Abstract
Objective
The current risk model for long-term prediction in coronary artery disease (CAD) is complicated, while a simple useful model is still lacking. We aim to investigate if CHADS2 and R2CHADS2 scores could predict long-term outcome for patients with CAD.
Patients and methods
We enrolled 3,700 patients with CAD between November 2010 and September 2014 at the Department of Cardiology from Chinese PLA General Hospital. The CHADS2 and R2CHADS2 scores were calculated. All cases were followed to track the incidence of composite end point consisting of cardiovascular (CV) death, myocardial infarction (MI), stroke, heart failure, and all-cause death.
Results
During a median 2.9-year follow-up, 443 patients experienced at least one element of the composite end point of CV death (n=168 [4.6%]), MI (n=59 [1.6%]), stroke (n=96 [2.6%]), heart failure (n=101 [2.8%]), and all-cause death (n=240 [6.6%]). Multivariate Cox regression analyses showed that the CHADS2 score (hazard ratio [HR]: 2.18, 95% CI: 2.00–2.38, p<0.0001) and the R2CHADS2 score (HR: 1.93, 95% CI: 1.83–2.04, p<0.0001) were independently associated with composite outcome. Receiver-operating characteristic analysis showed that compared with the CHADS2 score, the R2CHADS2 score had better discrimination for the prediction of long-term combined outcome (0.772 vs 0.791, p=0.0013).
Conclusion
CHADS2 and R2CHADS2 scores provide a quick and useful tool in predicting long-term outcome for patients with CAD.
Keywords: CHADS2 score, R2CHADS2 score, coronary artery disease, prognosis, risk factors, renal function
Introduction
Coronary artery disease (CAD) is the leading cause of morbidity and mortality worldwide.1 Therefore, risk factor assessment and risk stratification of patients with CAD became important aspects of current research. However, the current risk model for long-term survival prediction for these patients was complicated, while a simple useful model was still lacking.2,3
The CHADS2 (congestive heart failure, hypertension, age, diabetes, and stroke/ transient ischemic attack) score, which assigns one point each for a history of congestive heart failure, hypertension, age ≥75 years, diabetes mellitus (DM), and two points for prior stroke/transient ischemic attack (TIA), was used for embolic risk stratification and guidance in the treatment of anticoagulation for patients with non-valvular atrial fibrillation (AF).4 Recently, it was demonstrated that the CHADS2 score could predict clinical outcome in patients with acute myocardial infarction (MI),5 because the score included similar risk factors for poor prognosis.
A new risk model, the R2CHADS2 (renal dysfunction, congestive heart failure, hypertension, age, diabetes, and stroke/TIA) score, was proposed to be a powerful scoring scheme in predicting stroke or systemic embolism in AF patients.6 Renal dysfunction, the additional component of the R2CHADS2 score, was associated with worse clinical outcomes in CAD patients.7 Compared with the CHADS2 score, the R2CHADS2 score was believed to have better prognostic predictive value for stroke of AF patients.6 However, few studies have investigated the association of R2CHADS2 scores with long-term cardiovascular (CV) outcome in patients with CAD. Based on these experiences, we aimed to investigate if CHADS2 and R2CHADS2 scores could predict long-term outcome for patients with CAD.
Patients and methods
Study populations
We enrolled 3,700 patients with CAD between November 2010 and September 2014 at the Department of Cardiology from Chinese PLA General Hospital. Patients were included if they met the following criteria: age between 20 and 90 years, angiographic evidence of stenosis of 50% or greater in ≥1 coronary vessel and hemodynamically stable. Patients were excluded if they had cardiogenic shock, severe valvular heart disease, myocarditis, severe anemia, active inflammatory disease, or cancer. The Medical Ethics Committee of PLA General Hospital approved the research protocol. All participants provided written informed consent.
