Specialized phagocytes are a newly appreciated classification of phagocyte that currently encompasses Sertoli cells of the testes and the retinal pigment epithelial cells of the retina. While these cells support very different tissues, they have a striking degree of similarity both as phagocytes and in ways that go beyond cell clearance. The clearance of apoptotic germ cells, cell debris, and used photoreceptor outer segments are critical functions of these cells, and the unique nature of their clearance events make specialized phagocytes uniquely suited for studying the larger implications of cell clearance in vivo. The shared-functions of specialized phagocytes could provide novel insights in to how phagocytosis impacts tissue homeostasis and immune modulation. This review examines the remarkable similarities between Sertoli cells and retinal pigment epithelial cells as specialized phagocytes and the physiological effects of cell clearance within a tissue.
Physiological effects of apoptotic cell clearance
Everyday, billions of cells in our body undergo the programmed cell death process known as apoptosis. Apoptotic cell death is crucial for normal tissue development as well as homeostasis within developed tissues. While apoptosis occurs at astonishing rates throughout the body, apoptotic corpses are rarely detected in healthy tissues, as they are promptly cleared from the environment by phagocytes.
Phagocytes have been classified in to three broad categories: professional, non-professional and specialized (Box 1) [1]. While there are differences between these subsets, they also share commonalities. First, all phagocytes must be able to distinguish viable cells from cellular material designated for removal, both dying cells or pieces of live cells [1,2]. Second, all phagocytes must break down and process the ingested corpse(s) [3]. Third, all phagocytes must be able to adapt to the stress of absorbing a significant bio-mass [3]. Thus, the recognition and removal of apoptotic cells and cellular debris has significant effects on the phagocyte and interestingly, the surrounding tissue. Phagocytes responding to apoptotic cells have profound effects on their local environment and we are only beginning to understand the effects of phagocytosis on the phagocyte and the tissue within which it resides.
Box 1. Phagocyte categorization.
Phagocytes have been traditionally grouped in to families of either professional or non-professional phagocytes. Professional phagocytes are highly efficient scavengers, capable of rapidly ingesting multiple apoptotic corpses, cellular debris and other particles; phagocytosis is considered a primary function of these cells [23]. Professional phagocytes are hematopoietically derived phagocytes such as macrophages, immature dendritic cells, and microglia. Non-professional phagocytes are stromal cells that are capable of phagocytosis but this is usually not considered their primary function [8,23,33]. Furthermore, the rate and efficiency of phagocytosis by these cells is highly variable. Non-professional phagocytes include epithelial cells, fibroblasts, and smooth muscle cells. Specialized phagocytes are a newer classification of phagocytes that includes the retinal pigmented epithelium (RPE) and Sertoli cells (SCs) [1]. Like non-professional phagocytes, these are epithelial derived stromal cells and they have a multitude of functions beyond phagocytosis including maintenance of a blood-tissue barrier, vitamin A storage, glucose transport, cholesterol transport and immune modulation [34,42,46–51,57,58,64,65,69,76]. However, like professional phagocytes, specialized phagocytes routinely ingest apoptotic cells and cellular debris in addition to performing supportive and barrier functions. One other key feature shared by SCs and the RPE is that they are mitotically quiescent cells and persist for the entire lifespan of an organism. SCs and RPE support developing germ cells and photoreceptors, respectively. These are highly specialized cell-types and the phagocytes in their respective vicinities provide critical support for their development and long-term survival. Although SCs and RPE are currently categorized as specialized phagocytes, other cell types such as the choroid plexus epithelial cells and astrocytes may also fall in to the category of specialized phagocytes. Future investigations comparing the different tissue-specific specialized phagocytes may shed more light on their functional and mechanistic similarities and differences.
One of the best-understood effects of apoptotic cell clearance is the anti-inflammatory response. Phagocytes ingesting apoptotic cells actively suppress inflammation via multiple signaling pathways [4–6] (Box 2). Since the initial description of this phenomenon, much has been discovered about the anti-inflammatory effects of apoptotic cell clearance, specifically how clearance influences pathological states of inflammation. Failure to promptly or properly clear apoptotic cells, and the subsequent release of contents from the secondarily necrotic cells can promote a pro-inflammatory tissue environment. It has long been appreciated that apoptotic cells are repositories of autoantigens and that failure to remove them can drive autoimmune diseases such as systemic lupus erythematosus [6]. However, defects in apoptotic cell clearance exacerbate a multitude of other diseases, including atherosclerosis, colitis, allergic airway inflammation, and experimental autoimmune encephalitis (Box 2) [7–12].
Box 2. Defects in apoptotic cell clearance contribute to disease.
Apoptotic cell death is often referred to as “immunologically silent”; however, the term ‘silent’ is a bit of a misnomer. While apoptotic cell removal does not elicit a pro-inflammatory response per se, apoptotic cell clearance is actively anti-inflammatory [77]. This anti-inflammatory signaling induced within phagocytes engaging apoptotic cells ultimately leads to anti-inflammatory cytokine production and secretion [77]. Such anti-inflammatory mediators are important for preventing a potentially devastating inflammatory cascade, as apoptotic cells are a repository of autoantigens such as histones and DNA, and thereby apoptotic cell clearance helps to prevent autoimmune processes [78].
Failure to clear an apoptotic corpse results in secondary necrosis of the apoptotic cell [79]. Such secondarily necrotic cells, unlike apoptotic cells, often drive inflammatory responses by releasing damage associated molecular patterns while simultaneously releasing autoantigens [80,81]. In some cases, impaired apoptotic cell clearance can contribute to autoimmune diseases such as systemic lupus erythematosus by increasing the exposure of autoantigens to the immune system [82]. Interestingly, defective clearance drives other pathological states that are not autoimmune in nature. This suggests that perhaps the routine clearance of apoptotic cells establishes an anti-inflammatory milieu. Below are multiple pathologies worsened by defective apoptotic cell clearance.
Airway inflammation: Deletion of the GTPase Rac1 (facilitates actin cytoskeletal rearrangement for phagocytosis of apoptotic cells) within bronchial epithelial cells increases airway hyper-reactivity and inflammatory cytokine release [8].
Colitis: Deletion of the PtdSer receptor BAI1 increases severity of DSS-colitis [7]. Deletion of the PtdSer bridging molecule MFG-E8 increases severity of DSS-colitis [11]. Deletion of Axl and MerTK leads to increased colitis severity [83].
