4 (SQ1, SQ2 and PS). In all groups, RADIANT scores were higher for plans written in Slovenian compared to those written in English (Table 3).
Table 3.
Mean RADIANT scores* per case vignette in groups 1-4.
| Total | |||||||
|---|---|---|---|---|---|---|---|
| Vignette A | Vignette B | Vignette C | Vignette D | A | E | S | |
| Group 1 (n=8) | 1.9 | 3.3 | 2.0 | 1.5 | 2.2 | 1.7 (n=3) | 2.5 (n=5) |
| Group 2 (n=8) | 3.6 | 4.0 | 3.3 | 3.4 | 3.6 | 3.5 (n=4) | 3.6 (n=4) |
| Group 3 (n=5) | 1.8 | 3.0 | 2.2 | 2.8 | 2.5 | 2.0 (n=4) | 4.3 (n=1) |
| Group 4 (n=5) | 3.4 | 4.0 | 2.6 | 2.4 | 3.1 | 2.8 (n=4) | 4.3 (n=1) |
| Total (n = 26) | 2.7 | 3.6 | 2.5 | 2.5 | - | - | - |
Abbreviations: A: All participants in this study group; E: Participants in this study group that completed the survey in English (n=15); S: Participants in this study group that completed the survey in Slovenian (n=11).
RADIANT score: RADboudANTicipatory score, a scoring method to quantify the open text content of palliative care plans made by GPs.
The highest proportion of anticipatory care plans (40–80%) was made by GPs in group 4 (SQ1, SQ2 and PS), and the lowest (25–50%) in group 2 (SQ1) (Table 4). When comparing the proportions of anticipatory care in the different vignettes, the patient B (terminal oncology patient) scored highest (40–80%), and the patient C (frail elderly patient with dementia) scored lowest (20–40%).
Table 4.
Proportions of reactive and anticipatory palliative care plans.
| Vignette A | Vignette B | Vignette C | Vignette D | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R | A | R | A | R | A | R | A | R | A | |
| Group 1 (n=8) | 88% | 38% | 100% | 50% | 88% | 25% | 63% | 63% | 84% | 44% |
| Group 2 (n=8) | 100% | 50% | 100% | 50% | 100% | 25% | 100% | 38% | 100% | 41% |
| Group 3 (n=5) | 80% | 80% | 100% | 40% | 100% | 20% | 80% | 40% | 90% | 45% |
| Group 4 (n=5) | 100% | 40% | 100% | 80% | 100% | 40% | 80% | 40% | 95% | 50% |
| Total (n = 26) | 92% | 50% | 100% | 54% | 96% | 27% | 81% | 46% | - | - |
Shows the proportions of care plans that contain reactive and anticipatory aspects for each vignette per study group;
Abbreviations: R: reactive care; A: anticipatory care
The somatic dimension was most often included in the PC plans in all study groups (75%-95%) and all vignettes (65-100%), while the social/financial dimension was least mentioned within the different study groups (5-25%) and in vignettes B (terminal oncology patient) (19%) and D (advanced stage, but not yet terminal oncology patient) (12%). The existential/psychological dimension was least explored in vignettes A (organ failure patient) (4%) and C (frailty elderly with dementia) (8%). Group 1 (no SQs) shows the highest proportions of PC plans including all four aspects of multidimensional PC (9%), while none of the PC plans in group 4 (SQ1, SQ2 and PS) included all four aspects (Table 5).
Table 5.
Multidimensional care.
| Somatic | Social / financial | Care giving /ADL* | Existential /psychological | All domains | |
|---|---|---|---|---|---|
| Group 1 | 84% | 9% | 38% | 16% | 9% |
| Group 2 | 94% | 25% | 59% | 31% | 6% |
| Group 3 | 75% | 5% | 25% | 20% | 5% |
| Group 4 | 95% | 10% | 55% | 15% | 0% |
| Vignette A | 89% | 8% | 54% | 4% | |
| Vignette B | 100% | 19% | 54% | 35% | |
| Vignette C | 96% | 15% | 65% | 8% | |
| Vignette D | 65% | 12% | 8% | 35% |
Showing the proportion of care plans that include each of the four dimensions of palliative care for each study group and for each vignette separately and the proportion of care plans in each of the four study groups that include aspects of all four domains.
