Abstract
Background
Unfractionated heparin is commonly used for anticoagulation in extracorporeal membrane oxygenation (ECMO). Several studies have shown that nafamostat mesilate (NM) has comparable clinical outcomes to unfractionated heparin. This study compared anticoagulation with NM and heparin in a large-animal model.
Methods
Beagle dogs (n=8; weight, 6.5–9 kg) were placed on venovenous ECMO. Blood samples were taken every hour and the following parameters were compared: hemoglobin level, activated partial thromboplastin time (aPTT), thromboelastography (TEG) data, platelet function, and inflammatory cytokine levels.
Results
In both groups, the aPTT was longer than the baseline value. Although the aPTT in the NM group was shorter than in the heparin group, the TEG parameters were similar between the 2 groups. Hemoglobin levels decreased in both groups, but the decrease was less with NM than with heparin (p=0.049). Interleukin (IL)-1β levels significantly decreased in the NM group (p=0.01), but there was no difference in the levels of tumor necrosis factor alpha or IL-10 between the 2 groups.
Conclusion
NM showed a similar anticoagulant effect to that of unfractionated heparin, with fewer bleeding complications. NM also had anti-inflammatory properties during ECMO. Based on this preclinical study, NM may be a good alternative candidate for anticoagulation in ECMO.
Keywords: Extracorporeal membrane oxygenation, Anticoagulants, Nafamostat mesilate
Introduction
Effective anticoagulation with few bleeding complications is a major therapeutic goal during extracorporeal membrane oxygenation (ECMO). Unfractionated heparin is the most widely accepted anticoagulant in ECMO because it is cost-effective and easily reversed with protamine. While unfractionated heparin is safe, bleeding complications occur in 33% of patients [1].
Nafamostat mesilate (NM) is a serine protease inhibitor that inactivates coagulation, fibrinolysis, and platelet aggregation. It has been widely used for anticoagulation during hemodialysis [2]. Recently, several studies have proposed using NM for anticoagulation during ECMO as an alternative to unfractionated heparin, because it causes fewer bleeding episodes [3]. However, preclinical experimental data comparing the anticoagulant effects of NM and heparin have not yet been reported. The objective of this study was to compare the effects of NM and unfractionated heparin in a large-animal ECMO model.
Methods
1) Preparation of animal models
Eight 24-month-old male Beagle dogs weighing 6.5 to 9 kg were prepared for this experiment. Each animal was pre-medicated with midazolam and propofol before being placed in a supine position and intubated with an 8-mm internal diameter endotracheal tube. Anesthesia was maintained by the continuous infusion of propofol and remifentanil combined with inhalation of volatile isoflurane. Fluids were delivered with normal saline at a rate of 40 mL/hr. Vital signs, including continuous electrocardiography, pulse oximetry, and arterial blood pressure, were measured throughout the experiment.
2) Cannulation and extracorporeal membrane oxygenation maintenance
After the animals’ vital signs stabilized, an 8-Fr perfusion cannula and a 10-Fr drain cannula were inserted into the left internal jugular vein and the left femoral vein, respectively, using the Seldinger technique (Fig. 1). In accordance with the total body weight of the animals, pediatric membrane oxygenators (Medtronic Minimax Plus; Medtronic Inc., Minneapolis, MN, USA) and pumps (Medtronic Biomedicus 550; Medtronic Inc.) were used. Flow was maintained to achieve the target cardiac output of 80 mL/kg/min.
Fig. 1.
(A–D) Venovenous extracorporeal membrane oxygenation: jugular and femoral vein cannulation.
3) Anticoagulation treatment
One group (4 dogs) received anticoagulation with NM (the NM group) and the other group (4 dogs) received anticoagulation with unfractionated heparin (the heparin group). The NM group received intravenous NM continuously at a rate of 1 mg/kg/hr. The heparin group received a bolus injection of 100 units/kg of unfractionated heparin before cannulation. Additional doses of unfractionated heparin were infused according to the measured activated partial thromboplastin time (aPTT). Unfractionated heparin infusions were titrated to maintain a target aPTT of 1.5–2 times the baseline level according to a standard weight-based heparin dosing nomogram. Each hour, aPTT was checked in both groups using point-of-care testing.
