Abstract
Introduction
The src inhibitor Dasatinib has been widely studied as an anti-metastatic agent. The aims of this study were to examine the effect of Src inhibition on the metastatic potential of the 4T1 murine mammary carcinoma.
Context
Src is a non-receptor tyrosine kinase well-known to contribute to the metastatic potential of tumour cells. It does so through alteration of signalling pathways important to metastasis. Elevated levels of Src are common in many cancer types, and have been correlated with tumour progression and poor patient prognosis.
Aims
This study examined whether disruption of the Src signalling pathway could inhibit metastases formation.
Settings and Design
The Src inhibitor Dasatinib was evaluated in vitro and in vivo using the highly metastatic 4T1 murine mammary adenocarcinoma cell line.
Methods and Material
In vitro assays included growth curve, western blot, migration, and invasion assays. In vivo assays included intradermal and tail vein injection models.
Statistical analysis used
In vitro data were analysed using one-way ANOVA with Dunnett's multiple comparisons in GraphPad Prism 6.0. In vivo data were analysed using GraphPad Prism 6.0, using the Wilcoxon matched pairs test.
Results
Dasatinib is effective at inhibiting in vitro phosphorylation of Src, migration and invasion in the 4T1 cell line, as well as angiogenesis in vivo. In vitro treatment with Dasatinib impaired the metastatic ability of tumour cells as assessed by a tail vein injection model. However, both the syngeneic BALB/c and the athymic nu/nu mice receiving oral doses of the drug developed significantly higher numbers of 4T1 lung metastases. This effect was not seen in a different breast carcinoma cell line, the MDA-MB-231-4175-LM2, nor was this effect seen in the murine fibrosarcoma KHT cell line.
Conclusions
The 4T1 cell line is not an appropriate model to study Src inhibition.
Keywords: 4T1, Dasatinib, src, metastasis, cancer
Subjects and Methods
Background
Cancer is the second leading cause of death in America. It is estimated that 40% of all Americans will be diagnosed with cancer at some point in their lives.[1] Breast cancer is a particularly common type of cancer in America, with one in eight women receiving a diagnosis in her lifetime. The five-year survival rate for patients diagnosed with localized breast cancer is 98%. However, once the cancer has spread to distant locations throughout the body, treatment options become limited, and the five-year survival rate drops below 25%. [2] Metastasis is the primary cause of cancer-related death and is a complex, multi-step process.[3-6] The incidence of metastasis is inversely correlated with overall survival and quality of life.[7] Many developing therapeutics are aimed at the prevention of metastasis in order to improve survival and quality of life in cancer patients. One such target of interest is the oncoprotein Src.[8] Src is inactive in most normal cells. However, when Src is active it plays a role in a number of cellular processes, including survival, proliferation, adhesion, angiogenesis, motility and invasion.[8] Src protein and activity levels are elevated in a number of cancers, including breast cancer.[8] Additionally, protein and activity levels appear to positively correlate with disease progression.[8-10] Because of the critical role that Src plays in metastasis-related signaling pathways, inhibition of its activity may offer a valuable therapeutic strategy. [11] By inhibiting Src, it may be possible to reduce metastasis, and thereby improve overall host survival. Interest in Src has spurred the development of several small molecule Src inhibitors. These agents allow for targeting of the metastatic functions of tumor cells without the extreme cytotoxic side effects of traditional chemotherapy. Preclinical and clinical trials have tested the impact of Src inhibition therapy on the progression of breast cancer.[9-12]
One such targeted therapy is Dasatinib, an orally available small molecule Src inhibitor. [12] Dasatinib originally received FDA approval for its potent inhibition of the BCR/ABL fusion protein. In addition to BCR/ABL, it strongly binds to all the Src Family Kinase members, as well as c-KIT, PDGFR, c-FMS and Ephrin A receptor. It is currently used to treat chronic myelogenous leukemia and Philadelphia-positive acute lymphoblastic leukemia; both leukemias are driven by the BCR/ABL fusion protein. Dasatinib is currently in phase II clinical trials for the treatment of breast cancer, as well as prostate, colon, non-small cell lung cancer, melanoma, and many others. [12]
In order to study the impact of Src inhibition on metastasis, we chose the highly metastatic murine mammary carcinoma model 4T1. The 4T1 cell line is derived from a mammary adenocarcinoma tumor that spontaneously arose in a BALB/c mouse. Highly aggressive and invasive, it has many similarities to human metastatic breast cancer. After transplantation into the mammary fat pad of the mouse, tumor cells will spontaneously metastasize to the lymph nodes, lungs, liver, bone, and brain. Because of this, the 4T1 cell line is a valuable model to study human metastatic breast cancer.[13]
In this study, we utilize a murine mammary carcinoma model to explore the use of the small molecule Src inhibitor Dasatinib on phosphorylation of Src and several downstream effector proteins, on in vitro migration and invasion, and the impact of Dasatinib on angiogenesis and metastasis formation in the BALB/c and nu/nu mouse models.