Atherosclerosis: Expression of a mutant form of the PtdSer receptor MerTK increases lesion size in a murine model of atherosclerosis [12].
Experimental autoimmune encephalitis (model of multiple sclerosis): Deletion of the PtdSer receptor Axl increases the severity of inflammation and reduces myelin clearance [9].
The linkages between failed apoptotic cell clearance and inflammatory states have prompted multiple unique (and successful) attempts to enhance apoptotic cell clearance so as to alleviate disease. Several of these studies relied on enhancing cell clearance by increasing expression of phosphatidylserine (PtdSer) receptors, which recognize and initiate clearance of apoptotic cells. There are many varieties and families of PtdSer receptors, some of which recognize PtdSer directly and other that bind indirectly via bridging molecules (reviewed in [13]). In one instance, it was shown that inhibiting cleavage of the ectodomain of the indirect PtdSer receptor MerTK (effectively increasing the expression of MerTK) reduces lesion size and increases plaque stability in a model of atherosclerosis [14]. Similarly, transgenic overexpression of the direct PtdSer receptor BAI1, either globally or in colonic epithelial cells, reduces the severity of DSS-colitis [7]. Alternatively, apoptotic cell clearance can be enhanced via local injection of the opsonizing protein MFG-E8, which bridges PtdSer on apoptotic cells to integrins on the surface of phagocytes [15,16]. Indeed, myocardial injection of MFG-E8 improved cardiac outcomes post myocardial infarction [17]. While these studies focused on local enhancement of apoptotic cell clearance, systemic administration of apoptotic cells was also found to reduce disease severity in a model of colitis [5]. Autologous administration of apoptotic cells to human patients is currently being used as a medical therapy in the form of extracorporeal photopheresis (ECP). Though its use is limited to the treatment of cutaneous T cell lymphoma and graft-versus-host disease, it may be possible to extrapolate this technology for the treatment of other inflammatory diseases [18].
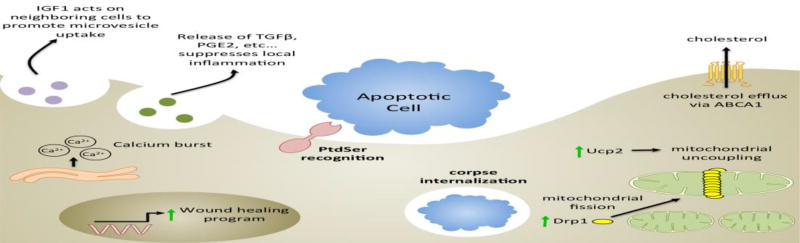
These advances in our understanding of the benefits of apoptotic cell clearance were due largely to extensive analysis of the anti-inflammatory effects of apoptotic cells. However, the effects of apoptotic cells and apoptotic cell clearance extend well beyond the suppression of inflammation (Figure 1). For instance, apoptotic cell clearance has metabolic effects on the phagocyte; apoptotic cell clearance increases mitochondrial membrane potential and potentiates mitochondrial fission [19,20]. Indeed, prolonged clearance necessitates expression of the uncoupling protein 2 (UCP2) and the fission protein, Drp1 [19,20]. Phagocytosis of apoptotic cells also affects cholesterol homeostasis. Apoptotic cell clearance drives ABCA1 upregulation and cholesterol efflux in macrophages [21,22]. This enhancement of cholesterol efflux is not only due to an increase in intracellular cholesterol but also due to a membrane-triggered signaling event dependent on PtdSer recognition [21,22]. Lastly, apoptotic cells drive macrophage production of insulin-like growth-factor-1, which acts on neighboring non-professional phagocytes to simultaneously suppress apoptotic cell clearance and enhance the uptake of anti-inflammatory microvesicles [23]. Interestingly, encountering an apoptotic cell can even have long term effects on phagocytes and their ability to influence wound healing [24]. Furthermore, recent studies have used elegant in vivo models to broaden our understanding of the genetic programs initiated by specific receptors within engulfing phagocytes [25–28]. Thus, apoptotic cell recognition and clearance affects the phagocyte and the local microenvironment in a myriad of ways that we are only beginning to comprehend.
Figure 1. Apoptotic cell clearance has a multitude of effects on phagocytes.
Upon recognition of an apoptotic cell via signaling-PtdSer receptors, multiple transcriptional events are initiated, which alter the biology of the phagocyte. One critical change is the upregulation of anti-inflammatory cytokines such as TGFβ with simultaneous suppression of pro-inflammatory mediators such as TNFα [6]. In addition, professional phagocytes, such as macrophages, which engulf apoptotic cells, increase expression and release of the growth factor IGF-1 [23]. IGF-1 secretion acts on neighboring cells to increase uptake of microvesicles [23]. Apoptotic cell recognition also enhances expression of the cholesterol transporter, ABCA1, in response to both PtdSer recognition and the increase in intracellular cholesterol from the apoptotic corpse [21,22]. Engulfment of apoptotic cells alters the metabolic profile of the phagocyte. Continued clearance of apoptotic cells results in uncoupling of the mitochondrial membrane, which is proposed to occur via increased expression of UCP2 [20]. Additionally, apoptotic cell engulfment increases translation of Drp1, which leads to increased mitochondrial fission [19]. The engulfment of corpses also leads to burst release of calcium from the endoplasmic reticulum [24]. Lastly, it was recently shown that apoptotic corpse engulfment potentiates wound healing both in the short and long term [10,24].
While significant advances have been made in comprehending the effects of apoptotic cell clearance, there are obstacles to assessing the effects of cell clearance in vivo. First, under homeostatic conditions, apoptosis and in turn, cell clearance, are asynchronous, making it difficult to precisely monitor these events. Second, the identity of the apoptotic corpse or the source of engulfed material can be varied. Interestingly, there are two tissues in which phagocytosis is routine, predictable, and the identity of the cargo is better known: the testes and the retina. In these tissues, routine phagocytic events are carried out by local ‘specialized’ phagocytes, the Sertoli cells (SCs) of the testes and the retinal pigmented epithelial cells (RPE) of the retina (Box 1) [1]. Despite supporting highly disparate tissues, these phagocytes have a striking level of functional overlap (Key figure 2). Furthermore, due to the unique nature of the phagocytic events that they participate in, RPE and SCs might provide an ideal in vivo system for assessing the many ways in which the clearance of cells and cellular debris influences the biology of the phagocyte, and in turn, how the phagocyte influences its environment.