4) Sampling and data acquisition
The dorsalis pedis artery was cannulated in each animal for blood pressure monitoring and blood sampling. Blood samples were taken every hour to analyze the hemoglobin level, aPTT, and platelet function.
5) Thromboelastography
Thromboelastography analyzes a number of factors associated with anticoagulation, and is an objective indicator of anticoagulant activity [4]. Baseline thromboelastography values were established by analyzing 1 mL of blood from each animal after commencing general anesthesia. After beginning ECMO, thromboelastography was conducted on an hourly basis using rotational thromboelastometry (ROTEM; Daeryun Instruments, Daejeon, Korea). Intrinsically activated thromboelastometric test (INTEM) data and extrinsically activated thromboelastometric test (EXTEM) data, including clotting time, the A10 value, and the alpha angle were statistically compared between the NM and heparin groups. INTEM data were acquired using a reagent containing phospholipids and ellagic acid, which activate the intrinsic anticoagulation pathway. Therefore, changes in INTEM show the degree to which an anticoagulant directly affects activation of the intrinsic pathway. EXTEM data were acquired using a reagent containing a tissue factor that activates the extrinsic pathway. Therefore, EXTEM shows the degree to which an anticoagulant affects the extrinsic pathway. In ROTEM, the clotting time is measured in seconds, and represents the time to reach a 2-mm amplitude (firmness) from the start of the test. Clotting time represents the degree to which anticoagulation influences clot generation. The A10 value is the clot firmness (amplitude in millimeters) 10 minutes after the clotting time, and represents the rate of clot production. It is affected by the degree of interaction between the anticoagulant and clotting factors. The alpha angle is the angle between the middle axis and a tangent to the clotting curve when the clot firmness reaches 2 mm in amplitude. The alpha angle represents the clot production rate and is influenced by platelet function, fibrinogen, and the presence of activated coagulants and anticoagulants.
6) Hemoglobin
Hemoglobin is an indicator of bleeding and anemia in clinical practice. The hemoglobin level, which was used here to quantitatively measure blood loss during ECMO, is an important index for investigating the complications caused by bleeding due to anticoagulation during treatment. After commencing general anesthesia, 1 mL of blood was collected from each animal to measure the baseline hemoglobin level. During ECMO, hemoglobin levels were measured hourly in a similar fashion.
7) Inflammatory cytokines
To assess changes in inflammatory cytokine levels during anticoagulant administration and ECMO, blood samples were collected at baseline and each hour thereafter, and stored at −70°C. Later, inflammatory cytokines were measured using interleukin (IL)-1β, IL-10, and tumor necrosis factor (TNF)-α enzymelinked immunosorbent assay (ELISA) kits (BD, San Jose, CA, USA).
8) Statistics
Statistical analysis was performed using IBM SPSS software ver. 21.0 (IBM Corp., Armonk, NY, USA). Multivariate analysis was done using repeated measures data analysis with 2 factors to analyze data collected repeatedly from the same subject. All p-values <0.05 were considered to indicate statistical significance.
9) Ethical approval
This experiment was conducted with approval from the Ethics Committee of Chungnam National University (IRB approval no., CNU-00352).
Results
1) Mortality
There was no mortality in either group and all animals recovered normally following treatment.
2) Bleeding
There were no major bleeding episodes in either group. However, 3 animals in the heparin group had minor cannulation site bleeding 2 hours after the initiation of ECMO. There was no cannulation site bleeding in the NM group.
3) Hemoglobin levels
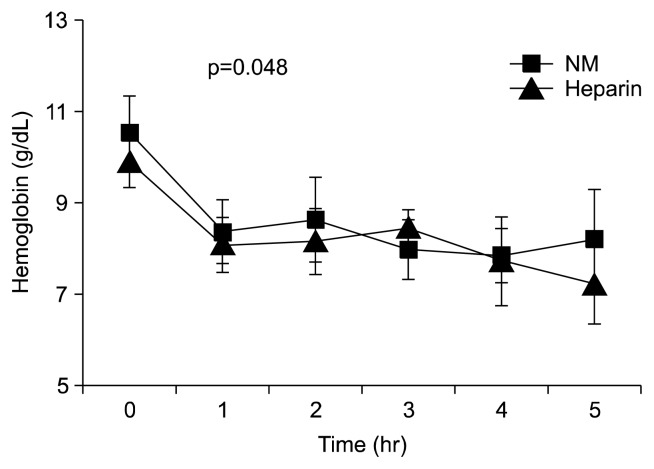
Hemoglobin levels were measured hourly and used to analyze the amount of blood loss during ECMO. No statistically significant difference was found in the baseline mean hemoglobin level between the groups. However, the degree of hemoglobin change per hour was significantly lower in the NM group (p=0.048). Furthermore, the mean absolute fall in hemoglobin was significantly lower in the NM group at 5 hours after ECMO initiation (Fig. 2). The simultaneous decline seen in both groups at 1 hour seems to have been due to the dilution effect of the ECMO priming solution.
