Abstract
Background:
The clinical consequences of hypothyroidism and hypothyroxinemia during pregnancy such as preterm birth are not still clear.
Objective:
The aim of this meta-analysis was to estimate the relation of clinical and subclinical hypothyroidism and hypothyroxinemia during pregnancy and preterm birth.
Materials and Methods:
In this meta-analysis, Preferred Reporting Items for Systematic review and Meta-Analysis were utilized. Searching the cohort studies were done by two researchers independently without any restrictions on Scopus, PubMed, Science Direct, Embase, Web of Science, CINAHL, Cochrane, EBSCO and Google Scholar databases up to 2017. The heterogeneity of the studies was checked by the Cochran's Q test and I2 index. Both random and fixed-effects models were used for combining the relative risk and 95% confidence intervals. Data were analyzed using Comprehensive Meta-Analysis software version 2.
Results:
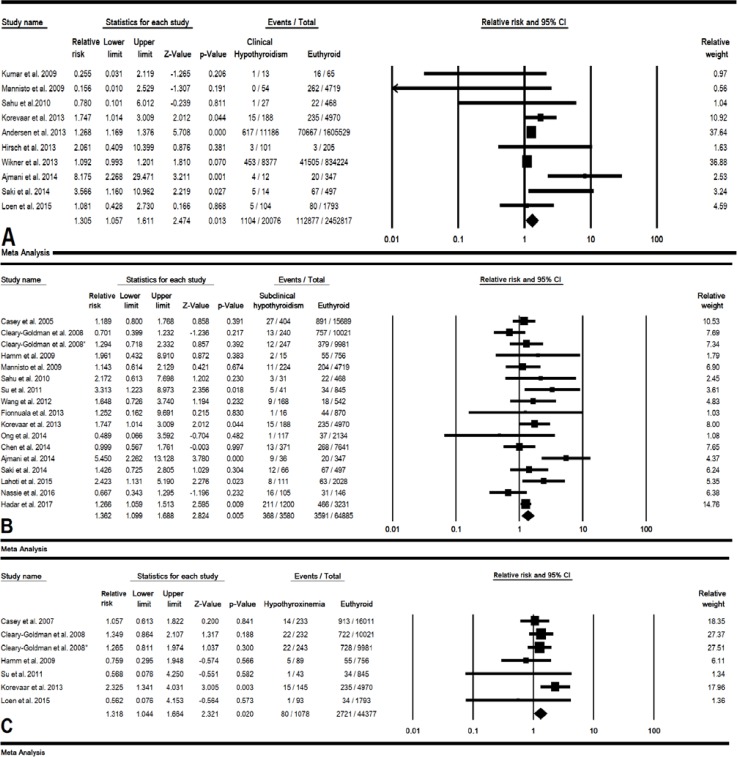
Twenty-three studies were included in the meta-analysis. The relative risks of the clinical hypothyroidism, subclinical hypothyroidism and hypothyroxinemia during pregnancy on preterm birth was estimated 1.30 (95% CI: 1.05-1.61, p=0.013, involving 20079 cases and 2452817 controls), 1.36 (95% CI: 1.09-1.68, p=0.005, involving 3580 cases and 64885 controls) and 1.31 (95% CI: 1.04-1.66, p=0.020, involving 1078 cases and 44377 controls), respectively.
Conclusion:
The incidence of preterm birth was higher among mothers with clinical and subclinical hypothyroidism or hypothyroxinemia during pregnancy compared to euthyroid mothers, and these relations were significant. Therefore, gynecologists and endocrinologists should manage these patients to control the incidence of adverse pregnancy outcomes such as preterm birth.
Key Words: Hypothyroidism, Pregnancy, Preterm birth, Meta-analysis, Cohort
Introduction
Thyroid hormones are needed for normal metabolism, regulation of body temperature, energy production, and fetal development (1). Changes in maternal thyroid function during pregnancy and lack of adequate adaptation to these changes will lead to thyroid dysfunction (2, 3). Some of these changes in thyroid function happen due to increased levels of thyroid binding globulin, an increase in renal clearance of iodine and thyrotrophic effect on human chorionic gonadotropin (4, 5). The prevalence of subclinical hypothyroidism during pregnancy is reported 1.5-4% and for clinical hypothyroidism 0.5-3% (6-8). However, the cutoff point, ethnicity, and the research design can be effective in this controversy. But generally, it is more prevalent in Asian countries (8).
In order to achieve the favorable result of pregnancy, which is a full-term and alive baby, all the conditions should be optimized in early pregnancy. Proper thyroid function of the mother, especially in the first trimester for fetus brain development and also when the fetus is not capable of producing thyroid hormones, is critical (9). The clinical consequences of hypofunction thyroid during pregnancy on adverse pregnancy outcomes such as premature birth are controversy (10-14). Systematic review and meta-analysis study by examining all relevant documentation and providing an overall estimate can present a full picture of problem in pregnant women (15, 16).
Given the importance of these disorders, especially hypothyroidism during pregnancy and also the inconsistent results of different studies in this field, this systematic review and meta-analysis study was conducted with the purpose of assessing the adverse effects of clinical hypothyroidism, subclinical hypothyroidism, and hypothyroxinemia during pregnancy on preterm birth.
The results obtained in this study could provide valuable information from the findings of multiple studies. It also can be a basis for creating new plans and programs to properly manage these disorders during pregnancy for prevention of preterm birth.