ADL: activities of daily living
3.4. Opinions
GPs were triggered to start PC in case of a terminal or incurable disease, like cancer or dementia, and symptoms, like pain, dyspnoea, weight loss and immobility. The second trigger were social aspects like ‘loss of independence,’ ‘absence of the next of kin,’ ‘powerlessness of relatives’ or ‘lack of home care and support.’
Seventeen of the eighteen GPs who were asked SQ1 and all ten GPs who were asked the combined SQ found the tools helpful. However, four GPs had some concerns about the usefulness of either SQ1 or the combined SQs in daily practice (Figure 4). All five GPs in group 4 (SQ1, SQ2, PS) considered the PS to be a helpful tool for planning multidimensional PC.
Figure 4.
Statements about the usefulness of the first and second surprise questions.
4. Discussion
In this pilot study, GPs were invited to plan care based on patient cases. GPs who were asked SQ1 and SQ2 before making the care plans, planned the most elaborate care for patients for whom they would not be surprised if they died within 12 months (the answer to SQ1 is ‘no’) and would be surprised if they were still alive after 12 months (the answer to SQ2 is ‘yes’). This is in concordance with our hypothesis that answering SQ1 with ‘no’ and SQ2 with ‘yes’ would lead to more elaborate PC than other answer combinations.
4.1. Disease Trajectories
In this study, the terminal oncology patient was most often identified as being likely to die within a year and, therefore, received the most elaborate care. The frail elderly patient suffering from dementia was least expected to die within 12 months and was allocated less anticipatory and multidimensional care. This is in concordance with a systematic review by Gardiner et al. that mentions the delayed recognition of the palliative transition in non-cancer patients (11). Evans et al. found that organ failure and old-age/dementia patients received PC less frequently than cancer patients. They also found that old-age/dementia patients, the group of patients most likely to lose decision-making capacity, had the least end-of-life discussions and anticipatory care planning (10).
4.2. PC in Slovenia
A study with the same methodology was recently performed among Dutch GPs. The mean RADIANT scores in this Dutch study ranged from 4.9 to 8.9 and are noticeably higher than the Slovenian mean scores ranging from 2.2 to 3.6 (21).
The mean RADIANT scores were higher for the care plans written in Slovenian than for the plans written in English. It is likely that some of the subtler treatment descriptions and nuances in the care plans were lost in translation or misinterpreted due to different meanings of words in different languages.
The differences in RADIANT scores between the Dutch and Slovenian studies lie within the anticipatory and psychosocial aspects of the care plans. Dutch GPs discussed patients’ aspects of quality of life, goals, and preferences more frequently than Slovenian GPs. In addition, Slovenian GPs scored fewer points on the social/financial and existential/psychological dimensions of multidisciplinary care. In this Slovenian study, treatment limitations, preferences for end-of-life care, dying scenarios and preferred places of death were never mentioned. This might be because Slovenian GPs did not consider discussing these topics as treatment and, therefore, did not include them in the care plan. Another possibility is that Slovenian GPs are less prone to discuss these topics due to differences between Dutch and Slovenian laws regarding matters as palliative sedation, euthanasia and advance directives. There are also cultural differences regarding health care, in general, and PC, in particular. In 2002, Lunder and Cerv wrote the following about PC in Slovenia: There has been long subjugation of the country to another’s rule. In the period of socialism, death was pushed into the sphere of the private, and the Church. There was no interest in the development of public institutions, like palliative care wards in hospitals or hospices (25). Ten years later, a study regarding psychosocial care in cancer patients concluded that the need for further development of psychosocial care in Slovenia is still underestimated, but first attempts are being made to fill this gap (26).