Methods
Cell Culture
4T1 murine mammary adenocarcinoma cells (ATCC) and MDA-MB-231-4175-LM2 human breast carcinoma cells (Memorial Sloan Kettering) were cultured in Dulbecco's Modified Eagle's Media (DMEM), supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 1% penicillin-streptomycin. KHT murine fibrosarcoma cells (Stanford) were cultured in Alpha Minimal Essential Media supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 1% penicillin-streptomycin. Cells were incubated at 37°C at 5% CO2. Cell culture supplies were obtained from Invitrogen Corporation.
Drug Preparation
For in vitro use, Dasatinib (Bristol-Myers Squibb) was dissolved in DMSO at a concentration of 10 mM and stored at -20°C. Subsequent dilutions were made in PBS immediately prior to use. For in vivo use, Dasatinib was dissolved in 80 mM citrate buffer and delivered by oral gavage at 0.01 mL/gram. A stock solution of 30 mg/mL was prepared weekly and stored at 4°C. Subsequent dilutions were made daily.
Growth Curve
4T1 cells were plated at 7×103 cells per 100 mm dish. Cells were treated with various doses of Dasatinib 24 hours after plating. On days two, three, six, and seven, plates were harvested and counted using trypan blue exclusion to determine viability (Figure 1).
Figure 1.
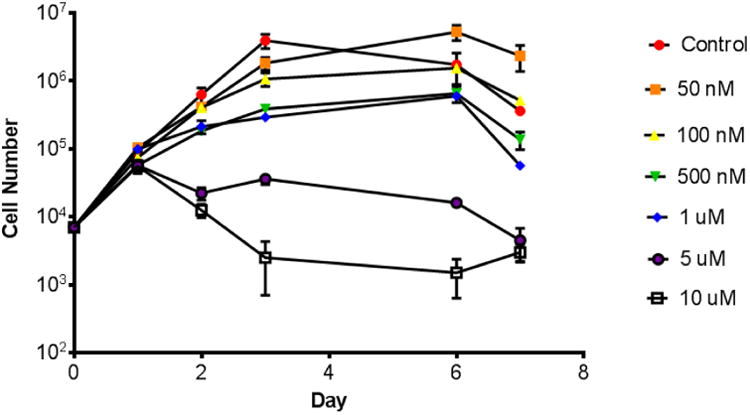
Effects of Dasatinib on growth of 4T1 cells. 4T1 cells were plated at 7×103 cells per 100 mm dish. Cells were treated with Dasatinib 24 hours after plating. On days two, three, six, and seven, plates were harvested and counted using trypan blue exclusion to determine viability.
Western Immunoblotting
Western immunoblotting was used to assess the effect of Dasatinib on Src, p-Src, p-Fak, p-Stat3, p-Pax, p-Met, and p-Akt. β-actin was used as a loading control. After a 24 hour Dasatinib treatment, cells were washed with cold PBS three times. Media and PBS rinses were centrifuged to recover non-adhered cells. Cells were harvested and lysed in RIPA buffer (50 mM HEPES, pH 7.4; 150 mM NaCl; 1% Triton X-100; 0.1% SDS; 0.5% sodium deoxycholate; 1 μM sodium orthovanadate; 5 μM EDTA; 5 μM sodium fluoride) containing a 1:20 dilution of mammalian protease inhibitor cocktail (Sigma-Aldrich). Cell lysates were centrifuged at 14,000 rpm, 4°C for 10 minutes. A Bradford assay (BioRad Laboratories) was used to assess total protein concentration. Lysates were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Proteins were transferred to Trans-Blot® Transfer Medium nitrocellulose membrane (BioRad Laboratories). Membranes were blocked in either 5% nonfat dry milk in TBS-T or 5% BSA in Tbs-t for 1 hour at room temperature on a shaker. Membranes were then incubated overnight at 4°C with primary antibodies for p-Src (Invitrogen #44660G, total Src (Invitrogen #AHO1152), p-FAK (Invitrogen #44626G), p-Stat3 (Cell Signaling #9145), p-Paxillin (Cell Signaling #2541), p-Met (Cell Signaling #3127), p-Akt (Cell Signaling #13038) and β-actin (Sigma #A1978). Membranes were incubated at room temperature for one hour with the appropriate secondary antibody (Jackson ImmunoResearch Laboratories) to allow band visualization with chemiluminescence (GE Healthcare) (Figures 2 and 3).
Figure 2.
