Abstract
The Δ1-pyrroline-5-carboxylate synthetase (P5CS; EC not assigned) is the rate-limiting enzyme in proline (Pro) biosynthesis in plants and is subject to feedback inhibition by Pro. It has been suggested that the feedback regulation of P5CS is lost in plants under stress conditions. We compared Pro levels in transgenic tobacco (Nicotiana tabacum) plants expressing a wild-type form of Vigna aconitifolia P5CS and a mutated form of the enzyme (P5CSF129A) whose feedback inhibition by Pro was removed by site-directed mutagenesis. Transgenic plants expressing P5CSF129A accumulated about 2-fold more Pro than the plants expressing V. aconitifolia wild-type P5CS. This difference was further increased in plants treated with 200 mm NaCl. These results demonstrated that the feedback regulation of P5CS plays a role in controlling the level of Pro in plants under both normal and stress conditions. The elevated Pro also reduced free radical levels in response to osmotic stress, as measured by malondialdehyde production, and significantly improved the ability of the transgenic seedlings to grow in medium containing up to 200 mm NaCl. These findings shed new light on the regulation of Pro biosynthesis in plants and the role of Pro in reducing oxidative stress induced by osmotic stress, in addition to its accepted role as an osmolyte.
Pro is known to play an important role as an osmoprotectant in plants subjected to hyperosmotic stresses such as drought and soil salinity (Delauney and Verma, 1993). Recent studies on Pro synthesis and catabolism genes have provided results that are consistent with diverse functions of Pro as a source of energy, nitrogen and carbon, and as an osmolyte in response to dehydration (Kohl et al., 1988; Kavi Kishor et al., 1995; Peng et al., 1996; Hua et al., 1997; Zhang et al., 1997). Synthesis, accumulation, and catabolism of Pro in plants are highly regulated processes. Pro is synthesized via two routes from either Glu or Orn (Adams and Frank, 1980; Delauney and Verma, 1993). We have demonstrated that the Glu pathway is predominant under the conditions of osmotic stress (Delauney et al., 1993). In Vigna aconitifolia and Arabidopsis, the first two steps of the Pro biosynthesis from Glu are catalyzed by Δ1-pyrroline-5-carboxylate synthetase (P5CS), a bifunctional enzyme with activities of γ-glutamyl kinase (γ-GK) and Glu-5-semialdehyde (GSA) dehydrogensae (or γ-glutamyl phosphate reductase; Hu et al., 1992; Savoure et al., 1995; Yoshiba et al., 1995). In tomato, it has been reported that there are two Pro loci in the nuclear genome: one specifies a bifunctional P5CS (tomPro2) and the other one (tomPro1) apparently encodes a polycistronic mRNA that directs the synthesis of γ-GK and GSA dehydrogenase as two separate peptides (Garcia-Rios et al., 1997). Two P5CS genes have also been shown to be present in both Arabidopsis and alfalfa (Strizhov et al., 1997; Ginzberg et al., 1998; Yoshiba et al., 1999). The Arabidopsis P5CS1 gene is expressed in most organs and is induced rapidly by stress (Strizhov et al., 1997; Zhang et al., 1997; Yoshiba et al., 1999). P5CS2 is expressed in dividing cell cultures and its induction by stress is dependent on protein synthesis (Ginzberg et al., 1998).
Earlier experiments suggested that Pro accumulation in plants under stress may involve the loss of feedback regulation due to a conformational change in the P5CS protein (Boggess et al., 1976a, 1976b). In bacteria, Pro biosynthesis has been shown to be regulated by the end product inhibition of γ-GK activity (Smith et al., 1984). A Salmonella typhimurium mutant resistant to a toxic Pro analog (3,4-dehydro-d,l-Pro) accumulated Pro and showed enhanced tolerance to osmotic stress (Csonka, 1981). The mutation was due to a change of an Asp residue (at position 107) to Asn, rendering the γ-GK much less sensitive to inhibition by Pro (Csonka et al., 1988; Dandekar and Uratsu, 1988). We showed that the conserved Asp residue (at position 128) in the V. aconitifolia P5CS is not involved in the feedback inhibition (Zhang et al., 1995). Using site-directed mutagenesis, a replacement of Phe at position 129 by Ala was made in V. aconitifolia P5CS (P5CSF129A). This mutant enzyme was shown to retain similar kinetic characteristics as wild-type P5CS, but its feedback inhibition was virtually eliminated (Zhang et al., 1995). In this report, we demonstrate that tobacco (Nicotiana tabacum) plants carrying P5CSF129A accumulate more Pro, produce fewer free radicals, and are more tolerant to osmotic stress than plants expressing the wild-type V. aconitifolia P5CS transgene only. The P5CS transgenic seeds germinated well in a high salinity (200 mm NaCl) environment, while the wild type did not. These results demonstrated that feedback regulation of P5CS by Pro plays a role in the control of Pro biosynthesis in plants, and that Pro accumulation reduces osmotic stress, which may be mediated by free radicals produced as a result of oxidative stress.