There is much to gain in terms of PC education since this subject takes up only 8 hours in the general curriculum of the University of Ljubljana, and 15 hours for the University of Maribor (27). Another point of attention is the content of PC education programs. Findings from an international review show that concepts of pain management are being well addressed, but current undergraduate curricula may not adequately explore issues of broader symptom control, and psychosocial and spiritual aspects of care (28). The need and wish for these educational initiatives is also mentioned by Chang et al. (29). Furthermore, they are reflected in the self-assessed scores for PC skills (6.5 out of 10), interest in PC (8.3 out of 10), and the proportion of GPs who indicated to have plans to improve their PC skills (90%). Unfortunately, the number of PC experts willing to work as PC providers or teachers is insufficient according to the EAPC (27). Nonetheless, in Slovenia, PC education initiatives have been developed in both undergraduate and postgraduate programs. Several courses are organized to provide doctors and other healthcare professionals with special knowledge and skills in PC (30).
4.3. Opinions
The participants mentioned multiple physical and social aspects that trigger them to initiate PC. Similar aspects were mentioned by Dutch and British GPs in two separate studies (1, 17). The SQs were considered to be helpful tools for the identification of patients in need of PC. Concerns about the usefulness of the SQs included the difficulty of the prediction of prognosis, especially in noncancer patients, and uncertainty about how to interpret the answers to the SQs. This uncertainty regarding interpretation is apparent in other studies as well. The SQ is often used as a prognostication tool to predict 1-year mortality while it was developed as a tool to identify patients with PC needs (15,16,17). The last concern was regarding the communication with the patient once the GP has considered the SQs. Communication has been mentioned before as a barrier for the early initiation of PC (8, 9, 31). The positive opinions regarding the PS are mirrored by recent research by Thoonsen et al., in which GPs stated that this PS helped them consider actual and possible future problems, needs and scenarios regarding all dimensions of PC (32). Surprisingly, this current study shows no effect of the use of the PS on the multidimensionality of the care plans. In fact, group 4 was the only group in which none of the care plans mentioned all four dimensions of PC.
4.4. Strengths and Limitations
The strength of this study lies in the fact that it combines the original SQ with a second SQ and a PS. This approach does not only help GPs to identify patients who are at high risk to deteriorate and die, but also triggers them to start multidisciplinary and anticipatory PC.
Unfortunately, due to the small number of participants, the results of this pilot study could not be subjected to significance testing, so any of the differences found might be due to chance. Another limitation is that the vignettes and score form were developed for use among Dutch GPs. This might explain the low RADIANT scores, low response rate and low number of GPs who actually completed the survey. The length of the survey also seemed to have a negative influence on participation, since less surveys were completed in groups 3 and 4, in which the survey contained more questions than in groups 1 and 2. The timing of the study was not ideal either, since the invitations and surveys were sent in July and August, when many GPs were on holidays.
5. Conclusion
This was one of the first studies to investigate the use of the combined SQs. The results of this pilot study seem promising, but further research is needed regarding the usefulness in daily practice. Furthermore, it seems worthwhile to continue exploring how GPs can be triggered to identify patients in need of PC, and to start multidisciplinary and anticipatory care. More attention could be given to the psychosocial aspects of care and to the discussion of patients’ goals and preferences for end-of-life care. In the future, PC should become more generally available for non-cancer patients. Further development of PC education programs and national guidelines could be the first step towards reaching these goals in Slovenia.
Appendix 1: vignettes
Vignette A
Mrs. A., 83 years old, is widowed and lives independently in a detached house on the outskirts of the village. She and her husband used to run several shops in the centre of the nearest city. She has six children, who are all closely involved. She enjoys life, and particularly the company of her children and grandchildren. Her cognitive functions are still excellent, but she has several relevant somatic problems, the most important of which are:
COPD Gold III-IV. She still enjoys several cigarettes per day.
Presbycusis, for which she wears bilateral hearing aids.
Diabetes type 2.
Kidney failure (MDRD <30).
Anaemia.
In 2011, she suffered a severe myocardial infarction, for which she underwent an emergency PTCA and coronary stent placement. Unfortunately, she developed severe heart failure (NYHA classification 3–4).