Procedures
Demographic characteristics including age, sex, history of DM, hypertension, current cigarette smoking, chronic heart failure, previous ischemic stroke or TIA, medical history of percutaneous coronary intervention and/or coronary artery bypass grafting, and biochemical and echocardiographic examination were obtained from the hospital records. Hypertension was defined as repeated measurements of systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or chronic treatment with antihypertensive medications. Diabetes was defined based on hospital records, hemoglobin A1c ≥7.0%, or the use of antidiabetic medications. Congestive heart failure was defined as left ventricular ejection fraction <40%. Data on prior stroke, TIA, AF, and chronic obstructive pulmonary disease (COPD) were collected from the hospital records. Smoking was defined as smoking 10 cigarettes a day for at least 1 year without quitting. Family history was defined as the presence of heart disease or sudden cardiac death in a male first-degree relative aged <55 years or in a female first-degree relative aged <65 years.
Calculation of CHADS2 and R2CHADS2 scores
Thereafter, two experienced cardiologists, without knowledge of the patients’ prognosis, calculated the CHADS2 and R2CHADS2 scores. The CHADS2 nomenclature represents congestive heart failure (C), HT (H), age (A), DM (D), and stroke (S). The CHADS2 score was calculated by assigning one point each for the presence of congestive heart failure, HT, and DM and by assigning two points for a history of stroke or TIA. The glomerular filtration rate (GFR) was calculated using the Chronic Kidney Disease Epidemiology (CKD-EPI) equation:
The CKD-EPI equation was suggested to offer a more precise assessment of glomerular filtration, as compared to previous equations.8 The R2CHADS2 score was derived by incorporating the components of the CHADS2 score and awarding two points for renal dysfunction, defined as a GFR <60 mL/min/1.73 m2. Scr is serum creatinine, k is 0.7 for female and 0.9 for male, a is −0.329 for female and −0.411 for male.
Clinical outcome
Follow-up data were collected by trained research coordinators through telephone interview and hospital records. We selected a composite end point consisting of MI, stroke, heart failure, and all-cause death. MI was defined as a clinical sign of infarction with recurrent chest pain and/or development of new electrocardiogram changes together with a rise of creatine kinase-MB or troponin-T measured following the chest pain. Stroke was defined as a new neurologic deficit, which could not be explained by other causes and with at least one image test (computed tomography or magnetic resonance imaging) compatible with the diagnosis, as well as confirmation from a neurologist. Heart failure was defined as hospitalization for signs and symptoms involving at least two of the following: orthopnea, paroxysmal nocturnal dyspnea, elevated jugular venous pressure, pulmonary rales, third heart sound, and pulmonary edema on radiography. Supportive documentation of reduced cardiac output and elevated pulmonary capillary wedge pressure was assessed when available. All-cause death included CV death and death caused by other reasons. CV death was defined as documentation of diagnoses involving ischemic heart disease, acute coronary syndrome (ACS), heart failure, cerebrovascular disease, arrhythmia, great vessel or peripheral vascular disease, valvular heart disease, or sudden death because of an unknown but presumed CV cause in high-risk patients. All patients were followed until they either reached the study end point or the end of study follow-up.
Statistical analysis
Descriptive statistics were performed for demographic and biomarker variables. The baseline data were summarized numerically as mean and SD and proportions for categorical variables. Differences between continuous values were assessed using ANOVA test for normally distributed variables. Differences between nominal variables were compared using the χ2 test. Cox proportional hazards models were used to identify the predictors of composite end point, CV death and all-cause mortality. Associations are reported as hazard ratios (HRs) with 95% CIs. Kaplan–Meier curves were used to illustrate event rates for each risk level defined by score. A log-rank test was used to compare the survival curves among different patient groups. The receiver-operating characteristic curve was also used to demonstrate the sensitivity and specificity of the CHADS2 score and the R2CHADS2 score and their cutoff values for predicting clinical events. The area under the curve (AUC) comparison of these scoring systems was performed using the Delong method. A p-value of 0.05 was considered significant, and all tests were two tailed. Data were analyzed with SPSS software (version 19.0; IBM Corporation, Armonk, NY, USA) and MedCalc Statistical Software version 12.2 (MedCalc Software bvba, Ostend, Belgium).