Material and methods
Study protocol
This meta-analysis was done in several detailed stages, including search strategy, determining the inclusion and exclusion criteria, quality evaluation of studies, data extraction, analysis and interpretation of findings by using the preferred reporting items for systematic reviews and meta-analyses protocol (PRISMA-P) (17). In order to avoid error and bias, all procedures were done by two researchers who were independent of each other.
Search strategy
Literature searching was done by two researchers independently who were familiar with search methods and information sources without any restrictions on Scopus, PubMed, Science Direct, Embase, Web of Science, CINAHL, Cochrane, EBSCO as well as Google scholar databases up to 2017 using keywords including thyroid disease, thyroid, hypothyroidism, subclinical hypothyroidism, clinical hypothyroidism, hypothyroxinemia, preterm delivery, premature delivery, preterm labor, premature labor, preterm birth and premature birth which being searched in combination using AND & OR operators. Combined search in PubMed is shown in Appendix 1. In order to achieve more studies, review articles and all relevant references were evaluated as well. Also, any encounters discussed by third expert researcher.
Inclusion and exclusion criteria
The study was considered to be eligible if the following criteria were met: 1) A prospective cohort study; 2) The mother suffered from clinical or subclinical hypothyroidism or hypothyroxinemia during pregnancy for case group; 3) Preterm birth was investigated in the outcome 4) The mother was not thyroid autoimmunity disease; 5) The mother was not receiving treatment for thyroid hypofunction and 6) Information about the number of preterm births in each generation was reported. In this investigation, data from review articles, case-controls, case reports, and letters to the editor were not reviewed.
Definitions
Preterm birth defined as a premature birth in less than 37 gestational weeks. A high thyroid-stimulating hormone (TSH) level with a low free thyroxine (FT4) level; a high TSH level with a normal FT4 level; and a low FT4 level with a normal TSH level was defined as clinical hypothyroidism, subclinical hypothyroidism, and hypothyroxinemia, respectively.
Quality evaluation
After determining the relevant investigations, selected papers were evaluated according to the STROBE checklist (18). The checklist consists of 22 sections which evaluate various aspects of the methodology. The researchers chose a simple method for scoring; 0-2 points were given to each question in the checklist, so maximum points attainable was considered to be 44. The papers were divided into three categories in terms of quality: Low (0-15), medium (16-30), and high quality (31-44). The articles that get a minimum score of 16 were gotten into the meta-analysis.
Data extraction
The researchers used a checklist containing the required information for studying the articles, including the author's name, article title, year of study, place of study, sample size, age, gestational age, any information on the incident of maternal thyroid disease, and preterm birth compared to a reference group.
Statistical analysis
Relative risk (RR) was used in order to determine the effect size of maternal hypothyroidism and hypothyroxinemia during pregnancy on preterm birth. RR with 95% confidence intervals (CIs) from selected studies was pooled. Heterogeneity among the investigated studies was determined using Cochran's Q test and I2 index. There are three categories for heterogeneity (less than 25% or low, between 25-50% considered as moderate, and above 50% as high heterogeneity) (19).
Therefore, in this study fixed-effects and random-effects were performed for low and high heterogeneity, respectively (20). Becuase of high heterogeneity, subgroup analysis based on the continent was performed to find sources of heterogeneity. Cumulative meta-analysis was performed based on the published year of the study to determine the year of acceptance or rejection of assumptions. Sensitivity analysis was conducted to assess the validity and reliability of results, and to show the effect of removing single study on the overall estimate at a time. Egger and Begg’s tests for checking Publication bias was used (21-22). The data was analyzed using meta-analysis specialized software, Comprehensive Meta-Analysis software version 2. The significance level was considered lower than 0.05.
Results
Search results
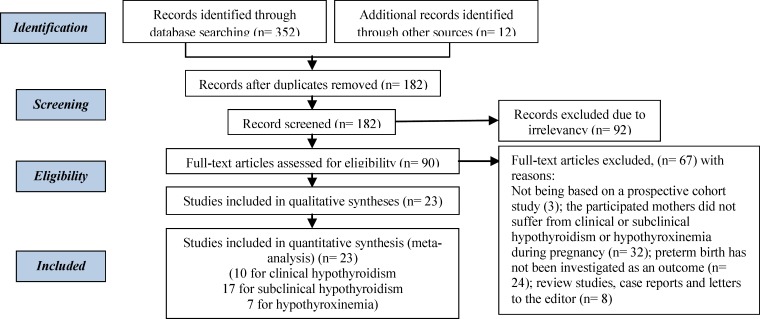
In this systematic search, 364 possible relevant studies were identified After further investigation, 342 studies were removed due to the lack of following criteria: duplication (182); irrelevant (92); not being based on a prospective cohort study (3); the participated mothers did not suffer from clinical or subclinical hypothyroidism or hypothyroxinemia during pregnancy (n=32); preterm birth has not been investigated as an outcome (n=24); review studies, case reports and letters to the editor (n=8) (Figure 1). Finally, 23 qualified studies (10 for clinical, 17 for subclinical hypothyroidism and 7 for hypothyroxinemia) entered into the quantitative meta-analysis (Table I).
Figure 1.
Study selection process
Table I.
