Classically, polymorphonuclear neutrophils (PMNs) are viewed as functioning to kill invading microorganisms via phagocytosis, reactive oxygen species, antimicrobial peptides, and neutrophil extracellular traps. However, accumulating evidence indicates that PMNs can also function to modulate the adaptive immune system (1). In fact, as with many cell types, there is increasing recognition of the heterogeneity of neutrophils, with distinct subsets performing specific functions. One subset, consisting of PMN myeloid-derived suppressor cells (PMN-MDSCs), emerges in pathologic conditions and functions to suppress T-cell proliferation and activation. By virtue of T-cell suppression, PMN-MDSCs modulate tumor immunity, autoimmune disease, and infection/sepsis (2–5). PMN-MDSCs are characterized by increased expression of CD11b, arginase 1, and reactive oxygen and nitrogen species, which mediate the suppressor effect (2, 3, 6, 7). Pillay and colleagues previously demonstrated that in humans exposed to endotoxin, a population of CD11bhi PMN-MDSCs emerged and suppressed T-cell proliferation (8).
During influenza, T cells become activated, proliferate, and differentiate into effector cells, including cytotoxic CD8 cells, which kill virus-infected cells, resulting in viral clearance but also weight loss and lung injury (9–11). Notably, MDSCs emerge in mice and humans during influenza infection (12).
In the context of these findings, Tak and colleagues (pp. 492–499) hypothesized that CD11b+ neutrophils may mitigate lung injury in influenza by suppressing T-cell proliferation. In this issue of the Journal, they demonstrate that Cd11b−/− mice suffer increased weight loss and lung permeability after influenza infection, accompanied by elevated T-cell counts in the lung with no difference in viral clearance (13). This enhanced lung injury appeared to be mediated by T cells, as T-cell depletion rescued the phenotype, restoring weight loss and lung permeability to the levels observed in wild-type mice. Finally, adoptive transfer of Cd11b+/+, but not Cd11b−/−, neutrophils into Cd11b−/− mice also mitigated lung injury with a trend toward diminished T-cell proliferation.
This simple yet well-executed study supports the conclusion that CD11b+ neutrophils suppress T-cell proliferation, in turn limiting lung injury, during influenza infection in mice (Figure 1). This finding is an important contribution to the field of myeloid-cell suppression of adaptive immunity. However, several questions remain unanswered.
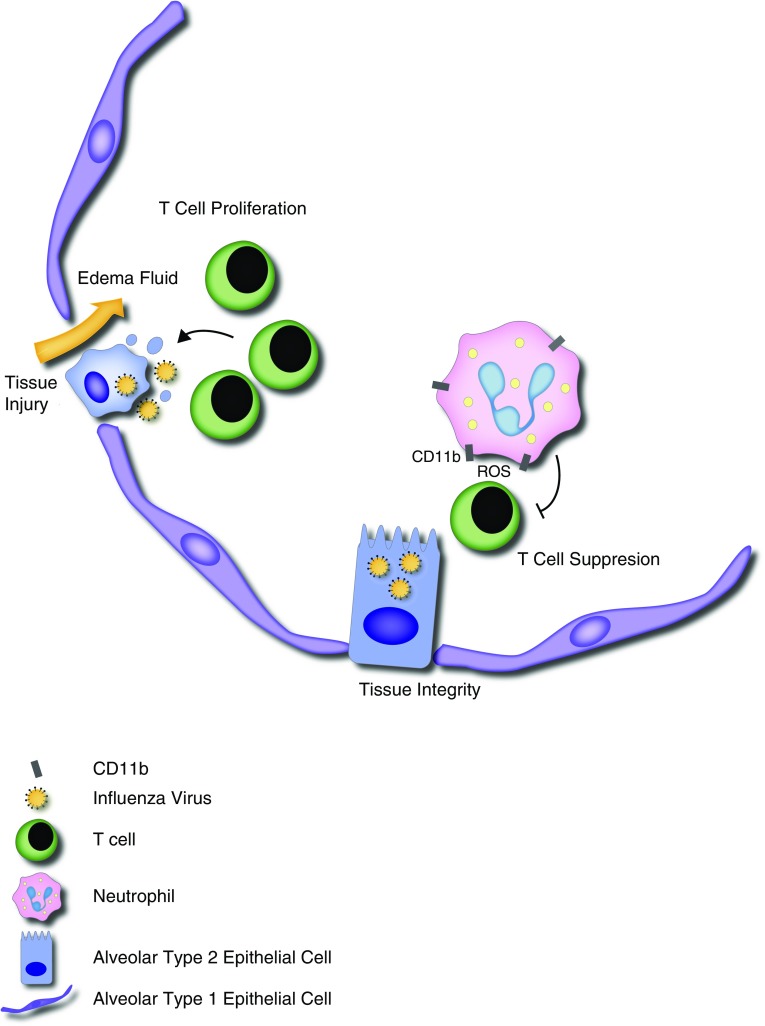
Figure 1.
