Abstract
In “strategy I” plants, several alterations in root physiology and morphology are induced by Fe deficiency, although the mechanisms by which low Fe levels are translated into reactions aimed at alleviating Fe shortage are largely unknown. To prove whether changes in hormone concentration or sensitivity are involved in the adaptation to suboptimal Fe availability, we tested 45 mutants of Arabidopsis defective in hormone metabolism and/or root hair formation for their ability to increase Fe(III) chelate reductase activity and to initiate the formation and enlargement of root hairs. Activity staining for ferric chelate reductase revealed that all mutants were responsive to Fe deficiency, suggesting that hormones are not necessary for the induction. Treatment of wild-type plants with the ethylene precursor 1-aminocyclopropane-1-carboxylic acid caused the development of root hairs in locations normally occupied by non-hair cells, but did not stimulate ferric reductase activity. Ectopic root hairs were also formed in −Fe roots, suggesting a role for ethylene in the morphological responses to Fe deficiency. Ultrastructural analysis of rhizodermal cells indicated that neither Fe deficiency nor 1-aminocyclopropane-1-carboxylic acid treatment caused transfer-cell-like alterations in Arabidopsis roots. Our data indicate that the morphological and physiological components of the Fe stress syndrome are regulated separately.
Root hairs are long, tubular-shaped outgrowths on the root surface derived from trichoblasts located in the rhizodermis. They are thought to play an important role in the uptake of water and nutrients from the environment by increasing the absorptive surface layer of the root. The development of root hairs is the result of intricate multiple genetic and environmental controls. Pharmacological experiments indicate that phytohormones, in particular ethylene and auxin, appear to be involved in root epidermal cell fate specification. For instance, treatment of seedlings with ethylene or the ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC) triggers the development of root hairs, and blocking either ethylene biosynthesis or perception causes a reduction in the frequency of root hairs (Masucci and Schiefelbein, 1994; Tanimoto et al., 1995). Other hormones, e.g. auxin and cytokinin, interact with ethylene metabolism. Alterations in the level or sensitivity of several hormones may therefore affect ethylene-mediated processes. Genetic approaches have also established the connection between plant hormones and root hair development. Auxin-resistant mutants form shorter and fewer root hairs or do not develop root hairs at all (Masucci and Schiefelbein, 1996). Likewise, ethylene overproducing (eto) mutants of Arabidopsis exhibit a higher frequency of root hairs (Smalle and van der Straeten, 1997). Similar results have been obtained with the constitutive triple response (ctr1) mutant, which is affected in ethylene signal transduction (Kieber, 1997, and refs. therein).
Fe is an essential mineral nutrient for plant growth and development. While Poaceae species (grasses) rely on secretion of phytosiderophores (PS) and subsequent uptake of the Fe(III)-PS complex (strategy II; Römheld and Marschner, 1986a), strategy I plants (dicots and non-grass monocots) respond to decreasing Fe in the environment by inducing a series of physiological responses that assist the mobilization of sparingly soluble Fe compounds (Schmidt, 1999). ATPase-mediated acidification of the rhizosphere (Toulon et al., 1992), enhanced activity of a plasma membrane-bound reductase (FRO2; Robinson et al., 1999), and increased expression of a Fe2+ transporter (IRT1; Eide et al., 1996; Fox et al., 1996) are ubiquitous responses of strategy I species upon Fe deficiency.
Morphological alterations that result in an increased surface area, such as the formation of root hairs and transfer cells in the rhizodermis, are thought to provide the basis for the physiological reactions. However, views conflict as to the role of root hairs and rhizodermal transfer cells in Fe uptake. While in Ficus benjamina, Fe reduction appeared to be limited to regions of root hair development (Rosenfield et al., 1991), Moog et al. (1995) found no difference with respect to the stimulation of root reduction activity between the root-hair-less Arabidopsis mutant RM57 and its wild type. The physiological importance of root hairs in Fe uptake was also questioned by Chaney et al. (1992). Using chelator-buffered nutrient solutions, the authors showed that root hairs were only developed at severe chlorosis, whereas other responses, e.g. reductase activity, occurred upon imposing intermediate levels of Fe stress severity. It was concluded that the formation of roots hairs is an effect of Fe-deficiency-induced chlorosis, rather than an integral part of the Fe stress syndrome. In contrast to these findings, Landsberg (1994) reported that in sugar beet, transfer cells and root hairs were also induced under latent Fe deficiency.
Ethylene and auxin have been implicated in the regulation of Fe deficiency responses. Fe-stress-induced increases in the synthesis of ethylene and auxin have been reported for both strategy I and strategy II species (Morgan and Hall, 1962; Römheld and Marschner, 1986b; Romera et al., 1999). Disruption of polar auxin transport from the shoot to the root by 2,3,5-triiodobenzoic acid resulted in the inhibition of rhizosphere acidification by Fe-deficient roots. Moreover, inhibitors of either ethylene synthesis or action repress the induction of all components of the Fe stress syndrome in cucumber (Romera et al., 1994) and have been shown to inhibit the uptake of phytosiderophores in barley (Welch et al., 1997). The morphological responses to Fe deficiency can be mimicked by the application of auxin, ethylene, and ABA, leading to enhanced formation of root hairs and, except for ABA, induction of transfer cells in the root epidermis (Romera et al., 1994; Landsberg, 1996; Schmidt and Bartels, 1996). The involvement of hormones in the physiological alterations induced by Fe deficiency is less clear. While Romera et al. (1994, 1999) found a stimulation of all Fe deficiency responses by ACC in several plant species, application of the auxin analog 2,4-dichlorophenoxyacetic acid (2,4-D) to Fe-sufficient Plantago lanceolata roots stimulated root hair growth and transfer cell formation, but their reduction capacity for ferric chelates appeared to be only slightly increased (Schmidt and Bartels, 1996).