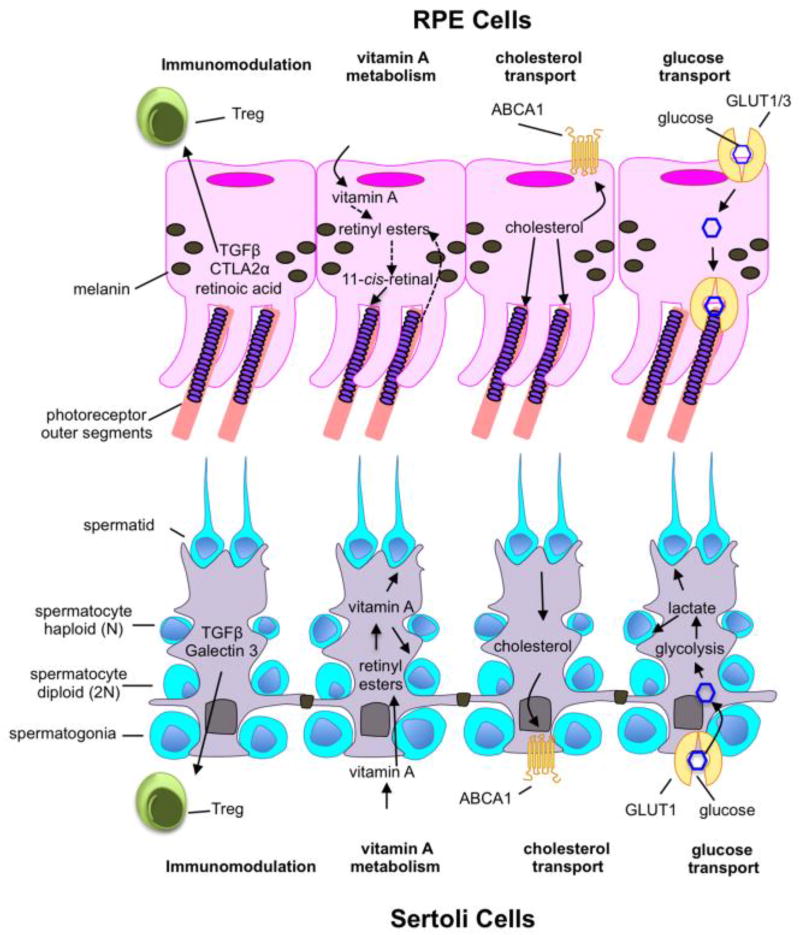
Key Figure 2. Specialized phagocytes share many functions beyond phagocytosis.
Specialized phagocytes have a multitude of functions within their respective tissues including immune regulation via the promotion Treg differentiation, vitamin A metabolism and storage, cholesterol transport, and glucose / nutrient transport.
Phagocytic functions of SCs and RPE
Sertoli Cells
SCs perform two major phagocytic functions. First, SCs phagocytose the developing germ cells that undergo apoptosis. Second, in the final phase of spermatogenesis, SCs prune the residual body of the sperm, which consists of excess cytoplasm [29,30]. These phagocytic events are dependent upon PtdSer recognition and multiple PtdSer receptors contribute to SC phagocytosis, including Tyro3, Axl, MerTK (an indirect PtdSer receptor family referred to as TAM receptors), and BAI1 [7,31].
Impaired SC phagocytosis can lead to accumulation of apoptotic germ cells in the seminiferous tubules and potentially, loss of fertility [31]. The importance of phagocytosis by SCs has been established by genetic deletion of PtdSer receptors, deletion of signaling proteins downstream of PtdSer receptors, and by blocking PtdSer in vivo [7,31–33]. Loss of all three TAM receptors leads to profound apoptotic corpse accumulation in the seminiferous tubules and male infertility [31]. The role of BAI1 and its downstream signaling partner ELMO in SC phagocytosis were demonstrated using Bai1−/− and Elmo1−/− knockout mice. Similar to Mertk−/− mice, Elmo1−/− mice exhibit corpse accumulation at baseline [33], while Bai1−/− mice exhibit corpse accumulation following testicular torsion [7].
Retinal Pigmented Epithelial Cells
In contrast to the Sertoli cells, which mediate apoptotic corpse clearance as well as the pruning of residual bodies during spermatogenesis, the RPE are thought to exclusively mediate PtdSer-dependent pruning events under homeostatic conditions. RPE cells prune photoreceptor outer segments (POS) to remove the photo-oxidative waste that accumulates during phototransduction [34]. While phagocytosis of POS by RPE (as well as residual bodies pruning by SC) is not a traditional corpse clearance event, it critically depends on the exposure of PtdSer and PtdSer receptors. The role of PtdSer in RPE phagocytosis has been demonstrated in multiple ways. First, PtdSer is exposed on outer segment membranes in a diurnal fashion that is coincident with the time of phagocytosis [35]. Second, blockade of PtdSer inhibits RPE phagocytosis of POS in vitro [35]. Third, the receptor MerTK is critical for POS uptake [36]. Loss of MerTK or its bridging molecules in mice and rats leads to a dramatic impairment in POS phagocytosis that is coincident with severe degeneration of photoreceptors, presumably due to impaired POS clearance [37]. In fact, loss of MerTK in humans is associated with early-onset rod-cone dystrophies such as retinitis pigmentosa [38,39]. While other PtdSer receptors, including αvβ5 integrin, Tyro3 and CD36 have been implicated in the process of POS phagocytosis, only MerTK is genetically linked to severe retinal degeneration phenotypes [36,40,41].
Specialized phagocytes: potential models for understanding the physiological effects of phagocytosis
SCs and RPE have a striking degree of functional overlap that extends beyond their phagocytic functions: both cell-types are epithelial-derived, mitotically-quiescent, barrier cells with active roles in the maintenance of immune privilege in their respective tissues (Figure 2). Due to their barrier function, both SCs and the RPE mediate extensive transport of critical nutrients from the blood stream to the cells they support. Furthermore, the SCs and RPE are both repositories of vitamin A, which is stored in the form of retinyl esters for future use in spermatogenesis and the visual cycle respectively [42]. Interestingly, several of these cellular processes have been linked, either directly or indirectly, to PtdSer recognition and apoptotic cell clearance. In this section, we explore other functions of specialized phagocytes that might be influenced by phagocytosis and propose novel ways in which these cell types could inform our understanding of phagocytosis in vivo.