MATERIALS AND METHODS
Transformation of Tobacco Plants
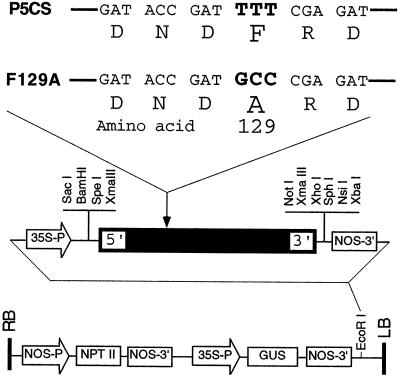
A plasmid (pBI-P5CSF129A, Fig. 1) containing mutagenized Vigna aconitifolia P5CSF129A cDNA (Zhang et al., 1995) under the control of the cauliflower mosaic virus 35S promoter was used for tobacco (Nicotiana tabacum cv Xanthi) transformation via Agrobacterium tumefaciens. All transgenic lines tested accumulated high levels of Pro (Fig. 2); line F129A-3 was used in this study. P5CS line 136, which expressed a V. aconitifolia wild-type P5CS gene described previously (Kavi Kishor et al., 1995), was used as a control along with plants transformed with vector pBI121 only.
Figure 1.
Mutation site of P5CS that removed feedback inhibition by Pro, and restriction map of pBI-P5CSF129A. Codon TTT at nucleotide positions 421 to 423 of the V. aconitifolia P5CS cDNA (Hu et al., 1992) was changed to GCC by site-directed mutagenesis so that Phe (F) at amino acid position 129 of the P5CS peptide is replaced by Ala (A), generating P5CSF129A. The mutant enzyme retains similar kinetic characteristics as the wild-type P5CS, except that its allosteric regulation by Pro is eliminated (Zhang et al., 1995).
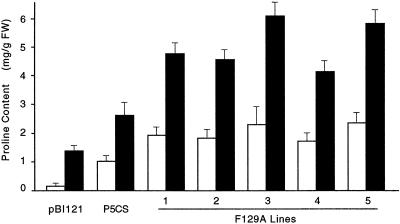
Figure 2.
Pro levels in independent P5CSF129A transgenic lines. Seeds of five independent P5CSF129A lines were germinated on MS medium containing no NaCl (control; white bars) or 200 mm NaCl (black bars). The plants were grown under constant light at 24°C in a growth room. Plants transformed with vector pBI121 and P5CS served as controls. Pro content was measured in leaf extracts.
Germination and Plant Growth
Seeds of wild-type tobacco and transgenic (pBI121, P5CS, and P5CSF129A) plants were germinated and maintained on Murashige and Skoog (MS) agar (4.3 g/L MS salts and 0.8% [w/v] Phytagar, Gibco-BRL, Cleveland) medium containing 0, 150, 200, 250, and 300 mm NaCl. The medium on which the transgenic seeds were plated contained kanamycin (200 μg/mL). Germination rate was recorded on d 14, 18, 20, and 24. Growth rate as measured by fresh weight (g) was recorded in 2-week-old seedlings. Three replicates containing about 60 seedlings each were taken for measurements. The data shown correspond to control and 200 mm NaCl-treated plants (in Fig. 5). Seeds were germinated on MS medium in a tissue culture room at 25°C ± 2°C under constant illumination (200 μmol m−2 s−1 from cool-white fluorescent tubes) and seedlings were grown in a growth chamber at 25°C ± 2°C with cycles of 16-h light and 8-h dark.
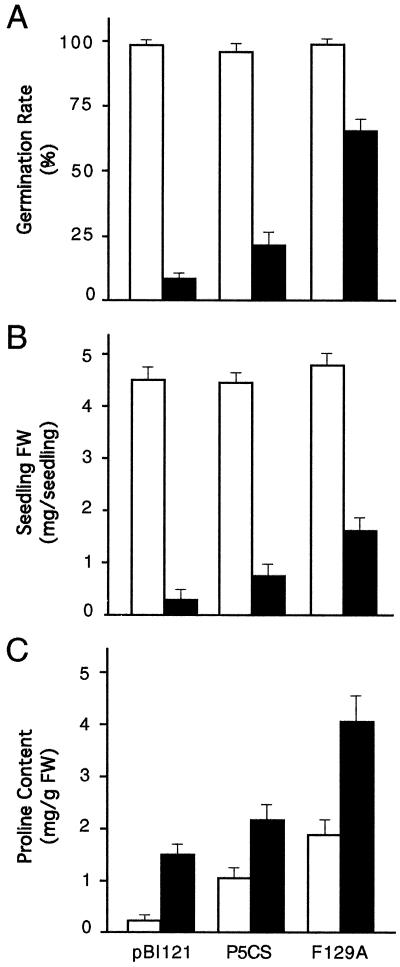
Figure 5.
Effect of V. aconitifolia P5CS and P5CSF129A gene expression on seed germination, seedling growth, and Pro accumulation in transgenic tobacco plants treated with 200 mm NaCl. Values presented are the average of three independent samples each containing about 200 seeds. White bars, Control; black bars, 200 mm NaCl.
Northern- and Western-Blot Analyses
Total RNA (15 μg) isolated from the transgenic and control tobacco seedlings was electrophoresed, blotted, and hybridized with the V. aconitifolia P5CS cDNA (Hu et al., 1992) as a probe. Hybridization and washing of the filters were carried out under high-stringency conditions (Kavi Kishor et al., 1995). Western blotting was performed using polyclonal antibodies to purified V. aconitifolia P5CS protein, as described previously (Kavi Kishor et al., 1995; Zhang et al., 1995).
Growth and Salinity Treatment of Tobacco Bright Yellow 2 (BY2) Cells
Tobacco BY2 cells were maintained in MS media (4.3 g/L MS salts from Gibco-BRL, 0.1 g/L inositol, 1.0 mg/L thiamine, 0.2 mg/L 2,4-dichlorophenoxyacetic acid [2,4-D], 255 mg/L KH2PO4, and 30 g/L Suc). For the salinity treatment, a 5-d-old cell suspension was used and the salt concentration was adjusted with a 5.0 m NaCl stock to final concentrations ranging from 50 to 400 mm. For time course induction of malondialdehyde (MDA) under salinity stress, the cell suspension was treated with 250 mm NaCl, and MDA contents were determined at time intervals of 5 h.