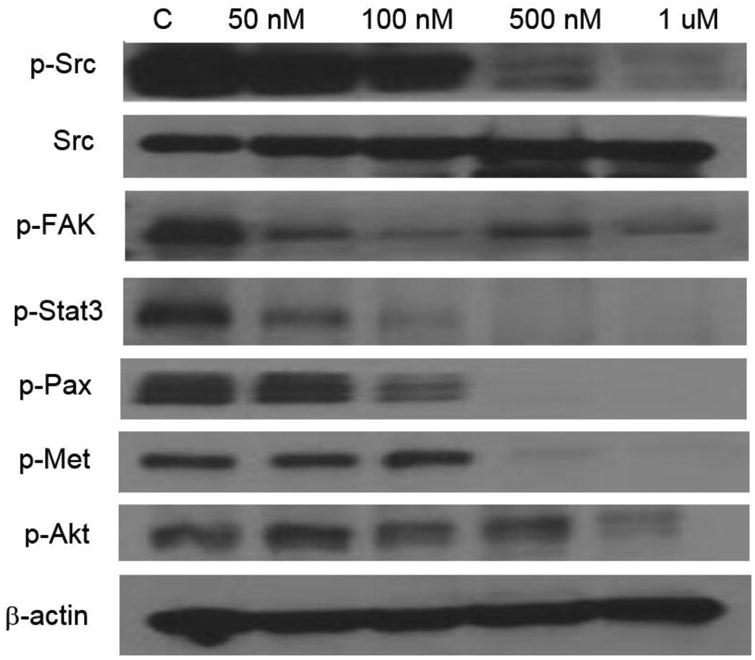
Effects of a 24 hour exposure of Dasatinib on phosphorylation of Src (Y418), FAK (Y861), Stat3 (Y705), Pax (Y118), Met (Y1234/1235), and Akt (T308) in 4T1 murine mammary carcinoma cells. B-actin was used as a loading control.
Figure 3.
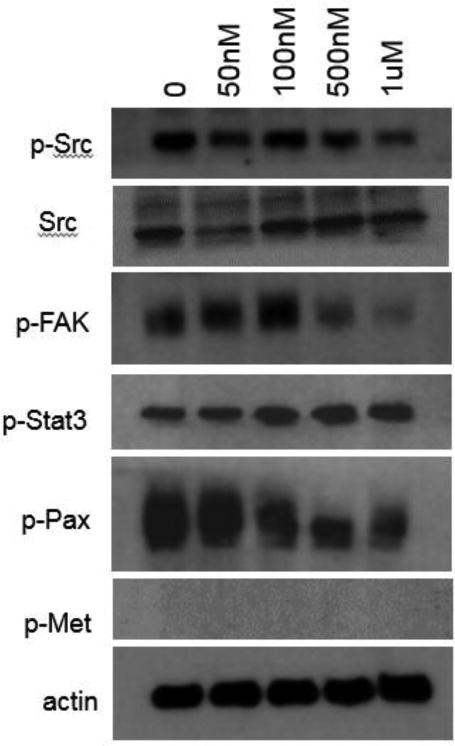
Effects of a 24 hour exposure of Dasatinib on phosphorylation of Src (Y418), FAK (Y861), Stat3 (Y705), Pax (Y118), and Met (Y1234/1235) in MDA-MB-231-LM2 human breast carcinoma cells. B-actin was used as a loading control.
Migration
4×104 4T1 cells plus drug were plated in 24 well Boyden chambers (BD Biosciences) in triplicate. After 24 hours, any cells remaining inside the insert were removed using a cotton swab. Inserts were stained with crystal violet, and cells that successfully migrated were counted using a light microscope at 5× magnification. Photos were taken on a Leica DMI 4000B using SPOT 5.2 software at 5× magnification. The results shown represent the average of three independent experiments (Figure 4a).
Figure 4.
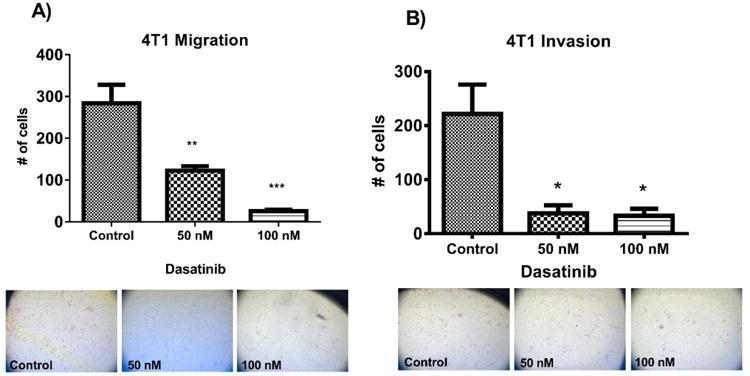
Effects of a 24 hour exposure of Dasatinib (50nM or 100 nM) on migration (A) and invasion (B) of 4T1 murine mammary carcinoma cells. Multiple comparisons ANOVA was used to analyse data in GraphPad Prism 6.0. *p<0.05, **p<0.01, ***p<0.001. Standard error of the mean is shown. Representative photos are shown.