Specialized phagocytes support immune privilege
Anatomical and physiological isolation from the immune system is critical for normal testicular and retinal function as both tissues house potent autoantigens [43,44]. The immunological privilege within the seminiferous tubules and retina are linked to the SCs and RPE, respectively; these cells form physical barriers that prevent immune cell infiltration and produce immunomodulatory molecules to actively suppress inflammation. Breakdown of this immunological privilege in the testes and eye results in devastating autoimmune disease [43,45].
The physical barriers formed by SCs and RPE are the first line of defense against inflammation and are composed tight junctions [46,47]. In addition to forming a physical barrier, SCs and RPE have robust anti-inflammatory effects on the local tissue milieu. SCs induce T regulatory cell differentiation and suppress immune responses via the production of TGFβ and JAGGED1, while RPE cells promote T regulatory cell differentiation via production of TGFβ, retinoic acid, and CTLA2α [48–51].
As phagocytosis promotes the production of anti-inflammatory cytokines such as TGFβ, the phagocytic function of SCs and RPE may drive their immunosuppressive profile. Further, SCs from TAM deficient mice exhibit phagocytic impairments and produce pro-inflammatory cytokines in response to apoptotic cells [45]. However, recent studies on SCs indicate that the role of cell clearance in immune modulation may extend beyond the production of anti-inflammatory mediators. Residual bodies, which are phagocytosed by SCs during spermiation, contain specific meiotic germ cell antigens that are permitted to egress the blood-testis-barrier [52]. These non-sequestered autoantigens drive tolerogenic responses that prevent autoimmune orchitis [52]. In this setting, phagocytosis provides the necessary tolerogenic self-antigen. Interestingly, mice lacking the PtdSer receptors Axl and MerTK exhibit increased susceptibility to autoimmune orchitis [53]. While this was attributed to the role of these receptors in antigen presenting cells, the TAM family of PtdSer receptors are also involved in residual body removal by SCs [54], raising the question of whether impaired residual body clearance contributes to this phenotype by limiting access to tolerogenic self-antigens. These observations raise the possibility that phagocytosis of apoptotic cells or cellular debris may similarly provide tolerogenic self-antigens in other settings. Indeed, the RPE are an analogous and potentially valuable system for assessing whether this occurs in other tissues.
In addition to promoting the anti-inflammatory and immunomodulatory effects of specialized phagocytes, phagocytosis may also support the physical blood-retina and blood-testis barriers. TAM deficient mice exhibit decreased integrity of the physical blood-testis-barrier [31], while MerTK-deficient RCS rats exhibit increased permeability of the blood-retina-barrier [55]. The potential linkage between physical barrier function and phagocytosis may be important for understanding the function of other cellular barriers, such as the intestinal epithelial barrier. Indeed, overexpression of the PtdSer receptor BAI1 in the colonic epithelium reduced disease in a model of DSS-colitis [7].
Specialized phagocytes contribute to local cholesterol homeostasis
Both the testes and the retina require complex regulation of cholesterol. Cholesterol in the testes is utilized for generation of steroid hormones while in the retina cholesterol is necessary for daily regeneration of POS membranes and rhodopsin discs. However, excessive accumulation of cholesterol in either tissue impairs function [56,57]. Importantly, both SCs and RPE mediate reverse cholesterol transport to prevent cholesterol accumulation in the testes and retina respectively [57,58]. In both SCs and RPE, cholesterol efflux is mediated by the ATP binding cassette ABCA1 and perturbations in ABCA1 expression have damaging effects on both the testes and the retina [57,59]. Loss of Abca1 in mice impairs spermatogenesis and leads to male infertility [57], while multiple SNPs in ABCA1 have a striking association with age-related macular degeneration [60].
Reverse cholesterol transport via ABCA1 is a critical physiological consequence of phagocytosis. While ABCA1 expression in specialized phagocytes has not been definitively linked to phagocytosis, both cell-types express MerTK, which drives ABCA1 upregulation during apoptotic cell clearance [61]. Additionally, SCs express BAI1, which upregulates ABCA1 expression upon binding to PtdSer [22]. The link between phagocytosis and ABCA1 in specialized phagocytes can be readily evaluated in vivo due to the temporal regulation of RPE phagocytosis and the frequency of SC phagocytosis. Such studies could help understand the timing, mechanism, and importance of cholesterol efflux as it pertains to apoptotic cell clearance in vivo, which could prove valuable for disease processes such as atherosclerosis.
Apoptotic cell clearance occurs unimpeded in the early atherosclerotic plaque but the lipid laden macrophages that result from this robust clearance undergo apoptosis, become secondarily necrotic and further contribute to inflammation [62]. Importantly, upregulation of ABCA1 protects against macrophage death by reducing oxidative stress [63]. Importantly, MerTK expression suppresses inflammation within atherosclerotic plaques via upregulation of ABCA1 [61]. Conversely, cleavage of MerTK promotes plaque necrosis and worsening of atherosclerosis [14]. Due to the complexity of atherosclerotic lesions and our current inability to reliably monitor apoptosis and phagocytosis in this setting, assessment of cholesterol transport in specialized phagocytes in vivo could serve as an alternative experimental system.
Clues from specialized phagocytes: identifying novel downstream effects of cell clearance
In addition to regulating immunological privilege and cholesterol homeostasis within the testes and retina, the SCs and RPE have a multitude of other functions. While many of these functions have yet to be associated with phagocytosis, the potential association of these cellular events with phagocytosis should be considered. In this section we have highlighted putative processes that phagocytosis may influence based on current functions ascribed to specialized phagocytes.
