Abstract
Background
Urine solute supersaturation leads to the formation of urinary tract caliceal stones. Many parameters can be involved in the supersaturation of solutes in urine, such as pH. Uric acid has pKa ≤5.5, and it is solubilized at pH ≥5.5. The objective of the study was to evaluate the effects of potassium citrate and lemonade supplementation in pediatric patients with urolithiasis.
Material/Methods
A total of 126 children who had lower ureteral stones calculi and fragments with severe colic pain participated in this cross-over study. Children drank lemonade (2 mEq/kg/day citrate) in 3 divided doses for 5 days. After a 15-day washout period, children drank 2 mEq/kg/day of potassium citrate in 3 divided doses for 5 days. On the sixth of the day of individual intervention, a 24-h urine sample was collected and evaluated for pH, urine volume, citrate level, uric acid level, magnesium, phosphorus, potassium, and sodium. Urinary parameters for 1-day urine collection measurements after each supplementation were compared with baseline using the Mann-Whitney test following Tukey post hoc test at 95% confidence level.
Results
Potassium citrate supplementation resulted in reduction of sodium concentration (p=0.0337; q=3.76) and increased pH of urine (p=0.0118; q=4.389). However, urine volume, citrate level, and uric acid level, as well as elemental magnesium, phosphorus, and potassium, remained unchanged after 5 days of supplementation with potassium citrate or lemonade.
Conclusions
Potassium citrate supplementation is an effective therapy for preventing pediatric urolithiasis, with acceptable adverse effects.
MeSH Keywords: Potassium Citrate, Uric Acid, Urinary Bladder Calculi, Urolithiasis
Background
The research on urolithiasis in children has not been as rigorously as that in adults [1] and their management is somewhat different than for adults [2]. There are several drug therapies available for urolithiasis. However, metabolic abnormalities play a key role in caliceal stones formation and recurrence [3]. Hypocitraturia, hyperuricosuria, hypercalciuria, and hyperoxaluria are common metabolic abnormalities seen in children with caliceal stones [4].
Supersaturation of solute formed in the urine leads to the formation of caliceal stones. Many parameters can be involved in the supersaturation of solutes in urine, such as pH. Uric acid has pKa ≤5.5, and it is solubilized at pH ≥5.5 [5]. Increased urinary pH exacerbates the situation, and supersaturation helps prevent the formation of caliceal stones [6]. For alkalization of the urine [7], hypocitraturia treatment is recommended in pediatric urolithiasis [8]. Citrate replacement is commonly recommended to decrease stone recurrence rates in children [9].
Changes in diet of pediatric patients may help to manage hypocitraturia. Several foods contain citric acid and their intake is recommended to prevent caliceal stones formation. A high fluid intake prevents caliceal stones formation by decreasing supersaturation and citrate prevents caliceal stones formation by ionization of urinary calcium [10]. Lemons, oranges, and grapes are recommended in pediatric urolithiasis [11,12]. Lemonade has 110 mg/kg calcium and 490 g/kg citric acid. However, oranges have 11 g/kg citric acid and 420 mg/kg calcium. Therefore, lemonade is more often used than orange juice in treating urolithiasis [3].
The objective of the study was to evaluate the effects of potassium citrate and lemonade supplementation on urinary parameters for 1-day urine collection in children with urolithiasis.
Material and Methods
Ethics approval and consent to participate
The study was registered in the Research Registry (www.researchregistry), UID No.: researchregistry3648, dated 10 February 2017. The protocol (CL/Ur/PQ/14/17, dated 1 February 2017) was approved by the First People’s Hospital of Tongxiang Review Board. The study adhered to the law of the P.R. China, CONSORT guidelines, and the 2013 Declaration of Helsinki. All patients signed an informed consent form regarding interventions and diagnosis before the commencement of the study. All data used and/or generated in the study are available from DCIOM files of the First People’s Hospital of Tongxiang, China and the Hangzhou Amcare Women’s and Children’s Hospital, China.
Inclusion criteria
We included all patients 4–16 years old, with lower ureteral stones calculi and fragment (diagnosed by color Doppler ultrasound, GE Healthcare, USA), admitted to the Department of Pediatrics of the First People’s Hospital of Tongxiang, China from 25 February 2017 to 1 November 2017 with severe colic pain. Patients who had stone size ≤12 mm were included in the trial. The demographical conditions of patients are reported in Table 1.
Table 1.