Basic characteristics of included cohort studies for A) clinical hypothyroidism, B) subclinical hypothyroidism, and C) hypothyroxinemia
| First author | Year | Country | Case (n) | Control (n) | Follow-up (yr) | Gestational age (wk) | RR for PB |
(95%CI)
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||||||||||||||
| A | ||||||||||||||||||||
| Korevaar et al (10) | 2013 | Netherlands | 188 | 4970 | - | <18 | 1.74 | 1.01 | 3.00 | |||||||||||
| Ajmani et al (11) | 2014 | India | 12 | 347 | 1 | 13-26 | 8.17 | 2.26 | 29.47 | |||||||||||
| Andersen et al (12) | 2013 | Denmark | 11186 | 1605529 | 11 | Before, during, and after pregnancy | 1.26 | 1.16 | 1.37 | |||||||||||
| Loen et al (13) | 2015 | Spain | 104 | 1793 | 11 | <13 | 1.08 | 0.42 | 2.73 | |||||||||||
| Wikner et al (14) | 2013 | Sweden | 8377 | 834224 | 1 | <13 | 1.09 | 0.99 | 1.20 | |||||||||||
| Sahu et al (23) | 2010 | India | 27 | 468 | 9 | 13-26 | 0.78 | 0.10 | 6.01 | |||||||||||
| Kumar et al (24) | 2009 | India | 13 | 65 | 3 | 5-39 | 0.25 | 0.031 | 2.11 | |||||||||||
| Saki et al (25) | 2014 | Iran | 14 | 497 | 1 | 15-28 | 3.56 | 1.16 | 10.96 | |||||||||||
| Hirsh et al (26) | 2013 | Israel | 34 | 92 | 3 | 29-41 | 2.06 | 0.40 | 10.33 | |||||||||||
| Mannisto et al (27) | 2009 | Finland | 54 | 4719 | 6 | ≥23 | 0.15 | 0.01 | 2.52 | |||||||||||
| B | ||||||||||||||||||||
| Wang et al (28) | 2012 | China | 168 | 542 | 3 | ≤12 | 3.35 | 1.3 | 3.75 | |||||||||||
| Cleary-Goldman et al (29) | 2008 | USA | 240 | 10021 | 3 | First trimester | 0.70 | 0.39 | 1.23 | |||||||||||
| Cleary-Goldman et al (29) | 2008 | USA | 247 | 9981 | 3 | Second trimester | 1.29 | 0.7 | 2.33 | |||||||||||
| Korevaar et al (10) | 2013 | Netherlands | 188 | 4970 | - | <18 | 1.74 | 1.01 | 3.00 | |||||||||||
| Casey et al(30) | 2005 | USA | 404 | 15689 | 4 | <20 | 1.18 | 0.8 | 1.76 | |||||||||||
| Su et al (31) | 2011 | China | 41 | 845 | 3 | <20 | 3.31 | 1.22 | 8.97 | |||||||||||
| Mannisto et al (27) | 2009 | Finland | 224 | 4719 | 2 | <20 | 1.14 | 0.61 | 2.12 | |||||||||||
| Ajmani et al (11) | 2014 | India | 36 | 347 | 1 | 13-26 | 5.45 | 2.26 | 13.12 | |||||||||||
| Ong et al (32) | 2014 | Australia | 117 | 2134 | 1 | 9-14 | 0.48 | 0.06 | 3.59 | |||||||||||
| Chen et al (33) | 2014 | China | 371 | 7641 | 2 | All trimester | 0.99 | 0.56 | 1.76 | |||||||||||
| Sahu et al (23) | 2010 | India | 31 | 468 | 3 | 13-26 | 2.17 | 0.61 | 7.69 | |||||||||||
| Saki et al (25) | 2014 | Iran | 14 | 497 | 1 | 15-28 | 1.42 | 0.72 | 2.80 | |||||||||||
| Lahoti et al (34) | 2015 | India | 111 | 2028 | 3 | All trimester | 2.42 | 1.13 | 5.19 | |||||||||||
