Abstract
We report longitudinal serum concentrations of select persistent organic pollutants (POPs) in children at ages 7 and 9 years and in their mothers prenatally and again when the children were 9 years old. The participating families were enrolled in the Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS), a longitudinal birth cohort study of low-income Hispanic families residing in the Salinas Valley, California. We observed decreasing concentrations in the mothers with year of serum collection (2009 vs 2011) for six out of seven polybrominated diphenyl ether (PBDE) congeners and for 2,2′,4,4′,5-pentachlorobiphenyl (CB-99; p < 0.05). The 9-year-old children had similarly decreasing serum concentrations of all seven PBDE congeners, CB-99, and 2,2′,3,4,4′,5′- and 2,3,3′,4,4′,6-hexachlorobiphenyl (CB-138/158) with year of serum collection (2009 vs 2011; p < 0.05). In mixed effect models accounting for weight gain as the children aged from 7 to 9 years, we observed an annual decrease ( 8.3% to 13.4%) in tri- to hexaBDE concentrations (p < 0.001), except for 2,2′,3,4,4′-tetrabromodiphenyl ether (BDE-85) and 2,2′,4,4′,5,5′-hexabromodiphenyl ether (BDE-153). The concentrations of these congeners were not associated with time of serum collection and instead showed an −0.9% to −2.6% decrease per kilogram of weight gain during the study period (p < 0.05). In the case of tetra- to heptachlorobiphenyls, we observed −0.5% to −0.7% decrease in serum concentration per kilogram of weight gain (p < 0.05) and −3.0% to −3.7% decrease in serum concentration per year of aging (p < 0.05), except for 2,3′,4,4′,5-pentachlorobiphenyl (CB-118) and 2,2′,4,4′,5,5′-hexachlorobiphenyl (CB-153), which were not associated with time of serum draw. 2,2-Bis(4-chlorophenyl)-1,1-dichloroethene (p,p′-DDE) decreased −2.4%/kg of weight gain between the two sampling points (p < 0.001). These findings suggest that as children grow, dilution in a larger body size plays an important role in explaining reductions in body burden in the case of traditional POPs such as PCBs and p,p′-DDE. By contrast, in the case of PBDEs, reductions are likely explained by reduction in exposure, as illustrated by decreased concentrations in more recent years, possibly amplified by presumed shorter biological half-life than other POPs.
Graphical Abstract
INTRODUCTION
Three commercial formulations of polybrominated diphenyl ethers (PBDEs), identified by their average bromine content, have been in commercial production since the 1970s. The ability of PBDEs to reduce flammability as well as stricter fire safety standards worldwide and increasing use of flammable materials in furniture were all factors that lead to increased use of flame-retardant chemicals, including PBDEs, over the last decades. PBDEs with 4–6 bromines on the diphenyl ether backbone (e.g., technical PentaBDE) were often used as flame retardants in polyurethane foam, commonly present in upholstered furniture and as padding under wall-to-wall carpets.1 PBDEs with 8–10 bromines (e.g., technical Octa-and DecaBDE) have been used in hard plastics such as casings of electrical appliances, TVs, and computers.1 Tetra- through hexaBDE congeners are present in common food items2 and almost universally detected in human biomonitoring studies in the United States.3–5 The Penta- and OctaBDE technical mixtures were voluntarily withdrawn from the U.S. market in 2004;6 while commercial use of DecaBDE ended in 2013,7 following concerns about their safety to humans due to their potential hormonal activity,8,9 neurodevelopmental toxicity,10,11 and decreased infant birth weight.12
However, the withdrawal of PBDEs from the market did not lead to an immediate reduction of exposure since historically produced PBDEs are still present in indoor environments, including older furniture and carpets and as residues in house dust. It is also possible that recycling of polyurethane may lead to the reintroduction of PBDEs into new products in our homes. Like exposure to lead, children have higher PBDE exposure than adults.13,14 This is most likely due to higher frequency of hand-to-mouth behavior in children compared to adults, resulting in higher nondietary ingestion of PBDEs from indoor dust15 containing PBDEs.16,17 Breastfeeding during infancy most likely is not a major contributing factor, due to the fact that the highest median 2,2′,4,4′-tetraBDE (BDE-47) concentration were observed at >4–6 years of age in a cohort of Texas children, that is, several years after cessation of breastfeeding. Concentrations then declined until >10–13 years of age, a fact likely explained by dilution with increasing body size and elimination.18
The objectives of the current study were to (1) expand the current limited information on the association of PBDE serum concentration and age in young children; (2) compare patterns of PBDE exposure with those of other persistent organic pollutants (POPs) such as polychlorinated biphenyls (PCBs) and 2,2-bis(4-chlorophenyl)-1,1-dichloroethene (p,p′-DDE), which are often present in indoor dust at lower concentrations than PBDEs; (3) explore whether dilution in a larger body size and/or elimination from the body are plausible explanations for decreasing levels of PBDEs and other POPs as the children age; (4) evaluate whether PBDE and other POPs are decreasing in concentration since the phase-out of PentaBDE in 2004; and (5) compare POP concentrations in the longitudinal birth cohort study of low-income Hispanic children and their mothers in California with concentrations in a national sample.3,19
MATERIALS AND METHODS
Study Population
Details of the CHAMACOS recruitment and follow-up have been published elsewhere.20,21 Briefly, pregnant women were recruited from six clinics serving low-income families between October 1999 and October 2000 in Monterey County, CA. Inclusion criteria included over 18 years old, less than 20 weeks gestation, eligible for low-income health insurance, planning to deliver at the local public hospital, and Spanish- or English-speaking. Of the participants, 527 (88%) were followed to delivery of a live-born, singleton infant; 294 (56%) of the children provided blood samples at 7 years of age and 264 (50%) at 9 years. Between January 2010 and October 2011, blood samples were collected from additional 9-year-old children (n = 290) and their mothers (n = 294) to supplement the original CHAMACOS cohort. The later recruited subjects were demographically comparable to the original cohort (Table S1). Subjects recruited later in the survey are hereafter referred to as CHAM2, while the original subjects are referred to as CHAM1. Serum samples available for measurements of POPs were collected from the mothers prenatally at 26 weeks gestation (n = 313) and 9 years after birth of the index child (n = 474); samples from the children were collected at 7 years of age (n = 275) and at 9 years (n = 554). The serum collection procedure has been described previously.22–24 Samples were immediately processed and stored at <−70 °C except when in transit on dry ice to the Centers for Disease Control and Prevention (CDC) in Atlanta, GA, for analysis. All study activities were approved by the institutional review boards at the University of California, Berkeley, and the CDC; written informed consent was obtained from mothers and oral assent from the child starting at age 7. No personally identifiable information on the research subjects was made available to CDC researchers.