The demographical conditions of enrolled patients.
| Characteristics | Patients | |
|---|---|---|
| Sample size | 126 | |
| Age | 9.85±3.24 | |
| Sex | Boy | 87 (69) |
| Girl | 39 (31) | |
| Weight (kg) | 25.1±3 | |
| Height (cm) | 138.24±5.6 | |
| Stone size (mm) | 5–10 | 99 (79) |
| 11–12 | 27 (21) | |
| Position of stone | Dominant side | 58 (46) |
| Non-dominant side | 68 (54) | |
| Stone location in ureter | Lower | 65 (52) |
| Upper | 49 (38) | |
| Middle | 12 (10) | |
| Ethnicity | Chinese | 124 (98) |
| Non-Chinese | 2 (2) | |
| History of ceftriaxone and/or cephalothin treatment | 15 (12) | |
Continuous data were represented as mean ±SD and constant data were represented as a number (percentage).
Exclusion criteria
Excluded criteria were: absence of stones (diagnosed by color Doppler ultrasound), anatomical abnormalities, voiding dysfunction, severe hydronephrosis, only urinary tract infection, history of open ureteral surgery, younger than 4 years and older than 16 years, failure to provide informed consent, stone size greater than 12 mm and less than 5 mm, and treatment with diuretics.
Design
A total of 126 children with pediatric urolithiasis participated in this randomized, experimental, cross-over study. A CONSORT flow diagram of the study is presented in Figure 1.
Figure 1.
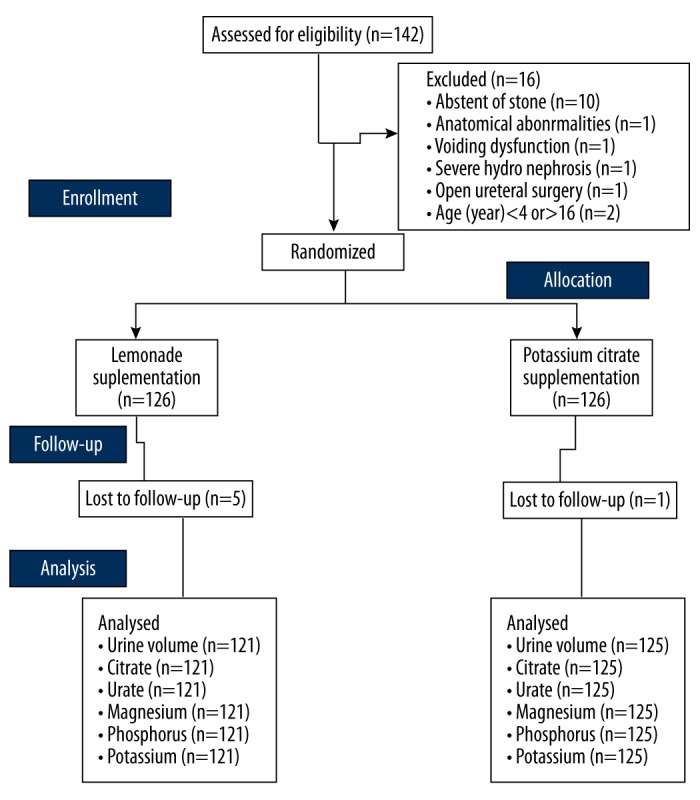
CONSORT flow diagram of the study with 15-day washout period.
Interventions
All enrolled children drank lemonade (2 mEq/kg/day citrate) in 3 divided doses for 5 days. After a 15-day washout period, all enrolled patients drank 2 mEq/kg/day of potassium citrate in 3 divided doses for 5 days [3]. Total fluid intake including water was not more than 4 L/day.
Urinary parameters measurements
On the sixth of the day of individual intervention, a 24-hour urine was collected and sent to the pathology laboratory for evaluation of pH, urine volume, citrate level, and uric acid level, as well as elemental magnesium, phosphorus, potassium, and sodium. Every child was evaluated at baseline, after drinking of lemonade and supplementation of potassium citrate (on the sixth day) [3].
Statistical analysis
Data are represented as mean ±SD of all. InStat (GraphPad, USA) was used for statistical analysis. Urinary parameters for 1-day urine collection measurements after each supplementation were compared with baseline using the Mann-Whitney test [13] following Tukey post hoc test (considering critical value [q] >3.328) [14] at 95% confidence level.