During influenza infection, viral particles infect epithelial cells. In response to antigen presentation, T cells become activated, proliferate, and differentiate into effector cells, including cytotoxic CD8 cells, which kill virus-infected cells, resulting in clearance of the virus but also breach of the lung barrier, resulting in permeability and the influx of edema fluid into the airspaces. In their current study, Tak and colleagues (13) demonstrate that neutrophils inhibit T-cell responses in a CD11b-dependent manner. The mechanism of T-cell suppression by CD11b+ neutrophils during influenza infection is still unknown. However, a prior study by this group demonstrated that CD11b-dependent release of reactive oxygen species (ROS) into the immunologic synapse between neutrophils and T cells mediated T-cell suppression (8). Other mechanisms of T-cell suppression by myeloid-derived suppressor cells include arginine depletion and release of reactive nitrogen species (2). Neutrophil-mediated T-cell suppression may be critical for limiting tissue damage but may also entail immunosuppression, resulting in vulnerability to secondary infections.
First, it is unclear whether the CD11b+ neutrophils that suppress T-cell–mediated lung injury in the murine model of influenza are PMN-MDSCs. By definition, PMN-MDSCs are rare or absent in health but emerge during cancer or inflammation, and are characterized by immature nuclear morphology and increased CD11b expression (3, 4, 6). Although no specific markers distinguish murine PMN-MDSCs from mature, terminally differentiated neutrophils, PMN-MDSCs localize with peripheral blood mononuclear cells in the low-density fraction after gradient centrifugation (4, 6). The neutrophils used for the adoptive transfer experiments in this study were isolated from naive mice, localized to the high-density fraction, and contained both mature and immature cells. Therefore, it remains to be determined whether mature neutrophils suppress T-cell proliferation in influenza or whether an immature CD11bhi population that emerges during influenza is responsible for T-cell suppression. Notably, mature and hypersegmented PMNs have been described to suppress T-cell responses (14). Ultimately, if PMN-MDSCs are explicitly defined as immature cells that only emerge in disease (6), and in fact preexisting mature or hypersegmented neutrophils suppress T-cell responses, the nomenclature and criteria for classification of these cells must be expanded.
Also still undefined is the mechanism underlying the suppressive effect of CD11b+ neutrophils on lung injury during influenza infection in mice. In this group’s human study, elegant ex vivo experiments demonstrated that CD11b+ neutrophils suppress T-cell proliferation via release of H2O2 into the immunologic synapse (8). In this setting, T-cell suppression by PMN-MDSCs requires CD11b, presumably because the integrin mediates cell–cell adhesion during formation of the immunologic synapse. Additional studies are needed to address whether the same mechanism mediates T-cell suppression by neutrophils during influenza infection in mice or whether other mechanisms, such as arginine depletion and reactive nitrogen species, induce the effect in a CD11b-dependent manner. Moreover, neutrophils tend to have a limited lifespan, and the Cd11b+/+ neutrophils that were adoptively transferred into the Cd11b−/− mice at Day 0 were not detectable at Day 8, the time point at which weight loss, permeability, and T-cell proliferation were mitigated. Therefore, the suppressive effect of the Cd11b+/+ neutrophils may be exerted at earlier time points via mechanisms other than direct interaction between neutrophils and proliferating T cells. Additionally, because adoptive transfer did not result in a statistically significant increase in T cells, the protective effect of the Cd11b+/+ neutrophils on lung injury and weight loss may be mediated by mechanisms other than T-cell suppression. Additional data showing the lifespan of the adoptively transferred neutrophils and absolute T-cell counts at early and late time points would elucidate these issues.
In addition to the mechanism underlying T-cell suppression by neutrophils, the signals that promote the expansion and activation of suppressor neutrophils in the setting of influenza remain to be determined. Candidates include granulocyte macrophage colony stimulating factor and Toll-like receptor ligands (2, 3). Because neutrophil-derived signals are also critical for T-cell recruitment and activation during influenza pneumonia (15), the mechanisms that drive neutrophils or distinct subsets of neutrophils to differentially activate or inhibit T-cell immunity should be explored.