Invasion
8×104 4T1 cells plus drug were plated onto Matrigel-coated invasion chambers (BD Biosciences), in triplicate. Media inside the chamber was serum-free. Chambers were placed into wells of a 24 well plate containing media supplemented with 10% FBS as a chemoattractant. After 24 hours, cells remaining inside the inserts were removed using a cotton swab and stained with crystal violet. Cells that successfully invaded through the Matrigel were stained and counted using a light microscope at 5× magnification. Photos were taken on a Leica DMI 4000B using SPOT 5.2 software at 5× magnification. The results shown represent the average of three independent experiments (Figure 4b).
Intradermal angiogenesis assay
All animals were cared for according to the University of Florida Institutional Animal Care and Use Committee. Five to six week old female athymic nu/nu mice were obtained from Frederick Laboratories, and provided with food and water ad libitum. Mice were injected intradermally at four ventral sites with 1×105 4T1 cells that had been treated with Dasatinib for 24 hours; or, mice were injected intradermally at four ventral sites with 1×105 untreated 4T1 cells, and treated with drug or vehicle at time of injection and once daily for two days thereafter. On the third day after cell inoculation, mice were euthanized and skin flaps were removed. Vessels growing towards tumor nodules were counted under a light microscope (Figure 5).
Figure 5.
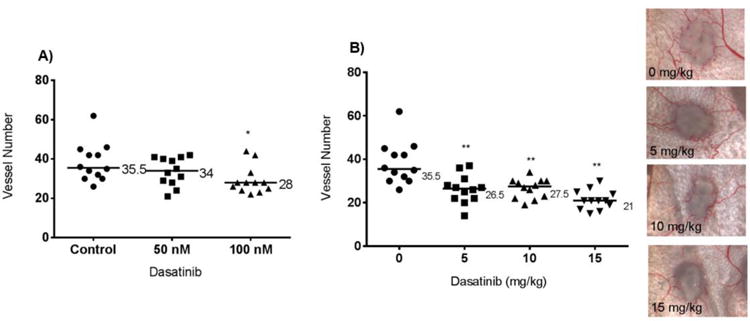
Intradermal angiogenesis assay: nu/nu mice inoculated with 4T1 tumour cells. (A) 4T1 tumour cells were treated in vitro with 50 or 100 nM Dasatinib for 24 hours, then injected intradermally into the mice. (B) Mice were injected intradermally with 4T1 cells, then treated with Dasatinib for three days. Each point on the graph represents the number of vessels intersecting a single tumour nodule. Median values are shown. Wilcoxon matched pairs test was used to analyse data in GraphPad Prism 6.0. *p<0.05, **p<0.01. Representative images are shown.
Lung metastasis assay with 4T1 cell line
Five to six week old female BALB/c or athymic nu/nu mice were obtained from Harlan Laboratories. Mice were injected via tail vein with 4T1 cells; 1×105or 5×104(BALB/c), or 5×104 (athymic nu/nu). One cohort of mice was injected with 1×1054T1 cells that had been pretreated in vitro with 100 nM Dasatinib for 24 hours prior to harvest (Figure 6A). A second cohort was injected with 5×104 untreated 4T1 cells and then treated daily with Dasatinib for five days (Figure 6B). A third cohort of mice was treated with Dasatinib for five days prior to injecting 1×105 4T1 tumor cells (Figure 7).
Figure 6.
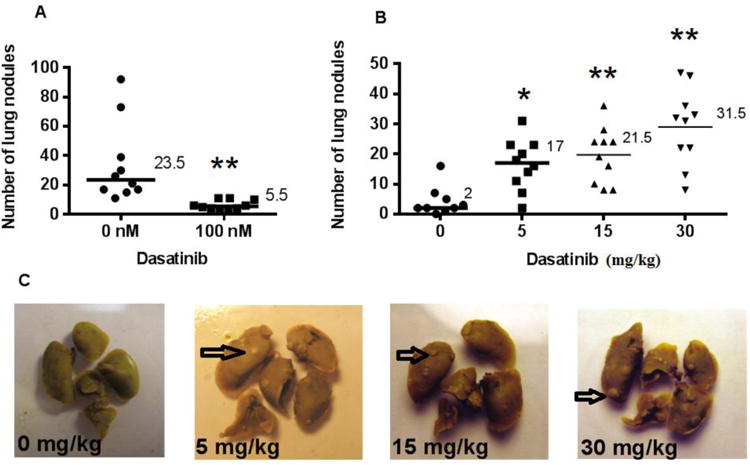
Lung metastasis assay: BALB/c mice inoculated with 4T1 tumour cells. (A) 1×1054T1 cells were pre-treated for 24 hours with vehicle or 100 nM Dasatinib, then injected via tail vein into BALB/c mice. (B) Mice were injected via tail vein with 5×1044T1 cells, then treated for five days with vehicle or 5, 15, or 30 mg/kg Dasatinib. (C) Representative photographs of lungs are shown. Arrows indicate representative metastasis. Median values are shown. Wilcoxon matched pairs test was used to analyse data in GraphPad Prism 6.0. *p<0.05, **p<0.01.