Results
Patient’s characteristics
After excluding patients with loss of follow-up (n=55) or missing data required for the calculation of CHADS2 and R2CHADS2 scores (n=12), the remaining 3,633 subjects were the subjects of this secondary data analysis. The follow-up rate was 98.5%. The median age was 61.5±11.7 years, and subjects comprised 2,625 (72.3%) men and 1,008 (27.7%) women. The CHADS2 score ranged from 0 to 6, with a mean±SD of 1.3±1.0 and a median of 1 (0–2), while the R2CHADS2 score ranged from 0 to 7, with a mean±SD of 1.6±1.3 and a median of 1 (0–2). Baseline clinical characteristics according to tertiles of R2CHADS2 score are presented in Table 1. Subjects in the highest tertiles were older and more likely to have a history of hypertension, stroke, DM, and MI. Demographic parameters such as systolic blood pressure, glucose, and creatinine were found to be significantly higher in patients with high R2CHADS2 score.
Table 1.
Baseline clinical characteristics of patients
| Characteristics | Overall N=3,633 |
0–2 n=2,970 |
3–5 n=610 |
6–8 n=53 |
p-value |
|---|---|---|---|---|---|
| Age, years | 61.5±11.7 | 59.6±10.7 | 69.9±12.0 | 73.2±12.4 | <0.001 |
| Male, n (%) | 2,625 (72.3) | 2,211 (74.4) | 378 (62.0) | 36 (67.9) | <0.001 |
| Smoking, n (%) | 1,091 (30.0) | 465 (15.7) | 103 (16.9) | 9 (17.0) | <0.001 |
| Previous stroke, n (%) | 349 (9.6) | 245 (8.2) | 93 (15.2) | 11 (20.8) | <0.001 |
| Previous MI, n (%) | 447 (12.3) | 329 (11.1) | 104 (17.0) | 14 (26.4) | <0.001 |
| Hypertension, n (%) | 2,373 (65.3) | 1,791 (60.3) | 533 (87.4) | 49 (92.5) | <0.001 |
| Hyperlipidemia, n (%) | 1,126 (31.0) | 961 (32.4) | 158 (25.9) | 7 (13.2) | <0.001 |
| Diabetes, n (%) | 1,163 (32.0) | 794 (26.7) | 329 (53.9) | 40 (75.5) | <0.001 |
| COPD, n (%) | 85 (2.3) | 50 (1.7) | 30 (4.9) | 5 (9.4) | <0.001 |
| AF, n (%) | 106 (2.9) | 66 (2.2) | 35 (5.7) | 5 (9.4) | <0.001 |
| Previous PCI, n (%) | 903 (24.9) | 702 (23.6) | 176 (28.9) | 25 (47.2) | <0.001 |
| Previous CABG, n (%) | 106 (2.9) | 73 (2.5) | 32 (5.2) | 1 (1.9) | <0.001 |
| SBP, mmHg | 135.2±22.0 | 134.3±21.9 | 139.0±22.3 | 142.8±23.0 | <0.001 |
| DBP, mmHg | 75.8±16.6 | 76.2±17.3 | 74.0±12.6 | 72.3±13.8 | 0.004 |
| BMI, kg/m2 | 25.6±3.4 | 25.7±3.4 | 25.1±3.6 | 25.9±3.3 | <0.001 |
| ABI | 1.1±0.2 | 1.1±0.2 | 1.1±0.2 | 0.9±0.3 | 0.087 |
| LVEF, % | 56.5±8.4 | 57.5±7.4 | 52.3±10.5 | 44.5±9.4 | <0.001 |
| TC, mg/dL | 4.0±1.1 | 4.0±1.1 | 4.0±1.1 | 3.8±1.3 | 0.209 |
