Abstract
Background
Triple negative breast cancer (TNBC) has a more aggressive recurrence. Previous reports have demonstrated that sphingosine kinase 1 (SphK1) is a crucial regulator of breast cancer progression. However, the correlation of SphK1 with clinical prognosis has been poorly investigated. Thus, we aimed to elaborate the role of SphK1 in TNBC metastasis.
Material/Methods
We first determined the level of SphK1 in breast cancer tissue samples and breast cancer cells. Furthermore, the expression of HER2 and phosphor-SphK1 (pSphK1) in human breast cancer tissue samples was determined by immunohistochemical analysis. Associations between SphK1 and clinical parameters of tumors were analyzed. The activity of SphK1 was measured by fluorescence analysis. Extracellular sphingosine-1-phosphate (S1P) was detected using an ELISA kit. Associations between SphK1 and metastasis potential were analyzed by Transwell assay.
Results
Levels of SphK1 in TNBC patients were significantly higher than levels in other patients with other breast tumors. The expression of SphK1 was positively correlated with poor overall survival (OS) and progression-free survival (PFS), as well as poor response to 5-FU and doxorubicin. The depression of SphK1 thus could repress the Notch signaling pathway, reduce migration, and invasion of TNBC cells in vivo and in vitro. Furthermore, silencing of SphK1 by Ad-SPHK1-siRNA or SphK1 inhibitor PF543 sensitized TNBCs to 5-FU and doxorubicin. Our results also indicated that SphK1 inhibition could effectively counteracts tumors metastasis via Notch signaling pathways, indicating a potentially anti-tumor strategy in TNBC.
Conclusions
We found that elevated levels of pSphK1 were positive correlation with high expression of S1P, which in turn promoted metastasis of TNBC through S1P/S1PR3/Notch signaling pathway.
MeSH Keywords: Neoplasm Metastasis; Receptors, Lysosphingolipid; Triple Negative Breast Neoplasms
Background
Breast cancer is the most aggressive malignant tumor in women [1,2]. Triple negative breast cancer (TNBC) accounts for approximately 15% of breast cancers, and possesses properties of serious invasion, poor prognosis, and short survival [3–5]. In addition, TNBC is a subtype of breast cancer in which progesterone receptor (PR) and estrogen receptor (ER) are not expressed, and human epidermal growth factor receptor 2 (EGFR-2) is not amplified or overexpressed. It is widely accepted that TNBC is a breast cancer that has less than 1% of tumor cells expressing ER and PR as measured by immunohistochemistry [6]. Compared with other subtypes of breast cancer, TNBC is common in young women. Moreover, both hormone therapies, such as tamoxifen, and targeted therapy against HER2 by Herceptin are not effective therapeutic agents against this type of tumor cells [7–9]. Accordingly, TNBC is characterized by more aggressive clinical manifestation than other subtypes of breast cancer.
Previous reports have demonstrated that the development of breast cancer usually undergoes a succession of processes, including ductal epithelial hyperplasia, atypical hyperplasia, carcinoma in situ, and invasive carcinoma [10,11]. There is a series of changes within molecular biology and biological behavior for breast cancer cell malignant transformation and progression; however, the mechanism to control the malignant transformation and progression remains unclear.
Sphingomyelin, cholesterol, and other phospholipids are important components in the cell membrane, and their most pivotal function is acting as bioactive signaling molecules. Among them, sphingosine-1-phosphate (S1P) produced by sphingomyelin, is not only used as intracellular second messengers to regulated the cell cycle, but also binds to the specific receptors on the cell surface to modulate cell proliferation, apoptosis, invasion, migration, and adhesion molecule expression [12–14]. In addition, S1P can promote the survival of many cell types and simultaneously inhibit its apoptosis, and the biological effect mediated by S1P is closely correlated with tumor formation [15].
Sphingosine kinase (SphK) plays a crucial role in the process of S1P biosynthesis. Previous reports have shown that SphK has two isoforms in humans and mice, SphK1 and SphK2 [16]. Among them, SphK1 is mainly distributed in brain, heart, lung, liver, spleen, and hematopoietic immune system, and it is important in regulating the generation of S1P and also participates in the proliferation of various tumor cells [17,18]. SphK1 forms a complex site of many cell surface receptors, in which lysophosphatidic acid (LPA), S1P, and epidermal growth factor (EGF) receptors regulate the migration and invasion in tumor cells [19]. Sato et al. reported that SphK1 can move actin from the binding site to the cell membrane to form ruffles lamellipodia according to the need for redistribution [20]. Moreover, SphK1 is also a necessary factor for the induction of nucleotide migration in mesangial cells; and upregulation of SphK1 leads to re-response to migration, and promotes migration in endothelial cells. Matula et al. also reported that upregulation of SphK1 expression was induced by the interaction of LPA1 and EGFR receptor, and promoted cancer cells migration and invasion in gastric cancer [21]. Subsequently, other studies have showed that silencing of SphK1 could inhibit the induction of EGF to MCF7 breast cancer cells, and decrease the migration of HEK293 cells induced by EGF [22,23]. These results suggested that SphK1/S1P may be a key regulator of cell migration, which can promote tumor cell invasion and metastasis. Nevertheless, despite previous evidence that indicated that higher expression of SphK1 was presented in ER-negative tumors compared with ER-positive tumors, the therapeutic implications and its molecular mechanism of SphK1 in TNBC metastasis have not been well explored [17,18].
In the current study, we systematically examined the expression of SphK1 in TNBC cells, then further explored the relationship between SphK1 expression and the invasion and metastasis capability in TNBC cells and the molecular mechanisms to provide an experimental basis for further revealing the biological function of SphK1 in malignant TNBC, and providing a potentially critical anti-tumor strategy for malignant tumors.