Structured Interviews
Interviews with the mothers were conducted in English or Spanish during pregnancy and when the children were 6 months and 1, 2, 3.5, 5, 7, and 9 years old. Collected information included maternal education, age, country of birth, and years in the United States prior to giving birth; family income, parity, and breastfeeding duration and sex of index child (Table S1).25 Height and weight of mothers and children were measured to compute body mass index.
Measurements of Persistent Organic Pollutants
The analytical methodology has been published elsewhere.26 Two grams of serum was used for the measurements of PBDEs, PCBs, and p,p′-DDE, and the samples were assigned to 24 sample batches for analysis; each analytical batch contained three quality control and three blank samples composed of bovine serum (Gibco Inc., Grand Island, NY) diluted 1:40 with water; this dilution was made to reduce any target analytes in the blank serum to a level less than 1 order of magnitude lower than the limit of detection (LOD). Bovine serum was tested prior to dilution for native presence of POPs by analyzing volumes from 0.5 to 4 mL and plotting any detectable POPs versus sample size used. A slope of zero for the resulting graphs shows that any detected POPs reflect method background and were not present at a concentration above the LOD in the bovine serum. No POPs were detected in the bovine serum prior to dilution. Measurements of serum POPs were made via gas chromatography/isotope dilution–high-resolution mass spectrometry.26,27 Analytical data were corrected by subtracting the median blank value. LOD was determined as the higher of (1) 3 times the standard deviation of the amount present in blanks or (2) the instrumental LOD, defined as the injected amount known to produce a signal/noise ratio >10. Concentrations below the LOD were substituted with LOD/√2. The target analytes measured and reported are given in Table 1.
Table 1.
Percentage Change between Ages 7 and 9 by Year and Change per Kilogram of Weight Gain for Selected PBDEs, PCBs, and p,p′-DDEa
| analyte | N | change per year
|
change per kilogram of weight gain
|
||||
|---|---|---|---|---|---|---|---|
| % change | 95% CIb | p-value | % change | 95% CI | p-value | ||
| Polybrominated Diphenyl Ethers (PBDEs) | |||||||
| BDE-28 | 228 | −9.6 | −14.4, −4.4 | <0.001 | −0.4 | −1.2, 0.5 | 0.44 |
| BDE-47 | 228 | −8.3 | −13.0, −3.4 | <0.001 | −0.4 | −1.3, 0.5 | 0.36 |
| BDE-85 | 228 | −4.2 | −9.3, 1.1 | 0.12 | −0.9 | −1.8, 0.0 | 0.04 |
| BDE-99 | 228 | −11.6 | −16.7, −6.2 | <0.001 | −0.4 | −1.4, 0.6 | 0.43 |
| BDE-100 | 228 | −10 | −14.0, −5.9 | <0.001 | −0.7 | −1.5, 0.2 | 0.11 |
| BDE-153 | 228 | 2.3 | −1.6, 6.4 | 0.25 | −2.6 | −3.3, −1.9 | <0.001 |
| BDE-154 | 228 | −13.4 | −17.7, −8.9 | <0.001 | −0.8 | −1.7, 0.0 | 0.06 |
| Polychlorinated Biphenyls (PCBs) | |||||||
| PCB-74 | 210 | −3.7 | −6.7, −0.7 | 0.02 | −0.5 | −0.9, −0.1 | 0.02 |
| PCB-99 | 224 | −3.2 | −6.1, −0.1 | 0.04 | −0.5 | −0.9, −0.1 | 0.01 |
| PCB-118 | 209 | −2.1 | −5.3, 1.2 | 0.21 | −0.7 | −1.1, −0.3 | <0.001 |
| PCB-138/158 | 224 | −3 | −6.0, 0.0 | 0.05 | −0.6 | −0.9, −0.2 | <0.001 |
| PCB-153 | 224 | −2.9 | −5.9, 0.2 | 0.07 | −0.6 | −1.0, −0.2 | <0.001 |
| PCB-180 | 224 | −3.2 | −6.1, −0.1 | 0.04 | −0.6 | −0.9, −0.2 | 0.01 |
| Persistent Pesticides | |||||||
| p,p′-DDE | 228 | −1.7 | −6.7, 3.6 | 0.53 | −2.4 | −3.4, −1.4 | <0.001 |
For participants with repeated measurements only.
95% confidence interval.
Statistical Methods
Statistical evaluation was limited to PBDE and PCB congeners having greater than 60% detection overall in the study (except maternal 26 weeks) and p,p′-DDE having 100% detection. 2,2′,3,4,4′,5′,6-Heptabromodiphenyl ether (BDE-183) and decabromodiphenyl ether (BDE-209) were also measured, but their detection frequencies were <25% (BDE-183) and <10% (BDE-209), so they were not included in the current data set.
Selected percentiles for the whole data set by sample category (children at ages 7 and 9 and mothers at 26 week of pregnancy and at child age 9) were calculated by the PROC UNIVARIATE procedure (Table S2). The longitudinal design of the study was not considered; differences between sample categories were assessed by Wilcoxon test using the PROC NPAR1WAY procedure.