| TG, mg/dL | 1.6±1.0 | 1.6±0.9 | 1.6±1.0 | 1.5±0.8 | 0.384 |
| HDL-C, mg/dL | 1.1±0.3 | 1.1±0.3 | 1.1±0.3 | 1.0±0.3 | 0.596 |
| LDL-C, mg/dL | 2.4±0.9 | 2.4±0.9 | 2.4±0.9 | 2.2±1.0 | 0.159 |
| Glucose, mg/dL | 7.0±4.8 | 6.8±4.5 | 7.8±5.9 | 7.9±3.5 | <0.001 |
| Creatinine, µmol/L | 86.1±67.9 | 76.1±34.1 | 128.7±132.6 | 156.3±131.1 | <0.001 |
| Multivessel disease, n (%) | 2,229 (61.4) | 1,807 (60.8) | 390 (63.9) | 32 (60.4) | 0.356 |
| CAD type, n (%) | |||||
| SA | 907 (25.0) | 786 (26.5) | 113 (18.5) | 8 (15.1) | <0.001 |
| UA | 2,297 (63.2) | 1,857 (62.5) | 405 (66.4) | 35 (66.0) | |
| STEMI | 125 (3.4) | 88 (3.0) | 35 (5.7) | 2 (3.8) | |
| NSTEMI | 304 (8.4) | 239 (8.0) | 57 (9.3) | 8 (15.1) | |
| Medication, n (%) | |||||
| Aspirin | 3,398 (93.5) | 2,825 (95.1) | 526 (86.2) | 47 (88.7) | <0.001 |
| Clopidogrel | 2,979 (82.0) | 2,441 (82.2) | 495 (81.3) | 43 (81.1) | 0.856 |
| ACEI | 1,540 (42.4) | 1,199 (40.4) | 309 (50.7) | 32 (60.4) | <0.001 |
| Statin | 3,431 (94.5) | 2,830 (95.3) | 555 (91.1) | 46 (86.8) | <0.001 |
| Beta blocker | 2,627 (72.5) | 2,134 (72.0) | 455 (74.7) | 38 (71.7) | 0.403 |
Notes: Data are presented as mean±SD or n (%). R2CHADS2, renal dysfunction, congestive heart failure, hypertension, age, diabetes, and stroke/transient ischemic attack.
Abbreviations: ABI, ankle brachial index; ACEI, angiotensin converting enzyme inhibitor; AF, atrial fibrillation; BMI, body mass index; CABG, coronary artery bypass grafting; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; HDL-C, high-density lipoprotein cholesterol; LDL-C, low- density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NSTEMI, non-ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; SA, stable angina; SBP, systolic blood pressure; STEMI, ST-segment elevation myocardial infarction; TC, total cholesterol; TG, triglycerides; UA, unstable angina.
Over a median follow-up of 2.9 years, 443 patients experienced at least one element of the composite end point of CV death (n=168 [4.6%]), MI (n=59 [1.6%]), stroke (n=96 [2.6%]), heart failure (n=101 [2.8%]), and all-cause death (n=240 [6.6%]). Kaplan–Meier plots showed that rates of composite outcome increased with increasing CHADS2 and R2CHADS2 scores (Figures 1 and 2). In terms of all-cause death and CV death, similar results were observed.
Figure 1.
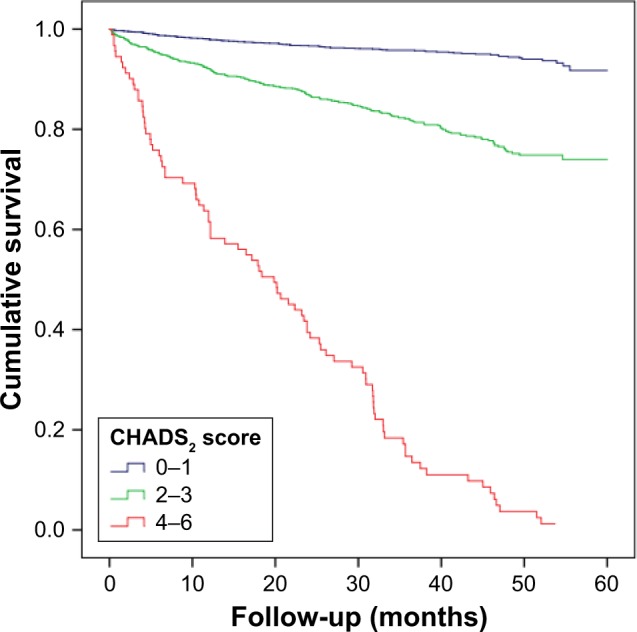
Event-free survival curve for patients according to the CHADS2 score.