Results
Urine samples of 1 child in the potassium citrate group and 5 children in the lemonade group were lost to evaluation during the follow-up study. After potassium citrate supplementation, urine sodium level was decreased (p=0.0337; q=3.76) and pH of urine was increased (p=0.0118; q=4.389). However, after lemonade supplimentation, urine sodium level was not decreased (p=0.239) and pH of urine was not increased (p=0.253). Urine volume, citrate level, and uric acid level, as well as elemental magnesium, phosphorus, and potassium, remained unchanged after 5-day supplementation with potassium citrate or lemonade (Table 2). Potassium citrate supplementation was more effective than lemonade (Table 3).
Table 2.
Urinary parameters for one-day urine collection measurements.
| Parameters | Baseline | After supplementation | ||||
|---|---|---|---|---|---|---|
| Potassium citrate | SA* | Lemonade | SA* | |||
| Sample size | 126 | 125 | p | 121 | p | |
| Urine volume (L) | Min | 0.75 | 0.74 | 0.845 | 0.8 | 0.586 |
| Max | 2.7 | 2.72 | 1.12 | |||
| Mean ±SD | 1.45±0.49 | 1.46±0.5 | 1.56±1 | |||
| Citrate (mg) | Min | 70 | 71 | 0.898 | 69 | 0.978 |
| Max | 1534 | 1537 | 1533 | |||
| Mean ±SD | 879.02±464.27 | 879.03±465.85 | 880.38±471.85 | |||
| Urate (mg) | Min | 101 | 99 | 0.858 | 97 | 0.655 |
| Max | 1021 | 1200 | 1195 | |||
| Mean ±SD | 701.83±321.26 | 697.71±322.14 | 684.42±325.23 | |||
| Magnesium (mEq/L) | Min | 35 | 33 | 0.848 | 32 | 0.929 |
| Max | 111 | 110 | 109 | |||
| Mean ±SD | 64.7±21.7 | 64.18±21.56 | 64.33±21.27 | |||
| Phosphorus (mEq/L) | Min | 101 | 99 | 0.863 | 97 | 0.688 |
| Max | 1201 | 1200 | 1195 | |||
| Mean ±SD | 699.94±321.83 | 695.85±322.76 | 684.42±325.23 | |||
| Potassium (mEq/L) | Min | 19 | 19 | 0.056 | 15 | 0.142 |
| Max | 81 | 81 | 77 | |||
| Mean ±SD | 39.02±17.43 | 40.94±16.18 | 36.54±17.09 | |||
SA – statistical analysis with respect to the baseline; Min – minimum; Max – maximum.
Mann-Whitney test following Tukey post hoc test was performed for statistical analysis. p<0.05 and q>3.328 values were considered for significant difference.
Table 3.
The treatment-emergent effects.
| Characteristics | Baseline# | After supplementation | ||||
|---|---|---|---|---|---|---|
| Potassium citrate | SA* | Lemonade | SA* | |||
| Sample size | 126 | 125 | p | q | 121 | p |
| Gastric discomfort | 1 (1) | 7 (6) | 0.4855 | N/A | 1(1) | 0.997 |
| Oropharyngeal discomfort | 0 (0) | 9 (7) | N/P | N/P | 1(1) | N/P |
| Number of hospital visits for pain in 5 days | 3.17±0.37 | 2.84±0.37 | <0.0001 | 12.084 | 3.05±0.36 | 0.119 |
| Analgesic use (Diclofenac sodium, mg) | 390.08±116.96 | 191.6±60.82 | <0.0001 | 29.199 | 376.03±103.1 | 0.401 |
| Numbers of colic pain episodes/day | 1.59±0.8 | 1.6±0.8 | 0.895 | N/A | 1.48±0.65 | 0.594 |
Continuous data were represented as mean ±SD and constant data were represented as a number (percentage).
SA – statistical analysis with respect to the baseline.
Mann-Whitney test following Tukey post hoc test was performed for statistical analysis. p<0.05 and q>3.328 values were considered for significant difference. N/A – not applicable, N/P – not possible;
Data of five days without treatment.
Discussion
This study was performed with lemonade and potassium citrate supplementation in 3 divided doses for 5 days. The advance non-surgical treatment available for upper-tract stones is ESWL (extracorporeal shock-wave lithotripsy) [15]. ESWL therapy is the first-line therapy for pediatric urolithiasis but has a high cost of treatment and does not always prevent recurrence [16]. Pediatric SWL is preferred for renal pelvic stones but not for caliceal stones [17]. It is debatable whether diet plays a crucial role in urinary tract stone formation [18]. However, oxalate, high protein, calcium, low liquid, and salt intakes can contribute to urinary tract stone formation [19]. Citrate is responsible for inhibition of caliceal stones, and increased citrate urine excretion leads to caliceal stones in the urinary tract [20]. Therefore, citrate supplementation is recommended in pediatric urolithiasis.