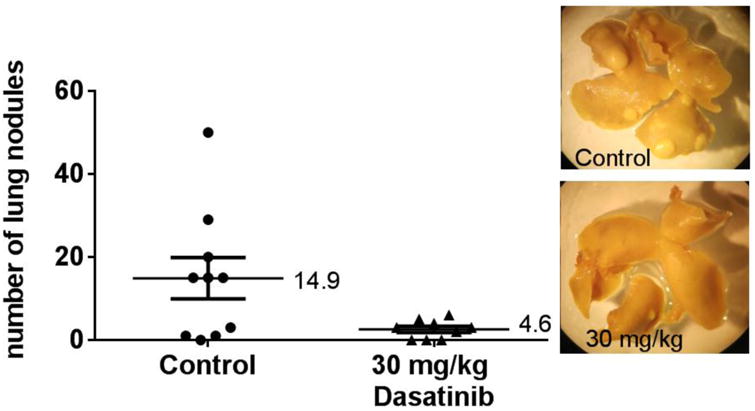
Figure 7.
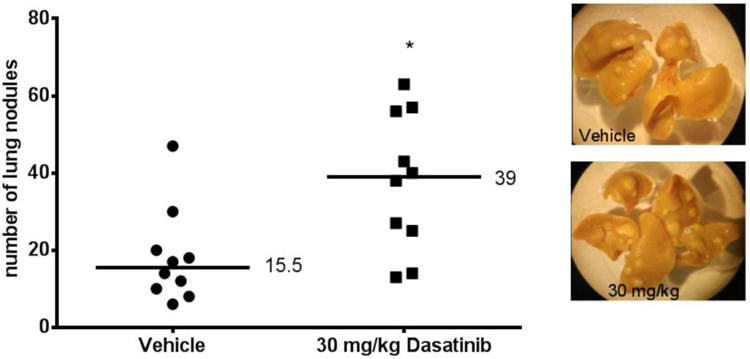
Lung metastasis assay: pre-treated BALB/c mice inoculated with 4T1 tumour cells. Mice were pre-treated with vehicle or 30 mg/kg Dasatinib for five days. On the sixth day, mice were injected via tail vein with 1×1054T1 cells. Each point on the graph represents the number of nodules found on a single set of lungs. Wilcoxon matched pairs test was used to analyse data in GraphPad Prism 6.0. *p<0.05. Median values are shown. Representative photographs of lungs are shown.
Mice were euthanized 15 (BALB/c mice) or 23 (athymic nu/nu mice) days after tumor cell injection. Lungs were removed and placed in Bouin's solution (Sigma-Aldrich). Twenty-four hours later, the Bouin's solution was replaced with 70% isopropyl alcohol. Lung nodules were counted under a light microscope.
Lung metastasis assay with MDA-MB-231-4175-LM2 cell line
Five to six week old female nude (athymic nu/nu) mice (Harlan) were injected with 2×104 MDA-MB-231-4175-LM2 breast carcinoma cells, then treated with Dasatinib or vehicle for five days following tumor cell injection. Eight weeks later, the mice were euthanized, the lungs removed, and evaluated for lung nodules as described above.
Lung metastasis assay with KHT cell line
Five to six week old C3H/HeJ mice (Jackson Laboratory) were injected via tail vein with 2×103 KHT fibrosarcoma tumor cells, along with 103 lethally irradiated cells. Mice were treated with vehicle, 15, or 30 mg/kg Dasatinib for five days following tumor cell inoculation. Seventeen days after tumor inoculation, the mice were euthanized and their lungs evaluated for lung nodule formation as described above.
Statistical Analysis
In vitro data were analyzed using one-way ANOVA in GraphPad Prism 5.0. In vivo data were analyzed using GraphPad Prism 6.0, using the Wilcoxon matched pairs test. A p value of less than 0.05 was considered statistically significant (*p<0.05).
Results
Dasatinib treatment at 1 μM and below has no negative effect on growth of 4T1 tumor cells in vitro
Sub-cytotoxic doses of Dasatinib in the 4T1 cell line were assessed using a growth curve assay. Up to 1 μM Dasatinib, 4T1 cell growth was not negatively impaired (Figure 1). Future functional assays utilized doses of 100 nM and below to assess functional effect at sub-cytotoxic dosing.
Dasatinib treatment inhibits phosphorylation of Src and downstream proteins in the 4T1 and MDA-MB-231-LM2 cell line
Dasatinib inhibits the phosphorylation of Src in a dose dependent manner without affecting total levels of Src, as indicated by western blot in the 4T1 and MDA-MB-231-LM2 cell lines. Dasatinib was also observed to inhibit the phosphorylation of several downstream proteins, including Fak, Stat3, Pax, Met, and Akt in 4T1 cells (Figure 2). Dasatinib reduced pathway activity of Fak and Pax, in addition to Src, in the MDA-MB-231-LM2 cell line (Figure 3). β-actin was used as a loading control. Dasatinib impacts molecular signaling pathways important for tumor cell metastasis.