Material and Methods
Human breast cancer tissue samples
In this study we selected 239 patients with identified breast cancer who underwent surgical resection in Southwest Hospital between January 2016 and December 2016. While in hospital, tumor tissue samples were collected from 76 breast cancer patients by surgical resection (patients provided informed consent), furthermore, these patients had invasive tumors with tumor diameter larger than 1.5 cm. We excluded 11 of the 76 patients (Table 1); the exclusion criteria included: patients who received adjuvant chemotherapy (four cases), patients with body mass index (BMI) greater than 35 (two cases), patients who suffered from bilateral breast cancer (three cases), and patients who suffered from other organ tumors (two cases). All collected tissue samples were processed by rapid cryopreservation using liquid nitrogen and then stored at −80°C. This research was approved by the Medical Ethics Committee of Southwest Hospital (No 2017041).
Table 1.
The clinical background of patients (n=65).
| Characteristics | Number of patients (%) |
|---|---|
| Age(years) | |
| <60 | 40 (61.5) |
| ≥60 | 25 (38.5) |
| Primary tumor | |
| T1 | 19 (29.2) |
| T2 | 38 (58.5) |
| T3 | 8 (12.3) |
| Molecular subtype | |
| TNBC | 16 (24.6) |
| Non-TNBC | 49 (75.4) |
| Menopause status | |
| Premenopausal | 38 (58.5) |
| Postmenopausal | 27 (41.5) |
| Regional lymph nodes* | |
| N0 | 35 (53.8) |
| N1 | 14 (21.5) |
| N2 | 10 (15.4) |
| N3 | 6 (9.3) |
| Stage* | |
| I | 9 (13.8) |
| II | 32 (49.2) |
| IIII | 24 (36.9) |
| Distant metastasis* | |
| M0 | 63 (96.9) |
| M1 | 2 (3.1) |
| Vascular invasion* | |
| Absent | 53 (81.5) |
| Present | 12 (18.5) |
| Lymphatic invasion* | |
| Absent | 48 (73.8) |
| Present | 17 (26.2) |
| HER2 overexpression/amplification | |
| Negative | 54 (83.1) |
| Positive | 11 (16.9) |
| Estrogen receptor expression | |
| Negative | 25 (38.5) |
| Positive | 40 (61.5) |
According to cancer grading system of AJCC.
Pathologic examination
All selected tissue samples were examined by at least two experienced pathologists. Paraffin-embedded blocks from each selected specimen were used for immunohistochemistry. Serial 4-mm paraffin sections were stained for H & E (hematoxylin and eosin), HER2, ER, PR, the proliferation index marker Ki-67, phosphorylation of SphK1 (pSphK1), and negative control. The protein expression of HER2 was scored. Ki-67 proliferation index marker was scored by counting positive and negative nuclei in selected tumor tissue specimens, and the proliferation index was acquired by counting the percentage of positive cells. At the same time, HER2 expression was also detected by immunohistochemistry. Furthermore, we determined the expression of pSphK1 in these HER2 positive and negative tumors. Immunohistochemistry and the staining scores were conducted as previously described [24].
Cell cultures and reagents
Human breast cancer cell lines, including MCF-7, SK-BR-3, MDA-MB-231, and LM2-4, were bought from American Type Culture Collection (ATCC, USA). MCF-7 cells were cultured in phenol-red free IMEM medium (Gibco BRL, USA). MDA-MB-231 and its metastatic variant LM2-4 cells were cultured in phenol-red free RPMI-1640 medium (Gibco BRL, USA). SK-BR-3 cells were maintained in DMEM (Dulbecco’s Modified Eagle’s Medium) (Gibco BRL, USA). All medium contained 10% heat-inactivated FBS (fetal bovine serum) (Gibco BRL, USA) and supplement 100x penicillin-streptomycin solution (LEAGENE), at 37°C in a 5% CO2 atmosphere. Human mammary cell line MCF-10A was used as the normal control. When breast cells or breast cancer cells grew to 80% to 90% convergence, the cells were used for passage or subsequent experiments. 5-FU and doxorubicin were purchased from Sigma (Sigma-Aldrich, USA). S1P was purchased from Enzo Life Sciences (Farmingdale, USA). Inhibitor of SphK1 PF543 was obtained from ApexBio (ApexBio, USA). TY52156, CAY10444, and JTE013 were purchased from Cayman Chemicals. In addition, LPA was obtained from Avanti Polar Lipids. SphK1 antibody was obtained from Exalpha Biologicals Inc.; GAPDH antibody was purchased from Cell Signaling Technology (CA, USA). Notch1 intracellular domain (N1ICD) antibody was obtained from Millipore (Merck Millipore, Germany).
Animal studies for in vivo metastasis
Animal research was approved by the Animal Ethics Committee of Third Military Medical University (No 20170193). In all, 15 healthy four to six week old female NOD/SCID mice were selected and were maintained in pathogen-free conditions at the Animal Facility of Third Military Medical University and received humane care, according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences. Then 15 NOD/SCID mice were randomly divided into control group, Ad-NC-siRNA group, or Ad-SPHK1-siRNA group, respectively. For in vivo metastasis assay, 2×105 MDA-MB-231 cells from the control, Ad-NC-siRNA, or Ad-SPHK1-siRNA #2 group with Matrigel at a ratio of 1: 1, then injected into the spleen of NOD/SCID female mice. Then four to six weeks after injection, all animals were sacrificed and imaged through an in vivo imaging system, examined for tumors metastatic nodules in different organs, and the weight of tumors recorded.
Measurement of SphK1 activity
The activity of SphK1 was detected as previously reported [25]. Briefly, 70 μg of protein from breast cancer cells was incubated with 20 μM of 15-NBDSph and ATP in SphK buffer (pH 7.4, containing 10 mM KCl, 15 mM MgCl2, 0.005% Triton X-100). After incubation for 30 minutes at 37°C, we added 100 μL potassium phosphate buffer (1 M, pH 8.5), then added 500 μL CHCL3 with MeOH at the ratio of 2: 1. After gently mixing and centrifugation for phase separation, the upper aqueous layer was removed to a new PE microplate (Greiner Bio-One), and then we added 75 μL of dimethylformamide (Merck). Fluorescence intensity was determined at 485/535 nm.