Glucose transport
The interior of the seminiferous tubules and the retina are physically sequestered from the blood stream by the SCs and RPE, respectively. In order to satisfy the energy demands of these tissues, SCs and RPE mediate transport of nutrients to the tissues they support. The retina and germ cells have high metabolic demands and thus both SCs and RPE express high levels of the glucose transporters GLUT1 and GLUT3 to support the energy requirements of the germ cells and retina, respectively [64,65]. RPE cells express these transporters on their apical and basolateral surfaces for efficient transcellular transport of glucose to the aerobically glycolytic photoreceptors [66]. In contrast, SCs absorb glucose via GLUT1 and GLUT3, metabolize it to lactate, which is then delivered to developing germ cells [67]. Continued corpse clearance by professional and non-professional phagocytes leads to uncoupling of the mitochondrial membrane potential [20]. Interestingly, mitochondrial uncoupling enhances glucose uptake by increasing expression of glucose transporters, including Glut1 [68]. Thus, it is possible that SC and RPE phagocytosis can similarly enhance glucose uptake via GLUT1, which could effectively increase glucose delivery to photoreceptors and lactate delivery to germ cells. While these questions could be investigated in vitro, specialized phagocytes provide a unique system for assessing whether apoptotic cell clearance influences glucose transport, and if this is critical for homeostasis within the retina and testes.
Vitamin A esterification and storage
SCs and the RPE are both repositories of Vitamin A [42,69]. Vitamin A is crucial for both spermatogenesis and vision and its local storage is integral to these processes [69,70]. Though vitamin A esterification has not been directly linked to phagocytosis, the RPE from MerTK-deficient, RCS rats exhibit a deficiency in vitamin A esterification [71]. Furthermore, MerTK-deficient mice have less retinyl ester accumulation in their retinas, even prior to the onset of retinal degeneration [27]. These findings suggest that phagocytosis may influence vitamin A esterification and thus vitamin A storage. While local storage of vitamin A is specific to the testes and retina, phagocytosis may influence esterification of alternative substrates in other tissues. For instance, phagocytosis might regulate the esterification of fatty acids. Should esterification be more broadly altered by phagocytosis, it could point to metabolic shifts common to apoptotic cell clearance that were repurposed in specialized phagocytes for vitamin A homeostasis. Such investigations would necessitate the use of other cell types in different contexts but knowledge of specialized phagocyte functions could inform and direct these studies.
Growing the family: do other cell-types fit the specialized phagocyte designation?
Thus far, only RPE and SCs have been incorporated under the designation of specialized phagocytes but perhaps there are more cell types that belong in this category. What other barrier cells in immune privileged sites actively nurture the surrounding tissue and mediate phagocytosis? The central nervous system houses two potential candidate cell-types: astrocytes and the cells of the choroid plexus epithelium. Astrocytes possess several characteristics that align with descriptions of SCs and RPE: they are phagocytic, they contribute to the blood-brain-barrier, they are long-lived, they support neighboring neurons, and they modulate immune responses within the immune privileged central nervous system [72–74]. Furthermore, like RPE and SCs, astrocytes exhibit ‘pruning’ of live cells by phagocytosing neuronal synapses in a MerTK dependent manner [74]. Similarly, the choroid plexus epithelium forms the blood-cerebrospinal fluid-barrier, modulates inflammation and immune cell migration to the central nervous system, and participates in inflammatory responses [75]. While phagocytic behavior has yet to be described, the choroid plexus epithelium does absorb certain substances, including amyloid β, which is commonly bound by scavenger receptors, including some that bind phosphatidylserine [13].
In classifying more cells as specialized phagocytes we may discover new and unexpected effects, complexities, and benefits of phagocytosis. One question that research on specialized phagocytes seems particularly well poised to answer is how these cells prune only select portions of their target, i.e. RPE engulfing only the distal most tips of rod outer segments, SCs engulfing the residual body during spermiation and astrocytes engulfing neuronal synapses. Although some of this may be attributed to restricted PtdSer exposure on parts of these structures, the totality of the mechanisms that limit a specialized phagocyte’s ‘bite-size’ remain a mystery. Our knowledge of what cellular factors mediate controlled phagocytosis is remarkably limited, and could have significant implications for understanding both accelerators and brakes of the phagocytic machinery.
Concluding remarks
The complexity of the phagocytic clearance of apoptotic cells and cellular debris has been increasingly appreciated over the past few years. New and exciting studies have begun to elucidate how this process influences phagocytes, and in turn, the local tissue environment. Specialized phagocytes present an as yet under-utilized system to further understand the myriad ways in which phagocytosis might influence the local tissue environment (see Outstanding questions). In fact, tremendous work has already been performed on the many functions of SCs and RPE, which could provide clues to other processes that phagocytosis may regulate. Furthermore, phagocytosis is a significant stressor, yet we do not fully understand how phagocytes manage the recycling and detoxification of engulfed cellular material. Specialized phagocytes are post-mitotic and do not renew, thus further evaluation of SCs and RPE could elucidate how phagocytes respond to repeated toxic insults. Additionally, the regularity with which specialized phagocytes engulf targets and the fact that the variety of targets is limited makes these cells uniquely suited to high-throughput screening (e.g. RNAseq and proteomics) of phagocytes and the changes elicited by phagocytosis. In addition to being a valuable tool to further our understanding of phagocytosis, SCs and RPE are fascinating with regard to their many similarities despite supporting seemingly different tissues. Perhaps specialized phagocytes are a shared feature of immune privileged sites. Intriguingly, astrocytes possess phagocytic properties, contribute to the blood-brain-barrier and participate in the regulation of immune responses within the central nervous system. By considering Sertoli cells, retinal pigmented epithelium, potentially astrocytes, and may be even the choroid epithelium, as a cellular ‘family’ we may gain further insight in to the broader mechanisms by which pathological states of inflammation are suppressed. Thus, further study of specialized phagocytes as a group has a tremendous potential to enhance our understanding of not only phagocytosis but also inflammation and immune regulation.
Outstanding questions.
Does phagocytosis of cells and cellular debris coordinate the other functions of specialized phagocytes e.g. vitamin A metabolism, immune modulation, glucose transport, regulation of the physical blood barrier?
What transcription and translation events in specialized phagocytes are influenced by phagocytosis?
Do other types of phagocytes share the functional characteristics of specialized phagocytes / does phagocytosis impart these properties on specialized as well as professional and non-professional phagocytes?
How do specialized phagocytes control ‘bite-size’? Or, what factors regulate the size of extent of phagocytosis mediated by specialized phagocytes?
How do specialized phagocytes manage the lifelong stress of phagocytosis?
Highlights.
Sertoli cells and the retinal pigmented epithelium are specialized phagocytes, a more recently appreciated category of phagocytes.