Migration and invasion are inhibited by Dasatinib treatment
To evaluate the functional effects of Dasatinib on tumor cells, migration and invasion assays were used. The ability of 4T1 cells to migrate and invade over 24 hours was significantly impaired by treatment with Dasatinib. At doses of 50 nM and 100 nM, Dasatinib inhibited migration by 56.8% and 90.6% respectively (Figure 4A). At these doses Dasatinib inhibited invasion by 83.0% and 85.0% respectively (Figure 4B). Dasatinib reduces in vitro migration and invasion, indicating a potential effect as an anti-metastatic agent.
4T1 cells treated in vitro have a reduced ability to induce angiogenesis in vivo
Initial studies examined the effect of pretreating 4T1 cells in vitro with 50 and 100 nM Dasatinib for 24 hours prior to injecting them intradermally into athymic nu/nu mice on their ability to induce blood vessels. 3 days after tumor cell injection, the mice were euthanized and the skin flaps were removed and the number of blood vessels growing into the tumor cell deposit were counted. The results showed that tumor cells pretreated with 100 nM Dasatinib had a significantly reduced ability to induce angiogenesis (Figure 5A).
Dasatinib inhibits tumor cell-induced angiogenesis in vivo
When 4T1 tumor cells were injected intradermally into mice and then the mice were given daily doses of Dasatinib (0, 5, 10, and 15 mg/kg) for three days, the number vessels were significantly reduced compared to mice given the drug carrier. Angiogenesis was significantly inhibited in dose dependent manner, with the highest dose (15mg/kg) leading to a 40% reduction in the number of blood vessels formed (Figure 5B). Treatment with Dasatinib reduced tumor cell-induced angiogenesis, a critical component of the metastatic cascade.
4T1 cells treated in vitro have a reduced ability to form lung metastases in vivo
4T1 tumor cells were pretreated in vitro with 100 nM Dasatinib for 24 hours, and then injected via tail vein into BALB/c mice in order to observe experimental lung metastasis formation. After 15 days, mice were euthanized, the lungs excised, and nodules on the lungs were counted. Tumor cells that had been pretreated with Dasatinib formed approximately 5-fold fewer lung nodules than did untreated tumor cells (Figure 6A). Pretreatment of tumor cells with Dasatinib impaired their ability to form experimental metastasis.
Dasatinib increases the number of lung metastases in the 4T1-BALB/c model
When BALB/c mice were treated with Dasatinib for 5 days post 4T1 cell injection, the number of lung nodules were dramatically increased. Based on knowledge from previous experiments, the cell number injected in Figure 6B was reduced from 6A, in order to quantify the lung metastasis produced in mice treated with 30 mg/kg Dasatinib. The 15 mg/kg dose of Dasatinib resulted in a roughly 10-fold increase in metastases, while the 30 mg/kg dose schedule resulted in a greater than 15-fold increase in metastases (Figure 6B). This experiment was subsequently repeated using a higher number of injected tumor cells. Consistent with the initial experiment, the Dasatinib-treated group was found to have developed significantly more metastases than the control group (data not shown).
Pretreatment of BALB/c mice facilitates 4T1 lung metastasis formation
BALB/c mice were treated with daily doses of 30 mg/kg Dasatinib for 5 days. Twenty-four hours after the last drug treatment, the mice were injected with 4T1 tumor cells and did not receive any further Dasatinib treatment. Dasatinib has a relatively short half-life: twenty-four hours after the last dose, the drug is undetectable in mouse plasma.[14] The results showed that mice that had been exposed to Dasatinib prior to tumor cell injection developed a significantly greater numbers of lung metastases than did the mice not pretreated with this agent (Figure 7).
Dasatinib increases the number of lung metastases in the 4T1-nu/nu model
To determine if the phenomenon of increased metastases would be observed in a strain of mouse other than the BALB/c, 4T1 tumor cells were injected into athymic nu/nu mice. Mice treated with daily 30 mg/kg doses of Dasatinib for 5 days post tumor cell inoculation developed approximately six-fold more 4T1 lung metastases than did the control group (Figure 8).
Figure 8.