We assessed the changes in serum concentration (nanograms per gram of lipid) by collection year of child samples at ages 7 and 9, as well as mother’s concentration sampled at child age 9, by using analysis of variance (ANOVA) models (Tables 2 and 3 and Table S3). To run the model, we first eliminated the outliers, which were defined as above 75th percentile plus 1.5 times the interquartile range (IQR) or below the 25th percentile minus 1.5 times IQR.28
Table 2.
Child Age 9 Serum Concentration by Collection Yeara
| compd | serum concentration (ng/g of lipid) of child age 9
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| year 2009
|
year 2010
|
year 2011
|
ANOVA level of significance
|
||||||||||
| N | GMb | 95% CI | N | GM | 95% CI | N | GM | 95% CI | F | 2009 vs 2010 | 2009 vs 2011 | 2010 vs 2011 | |
| Polybrominated Diphenyl Ethers (PBDEs) | |||||||||||||
| BDE-28 | 67 | 1.71 | 1.47–1.99 | 187 | 1.45 | 1.32–1.59 | 216 | 1.37 | 1.47–1.99 | 0.06 | 0.06 | <0.05 | 0.45 |
| BDE-47 | 67 | 44.7 | 37.7–53 | 187 | 35.8 | 32.3–39.6 | 215 | 32.2 | 37.7–53 | <0.01 | <0.05 | <0.01 | 0.17 |
| BDE-85 | 67 | 0.92 | 0.79–1.06 | 186 | 0.75 | 0.69–0.82 | 213 | 0.73 | 0.79–1.06 | <0.05 | <0.05 | <0.01 | >0.5 |
| BDE-99 | 68 | 10.9 | 9.02–13.1 | 187 | 7.59 | 6.79–8.5 | 215 | 7.2 | 9.02–13.1 | <0.01 | <0.01 | <0.001 | >0.5 |
| BDE-100 | 65 | 9.26 | 7.94–10.8 | 184 | 7.86 | 7.16–8.63 | 213 | 6.91 | 7.94–10.8 | <0.01 | 0.07 | <0.01 | 0.07 |
| BDE-153 | 68 | 10.8 | 9.33–12.5 | 181 | 9.46 | 8.64–10.4 | 213 | 8.47 | 9.33–12.5 | <0.05 | 0.13 | <0.01 | 0.10 |
| BDE-154 | 63 | 1.02 | 0.88–1.18 | 173 | 0.79 | 0.72–0.86 | 202 | 0.77 | 0.88–1.18 | <0.01 | <0.01 | <0.01 | >0.5 |
| Polychlorinated Biphenyls (PCBs) | |||||||||||||
| CB-74 | 60 | 0.68 | 0.61–0.75 | 185 | 0.72 | 0.68–0.77 | 214 | 0.7 | 0.61–0.75 | 0.49 | 0.24 | >0.5 | >0.5 |
| CB-99 | 68 | 0.89 | 0.8–0.99 | 186 | 0.87 | 0.81–0.92 | 214 | 0.66 | 0.8–0.99 | <0.001 | >0.5 | <0.001 | <0.001 |
| CB-118 | 66 | 0.99 | 0.9–1.08 | 186 | 1.05 | 0.99–1.11 | 212 | 0.9 | 0.9–1.08 | <0.01 | 0.26 | 0.1 | <0.001 |
| CB-138/158 | 67 | 1.55 | 1.38–1.74 | 186 | 1.53 | 1.43–1.65 | 210 | 1.34 | 1.38–1.74 | <0.05 | >0.5 | <0.05 | <0.05 |
| CB-153 | 65 | 1.91 | 1.69–2.16 | 187 | 1.89 | 1.75–2.04 | 213 | 1.66 | 1.69–2.16 | <0.05 | >0.5 | 0.06 | <0.05 |
| CB-180 | 65 | 1.15 | 1–1.32 | 188 | 1.18 | 1.09–1.29 | 212 | 1.01 | 1–1.32 | <0.05 | >0.5 | 0.13 | <0.05 |
| Persistent Pesticides | |||||||||||||
| p,p′-DDE | 66 | 136 | 115–163 | 182 | 146 | 131–162 | 208 | 128 | 115–163 | 0.27 | >0.5 | >0.5 | 0.11 |
The ANOVA p-value comparing collection years is given, as well as the total number of available samples by year.
GM, geometric mean.
Table 3.