Measurement of Pro and MDA Contents
Pro concentration was determined as described previously (Kavi Kishor et al., 1995) according to the procedure of Bates et al. (1973). Values were expressed as milligrams per gram fresh weight. MDA contents were measured using a thiobarbituric acid reaction (Heath and Packer, 1968). About 0.5 to 1.0 g of tissue was homogenized in 5 mL of 5% (w/v) trichloroacetic acid and the homogenate was centrifuged at 12,000g for 15 min at room temperature. The supernatant was mixed with an equal volume of thiobarbituric acid (0.5% in 20% [w/v] trichloroacetic acid), and the mixture was boiled for 25 min at 100°C, followed by centrifugation for 5 min at 7,500g to clarify the solution. Absorbance of the supernatant was measured at 532 nm and corrected for non-specific turbidity by subtracting the A600. MDA contents were calculated using an extinction coefficient of 155 m−1 cm−1. Values of Pro and MDA contents were taken from measurements of three independent samples, and ses of the means were calculated.
RESULTS
Expression of V. aconitifolia Mutated P5CS cDNA in Transgenic Tobacco Plants
Kinetics studies of V. aconitifolia P5CS enzyme showed that P5CS activity is inhibited by 6 mm Pro (Hu et al., 1992; Zhang et al., 1995). To remove this allosteric inhibition, a point mutation in the P5CS cDNA was introduced so that the Phe at position 129 was replaced by Ala (Fig. 1). This mutated protein (P5CSF129A) is enzymatically active, but its feedback inhibition by Pro is virtually eliminated (Zhang et al., 1995). We placed the V. aconitifolia mutated P5CS cDNA under the control of cauliflower mosaic virus 35S promoter (Fig. 1) and introduced this construct into tobacco plants by A. tumefaciens-mediated transformation. Seeds of five independent transgenic lines were germinated on MS media containing no NaCl or 200 mm NaCl. Pro levels in all P5CSF129A lines were almost 2-fold higher than the P5CS transgenic line (Fig. 2). The line (F129A-3) that produced the highest level of Pro both under control and salt-stressed conditions were chosen for further analyses on gene expression and oxidative damage due to osmotic stress.
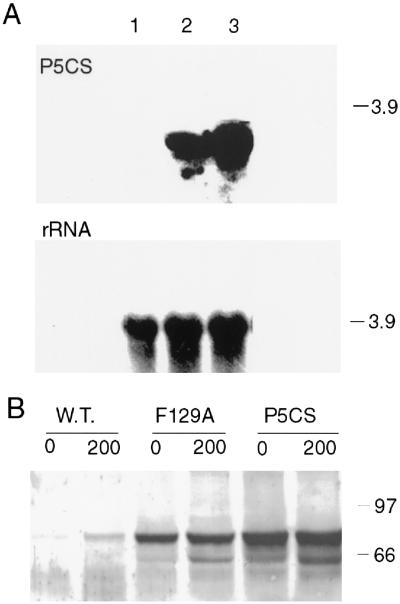
The expression of P5CSF129A in transgenic plants was monitored by northern blotting (Fig. 3A). High-stringency conditions were used for RNA hybridization and washing to eliminate any possible cross-reaction with the tobacco endogenous P5CS mRNA. Expression levels of the transgene in P5CSF129A lines (Fig. 3A, lane 2) were slightly lower than that in P5CS transgenic line 136 (Fig. 3A, lane 3) which had previously been shown to express high levels of the V. aconitifolia P5CS gene (Kavi Kishor et al., 1995). No cross-reaction with the tobacco endogenous P5CS mRNA was detected under these conditions (Fig. 3A, lane 1). Expression levels of V. aconitifolia P5CSF129A and the wild-type P5CS enzyme in transgenic tobacco plants were also determined by western blotting using P5CS antibodies (Fig. 3B). A weak cross reaction of the V. aconitifolia P5CS antibodies with the tobacco endogenous P5CS protein at the expected size (72 kD) appeared (Fig. 3B, lane 1–2) after prolonged exposure. High levels of P5CSF129A expression were detected in the transgenic plants (Fig. 3B, lane 3–4). In agreement with mRNA levels (Fig. 3A), protein expression in P5CSF129A line (F129A-3) was also slightly lower than that in P5CS line 136 (Fig. 3B, lane 5–6). Comparison of P5CS protein levels between control and salt-treated plants indicated an increase of about 50% in P5CS protein under stress conditions. These results are consistent with our earlier report that more P5CS protein is present in plants subjected to osmotic stress (Zhang et al., 1995).
Figure 3.
Expression of V. aconitifolia P5CSF129A in transgenic tobacco plants. A, Northern blot of total RNA from seedlings of 14-d-old wild-type (lane 1), P5CSF129A-3 (lane 2), and P5CS line 136 (lane 3). RNA blot was probed with 32P-labeled V. aconitifolia P5CS cDNA (top panel) or ribosomal DNA as internal loading control (bottom panel). Molecular masses of RNA markers are indicated (in kb). B, Western blot of total soluble proteins from 14-d-old wild-type (W.T.), P5CSF129A-3 (F129A), and P5CS line 136 (P5CS) seedlings. Seeds were germinated and maintained on MS medium containing 0 or 200 mm NaCl for 2 weeks. The nitrocellulose membrane was reacted with antibodies to purified V. aconitifolia P5CS protein. The top band corresponds to the expected size of P5CS protein, whereas the bottom band appears to be a degradation product. Molecular sizes of protein markers (Bio-Rad, Hercules, CA) are indicated in kD.