Measurement the levels of S1P
The levels of S1P were detected by S1P competitive ELISA kit, which had a sensitivity of 30 nM (Echelon Bioscience Inc.). Then 105 cells were plated into 6-wells plates cultured in RPMI-1640 medium without FBS for another 14 hours, then pretreated with inhibitors for 15 minutes before stimulation with 10% FBS for up to 240 minutes. The supernatant was collected for S1P analysis according to manufacturer’s instructions.
Adenovirus transfection
Breast cancer cells MDA-MB-231 and LM2-4 were seeded into 6-well plates at a density of 2×105 cells per well. One day after adherence, cells were cultured in RPMI-1640 medium supplemented with 2% FBS. These cells were infected with Ad-NC-siRNA or Ad-SPHK1-siRNA at 10 MOI, respectively. Virus-containing medium was changed with fresh RPMI-1640 medium containing 10% FBS after transfection 12 hours later. The transfection efficiency was detected by flow cytometry.
CCK-8 assay was used to detection cell viability
Breast cancer cells were seeded into 96-well plates at the concentration of 1×104 per well, then cells were treated with doxorubicin or 5-FU at different doses for 48 hours. CCK-8 assay was using for detecting breast cancer cell viability. After treatment for 48 hour, 10 μL/well CCK-8 solution was added to the 96-well plates, then incubated at 37°C, in a 5% CO2 humidified incubator for 2–4 hours. The absorbance value was read by a microplate reader (Bio-Rad, CA, USA) at 450 nm and cell viability was counted. The experiment was repeated at least three times.
Western blotting
Treated breast cancer cells were collected and lysis on ice for 30 minutes, the cell supernatant was obtained by centrifugation. The protein supernatant was carefully transferred to a new PE. Then 20 μg protein samples were taken for SDS-PAGE electrophoresis. N1ICD and GAPDH antibodies were respectively incubated at 4 °C overnight. The next day, secondary antibody was added and incubated at room temperature for two hours. The signals were detected by enhanced chemiluminescence (Pierce Biotechnology). GAPDH antibody was regard as an internal control. Analysis of the absorbance values of each band was done using QuantityOne software; the experiment was repeated three times and representative images were captured and analyzed.
Quantitative real-time PCR assay
Samples of total RNAs were isolated from breast cancer cells and breast cancer tissue samples using TRIzol agent (Invitrogen, USA) according to manufacturer’s instruction. NanoDrop-2000 was used to detect the purity and concentration of total RNAs. The integrity of RNAs was determined by electrophoresis. We took 1 μg total RNA for synthesizing the first strand cDNA according to the method of the reverse transcription kit (Promega Corporation, USA). Real-time (RT)-PCR was used to detect the mRNA expression of SPHK1, Hes1, Gli1, and DKK1. GAPDH mRNA was used as an internal control.
Migration and invasion assays
Migration and invasion assays were performed according to the previously reports [26]. In all, 3×104 breast cancer cells that were suspend in DMEM or RPMI-1640 medium without FBS were plated into the upper chamber of Transwell plates (Merck Millipore, Germany) and 20% FBS was added to medium in the lower chamber of 24-well plates (Costar, USA). For invasion assay, the upper side of the filter was covered with Matrigel (BD, Franklin Lakes, NJ, USA) and 1×105 cells were plated in the upper chamber. After 24 hours, cells on the upper chamber were removed and the filter membrane was fixed with 4% paraformaldehyde and stained with 0.5% crystal violet (Beyotime). The numbers of cells were counted in the membrane using 10 contiguous fields for each sample using a 40× objective.
Statistical analysis
SPSS version 16.0 statistic software was using for data processing. The results are presented with average ± standard deviation. The Student’s t-test was used for comparing the variables between two groups. A value of p<0.05 was marked as * and considered statistically significant. The correlation between SphK1 expression and metastatic potential was determined by Pearson correlation coefficient analysis.
Results
SphK1 expression was upregulated in human breast cancer tissue samples
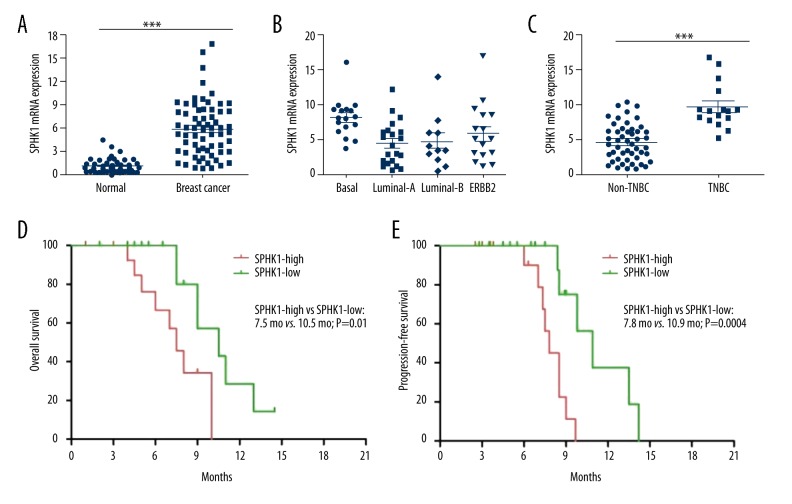
We detected the expression of SphK1 by RT-PCR in tumor tissue samples and adjacent normal tissue samples from 65 breast cancer patients. Our data showed that the mRNA level of SphK1 was more than three-fold higher in human breast cancer tissue samples compared to adjacent normal breast tissue samples in 76.92% of patients (50 of 65 cases) (Figure 1A). We further determined the expression of SphK1 in different molecular tumor subtypes, and the results indicated that basal-like subtype displayed the highest SphK1 gene expression of all the different molecular tumor subtypes (Figure 1B). Furthermore, we also detected the expression of SphK1 in TNBC and non-TNBC patients. Our results demonstrated that the expression of SphK1 was evidently higher in TNBC patients (16 cases) compared with non-TNBC patients (49 cases) (Figure 1C). All breast cancer patients were divided into SphK1-high group and SphK1-low group according to SphK1 expression, and our survival analysis further indicated an inverse correlation between SphK1 expression and overall survival (OS) (Figure 1D) and progression-free survival (PFS) (Figure 1E) in breast cancer patients (p=0.01 and p=0.004, respectively). These results demonstrated that high expression of SphK1 was correlated with poorer survival and prognosis in human breast cancer patients.