| Nassie et al(35) | 2016 | Israel | 105 | 146 | - | 23-34 | 0.66 | 0.34 | 1.29 | |||||||||||
| Hadar et al(36) | 2017 | Israel | 1200 | 3231 | 5 | First trimester | 1.26 | 1.05 | 1.51 | |||||||||||
| Fionnuala et al (37) | 2013 | Ireland | 16 | 870 | - | Second trimester | 1.25 | 0.16 | 9.69 | |||||||||||
| Hamm et al (38) | 2009 | Canada | 89 | 759 | 1 | 15-16 | 1.96 | 0.043 | 8.91 | |||||||||||
| C | ||||||||||||||||||||
| Cleary-Goldman et al (29) | 2008 | USA | 232 | 10021 | 3 | First trimester | 1.15 | 0.72 | 1.84 | |||||||||||
| Cleary-Goldman et al (29) | 2008 | USA | 243 | 9981 | 3 | Second trimester | 1.2 | 0.77 | 1.88 | |||||||||||
| Korevaar et al (10) | 2013 | Netherlands | 145 | 4970 | - | <18 | 2.54 | 1.42 | 4.54 | |||||||||||
| Hamm et al (38) | 2009 | Canada | 89 | 756 | 1 | 15-16 | 0.79 | 0.38 | 1.67 | |||||||||||
| Loen et al (13) | 2015 | Spain | 93 | 1793 | 11 | <13 | 0.92 | 0.28 | 3.01 | |||||||||||
| Su et al (31) | 2011 | China | 43 | 845 | 3 | <20 | 0.56 | 0.07 | 4.25 | |||||||||||
| Casey et al (39) | 2007 | USA | 233 | 16011 | 4 | <20 | 1.05 | 0.61 | 1.82 | |||||||||||
RR: Relative Risk
CI: Confidence Interval
PB: Preterm birth
Clinical hypothyroidism
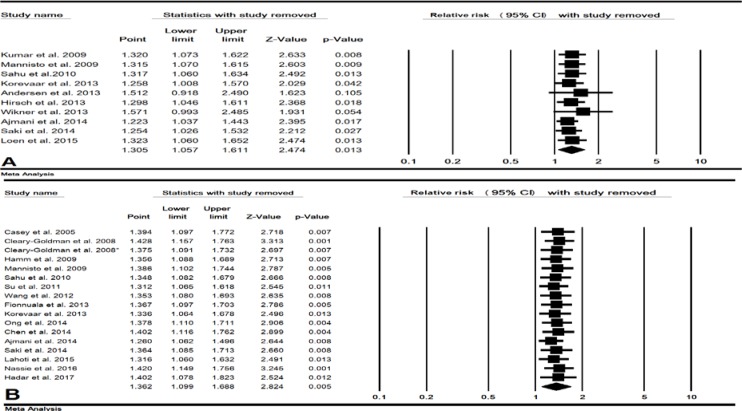
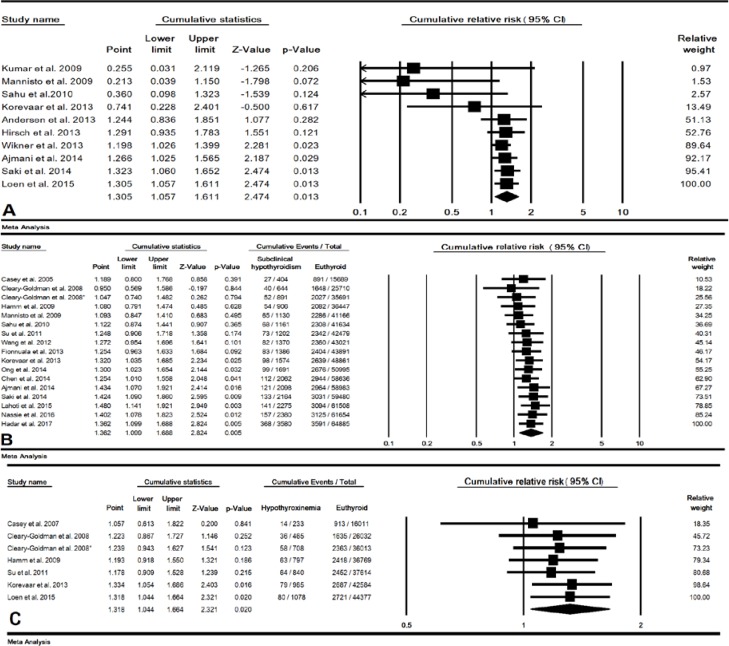
In 10 studies (20079 cases and 2452817 control pregnant women), the combined RR of maternal clinical hypothyroidism during pregnancy on preterm birth was estimated 1.30 (95% CI: 1.05-1.61, p=0.013) (Figure 2-A). In a sensitivity analysis, after removing single study of Andersen et al and Wikner et al the total p-value for this relationship increased to 0.105 and 0.054, respectively, indicating the low sensitivity of this meta-analysis (Figure 3-A). The cumulative meta-analysis for this relationship was shown in 2014, this relationship was statistically significant (Figure 4-A). The combined RR for this relationship by continent (Table II), and RR for Asian and European countries was estimated 2.06 (95% CI: 0.70-6.05, p=0.184) and 1.20 (95% CI: 1.03-1.39, p=0.016), respectively.
Figure 2.
Forest plot for relative risk (RR) in clinical hypothyroidism (A), subclinical hypothyroidism (B) and hypothyroxinemia (C) during pregnancy. For A and B according to High heterogeneity, random effects model and for C according to low heterogeneity, fixed effects model was used
Figure 3.
Forest plot for sensitivity analysis in clinical hypothyroidism (A) and subclinical hypothyroidism (B) during pregnancy on preterm.
Figure 4.
Forest plot for cumulative relative risk (RR) in clinical hypothyroidism (A), subclinical hypothyroidism (B) and hypothyroxinemia (C) during pregnancy on preterm
Table II.