Potassium citrate supplementation improves pH of urine, decreases urine sodium level, and increases the risk of gastric discomfort and oropharyngeal discomfort. Potassium citrate binds with sodium in urine and decreases sodium level in urine, increases pH of urine, and ultimately reduces stone formation [21]. These results were in line with available studies [11,12,20]. Potassium citrate is effective in treatment of pediatric urolithiasis.
The cost of 2 lemons in China is not more than 5 ¥, which is the daily dosage [22]. Lemonade has high patient acceptance and no adverse effects but did not increase urine volume or pH. Lemonade has low potassium content [23] and is a poor alkalinizing agent [3]. The treatment with lemonade has no any positive effects in pediatric urolithiasis [22]. Lemonade treatment is an alternative for mild hypocitraturia patients who are unable to tolerate first-line therapy [24] or for long-term prevention of recurrent pediatric urolithiasis [25]. However, these results were not in line with the available longitudinal study (3-months) [22], perhaps because the longitudinal studies were based on the incidence of symptomatic kidney stones as the main outcome measure [25], whereas the present study was a short-term study (5 days) and the objective was to assess effects of beverages on urinary parameters. Lemonade is not a good alternative to potassium citrate in pediatric urolithiasis.
Our study has certain limitations: the combination of lemonade and potassium citrate supplementation was not tried, a longitudinal study was not performed, we did not consider family history of stone formations, and unmeasured or residual confounding factors were not evaluated.
Conclusions
This randomized, experimental, cross-over study concluded that potassium citrate supplementation is a good therapy for preventing pediatric urolithiasis, with acceptable adverse effects. Further research is needed to assess effects of longer-duration interventions.
Acknowledgements
We thank all medical and non-medical staff of the First People’s Hospital of Tongxiang, China and Hangzhou Amcare Women’s and Children’s Hospital, China who made the study successful.
Footnotes
Conflict of Interest
None.
Source of support: Departmental sources
References
- 1.Matlaga BR, Schaeffer AJ, Novak TE, Trock BJ. Epidemiologic insights into pediatric kidney stone disease. Urol Res. 2010;38(6):453–57. doi: 10.1007/s00240-010-0327-9. [DOI] [PubMed] [Google Scholar]
- 2.Fulgham PF, Assimos DG, Pearle MS, Preminger GM. Clinical effectiveness protocols for imaging in the management of ureteral calculous disease: AUA technology assessment. J Urol. 2013;189(4):1203–13. doi: 10.1016/j.juro.2012.10.031. [DOI] [PubMed] [Google Scholar]
- 3.Koff SG, Paquette EL, Cullen J, et al. Comparison between lemonade and potassium citrate and impact on urine pH and 24-hour urine parameters in patients with kidney stone formation. Urology. 2007;69(6):1013–16. doi: 10.1016/j.urology.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Elmacı AM, Ece A, Akın F. Clinical characteristics and metabolic abnormalities in preschool-age children with urolithiasis in southeast Anatolia. J Pediatr Urol. 2014;10(3):459–99. doi: 10.1016/j.jpurol.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Abou-Elela A. Epidemiology, pathophysiology, and management of uric acid urolithiasis: A narrative review. J Adv Res. 2017;8(5):513–27. doi: 10.1016/j.jare.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanbara A, Seyama I. Effect of urine pH on uric acid excretion by manipulating food materials. Nucleosides Nucleotides Nucleic Acids. 2011;30(12):1066–71. doi: 10.1080/15257770.2011.596498. [DOI] [PubMed] [Google Scholar]
- 7.Pereira DJ, Schoolwerth AC, Pais VM. Cystinuria: Current concepts and future directions. Clin Nephrol. 2015;83(3):138–46. doi: 10.5414/cn108514. [DOI] [PubMed] [Google Scholar]
- 8.Zu’bi F, Sidler M, Harvey E, et al. Stone growth patterns and risk for surgery among children presenting with hypercalciuria, hypocitraturia, and cystinuria as underlying metabolic causes of urolithiasis. J Pediatr Urol. 2017;13(4):357.e1–7. doi: 10.1016/j.jpurol.2017.06.022. [DOI] [PubMed] [Google Scholar]