Serum Concentration of Mothers When Their Children Reached 9 Years of Age, by Collection Yeara
| compd | serum concentration (ng/g of lipid) of mother 9 years after birth of index child
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| year 2009
|
year 2010
|
year 2011
|
ANOVA level of significance
|
||||||||||
| N | GM | 95% CI | N | GM | 95% CI | N | GM | 95% CI | F | 2009 vs 2010 | 2009 vs 2011 | 2010 vs 2011 | |
| Polybrominated Diphenyl Ethers (Pbdes) | |||||||||||||
| BDE-28 | 91 | 1.4 | 1.19–1.64 | 248 | 1.22 | 1.11–1.34 | 214 | 1.14 | 1.04–1.25 | 0.1 | 0.16 | <0.05 | 0.31 |
| BDE-47 | 90 | 21.9 | 18.1–26.6 | 248 | 17.6 | 15.7–19.8 | 214 | 16.1 | 14.5–17.9 | <0.05 | 0.06 | <0.01 | 0.26 |
| BDE-85 | 90 | 0.51 | 0.44–0.6 | 245 | 0.41 | 0.37–0.45 | 213 | 0.42 | 0.38–0.45 | <0.05 | <0.05 | <0.05 | >0.5 |
| BDE-99 | 90 | 4.12 | 3.33–5.09 | 248 | 3.28 | 2.89–3.73 | 215 | 3.03 | 2.69–3.41 | <0.05 | 0.07 | <0.05 | 0.37 |
| BDE-100 | 90 | 4.27 | 3.59–5.08 | 247 | 3.33 | 3–3.69 | 211 | 3.07 | 2.79–3.38 | <0.01 | <0.05 | <0.01 | 0.27 |
| BDE-153 | 91 | 4.1 | 3.53–4.78 | 242 | 3.67 | 3.34–4.02 | 211 | 3.78 | 3.47–4.12 | 0.46 | 0.21 | 0.35 | >0.5 |
| BDE-154 | 91 | 0.52 | 0.46–0.59 | 245 | 0.43 | 0.4–0.46 | 211 | 0.42 | 0.39–0.45 | <0.05 | <0.05 | <0.01 | >0.5 |
| Polychlorinated Biphenyls (PCBs) | |||||||||||||
| CB-74 | 88 | 0.77 | 0.68–0.88 | 234 | 0.81 | 0.75–0.87 | 214 | 0.75 | 0.7–0.8 | 0.37 | >0.5 | >0.5 | 0.16 |
| CB-99 | 90 | 1.01 | 0.89–1.15 | 239 | 0.96 | 0.89–1.03 | 212 | 0.85 | 0.79–0.91 | <0.05 | 0.49 | <0.05 | <0.05 |
| CB-118 | 91 | 1.52 | 1.33–1.74 | 222 | 1.36 | 1.25–1.47 | 213 | 1.31 | 1.22–1.42 | 0.17 | 0.15 | 0.06 | >0.5 |
| CB-138/158 | 91 | 2.47 | 2.12–2.87 | 245 | 2.14 | 1.95–2.34 | 211 | 2.18 | 2–2.37 | 0.26 | 0.11 | 0.16 | >0.5 |
| CB-153 | 91 | 3.44 | 2.95–4.01 | 243 | 3.06 | 2.8–3.35 | 211 | 2.89 | 2.65–3.14 | 0.14 | 0.2 | 0.05 | 0.35 |
| CB-180 | 91 | 2.4 | 2.06–2.8 | 245 | 2.27 | 2.07–2.48 | 211 | 2.14 | 1.96–2.33 | 0.37 | >0.5 | 0.19 | 0.35 |
| Persistent Pesticides | |||||||||||||
| p,p ′-DDE | 88 | 290 | 227–370 | 231 | 291 | 251–337 | 204 | 249 | 217–286 | 0.27 | >0.5 | 0.28 | 0.13 |
The ANOVA p-value comparing collection years is given, as well as the total number of available samples by year.
Changes in child serum concentration from ages 7 to 9 years of age and their association with increasing body weight were examined by mixed effects models to control for non-independence of repeat samples. Statistical analysis was performed with SAS 9.3 or SAS Enterprise Guide 5.1 (SAS Institute Inc., Cary, NC) and SPSS (IBM, Armonk, NY)
RESULTS
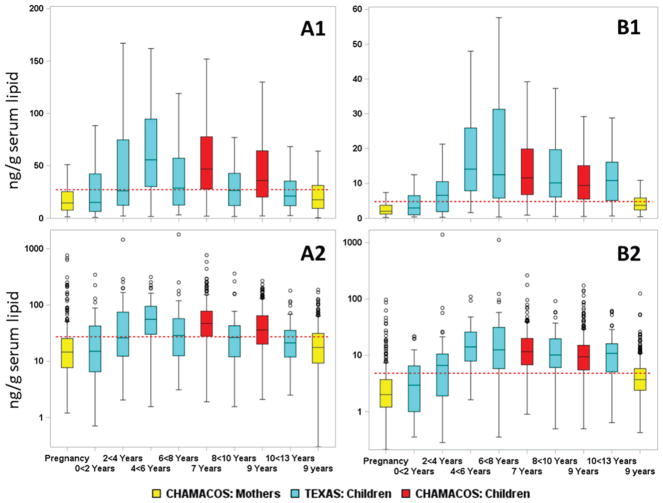
Our data on seven PBDE congeners, six PCB congeners, and p,p′-DDE provide useful information to evaluate the association of PBDE serum concentrations and age in young children. All of these POPs had detection frequencies ≥60% in children 7 and 9 years old and in their mothers 9 years after the children’s birth. Medians, interquartile range (IQR), and detection frequency are presented in Table S2 and Figure 1. The median concentration for the whole population (CHAM1 and CHAM2) of children decreased significantly for all measured POPs between 7 and 9 years [Table S2 and Figure 1 (BDE-47 and BDE-153) and Figure S1 (CB-153 and p,p′-DDE)]. The percentage reduction from 7 to 9 years was −23% for BDE-47, −25% for 2,2′,4,4′,5-pentabromodiphenyl ether (BDE-99), −19% for BDE-153, −18% for CB-153, and −19% for p,p′-DDE. On the other hand, PBDE concentrations were higher (except for BDE-99) while PCB and p,p′-DDE serum concentrations tended to be lower (−18.2% to −44.7%) in the mothers 9 years after the child was born compared to concentrations during pregnancy (Table S2 and Figure 1).
Figure 1.