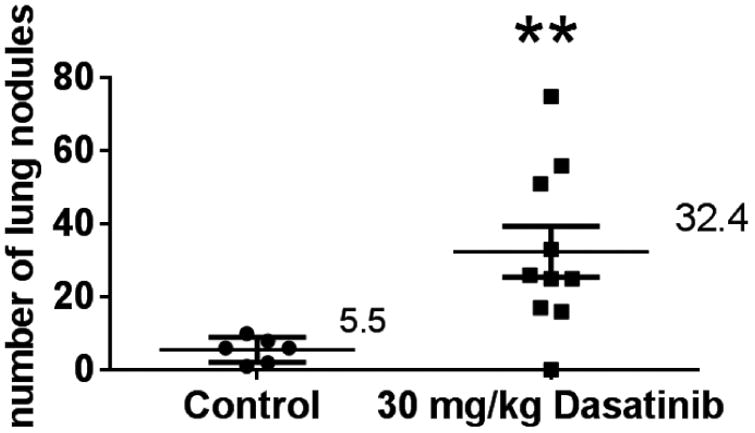
Lung metastasis assay: nu/nu mice inoculated with 4T1 tumour cells. Mice were injected via tail vein with 4T1 cells, then treated for five days with 30 mg/kg Dasatinib. Each point on the graph represents the number of nodules found on a single set of lungs. Mann-Whitney test was used to analyse data in GraphPad Prism 6.0. **p<0.01. Median values are indicated.
Dasatinib does not increase metastasis in a human breast carcinoma cell line
To determine if the increase in lung metastasis post-Dasatinib treatment observed in the 4T1 breast cancer model was a breast cancer related phenomenon, a second breast carcinoma cell line was assessed. The MDA-MB-231-4175-LM2 human breast carcinoma cell line was injected via tail vein into athymic nu/nu mice. Mice treated with Dasatinib did not develop more lung metastases than the control group, in contrast to the previously performed experiments utilizing the 4T1 cell line (Figure 9).
Figure 9.
Lung metastasis assay: nu/nu mice inoculated with MDA-MB-231-4175-LM2 tumour cells. Mice were injected via tail vein with MDA-MB-231-4175-LM2 cells, then treated for five days with 30 mg/kg Dasatinib. Each point on the graph represents the number of nodules found on a single set of lungs. Mann-Whitney test was used to analyse data in GraphPad Prism 6.0. Median values are indicated. Representative images are included.
Dasatinib does not increase metastasis in a murine fibrosarcoma model
To further assess the effects of Dasatinib on lung metastasis formation in a different tumor cell line and host, the syngeneic mouse model C3H/HeJ was inoculated via tail vein with the murine fibrosarcoma KHT. Treatment with 15 and 30 mg/kg Dasatinib did not have a significant impact on lung metastasis formation (Figure 10).
Figure 10.
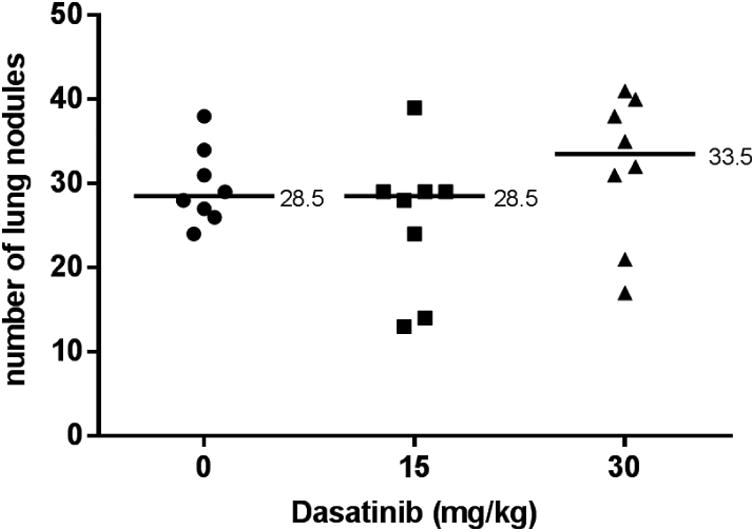
Lung metastasis assay: C3H/HeJ mice inoculated with KHT tumour cells. Mice were injected via tail vein with KHT cells, then treated for 5 days with vehicle, 15, or 30 mg/kg Dasatinib. Each point on the graph represents the number of nodules found on a single set of lungs. Mann-Whitney test was used to analyse data in GraphPad Prism 6.0. Median values are indicated.
Discussion
Elevated Src pathway activity is known to increase migration and invasion, therefore, inhibiting Src may inhibit these functions and decrease the metastatic potential of tumor cells. As observed by in vitro assays, Dasatinib treatment did result in decreased phosphorylation of Src at sub-cytotoxic doses, while inhibiting migration in a dose dependent manner. Invasion was potently inhibited at the lowest dose used, with no additional inhibitory effect at higher doses. Lower doses are likely required to see a dose-dependent effect on invasion in this cell line; however, these doses were chosen for sake of consistency. Furthermore, angiogenesis and lung metastasis formation were reduced in cells pretreated with Dasatinib.