Her main complaints are fatigue and some exertional dyspnoea. Her exercise capacity is clearly decreasing and walking longer distances within her house is sometimes challenging. In the past year, she has experienced several acute exacerbations of both heart failure and COPD, often combined. She regularly asks you about treatment options regarding her fatigue since she still very much enjoys life and does not want to say farewell to her children yet.
Vignette B
56-year-old Mr. W. is married and lives with his wife in an apartment on the edge of the forest. His wife is 52 years of age and very healthy. They have two daughters and five grandchildren. Both daughters are involved and live in the same area.
Mr W. is a manager at the university. He is rarely ill, but in the last few months, he has been having fluctuating, but sometimes severe pain of his upper abdomen. He also suffers from general malaise and an overall decline in his physical abilities. His condition was difficult to diagnose at first, but eventually a metastatic pancreatic tumour was found. Currently, he is suffering severe pain and he has lost several kilograms of weight. A coeliac plexus blockade has been planned. He is only moderately fit, but calm and resigned.
Vignette C
Mrs. C. is a 91-year old childless widow. She lives alone in a luxurious apartment, where additional care is provided. She has an 85-year-old sister in law, who cares for her.
Mrs. C. has been experiencing increasing problems in her daily life. She hardly leaves her house and tends to fail performing certain complex tasks. Her (short-term) memory seems to be intact. She is, however, increasingly disoriented in time and place (orientation in person is intact). A year and a half ago she was diagnosed with dementia by a geriatrician.
Several years ago, she had a heart attack, for which she was hospitalized and treated conservatively with medication. Next, she developed heart failure, which is currently stable. She also has atrial fibrillation, for which she takes oral anticoagulants.
She developed squamous cell carcinoma in her face several times. Recently, one of those carcinomas, situated at the right side of her mount, was surgically removed. Because of postoperative complications, she had to undergo a repeat surgery. This resulted in permanent dysfunction of the right side of her mouth causing eating difficulties and several kilograms of weight loss in the past months.
Finally, she suffers from bilateral coxarthrosis, which causes pain every now and then.
Lately, Mrs. C. has fallen regularly. Since her last fall, she has been immobilized due to severe lower back pain. She spends most of the day either dozing in her chair or lying in bed. Two years ago, she went through an episode during which she experienced the same problems, caused, at that time, by osteoporotic vertebral infraction. She recovered spontaneously from this previous episode in six months.
Vignette D
69-year-old Mr. T. is married and lives with his wife in a single-family home. He is quite healthy and hardly ever ill.
After a period of vague abdominal complaints, he was referred to an internist, who diagnosed him with colon cancer with hepatogenous and pulmonary metastasis. At first, Mr. T. was very emotional about this news, but after a while, he regained his calm.
Mr. T. would like to receive life-prolonging treatment. He is very fit and hardly experiences any complaints now. He has an appointment with a medical oncologist to discuss the possible options. He is very motivated to continue treatment.
Conflicts of interest: The authors declare that no conflicts of interest exist.
Funding: No funding was required.
Ethical approval: Received from the Slovene Medical Ethics Committee on 14-06-2016, consent number KME 126/06/16.