Growth of Seedlings Expressing Mutated P5CS Devoid of Feedback Control
Transgenic lines expressing V. aconitifolia wild-type P5CS and mutated enzyme (P5CSF129A) were subjected to different levels of salinity treatments. Uniform seed germination for all three groups of plants was observed in medium containing no NaCl. At concentrations of 250 and 300 mm NaCl, seed germination rate was very low for both transgenic lines, with no germination in control seeds. The differences observed with 200 mm salt were, however, highly significant (Fig. 4). The transgenic P5CSF129A plants exhibited highest germination rate, to an extent of 60% and 68% compared with 23% and 28% in P5CS lines at d 14 (Fig. 5A) and d 18 (data not shown), respectively. On the other hand, germination of pBI121 transgenic seeds (control) was severely inhibited (i.e. only 8% and 16% germination).
Figure 4.
Phenotype of 6-week-old wild-type, P5CS, and P5CSF129A seedlings as affected by salinity (200 mm NaCl) stress. Seeds were germinated and maintained on MS medium containing 200 mm NaCl. The plates were kept in a controlled environment at 24°C under constant light.
Growth of seedlings in terms of fresh weight (Fig. 5B) also showed no difference in the untreated control and transgenic plants. However, the P5CSF129A plants exhibited the least inhibition of growth over P5CS and pBI121 plants under 200 mm salt stress (Figs. 4 and 5B). The inhibition of plant growth over their respective controls at d 20 was approximately 95%, 82%, and 67% in the pBI121, the P5CS, and the mutant P5CSF129A plants, respectively (data not shown). Seedling growth in terms of root proliferation with abundant root hairs was observed in the transgenic P5CSF129A plants, whereas very few control seedlings exhibited root initiation and elongation, and root hairs were conspicuously absent.
Removal of Feedback Inhibition of P5CS Results in Higher Levels of Pro Accumulation in Transgenic Plants
To determine if the observed phenotypic differences in germination and seedling growth were related to Pro levels in these plants, we measured Pro contents in respective seedlings grown under both normal and salt-stressed conditions. When germinated on medium containing no salt, the P5CSF129A plants were found to produce about 2-fold more Pro than the P5CS plants, which in turn synthesized 5- to 6-fold more Pro than the pBI121 seedlings (Fig. 5C). Under salt stress conditions, the P5CSF129A plants accumulated about two times more Pro than the P5CS plants and 3-fold more than the pBI121 seedlings. This suggests that the wild-type P5CS enzyme is subject to feedback inhibition by the end product Pro under stress conditions, because removal of this feedback regulation rendered at least a 2-fold increase in Pro content.
Osmotic Stress Induces Free Radical Production in Plant Cells That Can Be Reduced by Pro
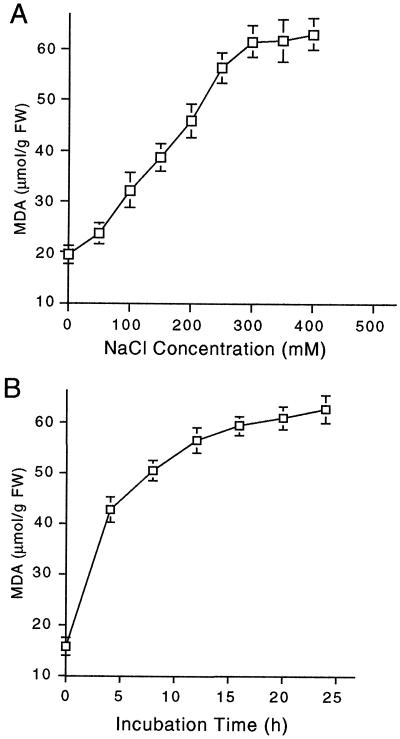
To further understand how Pro accumulation helps plant cells deal with osmotic stress, we examined the relationship between osmotic stress and oxidative stress. For this, we used tobacco BY2 cells because of the uniformity of the mass of tissue, and measured free radical levels in cells treated with different concentrations of NaCl. MDA is a major cytotoxic product of lipid peroxidation and has been used extensively as an indicator of free radical production (Kunert and Ederer, 1985). MDA levels increased linearly from 24 to 62 μg/g fresh weight of cells with the increase in NaCl concentration over the range from 50 to 300 mm NaCl (Fig. 6A). In cells treated with 250 mm NaCl, MDA accumulated within 5 h and continued to be produced at a slower rate over 24 h (Fig. 6B). These results suggest that oxidative stress accounts, at least in part, for the damage caused to the plant cells by osmotic stress.
Figure 6.
Free radical production as measured by MDA content in tobacco BY2 cells 5 d after subculturing. A, Effect of NaCl concentrations on MDA content measured after 8 h of salinity treatment. B, Time course of MDA accumulation in cells treated with 250 mm NaCl.