Sertoli cells and the retinal pigmented epithelium share a previously under appreciated level of functional similarity with shared functions that include phagocytosis, immune modulation, vitamin A metabolism, glucose transport and physical blood-barrier.
The phagocytic properties of Sertoli cells and retinal pigmented epithelium make them uniquely suited to the study of phagocytosis in vivo, and the effect of phagocytosis on the phagocyte and the local tissue environment.
The well-studied functions of specialized phagocytes provide clues to new processes that may be influenced or regulated by the phagocytosis of apoptotic cells and cellular debris.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arandjelovic S, Ravichandran KS. Phagocytosis of apoptotic cells in homeostasis. Nature Immunology. 2015;16:907–917. doi: 10.1038/ni.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Segawa K, Nagata S. An Apoptotic “Eat Me” Signal: Phosphatidylserine Exposure. Trends in Cell Biology. 2015;25:639–650. doi: 10.1016/j.tcb.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Han CZ, Ravichandran KS. Metabolic Connections during Apoptotic Cell Engulfment. Cell. 2011;147:1442–1445. doi: 10.1016/j.cell.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fadok VA, et al. Different populations of macrophages use either the vitronectin receptor or the phosphatidylserine receptor to recognize and remove apoptotic cells. The Journal of Immunology. 1992;149:4029–4035. [PubMed] [Google Scholar]
- 5.Grau A, et al. Apoptotic Cells Induce NF-κB and Inflammasome Negative Signaling. Plos ONE. 2015;10:e0122440. doi: 10.1371/journal.pone.0122440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poon IKH, et al. Apoptotic cell clearance: basic biology and therapeutic potential. Nature reviews Immunology. 2014;14:166–180. doi: 10.1038/nri3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee CS, et al. Boosting Apoptotic Cell Clearance by Colonic Epithelial Cells Attenuates Inflammation In Vivo. Immunity. 2016;44:807–820. doi: 10.1016/j.immuni.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juncadella IJ, et al. Apoptotic cell clearance by bronchial epithelial cells critically influences airway inflammation. Nature. 2012;493:547–551. doi: 10.1038/nature11714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinger JG, et al. Loss of the receptor tyrosine kinase Axl leads to enhanced inflammation in the CNS and delayed removal of myelin debris during experimental autoimmune encephalomyelitis. J Neuroinflammation. 2011;8:49. doi: 10.1186/1742-2094-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bosurgi L, et al. Macrophage function in tissue repair and remodeling requires IL-4 or IL-13 with apoptotic cells. Science. 2017;356:1072–1076. doi: 10.1126/science.aai8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kusunoki R, et al. Role of milk fat globule-epidermal growth factor 8 in colonic inflammation and carcinogenesis. J. Gastroenterol. 2015;50:862–875. doi: 10.1007/s00535-014-1036-x. [DOI] [PubMed] [Google Scholar]
- 12.Thorp E, et al. Mertk Receptor Mutation Reduces Efferocytosis Efficiency and Promotes Apoptotic Cell Accumulation and Plaque Necrosis in Atherosclerotic Lesions of Apoe−/− Mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28:1421–1428. doi: 10.1161/ATVBAHA.108.167197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Penberthy KK, Ravichandran KS. Apoptotic cell recognition receptors and scavenger receptors. Immunological Reviews. 2016;269:44–59. doi: 10.1111/imr.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai B, et al. MerTK receptor cleavage promotes plaque necrosis and defective resolution in atherosclerosis. J. Clin. Invest. 2017;127:564–568. doi: 10.1172/JCI90520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aziz MM, et al. MFG-E8 attenuates intestinal inflammation in murine experimental colitis by modulating osteopontin-dependent alphavbeta3 integrin signaling. The Journal of Immunology. 2009;182:7222–7232. doi: 10.4049/jimmunol.0803711. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, et al. Recombinant human MFG-E8 ameliorates colon damage in DSS- and TNBS-induced colitis in mice. Laboratory Investigation. 2015;95:480–490. doi: 10.1038/labinvest.2015.32. [DOI] [PubMed] [Google Scholar]
- 17.Nakaya M, et al. Cardiac myofibroblast engulfment of dead cells facilitates recovery after myocardial infarction. J. Clin. Invest. 2017;127:383–401. doi: 10.1172/JCI83822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martino M, et al. Extracorporeal photopheresis, a therapeutic option for cutaneous T-cell lymphoma and immunological diseases: state of the art. Expert Opin Biol Ther. 2012;12:1017–1030. doi: 10.1517/14712598.2012.688025. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, et al. Mitochondrial Fission Promotes the Continued Clearance of Apoptotic Cells by Macrophages. Cell. 2017;171:331–345.e22. doi: 10.1016/j.cell.2017.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park D, et al. Continued clearance of apoptotic cells critically depends on the phagocyte Ucp2 protein. Nature. 2011;477:220–224. doi: 10.1038/nature10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.A-Gonzalez N, et al. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity. 2009;31:245–258. doi: 10.1016/j.immuni.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fond AM, et al. Apoptotic cells trigger a membrane-initiated pathway to increase ABCA1. Journal of Clinical Investigation. 2015 doi: 10.1172/JCI80300DS1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han CZ, et al. Macrophages redirect phagocytosis by non-professional phagocytes and influence inflammation. Nature. 2016;539:570–574. doi: 10.1038/nature20141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weavers H, et al. Corpse Engulfment Generates a Molecular Memory that Primes the Macrophage Inflammatory Response. Cell. 2016;165:1658–1671. doi: 10.1016/j.cell.2016.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cummings RJ, et al. Different tissue phagocytes sample apoptotic cells to direct distinct homeostasis programs. Nature. 2016 doi: 10.1038/nature20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.A-Gonzalez N, et al. Phagocytosis imprints heterogeneity in tissue-resident macrophages. Journal of Experimental Medicine. 2017;214:1281–1296. doi: 10.1084/jem.20161375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Penberthy KK, et al. Context-dependent compensation among phosphatidylserine-recognition receptors. Sci Rep. 2017;7:14623. doi: 10.1038/s41598-017-15191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiang C-K, et al. Quantitative phosphoproteomics reveals involvement of multiple signaling pathways in early phagocytosis by the retinal pigmented epithelium. Journal of Biological Chemistry. 2017;292:19826–19839. doi: 10.1074/jbc.M117.812677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carr I, et al. Sertoli cells as phagocytes: an electron microscopic study. J. Anat. 1968;102:501–509. [PMC free article] [PubMed] [Google Scholar]