References
- 1.Claessen SJ, Francke AL, Engels Y, Deliens L.. How do GPs identify a need for palliative care in their patients? An interview study. BMC Fam Pract. 2013;14:42. doi: 10.1186/1471-2296-14-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Makivić I, Kersnik J, Klemenc-Ketiš Z.. The role of the psychosocial dimension in the improvement of quality of care: a systematic review. Zdr Varst. 2015;55:86–95. doi: 10.1515/sjph-2016-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abarshi E, Onwuteaka-Philipsen B, Donker G, Echteld M, Van den Block L, Deliens L.. General practitioner awareness of preferred place of death and correlates of dying in a preferred place: a nationwide mortality follow-back study in the Netherlands. J Pain Symptom Manage. 2009;38:568–77. doi: 10.1016/j.jpainsymman.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Gomes B, Calanzani N, Curiale V, McCrone P, Higginson IJ.. Effectiveness and costeffectiveness of home palliative care services for adults with advanced illness and their caregivers. Cochrane Database Syst Rev. 2013;6:CD007760. doi: 10.1002/14651858.CD007760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Švab I, Kravos A, Vidmar G.. Factors influencing home visits in Slovenian general practice. Fam Pract. 2003;20:58–60. doi: 10.1093/fampra/20.1.58. [DOI] [PubMed] [Google Scholar]
- 6.European Association for Palliative Care. Promoting palliative care in the community: producing a toolkit to improve and develop primary palliative care in different countries internationally. doi: 10.1177/0269216314522318. http://www.eapcnet.eu/LinkClick.aspx?fileticket=PXlXlRoSrXU%3D Accessed Juny 6th, 2016 at. [DOI] [Google Scholar]
- 7.Beernaert K, Deliens L, De Vleminck A, Devroey D, Pardon K, Van Den Block L. et al. Early identification of palliative care needs by family physicians: a qualitative study of barriers and facilitators from the perspective of family physicians, community nurses, and patients. Palliat Med. 2014;28:480–90. doi: 10.1191/0269216305pm937oa. [DOI] [PubMed] [Google Scholar]
- 8.Groot MM, Vernooij-Dassen MJFJ, Crul BJP, Grol RP.. General practitioners (GPs) and palliative care: perceived tasks and barriers in daily practice. Palliat Med. 2005;19:111–8. doi: 10.1177/0269216314526271. [DOI] [PubMed] [Google Scholar]
- 9.Evans N, Pasman HR, Donker GA, Deliens L, Van den Block L, Onwuteaka-Philipsen B.. End-of-life care in general practice: a cross-sectional, retrospective survey of ‘cancer’, ‘organ failure’ and ‘old-age/dementia’ patients. Palliat Med. 2014;28:965–75. doi: 10.1136/bmjspcare-2010-000001. [DOI] [PubMed] [Google Scholar]
- 10.Gardiner C, Ingleton C, Gott M, Ryan T.. Exploring the transition from curative care to palliative care: a systematic review of the literature. BMJ Support Palliat Care. 2011;1:56–63. doi: 10.1200/JCO.2008.17.7568. [DOI] [PubMed] [Google Scholar]
- 11.Thoonsen B, Engels Y, van Rijswijk E, Verhagen S, van Weel C, Groot M. et al. Early identification of palliative care patients in general practice: development of RADboud indicators for PAlliative Care Needs (RADPAC) Br J Gen Pract. 2012;62:625–31. doi: 10.1001/jama.2009.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hlebec V, Srakar A, Majcen B.. Determinants of unmet needs among Slovenian old population. Zdr Varst. 2016;55(1):78–85. doi: 10.1177/0269216314545006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Follwell M, Burman D, Le LW, Wakimoto K, Seccareccia D, Bryson J. et al. Phase II study of an outpatient palliative care intervention in patients with metastatic cancer. J ClinOncol. 2009;27:206–13. doi: 10.3399/bjgp12X654597. [DOI] [PubMed] [Google Scholar]
- 14.Bakitas M, Lyons KD, Hegel MT, Balan S, Brokaw FC, Seville J. et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA. 2009;302:741–9. doi: 10.1089/jpm.2010.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moss AH, Lunney JR, Culp S, Auber M, Kurian S, Rogers J. et al. Prognostic significance of the “surprise” question in cancer patients. J Palliat Med. 2010;13:837–40. doi: 10.3747/pdi.2011.00204. [DOI] [PubMed] [Google Scholar]