Figure 1.
SPHK1 expression was upregulated in breast cancer tissue samples. (A) RT-PCR assay was used to detect the SphK1 expression in human breast cancer tissue samples and the paired adjacent normal breast tissues. Two-tailed Student t-test was used for statistical significance. Each dot represents an individual patient. (B) The mRNA expression of SphK1 in different breast cancer subtypes. (C) The mRNA expression of SphK1 in triple negative breast cancer (TNBC) and non-TNBC. (D) Kaplan-Meier plots of overall survival (OS) in all breast cancer patients. (E) Kaplan-Meier plots of progression-free survival (PFS) in all breast cancer patients.
SphK1 expression was upregulated in TNBC patients, and S1P levels were correlated with high expression of p-SphK1
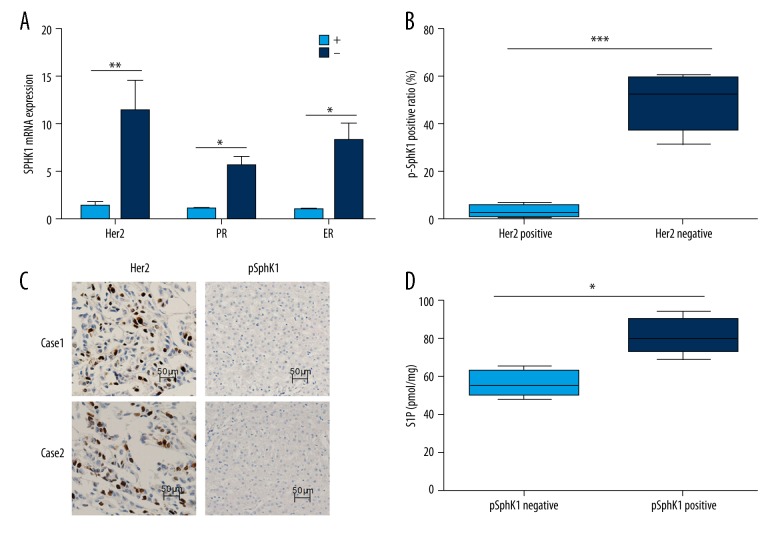
We further detected that HER2-negative (HER2-) tumors expressed higher SphK1 expression than HER2-positive (Her+) tumors; similar results were seen for PR-negative and ER-negative tumors (Figure 2A). Furthermore, previous reports have shown that pSphK1 (Ser-225) expression is involved in S1P export, and have detected the expression of pSphK1 in human breast cancer tissues by immunohistochemistry. Our results showed that pSphK1 had higher expression in HER2-negative tumors compared with HER-positive tumors (Figure 2B). All of HER2-positive patients had negative expression of pSphK1; representative images are shown in Figure 2C. We also observed much more S1P export in pSphK1 positive tumors than pSphK1 negative tumors, the result demonstrated that pSphK1 expression was evidently correlated with S1P expression in human breast cancer tissues (Figure 2D). The correlation between pSphK1 expression and clinicopathologic factors in human breast cancer tissue samples are shown in Table. 2.
Figure 2.
SPHK1 expression was upregulated in TNBC patients, and S1P levels are correlated with high expression of pSphK1. (A) The mRNA expression of SPHK1 in HER2-positive, HER2 (+), HER2-negative, HER2 (−), PR-positive, PR (+), PR-negative, PR (−) and ER-positive, ER (+), ER-negative, ER (−) breast cancer patients. (B) The positive ratio of pSphK1 was detected in HER2-positive and negative patients. (C) The expression of HER2 and pSphK1 were detected by immunohistochemistry, the represent images were shown. (D) S1P levels were detected in breast cancer patients with pSphK1 positive or negative.
Table 2.
The relationship between pSphK1 and clinicopathologic factors.
| Index | pSphK1 (n=65) | P value | |
|---|---|---|---|
| Negative | Positive | ||
| Regional lymph nodes* | |||
| Negative | 29 | 17 | 0.53 |
| Positive | 11 | 8 | |
| Primary tumor* | |||
| T1 | 13 | 6 | 0.87 |
| T2, T3, T4 | 32 | 14 | |
| HER2 overexpression/amplification | |||
| Negative | 31 | 23 | 0.017 |
| Positive | 10 | 1 | |
| Progesterone receptor expression | |||
| Negative | 10 | 4 | 0.39 |
| Positive | 35 | 16 | |
| Estrogen receptor expression | |||
| Negative | 19 | 6 | 0.032 |
| Positive | 24 | 16 | |
| Ki-67 index | |||
| <14 | 13 | 11 | 0.043 |
| ≥14 | 31 | 9 | |
| Vascular invasion* | |||
| Absent | 34 | 17 | 0.26 |
| Present | 9 | 5 | |
| Lymphatic invasion * | |||
| Absent | 30 | 18 | 0.037 |
| Present | 12 | 5 | |
According to cancer grading system of AJCC.
SPHK1 expression was upregulated human breast cancer cell lines and the metastatic ability of breast cancer cells were positively associated with SphK1 expression
RT-PCR assay was used to determine SphK1 expression on samples derived from human breast cancer cell lines MCF-7, SK-BR-3, MDA-MB-231, and LM2-4; breast epithelial cell line MCF-10A was regard as normal control. Our data demonstrated that compared to MCF-10A, the expression of SphK1 was evidently higher in breast cancer cell lines (Figure 3A and 3B). Furthermore, SphK1 activity was also higher in breast cancer cell lines compared to MCF-10A (Figure 3C). Moreover, TNBC cell lines, both MDA-MB-231 and its variant LM2-4 cells, exhibited the most pronounced metastatic abilities (Figure 3D). Further analysis indicated that the metastatic capability of breast cancer cells was positively associated with SphK1 expression (Figure 3E).
Figure 3.