- 30.Wang H, et al. Evaluation on the phagocytosis of apoptotic spermatogenic cells by Sertoli cells in vitro through detecting lipid droplet formation by Oil Red O staining. Reproduction. 2006;132:485–492. doi: 10.1530/rep.1.01213. [DOI] [PubMed] [Google Scholar]
- 31.Lu Q, et al. Tyro-3 family receptors are essential regulators of mammalian spermatogenesis. Nature. 1999;398:723–728. doi: 10.1038/19554. [DOI] [PubMed] [Google Scholar]
- 32.Maeda Y, et al. Inhibition of sperm production in mice by annexin V microinjected into seminiferous tubules: possible etiology of phagocytic clearance of apoptotic spermatogenic cells and male infertility. Cell Death and Differentiation. 2002;9:742–749. doi: 10.1038/sj.cdd.4401046. [DOI] [PubMed] [Google Scholar]
- 33.Elliott MR, et al. Unexpected requirement for ELMO1 in clearance of apoptotic germ cells in vivo. Nature. 2011;467:333–337. doi: 10.1038/nature09356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kevany BM, Palczewski K. Phagocytosis of Retinal Rod and Cone Photoreceptors. Physiology. 2010;25:8–15. doi: 10.1152/physiol.00038.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruggiero L, et al. Diurnal, localized exposure of phosphatidylserine by rod outer segment tips in wild-type but not Itgb5−/− or Mfge8−/− mouse retina. PNAS. 2012;109:8145–8148. doi: 10.1073/pnas.1121101109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prasad D, et al. TAM receptor function in the retinal pigment epithelium. Molecular and Cellular Neuroscience. 2006;33:96–108. doi: 10.1016/j.mcn.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 37.Burstyn-Cohen T, et al. Genetic Dissection of TAM Receptor-Ligand Interaction in Retinal Pigment Epithelial Cell Phagocytosis. Neuron. 2012;76:1123–1132. doi: 10.1016/j.neuron.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ostergaard E, et al. A novel MERTK deletion is a common founder mutation in the Faroe Islands and is responsible for a high proportion of retinitis pigmentosa cases. Molecular Vision. 2011;17:1485–1492. [PMC free article] [PubMed] [Google Scholar]
- 39.Mackay DS, et al. Novel mutations in MERTK associated with childhood onset rod- cone dystrophy. Molecular Vision. 2010;16:369–377. [PMC free article] [PubMed] [Google Scholar]
- 40.Finnemann SC, Silverstein RL. Differential Roles of CD36 and avb5 Integrin in Photoreceptor Phagocytosis by the Retinal Pigment Epithelium. Journal of Experimental Medicine. 2001;194:1289–1298. doi: 10.1084/jem.194.9.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greenberg ME, et al. Oxidized phosphatidylserine-CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. J Exp Med. 2006;203:2613–2625. doi: 10.1084/jem.20060370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hogarth CA, Griswold MD. The key role of vitamin A in spermatogenesis. J. Clin. Invest. 2010;120:956–962. doi: 10.1172/JCI41303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caspi RR, et al. Mouse models of experimental autoimmune uveitis. Ophthalmic Res. 2008;40:169–174. doi: 10.1159/000119871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silva CA, et al. Diagnosis and classification of autoimmune orchitis. Autoimmunity Reviews. 2014;13:431–434. doi: 10.1016/j.autrev.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y, et al. Breakdown of immune homeostasis in the testis of mice lacking Tyro3, Axl and Mer receptor tyrosine kinases. Immunology and Cell Biology. 2013;91:416–426. doi: 10.1038/icb.2013.22. [DOI] [PubMed] [Google Scholar]
- 46.Pelletier RM, Byers SW. The blood-testis barrier and Sertoli cell junctions: structural considerations. Microsc. Res. Tech. 1992;20:3–33. doi: 10.1002/jemt.1070200104. [DOI] [PubMed] [Google Scholar]
- 47.Rizzolo LJ. Development and role of tight junctions in the retinal pigment epithelium. Int. Rev. Cytol. 2007;258:195–234. doi: 10.1016/S0074-7696(07)58004-6. [DOI] [PubMed] [Google Scholar]
- 48.Suarez-Pinzon W, et al. Testicular sertoli cells protect islet beta-cells from autoimmune destruction in NOD mice by a transforming growth factor-beta1-dependent mechanism. Diabetes. 2000;49:1810–1818. doi: 10.2337/diabetes.49.11.1810. [DOI] [PubMed] [Google Scholar]
- 49.Campese AF, et al. Mouse Sertoli cells sustain de novo generation of regulatory T cells by triggering the notch pathway through soluble JAGGED1. Biology of Reproduction. 2014;90:53. doi: 10.1095/biolreprod.113.113803. [DOI] [PubMed] [Google Scholar]
- 50.Sugita S, et al. Retinal pigment epithelium-derived CTLA-2alpha induces TGFbeta-producing T regulatory cells. The Journal of Immunology. 2008;181:7525–7536. doi: 10.4049/jimmunol.181.11.7525. [DOI] [PubMed] [Google Scholar]
- 51.Kawazoe Y, et al. Retinoic acid from retinal pigment epithelium induces T regulatory cells. Experimental eye Research. 2012;94:32–40. doi: 10.1016/j.exer.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 52.Tung KSK, et al. Egress of sperm autoantigen from seminiferous tubules maintains systemic tolerance. J. Clin. Invest. 2017;127:1046–1060. doi: 10.1172/JCI89927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li N, et al. Mice lacking Axl and Mer tyrosine kinase receptors are susceptible to experimental autoimmune orchitis induction. Immunology and Cell Biology. 2015;93:311–320. doi: 10.1038/icb.2014.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen Y, et al. Functions of TAM RTKs in regulating spermatogenesis and male fertility in mice. Reproduction. 2009;138:655–666. doi: 10.1530/REP-09-0101. [DOI] [PubMed] [Google Scholar]