Subgroup analysis based on the continent for relative risk (RR) in A) clinical hypothyroidism and B) subclinical hypothyroidism during pregnancy on preterm
| Variable | No. of studies |
Sample size(N)
|
Heterogeneity
|
95% CI | RR | p-value | |||
|---|---|---|---|---|---|---|---|---|---|
| Case (n) | Control (n) | p-value | I 2 (%) | ||||||
| Clinical hypothyroidism | |||||||||
| Asia | 5 | 167 | 1582 | 0.052 | 57.44 | 0.70-6.05 | 2.06 | 0.184 | |
| Europe | 5 | 19909 | 2451235 | 0.051 | 57.69 | 1.03-1.39 | 1.20 | 0.016 | |
| Test for subgroup differences: P=0.324 | |||||||||
| Subclinical hypothyroidism | |||||||||
| Asia | 9 | 2129 | 15745 | 0.005 | 63.26 | 1.12-2.29 | 1.60 | 0.009 | |
| Australia | 1 | 117 | 2134 | - | 0 | 0.06-3.59 | 0.48 | 0.482 | |
| Europe | 3 | 428 | 10559 | 0.597 | 0 | 0.96-2.15 | 1.44 | 0.072 | |
| USA | 4 | 906 | 36447 | 0.328 | 12.90 | 0.79-1.47 | 1.08 | 0.628 | |
| Test for subgroup differences: P=0.269 | |||||||||
CI: Confidence Interval
RR: Relative Risk
Subclinical hypothyroidism
In 17 studies (3580 cases and 64885 control pregnant women), the combined RR of maternal subclinical hypothyroidism during pregnancy on preterm birth was estimated 1.36 (95% CI: 1.09-1.68, p=0.005) (Figure 2-B). The result of sensitivity analysis for total RR for this relationship was not affected by removing single study which meant this estimate had a good stability (Figure 3-A). The result of cumulative meta-analysis for this relationship was shown in Figure 4-B, the result was indicated in 2013, this relationship was statistically significant. The RR for this relationship in Asian studies was significant (p=0.009) but in American and European studies a significant relationship was not found (p=0.628 and p=0.072 respectively) (Table II).
Isolated hypothyroxinemia
In 7 studies (1078 cases and 44377 control pregnant women), the combined RR of maternal hypothyroxinemia during pregnancy on preterm birth was estimated was estimated 1.31 (95% CI: 1.04-1.66, p=0.020), and the association was statistically significant (Figure 2-C). Also, a cumulative meta-analysis was indicated in 2013, this relationship was statistically significant.
Publication bias
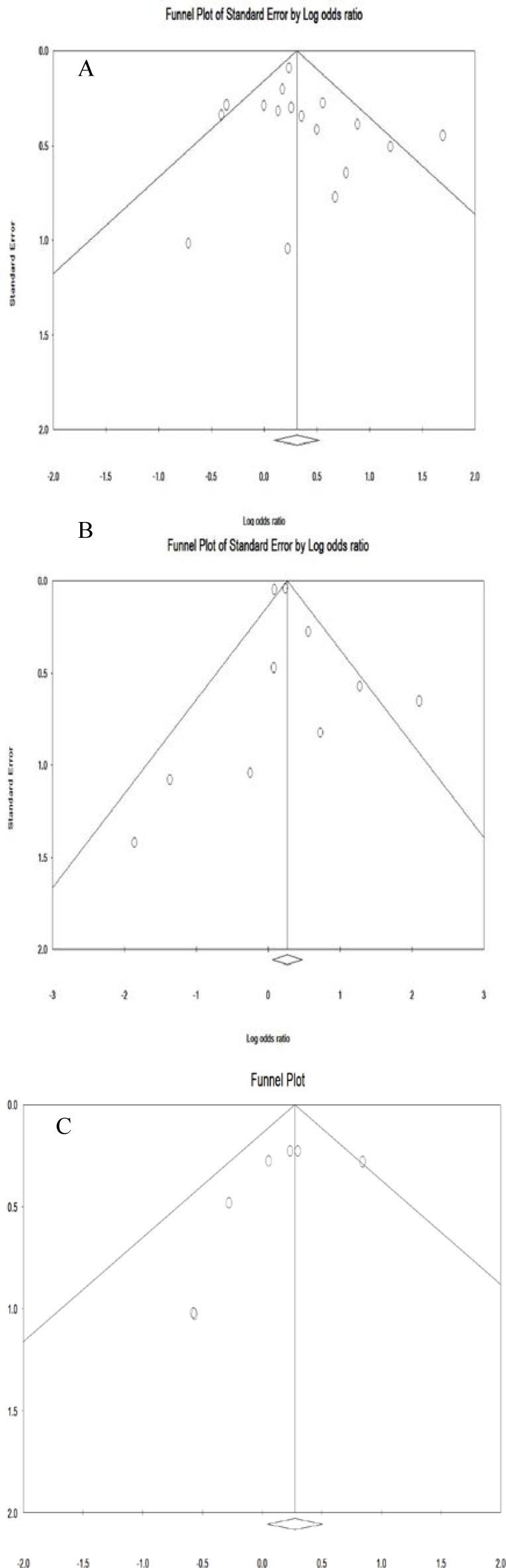
The Result for Egger test in clinical hypothyroidism (0.57), subclinical hypothyroidism (0.37) and hypothyroxinemia (0.22) was estimated. The Result for Begg’s test in clinical hypothyroidism (0.21), subclinical hypothyroidism (0.23) and hypothyroxinemia (0.76) was estimated. Publication bias in the obtained results is shown in Figure 4 which shows as symmetrical in Funnel Plot.
Figure 5.
Publications bias of included studies for clinical hypothyroidism (A), subclinical hypothyroidism (B) and hypothyroxinemia (C) during pregnancy on preterm
Discussion
In the present meta-analysis, the combined 17, 10 and 7 studies for subclinical hypothyroidism, clinical hypothyroidism and hypothyroxinemia during pregnancy showed that are related to preterm birth, with p-values of, 0.013, 0.005, and 0.020, respectively. The mechanism that hypothyroidism can increase the risk of premature birth may be affected by different paths. One possible explanation is that inflammatory process with a change in the regulation of cytokine networks in the uterus and omission of the pair-control inflammatory processes can be linked with premature birth (40-42). Another suggestion are that thyroid hormones may influence fetal development directly through action on maternal and fetal metabolism (43).