Concentrations (nanograms per gram of serum lipid) of (A) BDE-47 and (B) BDE-153, on a (1) linear and (2) log10 scale. Values for children (7 and 9 years) are given in red; those for their mothers, at week 26 of pregnancy and at age 9 of their children, are shown in yellow. Median and interquartile range (IQR) concentration (box) and outlier range (bars) are shown [outlier range is (25th percentile − 1.5 IQR) and (75th percentile + 1.5 IQR)]. A cohort of Texas children14 stratified by two-year age groups between <2 years and >10 years are included as a comparison; these values are shown in blue. The red horizontal dashed line represents the National Health and Nutrition Examination Study (NHANES) estimate for age category 12–19 years for survey years 2003/04.3,19
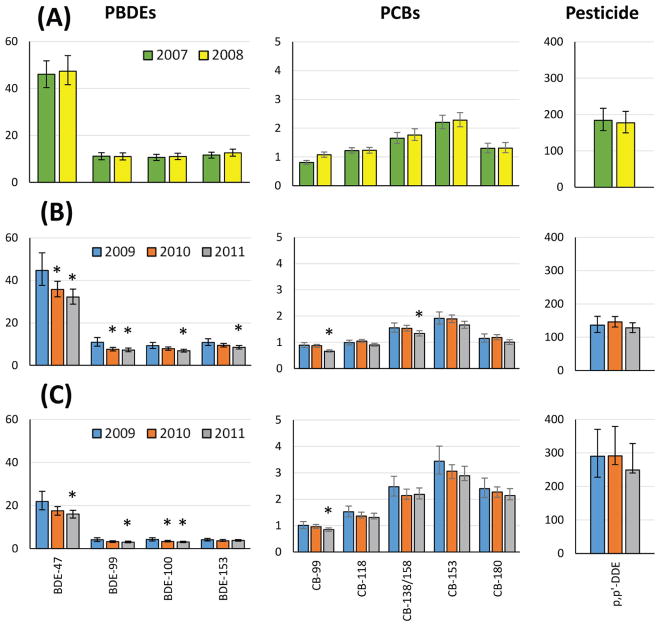
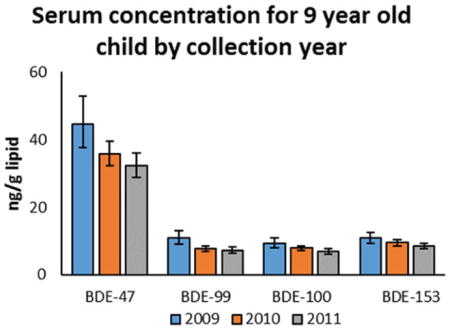
Tables 2 and 3 and Table S3 present geometric mean concentrations by collection year for the 7 and 9 year olds and their mothers to compare concentration patterns among these POPs (e.g., PBDE and PCBs) and to evaluate whether PBDE concentrations are declining since the phase-out of PentaBDE in 2004. We observed significantly decreasing concentrations for PBDEs and CB-99 and CB-138/158 by collection year between 2009 and 2011 among the 9-year-old children (Figure 2, Table 2) Similarly, concentrations of PBDEs (except BDE-153) decreased for maternal samples between 2009 and 2011, but among the organochlorines only CB-99 decreased significantly during the same time period (Table 3). By contrast, the concentrations of the 7-year-old children did not differ over the shorter collection period (2007–2008; Figure 2, Table S3).
Figure 2.
Geometric mean concentration (nanograms per gram of serum lipid) of samples from (A) child age 7, (B) child age 9, and (C) mother at child age 9 by sample collection year. Bars indicate 95% confidence interval. Significant differences compared to first year of sample collection are indicated with asterisks (ANOVA).
To assess whether dilution in a larger body size or elimination from the body may explain the decreasing concentrations of PBDEs and other POPs as children age, we present in Table 1 the percent change in congener concentration per kilogram increase in child weight between the blood collections at 7 and 9 years. We observed between −0.4% and −2.6% change for BDE congeners, with significant percent decreases per weight increase for BDE-85 and BDE-153. Similarly, there were significant decreases in congener concentration per kilogram increase in child weight for all PCBs (ranging from −0.5% to −0.7%) and for p,p′-DDE (−2.4%). In mixed effect models accounting for weight gain as the children aged from 7 to 9 years, we observed an annual decrease (−8.3% to −13.4%) in tri- to hexaBDEs concentration (p < 0.001), except for BDE-85 and BDE-153. These two congeners were not associated with time of serum collection and instead decreased by −0.9% to −2.6%/kg of weight gain during the study period (p < 0.05, Table 1). In the case of tetra- to heptachlorobiphenyls, we observed a decrease in serum concentration of −0.5% to −0.7%/kg of weight gain (p < 0.05) and a −3.0% to −3.7% decrease in serum concentration per year of aging (p < 0.05), except for CB-118 and CB-153, which were not associated with date of serum draw. p,p′-DDE decreased −2.4%/kg of weight gain between the two sampling points (p < 0.001, Table 1).
DISCUSSION
In this study, we report serum concentrations of select POPs in school-age children and their mothers residing in the Salinas Valley, California. These data expand the current limited information on the association of PBDE serum concentrations and age in young children.