- 55.Essner E, et al. Breakdown of blood. Retinal barrier in RCS rats with inherited retinal degeneration. Lab. Invest. 1980;43:418–426. [PubMed] [Google Scholar]
- 56.Saadane A, et al. Retinal and nonocular abnormalities in Cyp27a1(−/−)Cyp46a1(−/−) mice with dysfunctional metabolism of cholesterol. The American Journal of Pathology. 2014;184:2403–2419. doi: 10.1016/j.ajpath.2014.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Selva DM, et al. The ATP-binding cassette transporter 1 mediates lipid efflux from Sertoli cells and influences male fertility. J. Lipid Res. 2004;45:1040–1050. doi: 10.1194/jlr.M400007-JLR200. [DOI] [PubMed] [Google Scholar]
- 58.Fliesler SJ, Bretillon L. The ins and outs of cholesterol in the vertebrate retina. J. Lipid Res. 2010;51:3399–3413. doi: 10.1194/jlr.R010538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duncan KG, et al. Expression of reverse cholesterol transport proteins ATP-binding cassette A1 (ABCA1) and scavenger receptor BI (SR-BI) in the retina and retinal pigment epithelium. Br J Ophthalmol. 2009;93:1116–1120. doi: 10.1136/bjo.2008.144006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meyers KJ, et al. Genetic evidence for role of carotenoids in age-related macular degeneration in the Carotenoids in Age-Related Eye Disease Study (CAREDS) Investigative Ophthalmology & Visual Science. 2014;55:587–599. doi: 10.1167/iovs.13-13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim S-Y, et al. Liver X receptor and STAT1 cooperate downstream of Gas6/Mer to induce anti-inflammatory arginase 2 expression in macrophages. Sci Rep. 2016;6:571. doi: 10.1038/srep29673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thorp E, et al. The role of macrophages and dendritic cells in the clearance of apoptotic cells in advanced atherosclerosis. European Journal of Immunology. 2011;41:2515–2518. doi: 10.1002/eji.201141719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yvan-Charvet L, et al. ABCA1 and ABCG1 Protect Against Oxidative Stress-Induced Macrophage Apoptosis During Efferocytosis. Circulation Research. 2010;106:1861–1869. doi: 10.1161/CIRCRESAHA.110.217281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rato L, et al. Metabolic regulation is important for spermatogenesis. Nat Rev Urol. 2012;9:330–338. doi: 10.1038/nrurol.2012.77. [DOI] [PubMed] [Google Scholar]
- 65.Takata K, et al. Ultracytochemical localization of the erythrocyte/HepG2-type glucose transporter (GLUT1) in cells of the blood-retinal barrier in the rat. Investigative Ophthalmology & Visual Science. 1992;33:377–383. [PubMed] [Google Scholar]
- 66.Ng SK, et al. Cancer-like metabolism of the mammalian retina. Clin. Experiment. Ophthalmol. 2015;43:367–376. doi: 10.1111/ceo.12462. [DOI] [PubMed] [Google Scholar]
- 67.Oliveira PF, et al. Effect of insulin deprivation on metabolism and metabolism-associated gene transcript levels of in vitro cultured human Sertoli cells. Biochimica et Biophysica Acta. 2012;1820:84–89. doi: 10.1016/j.bbagen.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 68.Khayat ZA, et al. Rapid stimulation of glucose transport by mitochondrial uncoupling depends in part on cytosolic Ca2+ and cPKC. Am. J. Physiol. 1998;275:C1487–97. doi: 10.1152/ajpcell.1998.275.6.C1487. [DOI] [PubMed] [Google Scholar]
- 69.Kiser PD, Palczewski K. Retinoids and Retinal Diseases. Annu. Rev. Vis. Sci. 2016;2:197–234. doi: 10.1146/annurev-vision-111815-114407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Livera G, et al. Regulation and perturbation of testicular functions by vitamin A. Reproduction. 2002;124:173–180. [PubMed] [Google Scholar]
- 71.Berman ER, et al. Inherited retinal dystrophy in RCS rats: a deficiency in vitamin A esterification in pigment epithelium. Nature. 1981;293:217–220. doi: 10.1038/293217a0. [DOI] [PubMed] [Google Scholar]
- 72.Colombo E, Farina C. Astrocytes: Key Regulators of Neuroinflammation. Trends in Immunology. 2016;37:608–620. doi: 10.1016/j.it.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 73.Morizawa YM, et al. Reactive astrocytes function as phagocytes after brain ischemia via ABCA1-mediated pathway. Nature Communications. 2017;8:28. doi: 10.1038/s41467-017-00037-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chung W-S, et al. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature. 2013;504:394–400. doi: 10.1038/nature12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kaur C, et al. The Choroid Plexus in Healthy and Diseased Brain. J Neuropathol Exp Neurol. 2016;75:198–213. doi: 10.1093/jnen/nlv030. [DOI] [PubMed] [Google Scholar]
- 76.Karl J, Capel B. Sertoli cells of the mouse testis originate from the coelomic epithelium. Development. 1998;203:323–333. doi: 10.1006/dbio.1998.9068. [DOI] [PubMed] [Google Scholar]
- 77.Fadok VA, et al. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-b, PGE2, and PAF. Journal of Clinical Investigation. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rosen A, et al. Novel packages of viral and self-antigens are generated during apoptosis. J Exp Med. 1995;181:1557–1561. doi: 10.1084/jem.181.4.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vanden Berghe T, et al. Necroptosis, necrosis and secondary necrosis converge on similar cellular disintegration features. Cell Death and Differentiation. 2010;17:922–930. doi: 10.1038/cdd.2009.184. [DOI] [PubMed] [Google Scholar]
- 80.Maeda A, Fadeel B. Mitochondria released by cells undergoing TNF-a-induced necroptosis act as danger signal. Cell Death and Disease. 2014;5:e1312–9. doi: 10.1038/cddis.2014.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Scaffidi P, et al. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 82.Baumann I, et al. Impaired uptake of apoptotic cells into tingible body macrophages in germinal centers of patients with systemic lupus erythematosus. Arthritis Rheum. 2002;46:191–201. doi: 10.1002/1529-0131(200201)46:1<191::AID-ART10027>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 83.Bosurgi L, et al. Paradoxical role of the proto-oncogene Axl and Mer receptor tyrosine kinases in colon cancer. PNAS. 2013;110:13091–13096. doi: 10.1073/pnas.1302507110. [DOI] [PMC free article] [PubMed] [Google Scholar]