Fig. 2.
Effects of NM on hemoglobin concentrations in dogs. NM, nafamostat mesilate.
4) Activated partial thromboplastin time and thromboelastography
Each aPTT reading was more than 1.5 times longer than the baseline value in the heparin group, and the mean aPTT in the NM group was shorter than in the heparin group (Table 1). The INTEM data from thromboelastography revealed no significant between-group differences for clotting time (p=0.255), the alpha angle (p=0.379), or the A10 value (p=0.402). However, in both groups, the INTEM data changed between baseline and 1 hour, indicating that both NM and heparin caused anticoagulation by affecting the intrinsic pathway. The EXTEM data likewise showed no significant difference in clotting time (p=0.313), the alpha angle (p=0.577), or the A10 value (p=0.993) between groups. Furthermore, there was no change in the EXTEM data before versus after anticoagulation for either treatment (Fig. 3). Although the aPTT in the NM group was shorter than in the heparin group, the thromboelastography parameters were similar between the groups.
Table 1.
aPTT data in the heparin and NM groups
| Variable | Case | Baseline | Time (hr) | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| Heparin | 1 | 113.7 | 200.0 | 165.1 | 158.9 | 200.0 | 185.4 |
| 2 | 105.0 | 155.7 | 200.0 | 168.5 | 185.9 | 191.2 | |
| 3 | 111.0 | 185.0 | 190.0 | 188.0 | 192.0 | 169.0 | |
| 4 | 100.1 | 190.1 | 165.4 | 186.5 | 176.2 | 189.6 | |
| NM | 5 | 105.0 | 125.7 | 156.7 | 129.5 | 133.0 | 132.9 |
| 6 | 111.0 | 135.4 | 140.1 | 134.3 | 142.1 | 150.3 | |
| 7 | 113.7 | 200.0 | 165.1 | 129.9 | 128.7 | 147.5 | |
| 8 | 109.0 | 129.4 | 154.2 | 140.1 | 130.5 | 145.9 | |
Heparin group: mean aPTT=182.125s; NM group: mean aPTT=142.565s.
NM, nafamostat mesilate; aPTT, activated partial thromboplastin time.
Fig. 3.
Thromboelastogram data. (A, B) Clotting time. (C, D) Alpha angle. (E, F) A10. NM, nafamostat mesilate; INTEM, internal thromboelastometry; EXTEM, external thromboelastometry.
5) Inflammatory cytokines
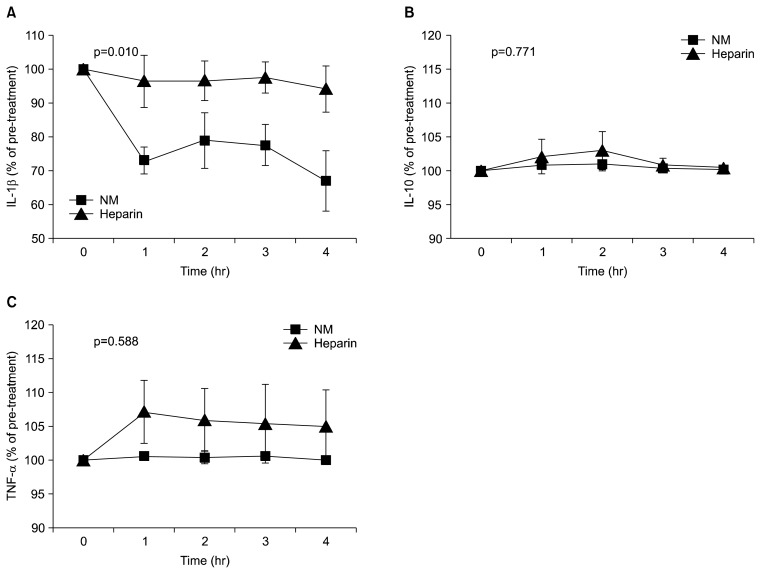
The NM group had significantly lower levels of the pro-inflammatory cytokine IL-1 than the heparin group (p=0.010). The degree of change in TNF-α did not differ between groups (p=0.588). However, the absolute values were lower in the NM group. No difference was observed in IL-10 levels between the groups (p=0.771) (Fig. 4).