Note: CHADS2, congestive heart failure, hypertension, age, diabetes, and stroke/transient ischemic attack.
Figure 2.
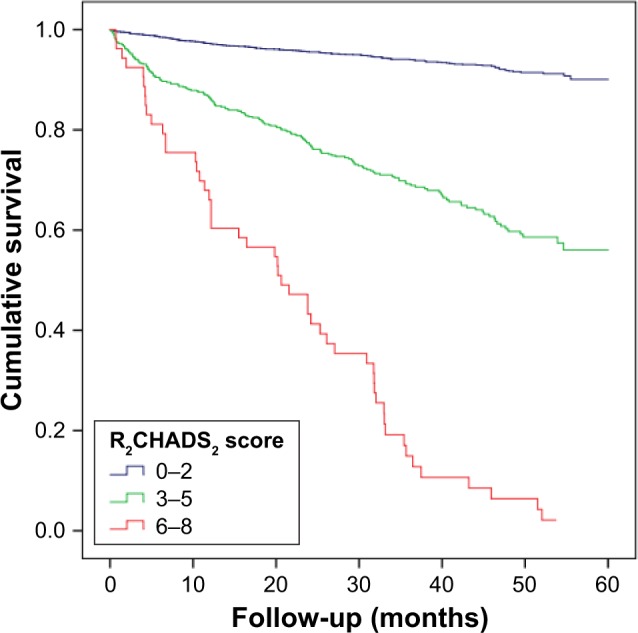
Event-free survival curve for patients according to the R2CHADS2 score.
Note: R2CHADS2, renal dysfunction, congestive heart failure, hypertension, age, diabetes, and stroke/transient ischemic attack.
Predictors of combined outcomes
Using two separate multivariate Cox regression analyses (model 1 was adjusted for sex, smoking, body mass index, hyperlipidemia, GFR, total cholesterol, triglycerides, low- density lipoprotein cholesterol and high-density lipoprotein cholesterol, medication at discharge; model 2 was adjusted for the aforementioned covariates except GFR), the CHADS2 and R2CHADS2 scores were strongly associated with the composite end point (Table 2).
Table 2.
Predictors for composite outcome in the entire cohort
| Variable | Model 1 | Model 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |||
| BMI | 0.9378 | 0.9116–0.9647 | <0.0001 | 0.9400 | 0.9131–0.9677 | <0.0001 | ||
| Hyperlipidemia | 0.5801 | 0.4527–0.7433 | <0.0001 | 0.5741 | 0.4478–0.7361 | <0.0001 | ||
| TG | 0.8784 | 0.7794–0.9900 | 0.0337 | 0.8791 | 0.7796–0.9913 | 0.0355 | ||
| HDL-C | 0.6389 | 0.4546–0.8979 | 0.0099 | 0.6444 | 0.4586–0.9053 | 0.0113 | ||
| GFR | 0.9807 | 0.9769–0.9846 | <0.0001 | |||||
| Aspirin | 0.7233 | 0.5372–0.9739 | 0.0328 | 0.6852 | 0.5159–0.9101 | 0.0090 | ||
| CHADS2 | 2.1848 | 2.0075–2.3777 | <0.0001 | |||||
| R2CHADS2 | 1.9299 | 1.8288 | 2.0365 | <0.0001 | ||||
Notes: Cox regression multivariate: model 1 was adjusted for sex, smoking, BMI, hyperlipidemia, GFR, TC, TG, LDL-C, HDL-C, and medication at discharge, while model 2 was adjusted for the aforementioned covariates except GFR. CHADS2, congestive heart failure, hypertension, age, diabetes, and stroke/transient ischemic attack; R2CHADS2, renal dysfunction, congestive heart failure, hypertension, age, diabetes, and stroke/transient ischemic attack.
Abbreviations: BMI, body mass index; GFR, glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; HR, hazard ratio; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglycerides.
Then, we separately evaluated the prognostic value of the two scores in patients with stable CAD and ACS. After adjustment for additional covariates (model 1 for CHADS2 score, model 2 for R2CHADS2 score), the CHADS2 and R2CHADS2 scores were independent predictors of composite outcome, all-cause death and CV death, in both stable CAD and ACS patients (Table 3).