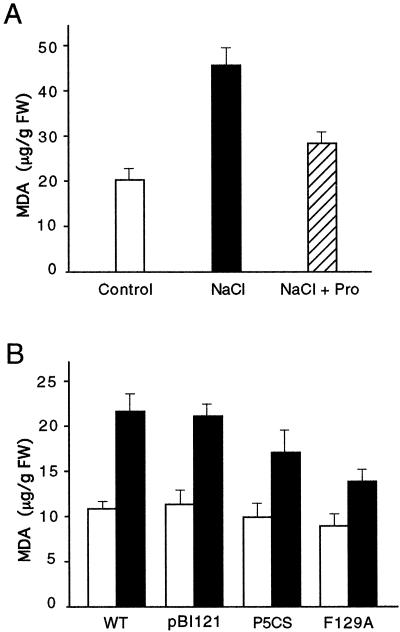
It has been proposed that Pro may act as a free radical scavenger to protect plants from damage by oxidative stress caused during osmotic stress (Alia et al., 1993; Smirnoff, 1993). To test this hypothesis, we measured the damage by free radicals to wild-type tobacco BY2 cells during salt stress with and without the addition of exogenous Pro. As shown in Figure 7A, salinity stress created by 250 mm NaCl for 8 h caused MDA accumulation from 20 to 46 μg/g fresh weight This value was effectively reduced by 40% in the presence of 120 mm Pro in the culture medium. These data indicate that the supply of exogenous Pro significantly reduces the accumulation of free radicals in cells under osmotic stress. Taking advantage of transgenic plants that produce high levels of endogenous Pro, we carried out measurements on free radical levels in these plants with and without salt stress.
Figure 7.
Effect of exogenous and endogenous Pro on free radical production in tobacco cells and transgenic plants. A, Effect of exogenous Pro (120 mm) on MDA content in tobacco BY2 cells treated with 250 mm NaCl for 8 h. B, Effect of endogenous Pro accumulation on MDA content in 14-d-old wild-type, pBI121, P5CS, and P5CSF129A transgenic seedlings treated with 0 (control; white bars) or 200 mm NaCl (black bars).
Minor differences in MDA levels were found among the three groups of plants when grown under normal conditions. Treatment with 200 mm NaCl caused about a 2-fold elevation of MDA in wild-type and pBI121- plants. A significantly lower MDA content was found in transgenic P5CSF129A plants (14.3 μg/g) than in control plants (Fig. 7B). The MDA level in P5CS plants were found to be intermediate (17.8 μg/g). These data indicate that high concentrations of Pro synthesized endogenously in transgenic plants may provide a means to reduce the levels of free radicals generated during osmotic stress. This observation demonstrated an additional role of Pro in reducing damage from oxidative stress generated by osmotic stress.
DISCUSSION
Biosynthesis of Pro in living cells is subject to several control mechanisms. In Escherichia coli and yeast, GK and GSADH are synthesized as separate peptides and form a heterodimer. GK is feedback inhibited by the end product of the pathway, Pro. In animals and plants, GK and GSADH are encoded by a bifunctional P5CS gene. Whereas human P5CS is feedback regulated by Orn (Hu et al., 1999), plant P5CS is subject to allosteric regulation by Pro (Hu et al., 1992; Zhang et al., 1995). The activity of V. aconitifolia P5CS is reduced to 50% by 6 mm Pro (Hu et al., 1992). Plant cells under osmotic stress can accumulate up to 129 mm Pro in the cytosol (Binzel et al., 1987; Delauney and Verma, 1993), a concentration that would almost completely turn off the P5CS enzyme. This is contradictory to the fact that plants under stress continue to synthesize Pro and build up the Pro pool. To explain this paradox, it has been assumed that the P5CS enzyme may undergo a conformational change and lose its feedback regulation property (Boggess et al., 1976a, 1976b). We reasoned that if the feedback regulation of the wild-type P5CS is completely lost during stress, then expression of the mutant P5CS, i.e. P5CSF129A, will not result in the synthesis of more Pro than expression of the wild-type P5CS transgene. On the other hand, if P5CS retains its feedback regulation under stress conditions, transgenic plants expressing the unregulated version of the enzyme, P5CSF129A, should accumulate much more Pro than those harboring the wild-type P5CS transgene.
Our results (Figs. 2 and 5C) show that removal of feedback inhibition in P5CSF129A resulted in a 2-fold increase in Pro compared with that in the P5CS transgenic line under both normal and stressed conditions. We conclude that feedback regulation of P5CS in plants is not completely eliminated under stress. Thus, Pro synthesis in plants under stress is regulated not only by transcriptional activation of P5CS (Hu et al., 1992; Garcia-Rios et al., 1997; Zhang et al., 1997), but also by feedback regulation by the end product of the pathway. Furthermore, a reciprocal increase in P5CS and Pro dehydrogenase during stress and recovery from stress controls the levels of Pro according to the environment (Peng et al., 1996).
In our previous report (Zhang et al., 1995), we performed site-directed mutagenesis to substitute each of the six amino acid residues between positions 126 and 131 of the P5CS peptide. Two residues were found to have a different degrees of effect on the allosteric property of the enzyme. Substitution of Phe at 129 with Ala (P5CSF129A) produced the most profound effect, with an increase in the 50% inhibition values from 6 mm Pro in the wild-type P5CS to 960 mm in P5CSF129A. Substitution of Asp at position 126 (P5CSD126A) resulted in a moderate change in feedback inhibition by 86 mm Pro (Zhang et al., 1995). This demonstrates that the feedback regulation property of P5CS can be changed to different degrees by modification of different amino acid residues. It remains to be determined if such a point mutation directly affects the allosteric site or if it brings about a conformational change in the protein. Under osmotic stress conditions, the wild-type P5CS enzyme may undergo some conformational change around the Pro feedback interaction site, leading to a partial loss of its allosteric regulation property. This “partial loss” hypothesis can explain the paradox of why plants under stress continue to build up Pro levels even after Pro concentrations reach the feedback inhibition levels. The differences between the levels of P5CS protein in both control and transgenic lines under normal and stress conditions indicate a contribution of native P5CS which is known to be induced under stress and cross-react with vigna P5CS antibody.