To understand how the expression of the physiological and morphological reactions to Fe deficiency stress are regulated, we examined several hormone-related Arabidopsis mutants for their ability to adapt to suboptimal Fe availability. The responses we focused on included root hair formation and root reductase activity. As a further objective, we sought to determine whether the formation of transfer cells in the root epidermis is coupled to the development of root hairs.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
The cin1 and cin2 Arabidopsis mutants were kindly provided by J. Kieber (University of Illinois, Chicago), the cri1-1 mutant was obtained from C. Bellini (Institut National de la Recherche Agronomique, Versailles, France), cpc from K. Okada (Kyoto University, Japan), ers1-1 from E.M. Meyerowitz (California Institute of Technology, Pasadena), eti5 from M.A. Hall (University of Wales, Aberystwyth), nit1-3 from B. Bartel (Rice University, Houston), rgr1 from D. Söll (Yale University, New Haven, CT), sur1 from M. van Montagu (Universiteit Gent, Belgium), and tir3-1 from M. Estelle (Indiana University, Bloomington). The other genetic stocks were obtained from the Arabidopsis Biological Resource Center (Ohio State University, Columbus). Plants were ecotype Columbia unless stated otherwise. All mutants have been described elsewhere (see Table I).
Table I.
Characteristics of root hairs and reductase activity in wild-type and mutant plants
| Gene | Mutant Phenotype | Root Ferric Reductase Activity +Fe/−Fe | Reference |
|---|---|---|---|
| Ecotypes | |||
| Col-0 | Columbia | −/++ | |
| Ler-0 | Landsberg erecta | −/++ | |
| La-0 | Landsberg | −/++ | |
| Ws-0 | Wassilewskija | −/++ | |
| Hormone-related mutants | |||
| aba1-1 (Ler) | Abscisic acid deficient | −/++ | Koorneef et al. (1982) |
| aba1-3 (Ler) | Abscisic acid deficient | −/++ | Koorneef et al. (1982) |
| aba1-4 (Ler) | Abscisic acid deficient | −/++ | Koorneef et al. (1982) |
| abi1-1 (Ler) | Abscisic acid insensitive | −/++ | Koorneef et al. (1984) |
| abi2-1 (Ler) | Abscisic acid insensitive | −/++ | Koorneef et al. (1984) |
| abi3-1 (Ler) | Abscisic acid insensitive | −/++ | Koorneef et al. (1984) |
| abi4 | Abscisic acid insensitive | −/++ | Finkelstein (1994) |
| abi5-1 (Ws) | Abscisic acid insensitive, fewer root hairs | −/++ | Finkelstein (1994) |
| amp1 | Altered meristem program, enhanced level of cyt | −/++ | Chaudhury et al. (1993) |
| aux1-7 | Auxin insensitive, display resistance to various hormones, reduced number of root hairs | −/+ | Pickett et al. (1990) |
| axr1-3 | Auxin resistant, display resistance to various hormones, decreased formation of root hairs | −/++ | Estelle and Sommerville (1987), Lincoln et al. (1990) |
| axr1-12 | Auxin resistant, display resistance to various hormones, very few root hairs | −/+ | Estelle and Sommerville (1987), Lincoln et al. (1990) |
| axr2 | Auxin resistant, display resistance to various hormones, nearly root hairless, cells with multiple root hairs | −/+ | Wilson et al. (1990), Lincoln et al. (1990), Evans et al. (1994) |
| axr4-1 | Auxin resistant, reduced number of root hairs | −/+(+) | Hobbie and Estelle (1995) |
| axr4-2 | Auxin resistant, reduced number of root hairs | −/+(+) | Hobbie and Estelle (1995) |
| aux1-7/axr4-2 | Double mutant, reduced number of root hairs | −/+(+) | Hobbie and Estelle (1995) |
| axr1-3/axr4-2 | Double mutant, reduced number of root hairs | −/+(+) | Hobbie and Estelle (1995) |
| cin1 | Cytokinin insensitive | −/+(+) | Vogel et al. (1998) |
| cin2 | Cytokinin insensitive | −/+(+) | Vogel et al. (1998) |
| ctr1-1 | Constitutive triple response, excessive root hairs, ectopic root hairs | −/++(+) | Kieber et al. (1993) |
| cri1-1 | Cristal-phenotype, cytokinin overaccumulator | −/+ | Santoni et al. (1997) |
| cyr1 | Cytokinin resistant | −/(+) | Deikman and Ulrich (1995) |
| ein2-1 | Ethylene insensitive | −/++ | Guzmán and Ecker (1990), Kieber et al. (1993) |
| ein3-1 | Ethylene insensitive | −/++ | Kieber et al. (1993), Roman et al. (1995) |
| ein4 | Ethylene insensitive | −/++ | Roman et al. (1995) |
| ein5-1 | Ethylene insensitive | −/++ | Roman et al. (1995) |
| ein6 (Ler) | Ethylene insensitive | −/++ | Roman et al. (1995) |
| ein7 | Ethylene insensitive | −/++ | Roman et al. (1995) |
| eir1-1 | Ethylene insensitive root, shoot is sensitive | −/+(+) | Roman et al. (1995) |
| ers1-1 | Ethylene response sensor, ethylene insensitive | −/++ | Hua et al. (1995) |
| eti5 | Ethylene insensitive | −/+(+) | Harpham et al. (1991) |
| eto1 | Ethylene overproducer, increased number of root hairs | −/++(+) | Guzmán and Ecker (1990) |
| eto2 | Ethylene overproducer, increased number of root hairs | −/+++ | Kieber et al. (1993) |
| eto3 | Ethylene overproducer, very much root hairs | −/+++(+) | Kieber et al. (1993) |
| etr1-1 | Ethylene resistant | −/++ | Bleecker et al. (1988), Chang et al. (1993) |
| etr1-3 | Ethylene resistant | −/++ | Bleecker et al. (1988) |
| fs1 (Ler) | Fass, auxin level enhanced | −/+ | Torres-Ruiz and Jürgens (1994)stp1 |
| stp1 | Stunted plant, low cyt response | −/+(+) | Baskin et al. (1995) |
| sur1 | Superroot, auxin overproducer | −/++ | Boerjan et al. (1995) |
| tir3-1 | (Auxin) transport inhibitor response, defect in polar auxin transport | −/++ | Ruegger et al. (1997) |
| Gene | Mutant Phenotype | Root Ferric Reductase Activity +Fe/−Fe | Reference |
|---|---|---|---|
| Root hair mutants | |||
| cpc | Caprice, few irregular root hairs | −/++ | Wada et al. (1997) |
| gl2-1 | Glabra, root hairs on almost every root epidermal cell | −/++(+) | Masucci et al. (1996) |
| rgr1 (Ws) | Reduced root gravitropism, resistant towards inhibitors of polar auxin trans | −/++ | Simmons et al. (1995) |
| gl1-1/rhd2-1 | Double mutant | −/++ | Schiefelbein and Somerville (1990) |
| ttg1 (Ler) | Transparent testa glabra, root hairs on almost every root epidermal cell | −/++(+) | Galway et al. (1994) |
The intensity of the staining by +Fe and −Fe plants is indicated by + and − symbols. Fe-sufficient wild-type roots, showing virtually no activity during the experimental period, was defined as −, the intensity of the reaction of −Fe wild-type and mutant roots was indicated by using the following scale in the order of increasing activity: + < ++ < +++ < ++++. Values in parentheses indicate intermediate levels of intensity.