Table 3.
Predictors for clinical outcome in stable CAD and ACS patients
| Stable CAD
|
ACS
|
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Composite outcome | ||||||
| CHADS2a | 2.6671 | 2.1660–3.2843 | <0.0001 | 2.1593 | 1.9830–2.3513 | <0.0001 |
| R CHADS2b | 2.0443 | 1.7664–2.3660 | <0.0001 | 1.9184 | 1.8178–2.0246 | <0.0001 |
| All-cause death | ||||||
| CHADS2a | 1.6533 | 1.1768–2.3228 | 0.0037 | 1.5993 | 1.4150–1.8077 | <0.0001 |
| R2CHADS2b | 1.5429 | 1.2284–1.9379 | <0.0001 | 1.6741 | 1.5519–1.8059 | <0.0001 |
| Cardiovascular death | ||||||
| CHADS2a | 1.5574 | 1.3347–1.8173 | <0.0001 | 1.5483 | 1.1757–3.0364 | 0.0086 |
| R2CHADS2b | 1.6492 | 1.4981–1.8156 | <0.0001 | 1.6282 | 1.4856–1.7846 | <0.0001 |
Notes: Cox regression multivariate:
model 1 was adjusted for sex, smoking, BMI, hyperlipidemia, GFR, TC, TG, LDL-C, HDL-C, and medication at discharge, while
model 2 was adjusted for the aforementioned covariates except GFR. CHADS2, congestive heart failure, hypertension, age, diabetes, and stroke/transient ischemic attack; R2CHADS2, renal dysfunction, congestive heart failure, hypertension, age, diabetes, and stroke/transient ischemic attack.
Abbreviations: ACS, acute coronary syndrome; BMI, body mass index; CAD, coronary artery disease; GFR, glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; HR, hazard ratio; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglycerides.
Model performances
Next, we performed receiver-operating characteristic analysis to determine the predictability of the CHADS2 score and the R2CHADS2 score to the composite end point. The AUC was 0.772 (95% CI: 0.758–0.785) for the CHADS2 score and 0.791 (95% CI: 0.777–0.804) for the R2CHADS2 score. With a cutoff value of 2, the CHADS2 score had a sensitivity of 75.8% and a specificity of 66.7% to identify patients with poor clinical outcome. Meanwhile, with a cutoff value of 3, the R2CHADS2 score had a sensitivity of 56.2% and a specificity of 87.0%. Compared with the CHADS2 score, the R2CHADS2 score showed better discrimination for the prediction of long-term combined outcome (0.772 vs 0.791, p=0.0013; Figure 3).
Figure 3.
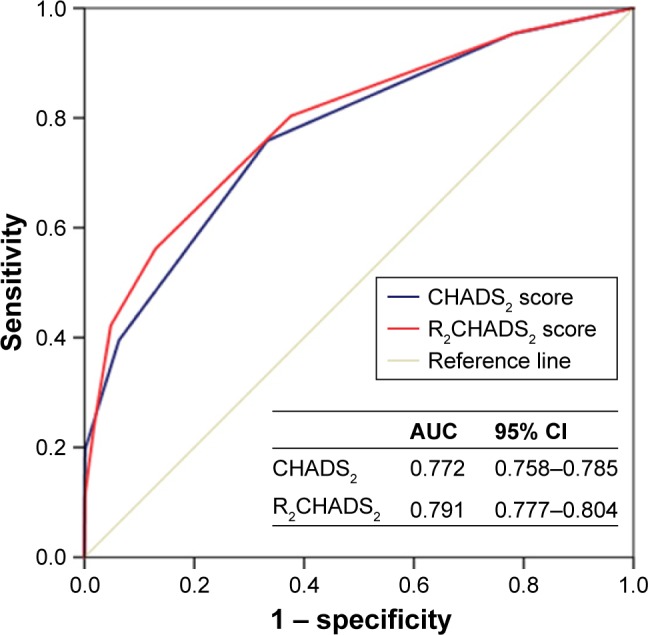
ROC curves for the CHADS2 and R2CHADS2 scores for predicting events.