Accumulation of Pro in plants under stress may offer multiple benefits to the cell. We showed that free radicals are formed during osmotic stress, as measured by an increase in the MDA production. These radicals can react with many cellular constituents, including DNA, proteins, and lipids, leading to radical chain processes, crosslinks, peroxidation, membrane leakage, and the production of toxic compounds (Davies, 1995). MDA, a lipid peroxidation product, has been used widely to assess the levels of free radicals in living cells (Kunert and Ederer, 1985). In this study, we found that MDA levels increased significantly with the NaCl concentration in tobacco BY2 cells (Fig. 6, A–B). Exogenously supplied Pro significantly reduced (by 40%) the levels of free radicals in the salt-treated BY2 cells (Fig. 7A). This confirms earlier observations by Alia et al. (1993) on the production of free radicals under salinity stress.
Measurements of MDA contents in transgenic plants producing high levels of endogenous Pro during salinity stress showed that P5CSF129A plants produced more Pro and accumulated less MDA than P5CS transgenic or wild-type plants (Fig. 7B). That Pro levels are increased as a result of free radical generation is indicated by treating BY2 cells with plumbagin, a known free radical generator (Z. Peng and D.P.S. Verma, unpublished data). These results clearly show a role of Pro in scavenging free radicals in cells exposed to salinity. Resistance to oxidative stress can also be increased by enhanced mannitol biosynthesis in transgenic plants (Shen et al., 1997). It is possible that the increased resistance to oxidative stress is due to some indirect metabolic or physiological consequence of the accumulation of Pro and other metabolites. Overproduction of Gly betaine results in the induction of two enzymes, ascorbate peroxidase and catalase, which are known to be involved in oxidative stress resistance in Arabidopsis (Alia et al., 1999). Intermediates in Pro biosynthesis and catabolism, such as Gln and P5C, have also been found to induce the expression of several osmotically regulated genes in rice (Iyer and Caplan, 1998).
Accumulation of Pro in plants under stress is a result of the reciprocal regulation of two pathways: increased expression of Pro synthetic enzymes (P5CS and P5CR) and repressed activity of Pro degradation (Delauney and Verma, 1993; Peng et al., 1996). This leads to a “proline cycle,” the homeostasis of which depends on the physiological state of the tissue (Verma, 1999). Pro catabolism is catalyzed by Pro dehydrogenase and P5C dehydrogenase (Hu et al., 1996; Peng et al., 1996). Suppression of Pro degradation has been demonstrated both in radiolabeling studies (Stewart and Boggess, 1978) and gene expression experiments (Kiyosue et al., 1996; Peng et al., 1996; Verbruggen et al., 1996). Recent studies have demonstrated that high Pro concentrations are present in the phloem sap of drought-stressed alfalfa (Girousse et al., 1996) and that the expression of a Pro-specific amino acid transporter is induced in response to water deficit and salt stress (Rentsch et al., 1996). Evidence for the transport of Pro to the root tip, where it accumulates during stress, has been reported (Verslues and Sharp, 1999). The data indicate that plants may have evolved a mechanism to coordinate synthesis, catabolism, and transport activities for the accumulation of Pro.
Plants well adapted to drought and saline environments manifest a variety of changes for sustained growth. The accumulation of Pro is one of the factors that facilitates this adjustment. The relative contribution of each step remains to be established. Our present results indicate that Pro synthesis in plants can be manipulated by eliminating feedback regulation of the key regulatory enzyme of the pathway, P5CS. The ability of plants to tolerate oxidative stresses imposed by osmotic stress can be significantly improved by expressing a mutant form of the enzyme in transgenic plants. Recent data have shown that expression of antisense P5CS inhibits Pro production and makes plants hypersensitive to osmotic stress (Najo et al., 1999). This is also consistent with a study on antisense Gln synthetase that reduces the Pro level and renders transgenic plants more sensitive to salt treatment (Brugiere et al., 1999). The present study also suggests that the role of Pro as a free radical scavenger may be more important in overcoming stress than in acting as a simple osmolyte. This opens a new avenue of research for metabolic engineering and stress tolerance in agriculturally important crops.
ACKNOWLEDGMENT
We thank Dr. Zhaohua Peng for help on MDA measurements.
Footnotes
This work was supported by grants from the U.S. Department of Agriculture National Research Initiative Competitive Grants Program.