Because neutrophils induce T-cell recruitment and activation, and both neutrophils and T cells are cytotoxic, generating a feed-forward mechanism of inflammation and lung injury (1), T-cell suppression by neutrophils is likely critical to limit tissue damage by an unchecked immune system during influenza infection. One might further speculate that as autoantigen exposure during tissue injury may trigger autoimmunity, the phenomenon described here may also prevent the development of autoimmune disease after influenza. On the other hand, as MDSC expansion after sepsis is associated with increased nosocomial infections (5), T-cell suppression by neutrophils after influenza may lead to an immunosuppressed state that facilitates the secondary bacterial infections that significantly contribute to the morbidity and mortality associated with influenza.
In summary, the current report by Tak and colleagues advances the field by demonstrating that during influenza infection in mice, CD11b+ neutrophils suppress T-cell–mediated lung injury, but it also raises additional questions. Future studies should explore the underlying mechanisms. As the field evolves, the full morphological, biochemical, and functional diversity of neutrophil-like cells that suppress T-cell function, as well as the ultimate physiologic and clinical consequences of these cells, will likely be elucidated.
Footnotes
Supported by National Institutes of Health (NIH) grants HL131608 and HL109517.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Mayadas TN, Cullere X, Lowell CA. The multifaceted functions of neutrophils. Annu Rev Pathol. 2014;9:181–218. doi: 10.1146/annurev-pathol-020712-164023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, et al. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J Exp Med. 2007;204:1463–1474. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gabrilovich DI. Myeloid-derived suppressor cells. Cancer Immunol Res. 2017;5:3–8. doi: 10.1158/2326-6066.CIR-16-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uhel F, Azzaoui I, Grégoire M, Pangault C, Dulong J, Tadié JM, et al. Early expansion of circulating granulocytic myeloid-derived suppressor cells predicts development of nosocomial infections in patients with sepsis. Am J Respir Crit Care Med. 2017;196:315–327. doi: 10.1164/rccm.201606-1143OC. [DOI] [PubMed] [Google Scholar]
- 6.Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. 2016;7:12150. doi: 10.1038/ncomms12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol. 2004;172:989–999. doi: 10.4049/jimmunol.172.2.989. [DOI] [PubMed] [Google Scholar]
- 8.Pillay J, Kamp VM, van Hoffen E, Visser T, Tak T, Lammers JW, et al. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. J Clin Invest. 2012;122:327–336. doi: 10.1172/JCI57990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLain L, Morgan DJ, Dimmock NJ. Protection of mice from lethal influenza by defective interfering virus: T cell responses. J Gen Virol. 1992;73:375–381. doi: 10.1099/0022-1317-73-2-375. [DOI] [PubMed] [Google Scholar]
- 10.Rygiel TP, Rijkers ES, de Ruiter T, Stolte EH, van der Valk M, Rimmelzwaan GF, et al. Lack of CD200 enhances pathological T cell responses during influenza infection. J Immunol. 2009;183:1990–1996. doi: 10.4049/jimmunol.0900252. [DOI] [PubMed] [Google Scholar]
- 11.La Gruta NL, Kedzierska K, Stambas J, Doherty PC. A question of self-preservation: immunopathology in influenza virus infection. Immunol Cell Biol. 2007;85:85–92. doi: 10.1038/sj.icb.7100026. [DOI] [PubMed] [Google Scholar]
- 12.De Santo C, Salio M, Masri SH, Lee LY, Dong T, Speak AO, et al. Invariant NKT cells reduce the immunosuppressive activity of influenza A virus-induced myeloid-derived suppressor cells in mice and humans. J Clin Invest. 2008;118:4036–4048. doi: 10.1172/JCI36264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tak T, Rygiel TP, Karnam G, Bastian OW, Boon L, Viveen M, et al. Neutrophil-mediated suppression of influenza-induced pathology requires CD11b/CD18 (MAC-1) Am J Respir Cell Mol Biol. 2018;58:492–499. doi: 10.1165/rcmb.2017-0021OC. [DOI] [PubMed] [Google Scholar]
- 14.Pillay J, Tak T, Kamp VM, Koenderman L. Immune suppression by neutrophils and granulocytic myeloid-derived suppressor cells: similarities and differences. Cell Mol Life Sci. 2013;70:3813–3827. doi: 10.1007/s00018-013-1286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim K, Hyun YM, Lambert-Emo K, Capece T, Bae S, Miller R, et al. Neutrophil trails guide influenza-specific CD8+ T cells in the airways. Science. 2015;349:aaa4352. doi: 10.1126/science.aaa4352. [DOI] [PMC free article] [PubMed] [Google Scholar]