Fig. 4.
Effects of NM and heparin on the concentrations of IL-1β, IL-10, and TNF-α in a beagle dog model of extracorporeal membrane oxygenation. NM, nafamostat mesilate; IL, interleukin; TNF, tumor necrosis factor.
Discussion
The use of ECMO to treat cardiopulmonary failure in patients for whom conventional treatment is ineffective is rapidly increasing worldwide [5], and proper anticoagulation is essential during ECMO. Currently, unfractionated heparin is the anticoagulant of choice, as it is cost-effective and easily reversed with protamine. However, bleeding related to systemic heparinization is a major complication during ECMO and is directly related to the patient’s prognosis [6]. While many alternative therapies to unfractionated heparin have been proposed, most have not been adequately investigated with respect to their efficacy and safety.
NM, a serine protease inhibitor, is one such alternative, and NM has been widely used for anticoagulation during continuous renal replacement therapy [7]. NM use during ECMO has been reported in patients at a high risk of bleeding due to its relatively short half-life [3]. In addition, a report described using NM for cardiopulmonary bypass during open heart surgery, and found that that NM inhibited fibrinolysis, maintained platelet function, and reduced bleeding [8].
In our study, we proved that NM is an acceptable alternative anticoagulant to unfractionated heparin in a large-animal ECMO model. No significant difference was found in the aPTT between the 2 groups. There was also no significant difference in the INTEM data between the groups, and both NM and heparin exerted anticoagulant activity by inhibiting the intrinsic pathway. The unfractionated heparin group had a significantly larger drop in hemoglobin at 5 hours after ECMO initiation (p=0.048).
The inflammatory reaction to ECMO was assessed using ELISA, which measured changes in inflammatory cytokine levels. There was no significant difference in the IL-10 or TNF-α levels between the groups. The absolute values were lower in the NM group, although this difference was also not statistically significant. Importantly, our study showed that the expression of IL-1β was significantly lower in the NM group (p=0.010). IL-1β, which is an important mediator of the inflammatory reaction induced by lymphocytes, is secreted from macrophages and is an important factor in the early stages of all inflammatory responses [9–11]. It is well known that extracorporeal circulation activates a systemic inflammatory response. Often, this inflammatory cascade causes systemic inflammatory response syndrome, and is closely related to complications such as sepsis, acute kidney injury, and bleeding disorders [12]. During ECMO, blood components come in direct contact with the non-endothelial surface of the circuit, which triggers the inflammatory cascade. This activation of the cellular and humoral cascades leads to the elevation of pro-inflammatory cytokines such as IL-1β [9–11]. NM treatment was associated with a significantly lower level of the expression of IL-1β, a pro-inflammatory cytokine, and can therefore be expected to minimize the systemic inflammatory response during ECMO.
This study had several limitations. First, the number of animals used was relatively small. Second, the 5-hour length of ECMO treatment may have been too short to evaluate both bleeding complications and the inflammatory response. Third, no consensus has been established regarding either the effective dose of NM for anticoagulation or the proper monitoring of anticoagulation with NM; this introduces uncertainty as to whether our regimen is the most effective for anticoagulation. Finally, NM treatment has been reported to cause electrolyte imbalances, such as hyperkalemia [13,14]. Electrolyte abnormalities were not monitored in this experiment. Serial assessments of electrolyte changes during NM anticoagulation should be included in future clinical studies.