The findings of one meta-analysis study on maternal thyroid dysfunction and its impact on pregnancy with combining only two studies concluded that there is no relationship between maternal thyroid disorder and unpleasant pregnancy outcomes (9). According to another meta-analysis, performed by Sheehan et al. on 6 studies and Hou et al. on 6 other studies, there is a significant relationship between clinical hypothyroidism and preterm birth (44, 45). Maraka et al. in their meta-analysis of 14 studies on the association between subclinical hypothyroidism in pregnant women with the incidence of preterm birth, indicated no association between maternal subclinical hypothyroidism and risk of preterm birth (46). In another meta-analysis study by Sheehan combining 10 studies, this relationship was not significant (p=0.32) (40). Also, Nazarpour et al. in a systematic review study suggested that further studies on neonatal outcomes of maternal subclinical hypothyroidism are essential (47). For hypothyroxinemia and preterm birth, the combined RR was 1.31 and the association was significant. In Sheehan et al. meta-analysis of 4 studies, this association was not significant (44). Hypothyroxinemia is a controversial management problem during pregnancy which can reassure practitioners that pregnant women with hypothyroxinemia have safety approach. It was revealed in a study that treatment of hypothyroxinemia in order to maintain FT4 above the normal top range may prevent preterm birth in multiparous women (48).
However, one important limitation of their studies compared to the present study was the lack of studies and less sample size, which can affect the results of the analysis. The results of the meta-analysis are reliable when all the investigations get through the quantitative analysis process which leads to lower variations and possibilities (16-17).
Conclusion
The incidence of preterm birth was higher among mothers with clinical hypothyroidism or subclinical hypothyroidism or hypothyroxinemia during pregnancy compared to euthyroid mothers, and these relations were significant. Therefore, gynecologists and endocrinologists should manage these patients to control the incidence of adverse pregnancy outcomes such as preterm birth. In future studies, clinical trial studies in the field of subclinical and clinical hypothyroidism, as well as the role of various treatments to prevent premature birth, is recommended.
Acknowledgements
We would like to thank Women's Reproductive Health Research Centre of Tabriz University of Medical Sciences for supporting this study.
Conflict of interest
All authors declare that there is no conflict of interest.
References
- 1.Laurberg P, Andersen SL, Pedersen IB, Andersen S, Carle A. Screening for overt thyroid disease in early pregnancy may be preferable to searching for small aberrations in thyroid function tests. Clin Endocrinol (Oxf) 2013;79:297–304. doi: 10.1111/cen.12232. [DOI] [PubMed] [Google Scholar]
- 2.Mullur R, Liu YY, Brent GA. Thyroid Hormone Regulation of Metabolism. Physiol Rev. 2014;94:355–382. doi: 10.1152/physrev.00030.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glinoer D. The regulation of thyroid function in pregnancy: pathways of endocrine adaptation from physiology to pathology. Endocr Rev. 1997;18:404–433. doi: 10.1210/edrv.18.3.0300. [DOI] [PubMed] [Google Scholar]
- 4.Karakosta P, Alegakis D, Georgiou V, Roumeliotaki T, Fthenou E, Vassilaki M, et al. Thyroid dysfunction and autoantibodies in early pregnancy are associated with increased risk of gestational diabetes and adverse birth outcomes. J Clin Endocrinol Metab. 2012;97:4464–4472. doi: 10.1210/jc.2012-2540. [DOI] [PubMed] [Google Scholar]
- 5.Skjoldebrand L, Brundin J, Carlstrom A, Pettersson T. Thyroid associated components in serum during normal pregnancy. Acta Endocrinol (Copenh) 1982;100:504–511. doi: 10.1530/acta.0.1000504. [DOI] [PubMed] [Google Scholar]
- 6.Vaidya B, Anthony S, Bilous M, Shields B, Drury J, Hutchison S, et al. Detection of thyroid dysfunction in early pregnancy: Universal screening or targeted high-risk case finding? J Clin Endocrinol Metab. 2007;92:203e7. doi: 10.1210/jc.2006-1748. [DOI] [PubMed] [Google Scholar]
- 7.Negro R, Mestman JH. Thyroid disease in pregnancy. Best Pract Res Clin Endocrinol Metab. 2011;25:927e43. doi: 10.1016/j.beem.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Allan WC, Haddow JE, Palomaki GE, Williams JR, Mitchell ML, Hermos RJ, et al. Maternal thyroid deficiency and pregnancy complications: implications for population screening. J Med Screen. 2000;7:127e30. doi: 10.1136/jms.7.3.127. [DOI] [PubMed] [Google Scholar]