Next, we assessed the impact of Dasatinib treatment on in vivo angiogenic and metastatic potential. In vivo drug doses were chosen to reflect the doses used in our in vitro studies, based on previously published pharmacokinetic data.[14] In vivo treatment of mice with Dasatinib had unexpected effects. While Dasatinib treatment did reduce angiogenesis, the Src inhibitor dramatically increased the frequency of lung metastases following tail vein injection of 4T1 breast cancer cells into both the BALB/c and athymic nu/nu mice. Furthermore, mice treated with Dasatinib for 5 days prior to tumor cell injection developed significantly higher numbers of lung metastases.
To determine if Dasatinib has a similarly augmenting effect in other tumor cell lines, we assessed the human breast MDA-MB-231-4175-LM2 and murine fibrosarcoma KHT cell lines. The results showed either a reduction or no significant change in metastasis formation in these cell lines. There are no published reports of Dasatinib causing an increase in metastasis formation. Additionally, there are no reports of other Src inhibitors besides Dasatinib causing this enhancing effect. Thus, we believe the augmenting effect on metastasis in the 4T1 cells to likely be cell line specific. We hypothesize that a pro-metastatic signaling pathway is being activated, perhaps through a feedback mechanism of some sort. However, until detailed analysis is performed, the authors can only speculate.
We conclude that the 4T1 murine mammary carcinoma model may not be an inappropriate model to study the effect of Src inhibition on metastasis. A major limitation of this study is the lack of the mechanism of action involved in the increased metastatic capacity of these cells. Further work must be done to clarify the underlying mechanism. Thus we caution the use of the 4T1 cell line in studies of Src inhibition on metastasis until the mechanisms responsible for the current findings are elucidated.
Key Messages.
The 4T1 cell line possesses an unknown mechanism that causes an increase in metastasis after treatment with Dasatinib, a src inhibitor. This effect was not seen in other cell lines treated with src inhibition. Further work to clarify the mechanism is critical to elucidate this phenomenon.
References
- 1.Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, et al. SEER Cancer Statistics Review, 1975-2008. 2011;2011 [Google Scholar]
- 2.Anonymous. American Cancer Society website [Google Scholar]
- 3.Mehlen P, Puisieux A. Metastasis: a question of life or death. Nat Rev Cancer. 2006;6:449–458. doi: 10.1038/nrc1886. doi:nrc1886[pii] [DOI] [PubMed] [Google Scholar]
- 4.Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–284. doi: 10.1038/nrc2622[doi]. [DOI] [PubMed] [Google Scholar]
- 5.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–18. doi: 10.1053/sonc.2002.37263[doi]. [DOI] [PubMed] [Google Scholar]
- 6.Folkman J. Seminars in Medicine of the Beth Israel Hospital, Boston. Clinical applications of research on angiogenesis. N Engl J Med. 1995;333:1757–1763. doi: 10.1056/NEJM199512283332608. [DOI] [PubMed] [Google Scholar]
- 7.Siddiqi A, Given CW, Given B, Sikorskii A. Quality of life among patients with primary, metastatic and recurrent cancer. Eur J Cancer Care (Engl) 2009;18:84–96. doi: 10.1111/j.1365-2354.2008.01021.x. [DOI] [PubMed] [Google Scholar]
- 8.Summy JM, Gallick GE. Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev. 2003;22:337–358. doi: 10.1023/a:1023772912750. [DOI] [PubMed] [Google Scholar]
- 9.Mayer EL, Krop IE. Advances in targeting SRC in the treatment of breast cancer and other solid malignancies. Clin Cancer Res. 2010;16:3526–3532. doi: 10.1158/1078-0432.CCR-09-1834. [DOI] [PubMed] [Google Scholar]
- 10.Yeatman TJ. A renaissance for SRC. Nat Rev Cancer. 2004;4:470–480. doi: 10.1038/nrc1366. [DOI] [PubMed] [Google Scholar]
- 11.Summy JM, Gallick GE. Treatment for advanced tumors: SRC reclaims center stage. Clin Cancer Res. 2006;12:1398–1401. doi: 10.1158/1078-0432.CCR-05-2692. [DOI] [PubMed] [Google Scholar]
- 12.Araujo J, Logothetis C. Dasatinib: a potent SRC inhibitor in clinical development for the treatment of solid tumors. Cancer Treat Rev. 2010;36:492–500. doi: 10.1016/j.ctrv.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pulaski BA, Ostrand-Rosenberg S. Mouse 4T1 breast tumor model. Curr Protoc Immunol. 2001;Chapter 20 doi: 10.1002/0471142735.im2002s39. Unit 20.2. [DOI] [PubMed] [Google Scholar]
- 14.Luo FR, Yang Z, Camuso A, Smykla R, McGlinchey K, Fager K, et al. Dasatinib (BMS-354825) pharmacokinetics and pharmacodynamic biomarkers in animal models predict optimal clinical exposure. Clin Cancer Res. 2006;12:7180–7186. doi: 10.1158/1078-0432.CCR-06-1112. [DOI] [PubMed] [Google Scholar]