We compared the POP levels in the CHAMACOS children and their mothers with levels reported in a group of Texas children14 and in a national sample.3,19 POP concentrations in the CHAMACOS children were generally comparable to a convenience sampling of Texas children, with slightly higher concentration of BDE-47 and similar median concentration of BDE-153 (Figure 1). The BDE-47 concentration in the mothers sampled at the child’s age 9 were likewise comparable with the oldest group of Texas children (10 to <13 years), while the BDE-153 concentration is about half that of the Texas children (Figure 1). However, in the Texas children we see a sharp increase in concentration from the youngest age group (<2 years) to an apex concentration at 4 to <6 years and 6 to <8 years for BDE-47 and BDE-153, respectively, which then declined at ages >8 years.14 Sjodin et al.14 speculated that this increase up to age 4–6 years was due to the hand-to-mouth behavior of young children exposing them to residential dust, which is known to contain high concentration of PBDEs16,17,29,30 but lower concentrations of other POPs investigated herein.31 Subsequent declining concentration may be caused by elimination of PBDEs and/or dilution in a growing body as the child ages and hand-to-mouth behaviors have ended or decreased. The current longitudinal study provides an opportunity to investigate these possibilities to a greater extent than what was possible in the 2014 publication of Sjodin et al.14
Among the CHAMACOS children, we observe a similar decline in serum concentration of organochlorine compounds as we see for the PBDEs, although these other POPs (e.g., PCBs and p,p′-DDE) are often present in indoor dust at lower concentrations than PBDEs. For example, CB-153 decreased 18% and p,p′-DDE decreased 19% when the 7- and 9-year-old samplings are compared, while the PBDEs decreased between 22% (BDE-85) and 32% (2,4,4′-tribromodiphenyl ether [BDE-28]). At first this appears inconsistent with the findings of Sjodin et al.14 However, in the case of traditional organo-chlorine POPs like PCBs and p,p′-DDE, we observed a significant decreased concentration with weight gain from age 7 to 9 as well as with year of serum draw, which is consistent with a dilution of POPs in a larger body mass as the child ages and grows (Table 1). On the other hand, for the PBDEs we do not see a significant decline with kilogram of weight increase (except for BDE-85 and BDE-153), but we do see on average about a 3-fold larger decrease with each year of aging compared to the traditional POPs (Table 1). The absence of significant associations with weight gain for most PBDE congeners is most likely explained by two factors: a reduction in exposure when hand-to-mouth behavior decreases at an older age and also, potentially, a relatively shorter biological half-life of PBDEs compared with PCBs. The biological half-life of the PBDEs present in commercial pentaBDE (tri- to hexaBDEs) has not yet been measured in humans. However, in electronics recycling workers in Sweden,32 BDE-183 and BDE-209 have been reported to have biological half-lives of 3 months and 2 weeks, respectively. It can thus be inferred that the lower brominated PBDE congeners may have shorter biological half-lives than PCBs, the half-lives of which are measured in years to decades.33 The hypothesis that BDE-153 has a longer biological half-life compared with other PBDEs is supported by the observation that in the Texas children14 the median ratio of BDE-153 to BDE-47 in the youngest age group (<2 years) was 0.18, while the age group >6–8 years had a median ratio of 0.50. This ratio did not change in a consistent direction for children over 8 years of age among the Texas children.14 In our cohort of CHAMACOS children, the median ratio of BDE-153 to BDE-47 was 0.23 at age 7 and 0.25 at age 9 and significantly different (Wilcoxon two-sided, p = 0.002), although, the difference was small in absolute terms. A longer biological half-life of BDE-153 and BDE-85 than that of BDE-183 and BDE-209 could potentially explain why a significant association between weight gain and percent reduction of PBDE serum concentration was observed for these congeners and not others (Table 1). Thus, a longer biological half-life of BDE-153 than other PBDEs would be consistent with the increase in BDE-153 to BDE-47 ratio with age, as observed in the Texas and CHAMACOS cohorts, and with the significant association between declining serum concentration and weight gain from age 7 to 9 in the CHAMACOS children.
The length of the sample collection, extending from March 2007 to November 2008 for collection of the samples from 7-year-old children and from April 2009 to October 2011 from the children and mothers when the children were 9 years of age, provided us with the opportunity to examine changes in concentration by collection year. In the 9-year-old children, we observed significantly decreasing concentrations by collection year for all PBDE congeners ranging from BDE-28 (−20%) to BDE-85 (−35%) and similarly decreasing concentrations of PCBs ranging from CB-118 (−9%) to CB-99 (−26%). This finding is consistent with decreasing exposures during the 19 months of collection of samples from 9-year-old children. No significant change was observed during the shorter collection of samples from 7-year-old children. In the case of the mothers, we did observe decreasing concentrations of all PBDEs except BDE-153, ranging from BDE-85 (−18%) to 2,2′,4,4′,6-pentabromodiphenyl ether (BDE-100) (−28%), but among the PCBs only CB-99 decreased significantly (−9%) during the same time period. These findings are consistent with an overall decline in exposure to POPs in the CHAMACOS cohort, which is consistent with the 2004 phase-out of commercial penta- and octaBDE.
This study has several limitations. It is possible that the later-recruited subjects in the cohort may have had different exposures and/or uncontrolled confounders that may have biased these findings (Table S1). For example, over half of the study population was overweight and/or obese, limiting the generalizability of our findings. However, we observed a significant correlation between decreasing concentrations of PCBs and p,p′-DDE and weight gain. This finding supports the conclusion that for these contaminants, with biological half-lives measured in years, dilution in a growing body size may be the main factor resulting in lower concentrations at older ages. The absence of such a correlation for most PBDEs suggests that lower exposure to PBDEs may be due to reduced dust ingestion from hand-to-mouth behavior at an older age and possibly lower exposures due to reduced use of these chemicals. Strengths of the investigation includes participation of avulnerable, socioeconomically underserved population with potentially high exposure to p,p′-DDE due to later phase-out of DDT in Mexico and a longitudinal study design.
Of interest, the geometric mean concentration of BDE-47 in the CHAMACOS 9-year-old children (44.7 ng/g of lipid) is almost twice the geometric mean in the National Health and Nutrition Examination Survey (NHANES) 2003/04 for the 12–19 year age category (28.2 ng/g lipid).3,19 On the other hand, the geometric mean of BDE-47 in the CHAMACOS mothers when their children were 9 years of age (21.9 ng/g of lipid) was similar to the 2003–2004 NHANES estimate for women over the age of 12 (19.6 ng/g of lipid).3,19 Looking at the 95th percentile of BDE-47 for 12–19 year olds in 2003/04 NHANES (174 ng/g of lipid),3,19 we observe that in the case of 7- and 9-year-old children we have 7.3% and 1.6%, respectively, of the children exceeding this level. The fact that the percentage of children in CHAMACOS with higher levels than the 95th percentile of NHANES BDE-47 concentration is not largely different from 5% indicates that we do not have unusual exposures in this socioeconomically underserved population. This observation is reassuring. However, it also suggests that PBDE concentrations in humans will remain elevated in the United States in comparison to other parts of the world for years to come, in large part because of the historical widespread use of these flame retardants in our country.1
Supplementary Material
Acknowledgments
We acknowledge the researchers who made this work possible: Emily McDonald for preparing all samples for gas chromatography/high-resolution mass spectrometry (HRMS) analysis; Sarah Anderson, Darlinda Harry, Carolyn Hodge, Susan Welch, and Yalin Zhang for HRMS analysis; Patricia McClure and Michelle Moore for data handling; and Pamela Olive for the serum lipids measurements. We also acknowledge the study participants, for making their time available and participating in this research study, and the CHAMACOS field office and CERCH personnel, for their contributions to the study. This research was supported by RO1-OH007400 (National Institute of Occupational Health), P01 ES009605 and RO1 ES015572 (National Institute of Environmental Health Sciences), and RD83451301 (U.S. Environmental Protection Agency).