- 9.van den Boogaard E, Vissenberg R, Land JA, van Wely M, Ven der Post JA, Goddijn Met all. Significance of (sub) clinical thyroid dysfunction and thyroid autoimmunity before conception and in early pregnancy: a systematic review. Hum Reprod Update. 2016;22:532–533. doi: 10.1093/humupd/dmw003. [DOI] [PubMed] [Google Scholar]
- 10.Korevaar TI, Schalekamp-Timmermans S, de Rijke YB, Visser WE, Visser W, de Muinck Keizer-Schrama SM, et al. Hypothyroxinemia and TPO-antibody positivity are risk factors for premature delivery: the generation R study. J Clin Endocrinol Metab. 2013;98:4382–4390. doi: 10.1210/jc.2013-2855. [DOI] [PubMed] [Google Scholar]
- 11.Ajmani SN, Aggarwal D, Bhatia P, Sharma M, Sarabhai V, Paul M. Prevalence of o vert and subclinical thyroid dysfunction among pregnant women and its effect on maternal and fetal outcome. J Obstet Gynaecol India. 2014;64:105–110. doi: 10.1007/s13224-013-0487-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersen SL, Olsen J, Wu CS, Laurberg P. Low birth weight in children born to mothers with hyperthyroidism and high birth weight in hypothyroidism, whereas preterm birth is common in both conditions: A danish national hospital register study. Eur Thyroid J. 2013;2:135–144. doi: 10.1159/000350513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.León G, Murcia M, Rebagliato M, Álvarez-Pedrerol M, Castilla AM, Basterrechea Met all. Maternalthyroiddysfunction during gestation, pretermdelivery, and birthweight The Infanciay Medio Ambiente Cohort, Spain. Paediatr Perinat Epidemiol. 2015;29:113–122. doi: 10.1111/ppe.12172. [DOI] [PubMed] [Google Scholar]
- 14.Wikner BN, Sparre LS, Stiller CO, Källén B, Asker C. Maternal use of thyroid hormones in pregnancy and neonataloutcome. Acta Obstet Gynecol Scand. 2008;87:617–627. doi: 10.1080/00016340802075103. [DOI] [PubMed] [Google Scholar]
- 15.Azami M, Nasirkandy MP, Mansouri A, Darvishi Z, Rahmati S, Abangah G, et al. Global Prevalence of Helicobacter pylori Infection in Pregnant Women: A Systematic Review and Meta-analysis Study. Int J Women's Health Reprod Sci. 2017;5:30–36. [Google Scholar]
- 16.Mansouri A, Adhami Mojarad MR, Badfar G, Abasian L, Rahmati S, Kooti W, et al. Epidemiology of Toxoplasma gondii among blood donors in Iran: A systematic review and meta-analysis. Transfus Apher Sci. 2017;56:404–409. doi: 10.1016/j.transci.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vandenbroucke JP, Elm Ev, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and Elaboration. PLoS Med. 2007;4:1628. doi: 10.1371/journal.pmed.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ades AE, Lu G, Higgins JP. The Interpretation of Random-Effects Meta-Analysis in Decision Models. Med Decis Making. 2005;25:646–654. doi: 10.1177/0272989X05282643. [DOI] [PubMed] [Google Scholar]
- 21.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 22.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sahu MT, Das V, Mittal S, Agarwal A, Sahu M. Overt and subclinical thyroid dysfunction among Indian pregnant women and its effect on maternal and fetal outcome. Arch Gynecol Obstet. 2010;281:215–220. doi: 10.1007/s00404-009-1105-1. [DOI] [PubMed] [Google Scholar]
- 24.Kumar A, Agarwal K, Gupta RK, Kar P. Obstetric outcome in women with hepatitis Cvirusinfection and thyroid dysfunction. Acta Obstet Gynecol Scand. 2009;88:1133–1137. doi: 10.1080/00016340903144220. [DOI] [PubMed] [Google Scholar]
- 25.Saki F, Dabbaghmanesh MH, Ghaemi SZ, Forouhari S, Ranjbar Omrani G, Bakhshayeshkaram M. Thyroid function in pregnancy and its influences on maternal and fetal outcomes. Int J Endocrinol Metab. 2014;12:e19378. doi: 10.5812/ijem.19378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirsch D, Levy S, Nadler V, Kopel V, Shainberg B, Toledano Y. Pregnancy outcomes in women with severe hypothyroidism. Eur J Endocrinol. 2013;169:313–320. doi: 10.1530/EJE-13-0228. [DOI] [PubMed] [Google Scholar]
- 27.Mannisto T, Vaarasmaki M, Pouta A, Hartikainen AL, Ruokonen A, Surcel HM, et al. Perinatal outcome of children born to mothers with thyroid dysfunction or antibodies: a prospective population-based cohort study. J Clin Endocrinol Metab. 2009;94:772–779. doi: 10.1210/jc.2008-1520. [DOI] [PubMed] [Google Scholar]
- 28.Wang S, Teng WP, Li JX, Wang WW, Shan ZY. Effects of maternal subclinical hypothyroidism on obstetrical outcomes during early pregnancy. J Endocrinol Invest. 2012;35:322–325. doi: 10.3275/7772. [DOI] [PubMed] [Google Scholar]
- 29.Cleary-Goldman J, Malone FD, Lambert-Messerlian G, Sullivan L, Canick J, Porter TF, et al. Maternal thyroid hypofunction and pregnancy outcome. Obstet Gynecol. 2008;112:85–92. doi: 10.1097/AOG.0b013e3181788dd7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Casey BM, Dashe JS, Wells CE, McIntire DD, Byrd W, Leveno KJ, et al. Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol. 2005;105:239–245. doi: 10.1097/01.AOG.0000152345.99421.22. [DOI] [PubMed] [Google Scholar]