Plants were grown in a growth chamber on an agar medium as described by Estelle and Somerville (1987). The seeds were surface-sterilized by immersing them in 96% ethanol for 7 min and 30% (v/v) NaOCl for 10 min, followed by four rinses in sterile distilled water. The medium was composed of 5 mm KNO3, 2 mm MgSO4, 2 mm Ca(NO3)2, 2.5 mm K2PO4, 70 μm H3BO3, 14 μm MnCl2, 1 μm ZnSO4, 0.5 μm CuSO4, 10 μm NaCl, 0.2 μm Na2MoO4, solidified with 0.7% agar. Suc (43 mm) and 2-(N-morpholino)-ethanesulfonic acid (MES; 4.7 mm) were included and the pH was adjusted to 6.0. Seeds were placed onto Petri dishes containing agar medium and kept for 3 d at 4°C in the dark, before the plates were transferred to a growth chamber and grown at 21°C in continuous light (150 μmol m−2 s−1, TL lamps, Philips, Eindhoven, The Netherlands). After 10 d, plants were grown for an additional 4 d either with 40 μm FeEDTA (+Fe plants) or without Fe and 100 μm 3-(2-pyridyl)-5,6-diphenyl-1,2,4-triazine sulfonate (FerroZine) (−Fe plants). 2,4-D was added to the medium after autoclaving from a stock dissolved in ethanol. ACC was added after autoclaving the medium.
Fe(III) Chelate Reductase
Root ferric reductase was quantified using FerroZine to chelate the reduced Fe. Spatial localization of Fe(III) reductase was determined by embedding the roots of seedlings in an agar (0.7%, w/v) medium containing 0.5 mm CaSO4, 0.5 mm FerroZine, and 0.5 mm FeEDTA for 20 min. Quantitative determination of root reduction activity was performed as described previously (Schmidt, 1994), except that FerroZine was used instead of bathophenanthrolinedisulfonate as a ferrous indicator. Reduction activity was measured by following the changes in A562. Reduction rates were calculated using an extinction coefficient of 25,200 m−1 cm−1.
Activity staining for ferric chelate reductase was performed for +Fe and −Fe plants of each genotype, with three independent runs with three plants per plate. The concentration of the agar medium allowed the removal of plants without any damage to root epidermal cells.
Microscopy
Analysis of root hair patterns was performed by light microscopy with and without differential interference contrast optics. Photomicrographs were recorded on Kodak 100 Gold negative film (Eastman-Kodak, Rochester, NY).
Electron Microscopy
Roots were cut into approximately 1-cm-long segments and then washed in 0.5 mm CaSO4. The segments were fixed overnight in 0.1 m potassium phosphate buffer, pH 7.4, containing 0.2% (w/v) glutaraldehyde and 1.5% (w/v) paraformaldehyde. After being rinsed three times in 0.1 m potassium phosphate buffer, pH 7.4, the tissue was dehydrated through a graded ethanol series of 20%, 40%, and postfixed in 0.25% (w/v) osmium tetroxide for 2 h in 40% ethanol at 4°C. Root segments were washed again in 40% ethanol (three times for 10 min) and treated in a solution of 0.3% (w/v) uranyl acetate in 40% ethanol for 2 h at 4°C. The material was washed again twice in 40% ethanol (10 min each) and once in 50% ethanol for 10 min. The samples were then dehydrated in an ethanol series of 75%, 90%, and two times 100% for 30 min each, infiltrated with LR White resin, and polymerized at 50°C for 24 h in vacuo.
Ultrathin sections were cut with a microtome (Ultracut E, Reichert, Vienna) and stained with uranyl acetate and lead citrate. Semithin sections were stained with toluidine blue. Sections used for electron microscopy were examined in an electron microscope (EM 902, Zeiss, Jena, Germany).
RESULTS
Fe Deficiency Affects Peculiarities and Patterning of Root Hairs
The patterns of root hairs differ among the genotypes under investigation. Under ordinary conditions, all auxin-related mutants and one of the ABA mutants, abi5-1, developed fewer root hairs than the wild type (Table I). An increased number of root hairs was observed in the ctr1 and eto mutants. In wild-type roots and most of the tested mutants, root hairs were almost exclusively located over anticlinal walls between adjacent cortical cells (Fig. 1). Ectopic hairs, i.e. hairs located over periclinal cortical cell walls, formed in the eto, ctr1, gl2, and ttg mutants. In gl2 and ttg, hairs were formed on almost every root epidermal cell. The TTG and GL2 genes are thought to be positive regulators of non-hair cell fate (Schiefelbein et al., 1997, and refs. therein).
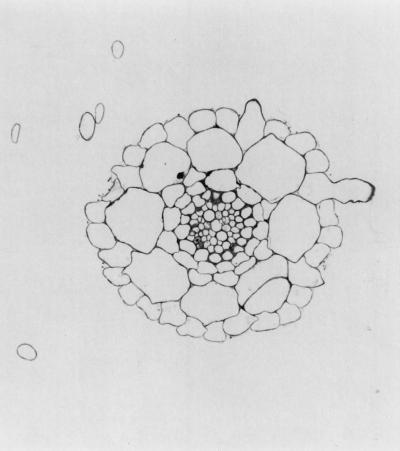
Figure 1.
Cross-section of an Fe-sufficient Arabidopsis root. Root hairs are located over anticlinal cortical cell walls. This section was stained with toluidine blue.