LITERATURE CITED
- Adams E, Frank L. Metabolism of proline and the hydroxyprolines. Annu Rev Biochem. 1980;49:1005–1061. doi: 10.1146/annurev.bi.49.070180.005041. [DOI] [PubMed] [Google Scholar]
- Alia, Kondo Y, Sakamoto A, Nonaka H, Hayashi H, Saradhi PP, Chen TH, Murata N. Enhanced tolerance to light stress of transgenic Arabidopsis plants that express the codA gene for a bacterial choline oxidase. Plant Mol Biol. 1999;40:279–288. doi: 10.1023/a:1006121821883. [DOI] [PubMed] [Google Scholar]
- Alia, Saradhi PP, Mohanty P. Proline in relation to free radical production in seedlings of Brassica juncea raised under sodium chloride stress. Plant Soil. 1993;155/156:497–500. [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207. [Google Scholar]
- Binzel ML, Hasegawa PM, Rhodes D, Handa S, Handa AV, Bressan RA. Solute accumulation in tobacco cells adapted to NaCl. Plant Physiol. 1987;84:1408–1415. doi: 10.1104/pp.84.4.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggess SF, Aspinall D, Paleg L. Stress metabolism IX. The significance of end-product inhibition of proline biosynthesis and of compartmentation in relation to stress-induced proline accumulation. Aust J Plant Physiol. 1976a;3:513–525. [Google Scholar]
- Boggess SF, Stewart CR, Aspinall D, Paleg L. Effect of water stress on proline synthesis from radioactive precursors. Plant Physiol. 1976b;58:398–401. doi: 10.1104/pp.58.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugiere N, Dubois F, Limami AM, Lelandais M, Roux Y, Sangwan RS, Hirel B. Glutamine synthetase in the phloem plays a major role in controlling proline production. Plant Cell. 1999;11:1995–2012. doi: 10.1105/tpc.11.10.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csonka LN. Proline over-production results in enhanced osmoregulation in Salmonella typhimurium. Mol Gen Gent. 1981;182:82–86. doi: 10.1007/BF00422771. [DOI] [PubMed] [Google Scholar]
- Csonka LN, Gelvin SB, Goodner BV, Orser CS, Siemieniak D, Slightom JL. Nucleotide sequence of a mutation in proB gene in Escherichia coli that confers proline overproduction and enhanced tolerance of osmotic stress. Gene (Amst) 1988;64:199–205. doi: 10.1016/0378-1119(88)90335-6. [DOI] [PubMed] [Google Scholar]
- Dandekar AM, Uratsu SL. A single base pair change in proline biosynthesis genes causes osmotic stress tolerance. J Bacteriol. 1988;170:5943–5945. doi: 10.1128/jb.170.12.5943-5945.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KJ. Oxidative stress: the paradox of aerobic life. Biochem Soc Symp. 1995;61:1–31. doi: 10.1042/bss0610001. [DOI] [PubMed] [Google Scholar]
- Delauney AJ, Hu C-AA, Kishor PBK, Verma DPS. Cloning of ornithine δ-aminotransferase cDNA from Vigna aconitifolia by trans-complementation in Escherichia coli and regulation of proline biosynthesis. J Biol Chem. 1993;268:18673–18678. [PubMed] [Google Scholar]
- Delauney AJ, Verma DPS. Proline biosynthesis and osmoregulation in plants. Plant J. 1993;4:215–223. [Google Scholar]
- Garcia-Rios M, Fujita T, LaRosa PC, Locy R, Clithero JM, Bressan RA, Csonka LN. Cloning of a polycistronic cDNA from tomato encoding γ-glutamyl kinase and γ-glutamyl phosphate reductase. Proc Natl Acad Sci USA. 1997;94:8249–8254. doi: 10.1073/pnas.94.15.8249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginzberg I, Stein H, Kapulnik Y, Szabados L, Strizhov N, Schell J, Koncz C, Zilberstein A. Isolation and characterization of two different cDNAs of delta1-pyrroline-5-carboxylate synthase in alfalfa, transcriptionally induced upon salt stress. Plant Mol Biol. 1998;38:755–764. doi: 10.1023/a:1006015212391. [DOI] [PubMed] [Google Scholar]
- Girousse C, Bournoville R, Bonnemain J-L. Water deficit-induced changes in concentrations in proline and some other amino acids in the phloem sap of alfalfa. Plant Physiol. 1996;111:109–113. doi: 10.1104/pp.111.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath RL, Packer L. Photoperoxidation in isolated chloroplasts. Arch Biochem Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Hu C-AA, Delauney AJ, Verma DPS. A bifunctional enzyme (Δ1-pyrroline-5-carboxylate synthetase) catalyzes the first two steps in proline biosynthesis in plants. Proc Natl Acad Sci USA. 1992;89:9354–9358. doi: 10.1073/pnas.89.19.9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C-AA, Lin WW, Obie C, Valle D. Molecular enzymology of mammalian Δ1-pyrroline-5-carboxylate synthase: alternative splice donor utilization generates isoforms with different sensitivity to ornithine inhibition. J Biol Chem. 1999;274:6754–6762. doi: 10.1074/jbc.274.10.6754. [DOI] [PubMed] [Google Scholar]
- Hu C-AA, Lin WW, Valle D. Cloning, characterization, and expression of cDNAs encoding human Δ1-pyrroline-5-carboxylate dehydrogenase. J Biol Chem. 1996;271:9795–9800. doi: 10.1074/jbc.271.16.9795. [DOI] [PubMed] [Google Scholar]
- Hua X-J, Van De Cotte B, Van Montagu M, Verbruggen N. Developmental regulation of pyrroline-5-carboxylate reductase gene expression in Arabidopsis. Plant Physiol. 1997;114:1215–1224. doi: 10.1104/pp.114.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer S, Caplan A. Products of proline catabolism can induce osmotically regulated genes in rice. Plant Physiol. 1998;116:203–211. [Google Scholar]