Footnotes
Notes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the U.S. Department of Health and Human Services.
The authors declare no competing financial interest.
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.est.7b05460.
Three tables listing demographic and descriptive statistics and child age 7 geometric mean concentrations by collection year; one figure showing concentrations of CB-153 and p,p′-DDE in the CHAMACOS cohort compared with that of Texas children14 by age category (PDF)
References
- 1.Alaee M, Arias P, Sjodin A, Bergman A. An overview of commercially used brominated flame retardants, their applications, their use patterns in different countries/regions and possible modes of release. Environ Int. 2003;29(6):683–689. doi: 10.1016/S0160-4120(03)00121-1. [DOI] [PubMed] [Google Scholar]
- 2.Schecter A, Haffner D, Colacino J, Patel K, Papke O, Opel M, Birnbaum L. Polybrominated diphenyl ethers (PBDEs) and hexabromocyclodecane (HBCD) in composite U.S. food samples. Environ Health Perspect. 2010;118(3):357–362. doi: 10.1289/ehp.0901345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sjodin A, Wong LY, Jones RS, Park A, Zhang Y, Hodge C, Dipietro E, McClure C, Turner W, Needham LL, Patterson DG., Jr Serum concentrations of polybrominated diphenyl ethers (PBDEs) and polybrominated biphenyl (PBB) in the United States population: 2003–2004. Environ Sci Technol. 2008;42(4):1377–1384. doi: 10.1021/es702451p. [DOI] [PubMed] [Google Scholar]
- 4.Sjodin A, Jones RS, Caudill SP, Wong LY, Turner W, Calafat AM. Polybrominated Diphenyl Ethers and Other Persistent Organic Pollutants in Serum Pools from the National Health and Nutrition Examination Survey: 2001–2002. Environ Sci Technol Lett. 2014;1:92–96. doi: 10.1021/ez400050w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sjodin A, Jones RS, Caudill SP, Wong LY, Turner WE, Calafat AM. Polybrominated diphenyl ethers, polychlorinated biphenyls, and persistent pesticides in serum from the national health and nutrition examination survey: 2003–2008. Environ Sci Technol. 2014;48(1):753–760. doi: 10.1021/es4037836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polybrominated Diphenyl Ethers (PBDEs) Action Plan. U.S. Environmental Protection Agency; Washington, DC: 2009. [accessed January 16, 2018]. https://www.epa.gov/sites/production/files/2015-09/documents/pbdes_ap_2009_1230_final.pdf. [Google Scholar]
- 7.DecaBDE Phase-out Initiative. U.S. Environmental Protection Agency; Washington, DC: 2012. [accessed January 16, 2018]. https://hero.epa.gov/hero/index.cfm/reference/download/reference_id/1003362. [Google Scholar]
- 8.Herbstman JB, Sjodin A, Apelberg BJ, Witter FR, Halden RU, Patterson DG, Panny SR, Needham LL, Goldman LR. Birth delivery mode modifies the associations between prenatal polychlorinated biphenyl (PCB) and polybrominated diphenyl ether (PBDE) and neonatal thyroid hormone levels. Environ Health Perspect. 2008;116(10):1376–1382. doi: 10.1289/ehp.11379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chevrier J, Harley KG, Bradman A, Sjodin A, Eskenazi B. Prenatal exposure to polybrominated diphenyl ether flame retardants and neonatal thyroid-stimulating hormone levels in the CHAMACOS study. Am J Epidemiol. 2011;174(10):1166–1174. doi: 10.1093/aje/kwr223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eskenazi B, Chevrier J, Rauch SA, Kogut K, Harley KG, Johnson C, Trujillo C, Sjodin A, Bradman A. In utero and childhood polybrominated diphenyl ether (PBDE) exposures and neurodevelopment in the CHAMACOS study. Environ Health Perspect. 2013;121(2):257–262. doi: 10.1289/ehp.1205597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herbstman JB, Mall JK. Developmental Exposure to Polybrominated Diphenyl Ethers and Neurodevelopment. Curr Environ Health Rep. 2014;1(2):101–112. doi: 10.1007/s40572-014-0010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harley KG, Chevrier J, Schall RA, Sjodin A, Bradman A, Eskenazi B. Association of prenatal exposure to polybrominated diphenyl ethers and infant birth weight. Am J Epidemiol. 2011;174(8):885–892. doi: 10.1093/aje/kwr212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lunder S, Hovander L, Athanassiadis I, Bergman A. Significantly higher polybrominated diphenyl ether levels in young U.S. children than in their mothers. Environ Sci Technol. 2010;44(13):5256–5262. doi: 10.1021/es1009357. [DOI] [PubMed] [Google Scholar]
- 14.Sjodin A, Schecter A, Jones R, Wong LY, Colacino JA, Malik-Bass N, Zhang Y, Anderson S, McClure C, Turner W, Calafat AM. Polybrominated diphenyl ethers, 2,2′,4,4′,5,5′-hexachlorobiphenyl (PCB-153), and p,p′-dichlorodiphenyldichloro-ethylene (p,p′-DDE) concentrations in sera collected in 2009 from Texas children. Environ Sci Technol. 2014;48(14):8196–8202. doi: 10.1021/es5016907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Exposure Factors Handbook 2011 Edition. U.S. Environmental Protection Agency; Washington, DC: 2011. [accessed: January 16, 2018]. http://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=236252. [Google Scholar]