NM produced a comparable anticoagulant effect to heparin, with fewer bleeding complications; although the aPTT in the NM group was shorter than in the heparin group, the thromboelastography parameters were similar between the 2 groups in a beagle dog ECMO model. NM also attenuated the inflammatory response to ECMO and can be expected to reduce the complications associated with this inflammation. Therefore, NM may be considered as an alternative to conventional treatment with unfractionated heparin during ECMO. However, large-scale preclinical and clinical studies are required before NM can be used in clinical practice.
Acknowledgments
This study was supported by the SK chemical research fund.
Footnotes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
References
- 1.Zangrillo A, Landoni G, Biondi-Zoccai G, et al. A meta-analysis of complications and mortality of extracorporeal membrane oxygenation. Crit Care Resusc. 2013;15:172–8. [PubMed] [Google Scholar]
- 2.Tolwani AJ, Wille KM. Anticoagulation for continuous renal replacement therapy. Semin Dial. 2009;22:141–5. doi: 10.1111/j.1525-139X.2008.00545.x. [DOI] [PubMed] [Google Scholar]
- 3.Han SJ, Kim HS, Kim KI, et al. Use of nafamostat mesilate as an anticoagulant during extracorporeal membrane oxygenation. J Korean Med Sci. 2011;26:945–50. doi: 10.3346/jkms.2011.26.7.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Traverso CI, Caprini JA, Arcelus JI. The normal thromboelastogram and its interpretation. Semin Thromb Hemost. 1995;21(Suppl 4):7–13. doi: 10.1055/s-0032-1313615. [DOI] [PubMed] [Google Scholar]
- 5.Marasco SF, Lukas G, McDonald M, McMillan J, Ihle B. Review of ECMO (extra corporeal membrane oxygenation) support in critically ill adult patients. Heart Lung Circ. 2008;17(Suppl 4):S41–7. doi: 10.1016/j.hlc.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 6.Sell LL, Cullen ML, Whittlesey GC, et al. Hemorrhagic complications during extracorporeal membrane oxygenation: prevention and treatment. J Pediatr Surg. 1986;21:1087–91. doi: 10.1016/0022-3468(86)90015-1. [DOI] [PubMed] [Google Scholar]
- 7.Hwang SD, Hyun YK, Moon SJ, Lee SC, Yoon SY. Nafamostat mesilate for anticoagulation in continuous renal replacement therapy. Int J Artif Organs. 2013;36:208–16. doi: 10.5301/ijao.5000191. [DOI] [PubMed] [Google Scholar]
- 8.Murase M, Usui A, Tomita Y, Maeda M, Koyama T, Abe T. Nafamostat mesilate reduces blood loss during open heart surgery. Circulation. 1993;88(5 Pt 2):II432–6. [PubMed] [Google Scholar]
- 9.Gery I, Gershon RK, Waksman BH. Potentiation of the T-lymphocyte response to mitogens: I. the responding cell. J Exp Med. 1972;136:128–42. doi: 10.1084/jem.136.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gery I, Handschumacher RE. Potentiation of the T lymphocyte response to mitogens: III. properties of the mediator(s) from adherent cells. Cell Immunol. 1974;11:162–9. doi: 10.1016/0008-8749(74)90016-1. [DOI] [PubMed] [Google Scholar]
- 11.Gery I, Waksman BH. Potentiation of the T-lymphocyte response to mitogens: II. the cellular source of potentiating mediator(s) J Exp Med. 1972;136:143–55. doi: 10.1084/jem.136.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bronicki RA, Hall M. Cardiopulmonary bypass-induced inflammatory response: pathophysiology and treatment. Pediatr Crit Care Med. 2016;17(8 Suppl 1):S272–8. doi: 10.1097/PCC.0000000000000759. [DOI] [PubMed] [Google Scholar]
- 13.Muto S, Imai M, Asano Y. Mechanisms of the hyperkalaemia caused by nafamostat mesilate: effects of its two metabolites on Na+ and K+ transport properties in the rabbit cortical collecting duct. Br J Pharmacol. 1994;111:173–8. doi: 10.1111/j.1476-5381.1994.tb14040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muto S, Imai M, Asano Y. Mechanisms of hyperkalemia caused by nafamostat mesilate. Gen Pharmacol. 1995;26:1627–32. doi: 10.1016/0306-3623(95)00072-0. [DOI] [PubMed] [Google Scholar]