- 16.Moroni M, Zocchi D, Bolognesi D, Abernethy A, Rondelli R, Savorani G. et al. The ‘surprise’ question in advanced cancer patients: a prospective study among general practitioners. Palliat Med. 2014;28:959–64. doi: 10.1177/0269216314526273. [DOI] [PubMed] [Google Scholar]
- 17.Elliott M, Nicholson C.. A qualitative study exploring use of the surprise question in the care of older people: perceptions of general practitioners and challenges for practice. BMJ Support Palliat Care. 2017;7:32–8. doi: 10.1136/bmjspcare-2014-000679. [DOI] [PubMed] [Google Scholar]
- 18.Downar J, Goldman R, Pinto R, Englesakis M, Adhikari NK.. The “surprise question” for predicting death in seriously ill patients: a systematic review and meta-analysis. CMAJ. 2017;189(13):E484–93. doi: 10.1186/1471-2296-12-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White N, Kupeli N, Vickerstaff V, Stone P.. How accurate is the ‘Surprise Question’ at identifying patients at the end of life? A systematic review and meta-analysis. BMC Med. 2017;15(1):139. doi: 10.1186/s12916-017-0907-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thoonsen B, Groot M, Engels Y, Prins J, Verhagen S, Galesloot C. et al. Early identification of and proactive palliative care for patients in general practice, incentive and methods of a randomized controlled trial. BMC Fam Pract. 2011;12:123. doi: 10.1186/1471-2296-12-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weijers F. Unpublished manuscript. Radboud University; Nijmegen, The Netherlands: 2016. Does adding the Surprise Questions to case vignettes help GPs to increase the thoroughness of palliative care planning? Results of a pilot RCT. [Google Scholar]
- 22.World Health Organization Definition of Palliative Care. http://www.who.int/cancer/palliative/definition/en/ Accessed Juny 10th, 2016 at. [Google Scholar]
- 23.Pallialine: general principles of palliative care. http://pallialine.nl/algemene-principes-van-palliatievezorg Accessed July 14th, 2016 at. [Google Scholar]
- 24.Information for patients about end-of-life decisions and treatment limitations. http://www.thuisarts.nl/levenseinde Accessed July 14th, 2016 at. [Google Scholar]
- 25.Lunder U, Cerv B.. Slovenia: status of palliative care and pain relief. J Pain Symptom Manage. 2002;24:233–5. doi: 10.1002/pon.3154. [DOI] [PubMed] [Google Scholar]
- 26.Grassi L, Watson M.. Psychosocial care in cancer: an overview of psychosocial programmes and national cancer plans of countries within the International Federation of Psycho-Oncology Societies. Psychooncology. 2012;21:1027–33. doi: 10.1002/pon.3154. [DOI] [PubMed] [Google Scholar]
- 27.Centeno C, Lynch T, Donea O, Rocafort J, Clark D. EAPC atlas of palliative care in Europe 2013. Milan: EAPC Press; 2013. [Google Scholar]
- 28.Fitzpatrick D, Heah R, Patten S, Ward H.. Palliative care in undergraduate medical education - how far have we come? Am J Hosp Palliat Care. 2017;34:762–73. doi: 10.1111/j.1440-172X.2008.01723.x. [DOI] [PubMed] [Google Scholar]
- 29.Chang E, Daly J, Johnson A, Harrison K, Easterbrook S, Bidewell J. et al. Challenges for professional care of advanced dementia. Int J Nurs Pract. 2009;15:41–7. doi: 10.1186/s12875-015-0342-6. [DOI] [PubMed] [Google Scholar]
- 30.Albreht T, Pribaković Brinovec R, Josar D, Poldrugovac M, Kostnapfel T, Zaletel M. et al. Slovenia: health system review. Health Syst Transit. 2016;18:1–207. doi: 10.1136/bmjspcare-2015-001031. [DOI] [PubMed] [Google Scholar]
- 31.Thoonsen B, Vissers K, Verhagen S, Prins J, Bor H, van Weel C. et al. Training general practitioners in early identification and anticipatory palliative care planning: a randomized controlled trial. BMC Fam Pract. 2015;16:126. doi: 10.1186/s12875-015-0342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thoonsen B, Gerritzen SH, Vissers KC, Verhagen S, van Weel C, Groot M. et al. Training general practitioners contributes to the identification of palliative patients and to multidimensional care provision: secondary outcomes of an RCT. BMJ Support Palliat Care. 2016; Epub ahead of print. doi: 10.1136/bmjspcare-2015-001031. [DOI] [PMC free article] [PubMed] [Google Scholar]