- 31.Su PY, Huang K, Hao JH, Xu YQ, Yan SQ, Li T, et al. Maternal thyroid function in the first twenty weeks of pregnancy and subsequent fetal and infant development: a prospective population-based cohort study in China. J Clin Endocrinol Metab. 2011;96:3234–3241. doi: 10.1210/jc.2011-0274. [DOI] [PubMed] [Google Scholar]
- 32.Ong GS, Hadlow NC, Brown SJ, Lim EM, Walsh JP. Does the thyroid-stimulating hormone measured concurrently with first trimester biochemical screening tests predict adverse pregnancy outcomes occurring after 20 weeks gestation? J Clin Endocrinol Metab. 2014;99:E2668–2672. doi: 10.1210/jc.2014-1918. [DOI] [PubMed] [Google Scholar]
- 33.Chen LM, Du WJ, Dai J, Zhang Q, Si GX, Yang H, et al. Effects of subclinical hypothyroidism on maternal and perinatal outcomes during pregnancy: a single-center cohort study of a Chinese population. PloS One. 2014;9:e109364. doi: 10.1371/journal.pone.0109364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lahoti SK, Toppo L. subclinical hypothyroidism and pregnancy outcomes. Ann Int Med Den Res. 2015;1:324–326. [Google Scholar]
- 35.Nassie DI, Ashwal E, Raban O, Ben-Haroush A, Wiznitzer A, Yogev Y, et al. Is there an association between subclinical hypothyroidism and preterm uterine contractions? A prospective observational study. J Matern Fetal Neonat Med. 2017;30:881–885. doi: 10.1080/14767058.2016.1191065. [DOI] [PubMed] [Google Scholar]
- 36.Hadar E, Arbib N, Krispin E, Chen R, Wiznitzer A, et al. First trimester thyroid stimulating hormone as an independent risk factor for adverse pregnancy outcome. Am J Obstet Gynecol. 2017;26:S435. doi: 10.1080/14767058.2016.1242123. [DOI] [PubMed] [Google Scholar]
- 37.Breathnach FM, Donnelly J, Cooley SM, Geary M, Malone FD. Subclinical hypothyroidism as a risk factor for placental abruption: Evidence from a low‐risk primigravid population. Aust N Z J Obstet Gynaecol. 2013;53:553–560. doi: 10.1111/ajo.12131. [DOI] [PubMed] [Google Scholar]
- 38.Hamm MP, Cherry NM, Martin JW, Bamforth F, Burstyn I. The impact of isolated maternal hypothyroxinemia on perinatal morbidity. J Obstet Gynaecol Can. 2009;31:1015–1021. doi: 10.1016/S1701-2163(16)34345-6. [DOI] [PubMed] [Google Scholar]
- 39.Casey BM, Dashe JS, Spong CY, McIntire DD, Leveno KJ, Cunningham GF. Perinatal significance of isolated maternal hypothyroxinemia identified in the first half of pregnancy. Obstet Gynecol. 2007;109:1129–1135. doi: 10.1097/01.AOG.0000262054.03531.24. [DOI] [PubMed] [Google Scholar]
- 40.Stagnaro-Green A. Thyroid antibodies and miscarriage: where are we at a generation later? J Thyroid Res. 2011;2011:841949. doi: 10.4061/2011/841949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Challis JR, Lockwood CJ, Myatt L, et al. Inflammation and pregnancy. Reprod Sci. 2009;16:206–215. doi: 10.1177/1933719108329095. [DOI] [PubMed] [Google Scholar]
- 42.Stagnaro-Green A. Maternal thyroid disease and preterm delivery. J Clin Endocr Metab. 2009;94:21–25. doi: 10.1210/jc.2008-1288. [DOI] [PubMed] [Google Scholar]
- 43.Forhead AJ, Fowden AL. Thyroid hormones in fetal growth and prepartum maturation. J Endocrinol. 2014;221:R87–R103. doi: 10.1530/JOE-14-0025. [DOI] [PubMed] [Google Scholar]
- 44.Sheehan PM, Nankervis A, Araujo Júnior E, Da Silva Costa F. Maternal Thyroid Disease and Preterm Birth: Systematic Review and Meta-Analysis. J Clin Endocrinol Metab. 2015;100:4325–4331. doi: 10.1210/jc.2015-3074. [DOI] [PubMed] [Google Scholar]
- 45.Hou J, Yu P, Zhu H, Pan H, Li N, Yang H, et al. The impact of maternal hypothyroidism during pregnancy on neonatal outcomes: a systematic review and meta-analysis. Gynecol Endocrinol. 2016;32:9–13. doi: 10.3109/09513590.2015.1104296. [DOI] [PubMed] [Google Scholar]
- 46.Maraka S, Ospina NM, O'Keeffe DT, Espinosa De Ycaza AE, Gionfriddo MR, et al. Subclinical Hypothyroidism in Pregnancy: A Systematic Review and Meta-Analysis. Thyroid. 2016;26:580–590. doi: 10.1089/thy.2015.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nazarpour S, Ramezani Tehrani F, Simbar M, Azizi F. Thyroid dysfunction and pregnancy outcomes. Int J Reprod BioMed. 2015;13:387–396. [PMC free article] [PubMed] [Google Scholar]
- 48.Torremante P, Flock F, Kirschner W. Free thyroxine level in the high normal reference range prescribed for nonpregnant women may reduce the preterm delivery rate in multiparous. J Thyroid Res. 2011;2011:905734. doi: 10.4061/2011/905734. [DOI] [PMC free article] [PubMed] [Google Scholar]