SPHK1 levels were higher in TNBC cells compared with non-TNBC cells, and the metastatic ability of breast cancer cells are positively correlated with SphK1 expression. (A) The mRNA expression of SphK1 was detected by RT-PCR in breast cancer cell lines and breast epithelial cell line MCF10A, respectively. (B) The activity of SphK1 were determined by fluorescence intensity at 485/535 nm. (C) Migration ability was determined in breast cancer cells derived from MCF-7, MDA-MB-231, and LM2-4, the breast epithelial cell line MCF10A was considered as normal control. (D) Pearson’s correlation analysis was used to determine the correlation between SphK1 expression and migration ability of BC cells (r2=0.968, p=0.004). All data was presents by mean ±SD; * p<0.05; ** p<0.01.
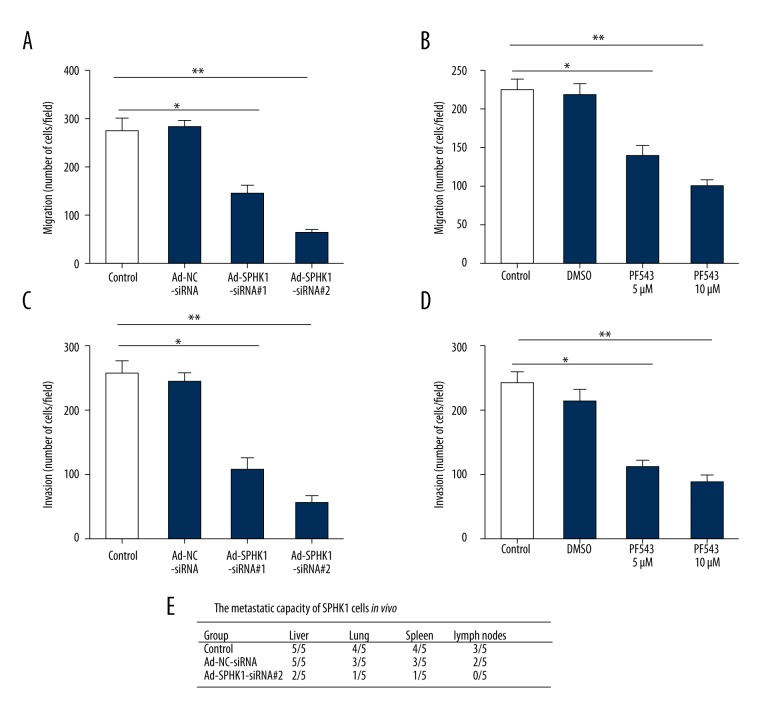
Inhibition of SphK1 impaired migration and invasion capability of MDA-MB-231 cells, and reduced the metastatic ability of MDA-MB-231 cells in NOD/SCID mice
To determine whether SphK1 expression was responsible for metastasis of TNBC cells, MDA-MB-231 cells were infected with adenovirus expressing SphK1 siRNAs (Ad-SPHK1-siRNA #1 and Ad-SPHK1-siRNA #2) (Figure 4A) or treatment with SphK1 inhibitor PF543 (5 μM and 10 μM) (Figure 4B) for 24 hours, Transwell assay was used to evaluated migration ability. Our results showed that the migration ability was remarkably reduced in Ad-SPHK1-siRNA and SphK1 inhibitor group compared with the control group (Figure 4A and 4B). A similar phenomenon was shown in invasion ability (Figure 4C, 4D). Intrasplenic injection has been indicated as a valid method for estimating the metastatic ability of MDA-MB-231 cells in vivo. In our study, cells from control, Ad-NC-siRNA or Ad-SPHK1-siRNA #2 group, respectively, were injected into the spleen of NOD/SCID mice; mice in the control group, and the Ad-NC-siRNA group all developed the primary tumors, with several of the injected mice having tumors occurring in the liver (5/5 for control and Ad-NC-siRNA group), the lung (4/5 for control group, 3/5 for Ad-NC-siRNA group), the spleen (2/5 for control group, 3/5 for Ad-NC-siRNA group) and the lymph node metastasis (3/5 for control group, 2/5 for Ad-NC-siRNA group). By contrast, mice injected with Ad-SPHK1-siRNA #2 cells displayed fewer metastatic nodules in different organs (Figure 4E). All together, these data indicated that the metastatic ability of TNBC cells was positively correlated with SphK1 expression.
Figure 4.
Inhibition of SphK1 impairs migration and invasion ability of MDA-MB-231 cells, and reduced the metastatic ability of MDA-MB-231 cells in NOD/SCID mice. (A) MDA-MB-231 cells were infected with Ad-NC-siRNA, Ad-SPHK1-siRNA #1, or Ad-SPHK1-siRNA #2 at 10 MOI for 24 hours, migration was determined. (B) MDA-MB-231 cells were treated with SphK1 inhibitor PF543 (5 μM and 10 μM) for 24 hours, migration was determined. (C) MDA-MB-231 cells were infected with Ad-NC-siRNA, Ad-SPHK1-siRNA #1, or Ad-SPHK1-siRNA #2 at 10 MOI for 24 hours, invasion was determined. (D) MDA-MB-231 cells were treated with SphK1 inhibitor PF543 (5 μM and 10 μM) for 24 hours, invasion was determined. All data was shown by mean ±SD; * p<0.05; ** p<0.01. (E) Silencing of SphK1 expression in MDA-MB-231 cells, the in vivo metastatic ability was determined by intrasplenic injection of MDA-MB-231 cells in female NOD/SCID mice. In four to six weeks after injection, animals were scarified for examination of tumor metastasis in different organs.