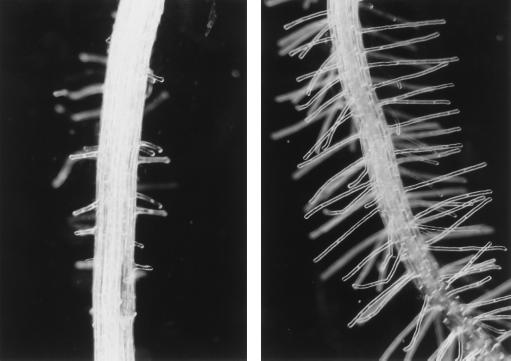
Similar to other species, Fe deficiency substantially increased both the length and number of hairs in Arabidopsis roots (Fig. 2). This response was already evident 24 h after the onset of Fe-deficient conditions. Root hair formation was increased in all of the mutants investigated, including the eto genotypes, which are densely covered with root hairs even under ordinary conditions. In the gl2 and ttg1 mutants, growth in Fe-free medium caused an elongation of root hairs. An extension of root hairs was also observed in the axr2 mutant, which had almost no hair-bearing cells at adequate Fe supply. In Fe-deficient plants, some extra root hairs in ectopic locations were observed, suggesting that ethylene might be involved in Fe-status-dependent patterning of root hairs (Figs. 3 and 7). Applying the ethylene precursor ACC exogenously to Fe-sufficient wild-type plants resulted in an increased formation of root hairs. Compared with those induced by Fe deficiency, ACC-induced root hairs were shorter and the root hair zone differed in shape. While in Fe-deficient roots, the length of the root hairs increased continuously from the apex, ACC-treated plants exhibited a nearly inverse pattern (Fig. 4). A similar shape of root hair formation was evident in the eto3 mutant. A small number of double root hairs was noted in roots of Fe-deficient plants, a feature characteristic of the auxin- and ethylene-resistant axr2 mutant (data not shown).
Figure 2.
Fe-Deficiency-induced alterations in root epidermis development. Left, Control root. Right, Root hairs of Fe-deficient roots after 4 d of −Fe treatment.
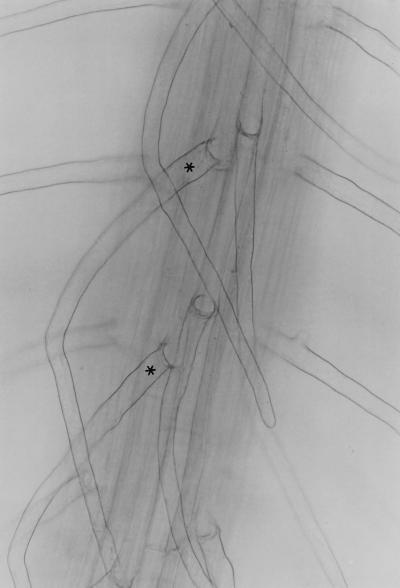
Figure 3.
Fe-deficiency-induced formation of root hairs in ectopic positions (*). Wild-type seedlings were grown for 4 d in Fe-free medium.
Figure 7.
A, Cross-sectioned root hair from an Fe-deficient plant; magnification ×13,860. B, Cross-section of epidermal cells of Fe-deficient roots. Note the root hair cell in ectopic position; magnification ×5,800.
Figure 4.
ACC-induced formation of root hairs. Wild-type seedlings were grown for 4 d on medium supplemented with 50 μm ACC.
Defects in Hormone Metabolism Do Not Inhibit the Induction of Fe(III) Reductase
Root-mediated reduction of mutants was estimated by an arbitrary scale (Table I). This scale was calibrated using the Fe(III) chelate reductase activity of wild-type roots as a standard. The visualization method was used instead of quantitative determination to avoid incorrect results due to a non-linear relationship between reduction activity and unit root weight, which was observed when assaying mutants with extremely small root systems. A further advantage of this method is the ability to examine the pattern of reduction activity in the various mutants. A visualization of ferric reduction by +Fe and −Fe plants is shown in Figure 5. No major deviations from this pattern of reduction activity was observed in the mutant ecotypes under investigation. Quantitative analysis of Fe chelate reductase activity of the col-0 ecotype revealed a more than 10-fold increase in reduction rate by Fe deficiency (i.e. from 0.38 ± 0.08 to 4.36 ± 0.67 μmol g−1 fresh weight h−1).
Figure 5.
Visualization of ferric reduction activity of Fe-sufficient (A) and Fe-deficient (B) Arabidopsis roots. The resulting Fe(II) is trapped by FerroZine to produce a red product.
When grown under Fe-deficient conditions, Fe(III) chelate reductase activity was up-regulated in all genotypes under investigation (Table I). However, a number of mutants displayed either a more or a less pronounced reaction compared with the wild type. Interestingly, most of the cytokinin-related mutants exhibited a slightly (cin1 and cin2) or markedly (cyr1 and cri1) reduced coloration of the test assay. Since the cin mutations negatively affect cytokinin-induced ethylene production (Kakimoto, 1998), the response of the cin1 and cin2 mutants might be interpreted in terms of an involvement of ethylene in the induction of ferric chelate reductase activity. However, amp1, which is characterized by an enhanced cytokinin level, was indistinguishable from the wild type with respect to root reduction induced by Fe deficiency. The extremely small root system developed by the cri1 and cyr1 mutants, which may lead to an underestimation of the activity in the agar plates, is a more plausible explanation for the observed effects. This conclusion may also apply to stp1, which also possesses an abbreviated root system.
Of the auxin mutants, those exhibiting a reduced number of root hairs (aux1, axr1, axr4, and aux/axr double mutants) exhibited only a slightly increased reductase activity when grown under −Fe conditions. This was particularly true for axr2 roots, which almost lack hairs when grown under adequate Fe conditions. Neither the tir3 mutant (with a defect in polar auxin transport) nor sur1 (with elevated free indole-3-acetic acid levels) showed marked deviations from the wild type, suggesting that auxin is not important for the regulation of ferric reduction. Decreased activity was also observed in fs1, which displays enhanced auxin levels. As in the case of the cytokinin-related mutants, this may be explained by the phenotype of this mutant, which has a very small root system. It should be noted that aux and axr mutants display resistance to various hormones.
Concerning the ethylene mutants, those altered in ethylene sensitivity, such as ein, ers1, and etr1, showed no differences from the wild type with regard to either morphological or physiological responses to low Fe. A slightly less intense staining was noted in the insensitive mutants eir1 and eti5. In eir1, fewer root hairs shorter than those of wild-type plants under −Fe conditions developed. All ethylene-overproducing eto mutants and ctr1, which displays a constitutive ethylene response, exhibited a more pronounced Fe-stress reaction. This was true, in particular, for eto3, which was characterized by extremely dense hairs in both growth types (+Fe and −Fe). A slightly higher stress response was also observed in the root hair mutants gl2 and ttg, which possessed hairs on nearly all epidermal cells. No significant deviations from the wild type were noted for the ABA mutants (aba and abi).