- 9.Jhagroo RA, Wertheim ML, Penniston KL. Alkali replacement raises urinary citrate excretion in patients with topiramate-induced hypocitraturia. Br J Clin Pharmacol. 2016;81(1):131–36. doi: 10.1111/bcp.12751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siener R. Can the manipulation of urinary pH by beverages assist with the prevention of stone recurrence? Urolithiasis. 2016;44(1):51–56. doi: 10.1007/s00240-015-0844-7. [DOI] [PubMed] [Google Scholar]
- 11.Kern A, Grimsby G, Mayo H, Baker LA. Medical and dietary interventions for preventing recurrent urinary stones in children. Cochrane Database Syst Rev. 2017;11:CD011252. doi: 10.1002/14651858.CD011252.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rahman F, Birowo P, Widyahening IS, Rasyid N. Effect of citrus-based products on urine profile: A systematic review and meta-analysis. F1000Res. 2017;6:220. doi: 10.12688/f1000research.10976.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar S, Jayant K, Agrawal MM, et al. Role of tamsulosin, tadalafil, and silodosin as the medical expulsive therapy in lower ureteric stone: A randomized trial (a pilot study) Urology. 2015;85(1):59–63. doi: 10.1016/j.urology.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 14.Otunctemur A, Ozbek E, Polat EC, et al. Increasing urinary calcium excretion after ceftriaxone and cephalothin therapy in adults: Possible association with urolithiasis. Urolithiasis. 2014;42(2):105–8. doi: 10.1007/s00240-013-0627-y. [DOI] [PubMed] [Google Scholar]
- 15.Jayant K, Agrawal R, Agrawal S. Tamsulosin versus tamsulosin plus tadalafil as medical expulsive therapy for lower ureteric stones: A randomized controlled trial. Int J Urol. 2014;21(10):1012–15. doi: 10.1111/iju.12496. [DOI] [PubMed] [Google Scholar]
- 16.Lu P, Wang Z, Song R, et al. The clinical efficacy of extracorporeal shock wave lithotripsy in pediatric urolithiasis: A systematic review and meta-analysis. Urolithiasis. 2015;43(3):199–206. doi: 10.1007/s00240-015-0757-5. [DOI] [PubMed] [Google Scholar]
- 17.Hammad FT, Kaya M, Kazim E. Pediatric extracorporeal shockwave lithotripsy: Its efficiency at various locations in the upper tract. J Endourol. 2009;23(2):229–35. doi: 10.1089/end.2008.0133. [DOI] [PubMed] [Google Scholar]
- 18.Seeger H, Kaelin A, Ferraro PM, et al. Changes in urinary risk profile after short-term low sodium and low calcium diet in recurrent Swiss kidney stone formers. BMC Nephrol. 2017;18(1):349. doi: 10.1186/s12882-017-0755-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferraro PM, Curhan GC, Gambaro G, Taylor EN. Total, dietary, and supplemental vitamin C intake and risk of incident kidney stones. Am J Kidney Dis. 2016;67(3):400–7. doi: 10.1053/j.ajkd.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prezioso D, Strazzullo P, Lotti T, et al. CLU Working Group. Dietary treatment of urinary risk factors for renal stone formation. A review of CLU Working Group. Arch Ital Urol Androl. 2015;87(2):105–20. doi: 10.4081/aiua.2015.2.105. [DOI] [PubMed] [Google Scholar]
- 21.Robinson MR, Leitao VA, Haleblian GE, et al. Impact of long-term potassium citrate therapy on urinary profiles and recurrent stone formation. J Urol. 2009;181(3):1145–50. doi: 10.1016/j.juro.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 22.Aras B, Kalfazade N, Tugcu V, et al. Can lemon juice be an alternative to potassium citrate in the treatment of urinary calcium stones in patients with hypocitraturia? A prospective randomized study. Urol Res. 2008;36(6):313–17. doi: 10.1007/s00240-008-0152-6. [DOI] [PubMed] [Google Scholar]
- 23.Tracy CR, Pearle MS. Update on the medical management of stone disease. Curr Opin Urol. 2009;19(2):200–4. doi: 10.1097/MOU.0b013e328323a81d. [DOI] [PubMed] [Google Scholar]
- 24.Kang DE, Sur RL, Haleblian GE, et al. Long-term lemonade based dietary manipulation in patients with hypocitraturic nephrolithiasis. J Urol. 2007;177(4):1358–62. doi: 10.1016/j.juro.2006.11.058. [DOI] [PubMed] [Google Scholar]
- 25.Odvina CV. Comparative value of orange juice versus lemonade in reducing stone-forming risk. Clin J Am Soc Nephrol. 2006;1(6):1269–74. doi: 10.2215/CJN.00800306. [DOI] [PubMed] [Google Scholar]