Inhibition of SphK1 induced breast cancer cells re-sensitized to 5-FU and doxorubicin
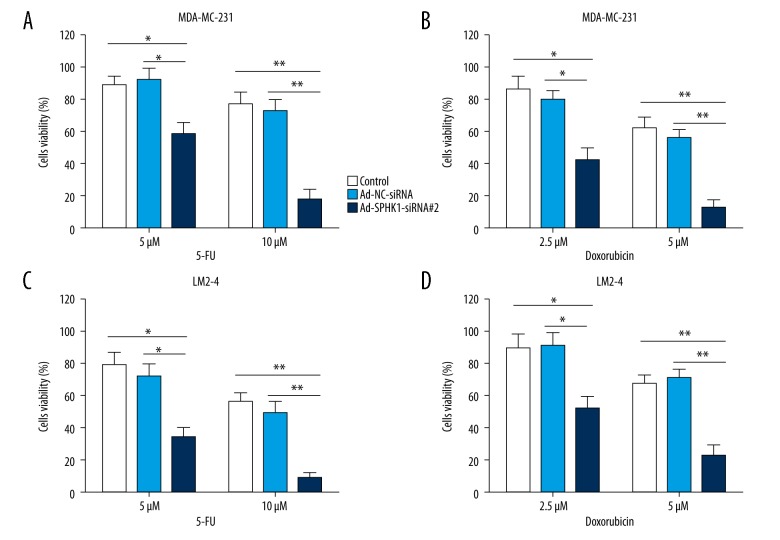
We further determined the effect of inhibiting SphK1 levels on the viability of TNBC cells MDA-MB-231 and its variant LM2-4. MDA-MB-231 cells transfected with Ad-NC-siRNA or Ad-SPHK1-siRNA #2 for 24 hours, followed by treatment with 5 μM or 10 μM 5-FU for 48 hours (Figure 5A), or treatment with 2.5 μM or 5 μM doxorubicin for 48 hours (Figure 5B); CCK-8 assay was used for evaluating cell viability. The data indicated that cell viability was predominantly decreased in the Ad-SPHK1-siRNA #2 group compared with the control or the Ad-NC-siRNA group (Figure 5A, 5B). A similar result was shown in MDA-MB-231 variant LM2-4 cells (Figure 5C, 5D). These data suggested that induction of cell death was a major mechanism for inhibition of SphK1 in modulating sensitivity of TNBC cells to 5-FU or doxorubicin.
Figure 5.
Inhibition of SphK1 induced breast cancer cells re-sensitized to 5-FU and doxorubicin. (A, B) Cell viability by CCK-8 assay in MDA-MB-231 cells transfected with Ad-NC-siRNA or Ad-SPHK1-siRNA #2 at 10 MOI for 24 hours, followed by treatment with 5 μM or 10 μM 5-FU for 48 hours (A), or treatment with 2.5 μM or 5 μM doxorubicin for 48 hours (B). (C, D) Cell viability by CCK-8 assay in LM2-4 cells transfected with Ad-NC-siRNA or Ad-SPHK1-siRNA#2 at 10 MOI for 24 hours, followed by treatment with 5 μM or 10 μM 5-FU for 48 hours (C), or treatment with 2.5 μM or 5 μM doxorubicin for 48 hours (D). All data presents the mean ±SD of at least three independent experiments; * p<0.05; ** p<0.01.
SphK1 lead to increase S1P levels and activated Notch signaling via S1PR3
Much evidence has demonstrated that S1PR plays an important role in SphK1 induced cell survival, so, we next focused on Wnt, Notch, and Hedgehog signaling pathway as candidates downstream of S1PR. In MDA-MB-231 cells, silencing the expression of SphK1 with Ad-SPHK1-siRNA #2 for 24 hours could obviously reduce the levels of Notch target gene Hes1 (Figure 6A). To the contrary, stimulation with S1P could evidently induce the expression of the Hes1 in MDA-MB-231 cells (Figure 6B). However, neither Gli1 (Hedgehog target gene) nor Dkk1 (Wnt target gene) was induced (Figure 6A and 6B). Furthermore, S1P-stimulated Hes1 expression was significantly repressed by S1PR3 antagonists Y52156 (2 mM), or 10444 (5 mM), but not for S1PR2 antagonists JTE013 (2 mM) (Figure 6C). To investigate whether S1P could activate the Notch signaling pathway, we also detected the cleavage of Notch in MDA-MB-231 cells. Treatment of MDA-MB-231 cells with S1P could remarkably upregulated Notch intracellular domain (N1ICD), which suggested induced activation of the Notch signaling pathway (Figure 6D). Collectively, these results demonstrated that SphK1 mediated S1P-induced Notch signaling pathway activation via S1PR3.
Figure 6.
SphK1 leads to increase S1P levels and enhances Notch signaling pathway via S1PR3. (A) TNBC cells MDA-MB-231 were transfected with Ad-NC-siRNA or Ad-SPHK1-siRNA #2 at 10 MOI for 24 hours, RT-PCR assay was using for detection the expression of Hes1 (Notch signaling target gene), Gli1 (Hedgehog signaling target gene), and Dkk1 (Wnt signaling target gene). (B) MDA-MB-231 cells were treatment with S1P (200 nM) or LPA (200 nM) for 24 hours, expression levels of Hes1, Gli1, and Dkk1 were detected by RT-PCR. (C) Effects of TY52156 (2 mM), CAY10444 (5 mM) or JTE013 (2 mM) on S1P-induced Hes1 expression by RT-PCR. Data represent mean ±SD (n=3). (D) Effects of S1P, LPA, and N1ICD overexpression (N1ICD OE) on N1ICD production by western blotting, GAPDH was regard as control.