Fe Deficiency Does Not Induce the Formation of Transfer Cells in Arabidopsis Roots
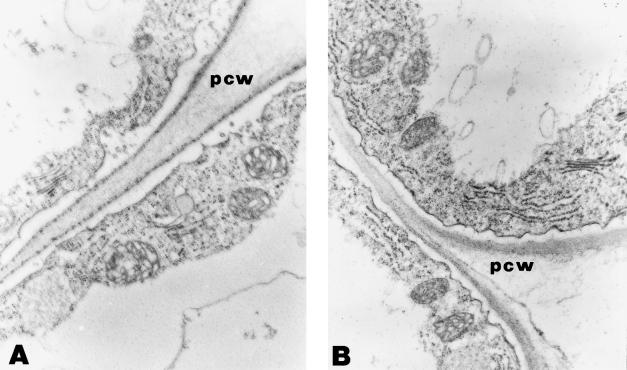
The differentiation of transfer cells in the rhizodermis is a common response of strategy I plants to suboptimal Fe availability (Landsberg, 1982, 1994; Schmidt and Bartels, 1996). This cell type is characterized by ingrowths of the cell wall resulting in an enhanced surface-area-to-volume ratio. Enrichment of mitochondria and smaller vacuoles relative to ordinary root epidermal cells are additional peculiarities of transfer cells. Representative micrographs of rhizodermal cells in the root hair zone of +Fe and −Fe roots are shown in Figure 6. Epidermal cells of both growth types are highly vacuolated. Roots subjected to Fe deficiency did not reveal any major structural differences and exhibited no transfer-cell-like protuberances. Serial sectioning confirmed that transfer cells were neither formed in the meristematic zone nor in the proximal root part. Like non-hair epidermal cells, root hairs of Fe-deficient Arabidopsis plants did not differentiate into transfer cells nor show any wall ingrowths (Fig. 7). Furthermore, wall ingrowths were not observed in ACC- or 2,4-D-treated roots (data not shown).
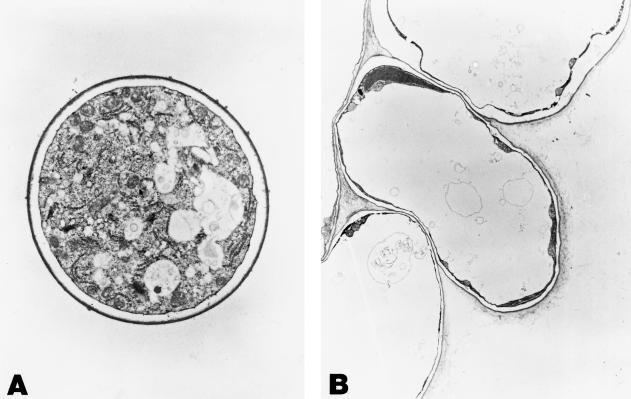
Figure 6.
Epidermal cells of control (A) and Fe-deficient (B) roots; magnification ×28,560. pcw, Peripheral cell wall.
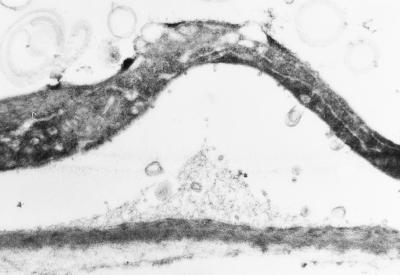
In some cases appositions of secondary wall material were observed in epidermal cells of −Fe roots (Fig. 8). In no case was the occurrence of these structures coupled with other characteristics of transfer cells.
Figure 8.
Cell wall apposition in an Fe-deficient epidermal cell; magnification ×43,400.
DISCUSSION
Role of Hormones in Fe-Deficiency-Induced Root Hair Formation
Increased root hair density is a common response of higher plants to low Fe supply (Schmidt, 1999). Such morphological alterations are also evident in Fe-deficient Arabidopsis roots, indicating that, despite the regular pattern of hair-forming and hairless cells in the root epidermis, cell fate is influenced by environmental Fe availability. In Arabidopsis, root hairs are formed in a position-dependent pattern relative to the underlying cortical cells (Schiefelbein et al., 1997). In wild-type roots, hair-forming cells are always located over the anticlinal walls of two underlying cortical cells. Ectopic hairs, i.e. hairs over periclinal cortical cell walls, are formed in roots of ctr1 and in the eto mutants, due to increased ethylene synthesis (Table I; Kieber, 1997). In −Fe-grown plants root hairs are present at the same relative position, implying that ethylene is involved in the regulation of morphological changes associated with the adaptation to low Fe availability. A role for ethylene can also be inferred from the fact that root appearance under Fe stress can be mimicked by supplementing the medium with the ethylene precursors ACC or ethephon (Romera and Alcántara, 1994; Landsberg, 1996; this study). Similar to −Fe conditions, ACC treatment causes the formation of ectopic root hairs (Tanimoto et al., 1995; Landsberg, 1996).
Since exogenously applied auxin also mimics the morphology of Fe-deficient roots (Landsberg, 1996; Schmidt and Bartels, 1996), a role for auxin in the induction of morphological alterations in response to low Fe availability may be inferred. The root-hair-less axr2 mutant forms root hairs under both high exogenous auxin (Wilson et al., 1990) and low-phosphorus media (Bates and Lynch, 1996). This holds also true for growth under −Fe conditions, although the main response under the latter conditions is root hair elongation rather than an increase in number. Whether auxin acts directly or indirectly via an increase in ethylene production cannot be deduced from the data. Interestingly, ethylene levels are enhanced under both −Fe and −P conditions, suggesting similarities in the transduction sequence (Lynch, 1998; Romera et al., 1999).
Role of Hormones in the Induction of Fe(III) Chelate Reductase Activity
All surveyed mutants appeared to be capable of responding to Fe-deficiency stress by elevated Fe(III) chelate reductase activity (Table I). The intensity of staining produced by most −Fe-grown mutants was indistinguishable from the wild type, suggesting that hormones are not required for the induction of reduction activity. Mutants exhibiting deviations from the wild type are further characterized by alterations in length and density of root hairs. This was particularly evident for axr2, which has almost no root hairs and displayed a small increase in reduction activity, and eto3, which represents the other extreme with respect to root hair density and intensity of reductase staining (Table I). Thus, reductase activity seems to be associated with root surface area rather than with alterations in hormone levels or sensitivity. Exceptions to this pattern were only noted in cases where the mutation causes a highly reduced root system (e.g. cri1, cyr1, and stp1).