- Kavi Kishor PB, Hong Z, Miao G, Hu C, Verma DPS. Overexpression of Δ1-pyrroline-5-carboxylate synthetase increases proline overproduction and confers osmotolerance in transgenic plants. Plant Physiol. 1995;108:1387–1394. doi: 10.1104/pp.108.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyosue T, Yoshiba Y, Yamaguchi-Shinozaki K, Shinozaki K. A nuclear gene encoding mitochondrial proline dehydrogenase, an enzyme involved in proline metabolism, is upregu-lated by proline but downregulated by dehydration in Arabidopsis. Plant Cell. 1996;8:1323–1335. doi: 10.1105/tpc.8.8.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl DH, Schubert KR, Carter MB, Hagedorn CH, Shearer G. Proline metabolism in N2-fixing root nodules: energy transfer and regulation of purine synthesis. Proc Natl Acad Sci USA. 1988;85:2036–2040. doi: 10.1073/pnas.85.7.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunert KJ, Ederer M. Leaf aging and lipid peroxidation: the role of antioxidants vitamin C and E. Physiol Plant. 1985;65:85–88. [Google Scholar]
- Nanjo T, Kobayashi M, Yoshiba Y, Sanada Y, Wada K, Tsukaya H, Kakubari Y, Yamaguchi-Shinozaki K, Shinozaki K. Biological functions of proline in morphogenesis and osmotolerance revealed in antisense transgenic Arabidopsis thaliana. Plant J. 1999;18:185–193. doi: 10.1046/j.1365-313x.1999.00438.x. [DOI] [PubMed] [Google Scholar]
- Peng Z, Lu Q, Verma DPS. Reciprocal regulation of Δ1-pyrroline-5-carboxylate synthetase and proline dehydrogenase genes control levels during and after osmotic stress in plants. Mol Gen Genet. 1996;253:334–341. doi: 10.1007/pl00008600. [DOI] [PubMed] [Google Scholar]
- Rentsch D, Hirner B, Schmelzer E, Frommer WB. Salt stress-induced proline transporters and salt stress-repressed broad specificity amino acid permeases identified by suppression of a yeast amino acid permease-targeting mutant. Plant Cell. 1996;8:1437–1446. doi: 10.1105/tpc.8.8.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoure A, Jaoua S, Hua XJ, Ardiles W, Van Montagu M, Verbruggen N. Isolation, characterization, and chromosomal location of a gene encoding the Δ1-pyrroline-5-carboxylate synthetase in Arabidopsis thaliana. FEBS Lett. 1995;372:13–19. doi: 10.1016/0014-5793(95)00935-3. [DOI] [PubMed] [Google Scholar]
- Shen B, Jensen RG, Bohnert HJ. Increased resistance to oxidative stress in transgenic plants by targeting mannitol biosynthesis to chloroplasts. Plant Physiol. 1997;108:1387–1394. doi: 10.1104/pp.113.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnoff N. The role of active oxygen in the response of plants to water-deficit and desiccation. New Phytol. 1993;125:27–58. doi: 10.1111/j.1469-8137.1993.tb03863.x. [DOI] [PubMed] [Google Scholar]
- Smith CJ, Deutch AH, Rushlow KE. Purification and Characteristics of a γ-glutamyl kinase involved in Escherichia coli proline biosynthesis. J Bacteriol. 1984;157:545–551. doi: 10.1128/jb.157.2.545-551.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CR, Boggess SF. Metabolism of [5-3H]proline by barley leaves and its use in measuring the effects of water stress on proline oxidation. Plant Physiol. 1978;61:654–657. doi: 10.1104/pp.61.4.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strizhov N, Abraham E, Okresz L, Blickling S, Zilberstein A, Schell J, Koncz C, Szabados L. Differential expression of two P5CS genes controlling proline accumulation during salt-stress requires ABA and is regulated by ABA1, ABI1 and AXR2 in Arabidopsis. Plant J. 1999;12:557–569. doi: 10.1046/j.1365-313x.1997.00557.x. [DOI] [PubMed] [Google Scholar]
- Verbruggen N, Hua XJ, May M, Van Montagu M. Environmental and developmental signals modulate proline homeostasis: evidence for a negative transcriptional regulator. Proc Natl Acad Sci USA. 1996;93:8787–8791. doi: 10.1073/pnas.93.16.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma DPS. Osmotic stress tolerance in plants: role of proline and sulfur metabolisms. In: Shinozaki K, Yamaguchi-Shinozaki K, editors. Molecular Responses to Cold, Drought, Heat and Salt Stress in Higher Plants. R.G. Austin, TX: Landes Company; 1999. pp. 153–168. [Google Scholar]
- Verslues PE, Sharp RE. Proline accumulation in maize (Zea mays L.) primary roots at low water potentials. II. Metabolic source of increased proline deposition in the elongation zone. Plant Physiol. 1999;119:1349–1360. doi: 10.1104/pp.119.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiba Y, Kiyosue T, Katagiri T, Ueda H, Mizoguchi T, Yamaguchi-Shinozaki K, Wada K, Harada Y, Shinozaki K. Correlation between the induction of a gene for Δ1-pyrroline-5-carboxylate synthetase and the accumulation of proline in Arabidopsis thaliana under osmotic stress. Plant J. 1995;7:751–760. doi: 10.1046/j.1365-313x.1995.07050751.x. [DOI] [PubMed] [Google Scholar]
- Zhang C-S, Lu Q, Verma DPS. Removal of feedback inhibition of Δ1-pyrroline-5-carboxylate synthetase, a bifunctional enzyme catalyzing the first two steps of proline biosynthesis in plants. J Biol Chem. 1995;270:20491–20496. doi: 10.1074/jbc.270.35.20491. [DOI] [PubMed] [Google Scholar]
- Zhang C-S, Lu Q, Verma DPS. Characterization of Δ1-pyrroline-5-carboxylate synthetase gene promoter in transgenic Arabidopsis thaliana subjected to water stress. Plant Sci. 1997;129:81–89. [Google Scholar]