Discussion
Unlike the anti-proliferative effects of ceramide and sphingosine, S1P can promote cells proliferation, migration, and cell survival, stimulate angiogenesis, and play a crucial role in the pathogenesis of inflammation as well as chemotaxis of lymphocytes [27]. SphK1 is mainly responsible for the key enzyme responsible for the formation of S1P in vivo, including two subtypes, SphK1, and SphK2; in which SphK1, activated by several extracellular receptor agonists, acts as the main factor for the regulation of the levels of extracellular S1P. Previous reports have shown that SphK1 expression in tumor tissue samples was significantly higher than normal tissue samples, tumor tissue included lung cancer, gastric cancer, endometrial cancer, prostate cancer, thyroid cancer, head and neck squamous cell carcinoma, intracranial tumor, and colon cancer [28–30]. Inhibiting expression of SphK1 can depress the proliferation of tumor cells and promote apoptosis. Lu et al. reported that inhibition of SphK1 expression can lead to significant decrease in migration capacity of HCC cells [31]. Recently, Zhu et al. found that patients with amplified SphK1 protein expression had shorter OS and DFS times compared with patients with lower SphK1 protein expression, which indicated that SphK1 may be act as a predictive factor in breast cancer patients [32]. Our data showed that there was rarely any SphK1 expression in normal breast tissue, weak expression in atypical hyperplasia tissues, and high expression in breast carcinoma tissues, suggesting that SphK1 could play a key role in development of breast cancer; which was consistent with previous reports [33]. Furthermore, our results showed that the cytotoxic effects of doxorubicin and 5-FU in TNBC cells were enhanced by SphK1 siRNA knockdown in vitro; which was consistent with results published by Arpita et al. [34]. In addition, our data also indicated that the combination of SphK1 inhibitors and conventional chemotherapeutic drugs was effective against TNBC cells. Indeed, cells cytotoxicity was appreciably enhanced by the combination of conventional chemotherapeutic drugs with PF543. This view has also been supported by cases studies in other cancers. Accordingly, to prolong the clinical application of this strategy, breast cancer patients who poorly respond to doxorubicin may benefit from SphK1-targeted therapy due to the obviously higher expression of SphK1 in breast tumors, thus presenting a promising anti-tumor strategy.
In this study, the data showed that the expression of SphK1 in TNBC was obviously upregulated by comparative determination of SphK1 expression in breast cancer tissue samples and cell lines. Furthermore, our results demonstrated that the upregulation of SphK1 plays a crucial role in TNBC cells invasion and metastasis. It has been reported that SKI-5C (a chemotherapeutic drug) could significantly depress the serum-induced growth and survival of TNBC cells by ERK/AKT pathways [25,35], which, therefore, implied that TNBC cells are re-sensitive to SphK1 inhibitor. Our data also indicated that the activity of SphK1 was crucial for S1P export in TNBC cells in response to serum stimulation. However, the downstream mechanism of the activated SphK1/S1P pathway that promotes TNBC cells invasion and migration is still unclear. Accordingly, our results will provide further evidence for the pharmacological repression of SphK1 as an effective therapeutic strategy in treatment of TNBC.
In the current study, the focus was on the candidates downstream of the S1PR, such as Notch, Hedgehog, and Wnt. Our data showed that the Notch target gene Hes1 was obviously upregulated by stimulation with S1P in MDA-MB-231 cells, and S1P-induced Hes1 expression was obviously inhibited by S1PR3 antagonists, which indicated that the Notch signaling pathway was involved in the activation of the SphK1/S1P pathway to promote the invasion and migration of TNBC cells.
Conclusions
All in all, our study indicated that SphK1 represented a valid therapeutic target in breast cancers with ER-positive tumors, especially in TNBC. Our data also validated that the upregulation of SphK1 mRNA in TNBC was closely correlated with poor OS and PFS in breast cancer patients. Indeed, we also demonstrated that patients with TNBC who poorly respond to treatment with doxorubicin were closely correlated to the high level of SphK1. Furthermore, we also demonstrated by in vitro and in vivo models, that the depression of SphK1 in TNBC cells could inhibit the signaling mechanism through S1P, Notch signaling pathway. In addition, the downregulation of SphK1 could sensitize TNBC cells to chemotherapeutic drugs such as doxorubicin and 5-FU. Accordingly, the development of SphK1-targeted therapeutic strategies may be expected to further promote treatment and prognosis of patients with TNBC.
Footnotes
Conflict of interests
None.
Source of support: Departmental sources
References
- 1.Massarotti C, Scaruffi P, Lambertini M, et al. State of the art on oocyte cryopreservation in female cancer patients: A critical review of the literature. Cancer Treat Rev. 2017;57:50–57. doi: 10.1016/j.ctrv.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 2.Shukla G, Khera HK, Srivastava AK, et al. Therapeutic potential, challenges and future perspective of cancer stem cells in translational oncology: A critical review. Curr Stem Cell Res Ther. 2017;12(3):207–24. doi: 10.2174/1574888X11666161028143224. [DOI] [PubMed] [Google Scholar]
- 3.Fleisher B, Clarke C, Ait-Oudhia S. Current advances in biomarkers for targeted therapy in triple-negative breast cancer. Breast Cancer (Dove Med Press) 2016;8:183–97. doi: 10.2147/BCTT.S114659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balduzzi S, Mantarro S, Guarneri V, et al. Trastuzumab-containing regimens for metastatic breast cancer. Cochrane Database Syst Rev. 2014;(6):CD006242. doi: 10.1002/14651858.CD006242.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Afghahi A, Telli ML, Kurian AW. Genetics of triple-negative breast cancer: Implications for patient care. Curr Probl Cancer. 2016;40(2–4):130–40. doi: 10.1016/j.currproblcancer.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Hubalek M, Czech T, Müller H. Biological subtypes of triple-negative breast cancer. Breast Care (Basel) 2017;12(1):8–14. doi: 10.1159/000455820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herold CI, Anders CK. New targets for triple-negative breast cancer. Oncology (Williston Park) 2013;27(9):846–54. [PubMed] [Google Scholar]
- 8.Boyle P. Triple-negative breast cancer: Epidemiological considerations and recommendations. Ann Oncol. 2012;23(Suppl 6):vi7–12. doi: 10.1093/annonc/mds187. [DOI] [PubMed] [Google Scholar]