These findings support a model in which, unlike the morphological changes, induction of the physiological responses to Fe deficiency stress are not mediated by hormones. This model is supported by the normal Fe stress response of ethylene insensitive mutants (ein2–ein7 and etr) and other hormone-related mutants. The results presented here are at variance with those reported by Romera et al. (1994). However, while the data of the present study do exclude an absolute requirement of hormones for the induction of physiological responses to Fe deficiency, they do not exclude that ethylene can act as enhancer in the transduction of environmental stimuli. Such an enhancer function might be inferred from the relatively low Fe-stress-induced increase in activity of glabra and ttg phenotypes and the normal response of the caprice mutants, which possess fewer root hairs than the wild type under −Fe conditions (Table I).
An essential function for auxin in the induction of ferric reductase activity seems unlikely with regard to the present data. Since inhibitors of polar auxin transport have been suggested to interfere with the induction of Fe-deficiency-induced responses (Landsberg, 1982), the normal response of tir3-1, a defect in polar auxin transport, is of particular interest and can be interpreted in this way: The staining of the medium by −Fe-grown roots of the auxin overproducer sur1 is indistinguishable from the wild type, which may be judged as further evidence against a possible role of auxin in the regulation of root reduction activity.
Function of Root Hairs in Fe Reduction
Determinations of Fe(III) chelate reductase activity in vivo are usually based on a root fresh weight basis (for an overview, see Moog and Brüggemann, 1994). This is also true for the half-quantitative visualization of root-mediated Fe(III) reduction in agar medium used in the present study. Since the reduction activity is restricted to young, non-lignified root zones, it may be assumed that an increase in surface area by enhanced root hair formation increases the reduction of Fe(III) in the medium. The present results appear to support this assumption. Further evidence comes from a study by Moog et al. (1995) showing that the activity of wild-type Arabidopsis roots was about 2-fold higher than the root-hair-less mutant RM57. With respect to the temporal pattern of induction of the Fe stress syndrome in Arabidopsis, the occurrence of extra root hairs always preceded reduction activity and the development of chlorosis symptoms. Fe-deficiency-induced root hair formation may therefore be regarded as a means to enhance the physiological reactions, and not as a secondary effect of chlorosis, as suggested by Chaney et al. (1992). It should be noted, however, that the present data do not support the idea that the formation of root hairs is a prerequisite for the development of enhanced ferric reduction activity. Even mutants with extremely reduced surface area, e.g. axr2, showed this response under the conditions in our study.
Function of Rhizodermal Transfer Cells in Fe Uptake
The function of transfer cells in the rhizodermis has mainly been inferred from their position and structural features. Aside from the formation of root hairs, the labyrinth-like cell wall of rhizodermal transfer cells provides a possibility to increase the absorptive surface of the root. Epidermal cells in Fe-deficient and ACC-treated roots were indistinguishable from Fe-sufficient control roots, indicating that Arabidopsis cannot initiate transfer cells in the root epidermis. This finding supports our assumption that transfer cells are not necessary for root-mediated reduction of Fe(III) chelates (Schmidt and Bartels, 1996). Appositions of cell wall material have been observed exclusively in −Fe roots (Fig. 8). Since the enlargement of the absorptive surface is minor, an adaptive nature of these alterations is not presumed. It is interesting that some plants are genetically equipped to form transfer cells under −Fe conditions while others are not. The fine root system of Arabidopsis may be sufficient with respect to the surface available for the uptake of Fe, and therefore it is tempting to speculate that the formation of extra root hairs and transfer cell differentiation represents alternative strategies. More data are necessary to verify this assumption.
CONCLUSIONS
Our data strongly suggest that ethylene (and possibly auxin) mediates Fe-deficiency-induced root hair formation. Evidence for this assumption comes from the −Fe-like phenotype of roots treated with ACC and the ectopically placed extra root hairs formed under −Fe conditions, ACC treatment, and in ethylene-overproducing mutant seedlings. The root hairs induced by Fe deficiency may act as an enhancer for the reduction of ferric Fe, but this does not represent a prerequisite for physiological adaptations. The results described here also indicate that hormones are not required for an increase of Fe(III) chelate reductase activity. We interpret our results to mean that morphological peculiarities induced by Fe deficiency are regulated independently of physiological responses.
ACKNOWLEDGMENTS
We thank J. Kieber, C. Bellini, K. Okada, E.M. Meyerowitz, M.A. Hall, B. Bartel, D. Söll, M. van Montagu, M. Estelle, and the Arabidopsis Biological Resource Center at Ohio State University for kindly providing the Arabidopsis mutants used in this work. We thank also Prof. W. Eber (University of Oldenburg) for allowing us to use the microscopes in his laboratory.
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft.