- 16.Stapleton HM, Eagle S, Sjodin A, Webster TF. Serum PBDEs in a North Carolina toddler cohort: associations with handwipes, house dust, and socioeconomic variables. Environ Health Perspect. 2012;120(7):1049–1054. doi: 10.1289/ehp.1104802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sjodin A, Papke O, McGahee E, Focant JF, Jones RS, Pless-Mulloli T, Toms LM, Herrmann T, Muller J, Needham LL, Patterson DG., Jr Concentration of polybrominated diphenyl ethers (PBDEs) in household dust from various countries. Chemosphere. 2008;73(1 Suppl):S131–S136. doi: 10.1016/j.chemosphere.2007.08.075. [DOI] [PubMed] [Google Scholar]
- 18.Bradman AS, Schwartz JM, Fenster L, Barr DB, Holland NT, Eskenazi B. Factors predicting organochlorine pesticide levels in pregnant Latina women living in a United States agricultural area. J Exposure Sci Environ Epidemiol. 2007;17(4):388–399. doi: 10.1038/sj.jes.7500525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fourth National Report on Human Exposure to Environmental Chemicals. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, GA: 2009. https://www.cdc.gov/exposurereport/pdf/fourthreport.pdf. [Google Scholar]
- 20.Eskenazi B, Bradman A, Gladstone EA, Jaramillo S, Birch K, Holland NT. CHAMACOS, a longitudinal birth cohort study: lessons from the fields. J Child Health. 2003;1(1):3–27. [Google Scholar]
- 21.Eskenazi B, Harley K, Bradman A, Weltzien E, Jewell NP, Barr DB, Furlong CE, Holland NT. Association of in utero organophosphate pesticide exposure and fetal growth and length of gestation in an agricultural population. Environ Health Perspect. 2004;112(10):1116–1124. doi: 10.1289/ehp.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bradman A, Fenster L, Sjodin A, Jones RS, Patterson DG, Jr, Eskenazi B. Polybrominated diphenyl ether levels in the blood of pregnant women living in an agricultural community in California. Environ Health Perspect. 2007;115(1):71–74. doi: 10.1289/ehp.8899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castorina R, Bradman A, Sjodin A, Fenster L, Jones RS, Harley KG, Eisen EA, Eskenazi B. Determinants of serum polybrominated diphenyl ether (PBDE) levels among pregnant women in the CHAMACOS cohort. Environ Sci Technol. 2011;45(15):6553–6560. doi: 10.1021/es104295m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eskenazi B, Fenster L, Castorina R, Marks AR, Sjodin A, Rosas LG, Holland N, Guerra AG, Lopez-Carillo L, Bradman A. A comparison of PBDE serum concentrations in Mexican and Mexican-American children living in California. Environ Health Perspect. 2011;119(10):1442–1448. doi: 10.1289/ehp.1002874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosas LG, Harley K, Fernald LC, Guendelman S, Mejia F, Neufeld LM, Eskenazi B. Dietary associations of household food insecurity among children of Mexican descent: results of a binational study. J Am Diet Assoc. 2009;109(12):2001–2009. doi: 10.1016/j.jada.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sjodin A, Jones RS, Lapeza CR, Focant JF, McGahee EE, III, Patterson DG., Jr Semiautomated high-throughput extraction and cleanup method for the measurement of polybrominated diphenyl ethers, polybrominated biphenyls, and polychlorinated biphenyls in human serum. Anal Chem. 2004;76(7):1921–1927. doi: 10.1021/ac030381+. [DOI] [PubMed] [Google Scholar]
- 27.Barr JR, Maggio VL, Barr DB, Turner WE, Sjodin A, Sandau CD, Pirkle JL, Needham LL, Patterson DG., Jr New high-resolution mass spectrometric approach for the measurement of polychlorinated biphenyls and organochlorine pesticides in human serum. J Chromatogr B: Anal Technol Biomed Life Sci. 2003;794(1):137–148. doi: 10.1016/s1570-0232(03)00451-3. [DOI] [PubMed] [Google Scholar]
- 28.Tukey J. Exploratory Data Analysis. Addison-Wesley; 1977. [Google Scholar]
- 29.Hilton DC, Jones RS, Sjodin A. A method for rapid, non-targeted screening for environmental contaminants in household dust. J Chromatogr A. 2010;1217(44):6851–6856. doi: 10.1016/j.chroma.2010.08.039. [DOI] [PubMed] [Google Scholar]
- 30.Watkins DJ, McClean MD, Fraser AJ, Weinberg J, Stapleton HM, Sjodin A, Webster TF. Impact of dust from multiple microenvironments and diet on PentaBDE body burden. Environ Sci Technol. 2012;46(2):1192–1200. doi: 10.1021/es203314e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitehead TP, Brown FR, Metayer C, Park JS, Does M, Dhaliwal J, Petreas MX, Buffler PA, Rappaport SM. Polychlorinated biphenyls in residential dust: sources of variability. Environ Sci Technol. 2014;48(1):157–164. doi: 10.1021/es403863m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thuresson K, Hoglund P, Hagmar L, Sjodin A, Bergman A, Jakobsson K. Apparent half-lives of hepta- to decabrominated diphenyl ethers in human serum as determined in occupationally exposed workers. Environ Health Perspect. 2006;114(2):176–181. doi: 10.1289/ehp.8350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aylward L, Collins J, Bodner K, Wilken M, Bodnar C. “Intrinsic” elimination rate and dietary intake estimates for selected indicator PCBs: toxicokinetic modeling using serial sampling data in US subjects, 2005–2010. Chemosphere. 2014;110:48–52. doi: 10.1016/j.chemosphere.2014.03.070. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.