- 9.Harbeck N, Gnant M. Breast cancer. Lancet. 2017;389(10074):1134–50. doi: 10.1016/S0140-6736(16)31891-8. [DOI] [PubMed] [Google Scholar]
- 10.Buttigliero C, Monagheddu C, Petroni P, et al. Prognostic role of vitamin d status and efficacy of vitamin D supplementation in cancer patients: A systematic review. Oncologist. 2011;16(9):1215–27. doi: 10.1634/theoncologist.2011-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernier J, Poortmans PM. Surgery and radiation therapy of triple-negative breast cancers: From biology to clinics. Breast. 2016;28:148–55. doi: 10.1016/j.breast.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 12.Wollny T, Wątek M, Durnaś B, et al. Sphingosine-1-phosphate metabolism and its role in the development of inflammatory bowel disease. Int J Mol Sci. 2017;18(4) doi: 10.3390/ijms18040741. pii: E741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mao-Draayer Y, Sarazin J, Fox D, Schiopu E. The sphingosine-1-phosphate receptor: A novel therapeutic target for multiple sclerosis and other autoimmune diseases. Clin Immunol. 2017;175:10–15. doi: 10.1016/j.clim.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez YI, Campos LE, Castro M, et al. Sphingosine-1 phosphate: A new modulator of immune plasticity in the tumor microenvironment. Front Oncol. 2016;6:218. doi: 10.3389/fonc.2016.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Proia RL, Hla T. Emerging biology of sphingosine-1-phosphate: Its role in pathogenesis and therapy. J Clin Invest. 2015;125(4):1379–87. doi: 10.1172/JCI76369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aoki H, Aoki M, Katsuta E, et al. Host sphingosine kinase 1 worsens pancreatic cancer peritoneal carcinomatosis. J Surg Res. 2016;205(2):510–17. doi: 10.1016/j.jss.2016.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu H, Ma Y, He HW, et al. SPHK1 (sphingosine kinase 1) induces epithelial-mesenchymal transition by promoting the autophagy-linked lysosomal degradation of CDH1/E-cadherin in hepatoma cells. Autophagy. 2017;13(5):900–13. doi: 10.1080/15548627.2017.1291479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dai L, Xia P, Di W. Sphingosine 1-phosphate: A potential molecular target for ovarian cancer therapy? Cancer Invest. 2014;32(3):71–80. doi: 10.3109/07357907.2013.876646. [DOI] [PubMed] [Google Scholar]
- 19.Ruckhäberle E, Rody A, Engels K, et al. Microarray analysis of altered sphingolipid metabolism reveals prognostic significance of sphingosine kinase 1 in breast cancer. Breast Cancer Res Treat. 2008;112(1):41–52. doi: 10.1007/s10549-007-9836-9. [DOI] [PubMed] [Google Scholar]
- 20.Sato M, Ikeda H, Uranbileg B, et al. Sphingosine kinase-1, S1P transporter spinster homolog 2 and S1P2 mRNA expressions are increased in liver with advanced fibrosis in human. Sci Rep. 2016;6:32119. doi: 10.1038/srep32119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matula K, Collie-Duguid E, Murray G, et al. Regulation of cellular sphingosine-1-phosphate by sphingosine kinase 1 and sphingosine-1-phopshate lyase determines chemotherapy resistance in gastroesophageal cancer. MC Cancer. 2015;15:762. doi: 10.1186/s12885-015-1718-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albinet V, Bats ML, Huwiler A, et al. Dual role of sphingosine kinase-1 in promoting the differentiation of dermal fibroblasts and the dissemination of melanoma cells. Oncogene. 2014;33(26):3364–73. doi: 10.1038/onc.2013.303. [DOI] [PubMed] [Google Scholar]
- 23.Nakahara T, Iwase A, Nakamura T, et al. Sphingosine-1-phosphate inhibits H2O2-induced granulosa cell apoptosis via the PI3K/Akt signaling pathway. Fertil Steril. 2012;98(4):1001–8. doi: 10.1016/j.fertnstert.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 24.Rossmeisl JH, Hall-Manning K, Robertson JL, et al. Expression and activity of the urokinase plasminogen activator system in canine primary brain tumors. Onco Targets Ther. 2017;10:2077–85. doi: 10.2147/OTT.S132964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Datta A, Loo SY, Huang B, et al. SPHK1 regulates proliferation and survival responses in triple-negative breast cancer. Oncotarget. 2014;5(15):5920–33. doi: 10.18632/oncotarget.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu Y, Huang J, Ma L, et al. MicroRNA-122 confers sorafenib resistance to hepatocellular carcinoma cells by targeting IGF-1R to regulate RAS/RAF/ERK signaling pathways. Cancer Lett. 2016;371(2):171–81. doi: 10.1016/j.canlet.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 27.Panneer Selvam S, De Palma RM, Oaks JJ, et al. Binding of the sphingolipid S1P to hTERT stabilizes telomerase at the nuclear periphery by allosterically mimicking protein phosphorylation. Sci Signal. 2015;8(381):ra58. doi: 10.1126/scisignal.aaa4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohammed S, Harikumar KB. Sphingosine 1-phosphate: A novel target for lung disorders. Front Immunol. 2017;8:296. doi: 10.3389/fimmu.2017.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maczis M, Milstien S, Spiegel S. Sphingosine-1-phosphate and estrogen signaling in breast cancer. Adv Biol Regul. 2016;60:160–65. doi: 10.1016/j.jbior.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Maceyka M, Harikumar KB, Milstien S, Spiegel S. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 2012;22(1):50–60. doi: 10.1016/j.tcb.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu PH, Chen MB, Liu YY, et al. Identification of sphingosine kinase 1 (SphK1) as a primary target of icaritin in hepatocellular carcinoma cells. Oncotarget. 2017;8(14):22800–10. doi: 10.18632/oncotarget.15205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu YJ, You H, Tan JX, et al. Overexpression of sphingosine kinase 1 is predictive of poor prognosis in human breast cancer. Oncol Lett. 2017;14(1):63–72. doi: 10.3892/ol.2017.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J, Song Z, Wang Y, et al. Overexpression of SphK1 enhances cell proliferation and invasion in triple-negative breast cancer via the PI3K/AKT signaling pathway. Tumour Biol. 2016;37(8):10587–93. doi: 10.1007/s13277-016-4954-9. [Retracted article] [DOI] [PubMed] [Google Scholar]
- 34.Datta A, Loo SY, Huang B, et al. SPHK1 regulates proliferation and survival responses in triple-negative breast cancer. Oncotarget. 2014;5(15):5920–23. doi: 10.18632/oncotarget.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madhunapantula SV, Hengst J, Gowda R, et al. Targeting sphingosine kinase-1 to inhibit melanoma. Pigment Cell Melanoma Res. 2012;25(2):259–74. doi: 10.1111/j.1755-148X.2012.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]