LITERATURE CITED
- Baskin TI, Cork A, Williamson RE, Gorst JR. STUNTED PLANT1, a gene required for expansion in rapidly elongating but not in dividing cells and mediating root growth responses to applied cytokinin. Plant Physiol. 1995;107:233–243. doi: 10.1104/pp.107.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates TR, Lynch JP. Stimulation of root hair elongation in Arabidopsis thaliana by low phosphorus availability. Plant Cell Environ. 1996;19:529–538. [Google Scholar]
- Bleecker AB, Estelle MA, Somerville C, Kende H. Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science. 1988;241:1086–1089. doi: 10.1126/science.241.4869.1086. [DOI] [PubMed] [Google Scholar]
- Boerjan W, Cervera M-T, Delarue M, Beeckman T, Dewitte W, Bellini C, Caboche M, Van Onckelen H, Van Montagu M, Inzé D. superroot, a recessive mutation in Arabidopsis, confers auxin overproduction. Plant Cell. 1995;7:1405–1419. doi: 10.1105/tpc.7.9.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaney RL, Chen Y, Green CE, Holden MJ, Bell PF, Luster DG, Angle JS. Root hairs on chlorotic tomatoes are an effect of chlorosis rather than part of the adaptive Fe-stress-response. J Plant Nutr. 1992;15:1857–1875. [Google Scholar]
- Chang C, Kwok S, Bleeker AB, Meyerowitz EM. Arabidopsis ethylene-response gene ETR1: similarity of product to two-component regulators. Science. 1993;262:539–544. doi: 10.1126/science.8211181. [DOI] [PubMed] [Google Scholar]
- Chaudhury AM, Letham S, Craig S, Dennis ES. amp1: a mutant with high cytokinin levels and altered embryonic pattern, faster vegetative growth, constitutive photomorphogenesis and precocious flowering. Plant J. 1993;4:907–916. [Google Scholar]
- Deikman J, Ulrich M. A novel cytokinin-resistant mutant of Arabidopsis with abbreviated shoot development. Planta. 1995;195:440–449. doi: 10.1007/BF00202603. [DOI] [PubMed] [Google Scholar]
- Eide D, Broderius M, Feit J, Guerinot ML. A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc Natl Acad Sci USA. 1996;93:5624–5628. doi: 10.1073/pnas.93.11.5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estelle MA, Somerville C. Auxin-resistant mutants of Arabidopsis thaliana with an altered morphology. Mol Gen Genet. 1987;206:200–206. [Google Scholar]
- Evans ML, Ishikawa H, Estelle MA. Responses of Arabidopsis roots to auxin studied with high temporal resolution: comparison of wild type and auxin-response mutants. Planta. 1994;194:215–222. [Google Scholar]
- Finkelstein RP. Mutations at two new Arabidopsis ABA response loci are similar to the abi3 mutations. Plant J. 1994;5:765–771. [Google Scholar]
- Fox TC, Shaff JE, Grusak MA, Norvell WA, Chen Y, Chaney RL, Kochian LV. Direct measurement of 59labeled Fe2+ influx in roots of Pisum sativum using a chelator buffer system to control free Fe2+ in solution. Plant Physiol. 1996;111:93–100. doi: 10.1104/pp.111.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galway ME, Masucci JD, Lloyd AM, Walbot V, Davis RW, Schiefelbein JW. The TTG gene is required to specify epidermal cell fate and cell patterning in the Arabidopsis root. Dev Biol. 1994;166:740–754. doi: 10.1006/dbio.1994.1352. [DOI] [PubMed] [Google Scholar]
- Guzmán P, Ecker JR. Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell. 1990;2:513–523. doi: 10.1105/tpc.2.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpham NVJ, Bery AW, Knee EM, Roveda-Hoyos G, Raskin I, Sanders IO, Smith AR, Wood CK, Hall MA. The effect of ethylene on the growth and development of wild-type and mutant Arabidopsis thaliana (L.) Heynh. Ann Bot. 1991;68:55–61. [Google Scholar]
- Hobbie L, Estelle M. The axr4 auxin-resistant mutants of Arabidopsis thaliana define a gene important for root gravitropism and lateral root initiation. Plant J. 1995;7:211–220. doi: 10.1046/j.1365-313x.1995.7020211.x. [DOI] [PubMed] [Google Scholar]
- Hua J, Chang C, Sun Q, Meyerowitz EM. Ethylene insensitivity conferred by Arabidopsis ERS gene. Science. 1995;269:1712–1714. doi: 10.1126/science.7569898. [DOI] [PubMed] [Google Scholar]
- Kakimoto T. Cytokinin signaling. Curr Opin Plant Biol. 1998;1:399–403. doi: 10.1016/s1369-5266(98)80263-x. [DOI] [PubMed] [Google Scholar]
- Kieber JJ. The ethylene signal transduction pathway in Arabidopsis. J Exp Bot. 1997;48:211–218. doi: 10.1093/jxb/48.2.211. [DOI] [PubMed] [Google Scholar]
- Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR. CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the Raf family of protein kinases. Cell. 1993;72:427–441. doi: 10.1016/0092-8674(93)90119-b. [DOI] [PubMed] [Google Scholar]
- Koorneef M, Jorna ML, Brinkhorst-van der Swan DLC, Karssen CM. The isolation of abscisic acid (ABA) deficient mutants by selection of induced revertants in non-germinating gibberellin sensitive lines of Arabidopsis thaliana (L.) Heynh. Theor Appl Genet. 1982;61:385–393. doi: 10.1007/BF00272861. [DOI] [PubMed] [Google Scholar]
- Koorneef M, Reutling G, Karssen CM. The isolation and characterization of abscisic acid-insensitive mutants of Arabidopsis thaliana. Physiol Plant. 1984;61:377–383. [Google Scholar]
- Landsberg EC. Transfer cell formation in the root epidermis: a prerequisite for Fe-efficiency? J Plant Nutr. 1982;5:415–432. [Google Scholar]
- Landsberg EC. Transfer cell formation in sugar beet roots induced by latent Fe deficiency. Plant Soil. 1994;165:197–205. [Google Scholar]
- Landsberg EC. Hormonal regulation of iron-stress response in sunflower roots: a morphological and cytological investigation. Protoplasma. 1996;194:69–80. [Google Scholar]
- Lincoln C, Britton JH, Estelle M. Growth and development of the axr1 mutants of Arabidopsis. Plant Cell. 1990;2:1071–1080. doi: 10.1105/tpc.2.11.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP. Root architecture and phosphorus acquisition efficiency in common bean. In: Flores HE, Lynch JP, Eisenstat D, editors. Radical Biology: Advances and Perspectives on the Function of Plant Roots. Rockville, MD: American Society of Plant Physiologists; 1998. pp. 81–91. [Google Scholar]
- Masucci JD, Rerie WG, Foreman DR, Zhang M, Galway ME, Marks DM, Schiefelbein JW. The homeobox gene GLABRA2 is required for position dependent cell differentiation in the root epidermis of Arabidopsis thaliana. Development. 1996;122:1253–1260. doi: 10.1242/dev.122.4.1253. [DOI] [PubMed] [Google Scholar]
- Masucci JD, Schiefelbein JW. The rhd6 mutation of Arabidopsis thaliana alters root-hair initiation through an auxin- and ethylene-associated process. Plant Physiol. 1994;106:1335–1346. doi: 10.1104/pp.106.4.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masucci JD, Schiefelbein JW. Hormones act downstream of TTG and GL2 to promote root hair outgrowth during epidermis development in the Arabidopsis root. Plant Cell. 1996;8:1505–1517. doi: 10.1105/tpc.8.9.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moog PR, Brüggemann W. Iron reductase systems on the plant plasma membrane: a review. Plant Soil. 1994;165:241–260. [Google Scholar]
- Moog PR, van der Kooij TAW, Brüggemann W, Schiefelbein JW, Kuiper PJC. Responses to iron deficiency in Arabidopsis thaliana: the turbo iron reductase does not depend on the formation of root hairs and transfer cells. Planta. 1995;195:505–513. doi: 10.1007/BF00195707. [DOI] [PubMed] [Google Scholar]
- Morgan PW, Hall WC. Effect of 2,4-dichlorophenoxyacetic acid on the production of ethylene by cotton and grain sorghum. Physiol Plant. 1962;15:420–427. [Google Scholar]
- Pickett FB, Wilson AK, Estelle M. The aux1 mutation of Arabidopsis confers both auxin and ethylene resistance. Plant Physiol. 1990;94:1462–1466. doi: 10.1104/pp.94.3.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson NJ, Procter CM, Conolly EL, Guerinot ML. A ferric-chelate reductase for iron uptake from soils. Nature. 1999;397:694–697. doi: 10.1038/17800. [DOI] [PubMed] [Google Scholar]
- Roman G, Lubarsky B, Kieber JJ, Rothenberg M, Ecker JR. Genetic analysis of ethylene signal transduction in Arabidopsis thaliana: five novel mutant loci integrated into a stress response pathway. Genetics. 1995;139:1393–1409. doi: 10.1093/genetics/139.3.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romera FJ, Alcántara E. Iron-deficiency stress responses in cucumber (Cucumis sativus L.) roots: a possible role for ethylene? Plant Physiol. 1994;105:1133–1138. doi: 10.1104/pp.105.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romera FJ, Alcántara E, De la Guardia MD. Ethylene production by Fe-deficient roots and its involvement in the regulation of Fe-deficiency stress responses by strategy I plants. Ann Bot. 1999;83:51–55. [Google Scholar]
- Römheld V, Marschner H. Evidence for a specific uptake system for iron phytosiderophores in roots of grasses. Plant Physiol. 1986a;80:175–180. doi: 10.1104/pp.80.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römheld V, Marschner H. Mobilization of iron in the rhizosphere of different plant species. In: Tinker B, Läuchli A, editors. Advances in Plant Nutrition. Vol. 2. New York: Praeger; 1986b. pp. 155–204. [Google Scholar]
- Rosenfield CL, Reed DW, Kent MW. Dependency of iron reduction on development of a unique root morphology in Ficus benjamina L. Plant Physiol. 1991;95:1120–1124. doi: 10.1104/pp.95.4.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruegger M, Dewey E, Hobbie L, Brown D, Bernasconi P, Turner J, Muday G, Estelle M. Reduced naphthylphthalamic acid binding in the tir3 mutant of Arabidopsis is associated with a reduction in polar auxin transport and diverse morphological defects. Plant Cell. 1997;9:745–757. doi: 10.1105/tpc.9.5.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoni V, Delarue M, Caboche M, Bellini C. A comparison of two-dimensional electrophoresis data with phenotypical traits in Arabidopsis leads to the identification of a mutant (cri1) that accumulates cytokinins. Planta. 1997;202:62–69. doi: 10.1007/s004250050103. [DOI] [PubMed] [Google Scholar]
- Schiefelbein JW, Masucci JD, Wang H. Building a root: the control of patterning and morphogenesis during root development. Plant Cell. 1997;9:1089–1098. doi: 10.1105/tpc.9.7.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefelbein JW, Somerville C. Genetic control of root hair development in Arabidopsis thaliana. Plant Cell. 1990;2:235–243. doi: 10.1105/tpc.2.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt W. Effects of various inhibitors on in vivo reduction by Plantago lanceolata L. roots. Plant Soil. 1994;165:207–212. [Google Scholar]
- Schmidt W. Mechanisms and regulation of reduction-based iron uptake in plants. New Phytol. 1999;141:1–26. [Google Scholar]
- Schmidt W, Bartels M. Formation of root epidermal transfer cells in Plantago. Plant Physiol. 1996;110:217–225. doi: 10.1104/pp.110.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons C, Migliaccio F, Masson P, Caspar T, Söll D. A novel root gravitropism mutant of Arabidopsis thaliana exhibiting altered auxin physiology. Physiol Plant. 1995;93:790–798. [PubMed] [Google Scholar]
- Smalle J, van der Straeten D. Ethylene and vegetative development. Physiol Plant. 1997;100:593–605. [Google Scholar]
- Tanimoto M, Roberts K, Dolan L. Ethylene is a positive regulator of root hair development in Arabidopsis thaliana. Plant J. 1995;8:943–948. doi: 10.1046/j.1365-313x.1995.8060943.x. [DOI] [PubMed] [Google Scholar]
- Torres-Ruiz R, Jürgens G. Mutations in the FASS gene uncouple pattern formation and morphogenesis in Arabidopsis development. Development. 1994;120:2967–2978. doi: 10.1242/dev.120.10.2967. [DOI] [PubMed] [Google Scholar]
- Toulon V, Sentenac H, Thibaud JB, Davidian JC, Moulineaz C, Grignon C. Role of apoplast acidification by the H+ pump: effect on the sensitivity to pH and CO2 of iron reduction by roots of Brassica napus L. Planta. 1992;186:212–218. doi: 10.1007/BF00196250. [DOI] [PubMed] [Google Scholar]
- Vogel JP, Schuerman P, Woeste K, Brandstater I, Kieber JJ. Isolation and characterization of Arabidopsis mutants defective in the induction of ethylene biosynthesis by cytokinin. Genetics. 1998;149:417–427. doi: 10.1093/genetics/149.1.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada T, Tachibana T, Shimura Y, Okada K. Epidermal cell differentiation in Arabidopsis determined by a Myb homolog, CPC. Science. 1997;277:1113–1116. doi: 10.1126/science.277.5329.1113. [DOI] [PubMed] [Google Scholar]
- Welch RM, Norvell WA, Gesuwan P, Schaefer S. Possible role of root-ethylene in Fe(III)-phytometallophore uptake in strategy II species. Plant Soil. 1997;196:229–232. [Google Scholar]
- Wilson AK, Pickett FB, Turner JC, Estelle M. A dominant mutation in Arabidopsis confers resistance to auxin, ethylene and abscisic acid. Mol Gen Genet. 1990;222:377–383. doi: 10.1007/BF00633843. [DOI] [PubMed] [Google Scholar]