Note: CHADS2, congestive heart failure, hypertension, age, diabetes, and stroke/ transient ischemic attack; R2CHADS2, renal dysfunction, congestive heart failure, hypertension, age, diabetes, and stroke/transient ischemic attack.
Abbreviations: AUC, area under the curve; ROC, receiver operating characteristic.
Discussion
In this study, we found that the CHADS2 score was a useful tool in risk stratification and long-term prognosis for patients with coronary heart disease. In particular, the new risk model, R2CHADS2 score, had better predictability than the CHADS2 score of composite outcome for patients with CAD. Both CHADS2 and R2CHADS2 scores could predict long-term clinical outcome for patients with stable CAD and ACS.
The original purpose of the CHADS2 score was for risk stratification in stroke prevention of AF. Among several risk stratification indices, the CHADS2 score is the most commonly used because it is simple to calculate, well validated, and endorsed in practice guidelines. Later, the utility of the CHADS2 score in other CV fields attracted increasing attention.9 Tasolar et al enrolled 252 non-ST-segment elevation acute coronary syndrome (NSTE-ACS) patients and found that the CHA2DS2-VASc-HS score, which incorporated hyperlipidemia and smoking, was positively correlated with the severity and complexity of CAD.10 They also found that CHA2DS2-VASc-HS was comparable with other risk scores for the risk stratification of the in-hospital major adverse cardiac events of NSTE-ACS patients. Welles et al enrolled 916 patients with stable CAD and no AF and reported that the CHADS2 score was strongly predictive of ischemic stroke/TIA (AUC: 0.65).11 Poci et al enrolled 2,335 participants with ACS and reported that long-term mortality was associated with the CHADS2 score (HR: 1.38, 95% CI: 1.28–1.48).12
In line with these findings, this study demonstrated that higher CHADS2 score was associated with higher risk of combined outcome, all-cause death, and CV death in patients with CAD. It is of great importance to assess the risk of CAD patients to provide appropriate medical treatment and reduce CV events and mortality. Thus, several risk prediction scoring systems, including various CAD risk factors, have been developed.2,13,14 However, simple and reliable tools to identify CAD patients’ risks are needed for routine practice. CHADS2 score itself is proved to be a reliable and convenient tool to predict outcome for CAD patients. In fact, it is reasonable to expand the role of CHADS2 score in CAD, since each component of the CHADS2 score is also a risk factor of coronary heart disease and stroke itself also causes disability, further contributing to mortality.
Renal function is a powerful risk factor for mortality in patients with AF. Thus, Piccini et al have made attempts to combine CrCl with the CHADS2 score and created R2CHADS2 score.6 The CrCl was calculated with the Cockcroft–Gault formula. In this study, when calculating GFR, we used the CKD-EPI equation instead of the Cockcroft–Gault formula; it was because a previous study showed that GFR-based scheme, R2(GFR)CHADS2, provided a significant improvement of predictive ability for mortality risk in older patients with AF.15 In a recent study, Huang et al enrolled 3,295 subjects with CAD and found that R2CHADS2 had comparable predictive ability of mortality to the Global Registry of Acute Coronary Events score.16 Compared with the CHADS2 score (c-statistic =0.61), the R2CHADS2 (c-statistic =0.66, p<0.05) score provides better discrimination for mortality. The results of this study suggested that the R2CHADS2 score could be used to predict composite events for patients with CAD, and the AUC of the R2CHADS2 was statistically larger than of the CHADS2 score. The clinical utility of the R2CHADS2 score should be emphasized, for those with a R2CHADS2 score of ≥3 had a rate of adverse events as high as 37.6%. These results indicated that reduced renal function played a critical role in the prognosis of CV outcomes in patients with CAD. Several potential mechanisms may explain these findings. Patients with reduced renal function often have consequences such as anemia, volume overload, and oxidative stress, which contribute to the poor outcomes.17 In addition, it has been reported that impaired renal function causes decreased number of smooth muscle cells within the plaque, which may accelerate the formation of vulnerable plaque and increase the possibility of plaque disruption.18,19
To our knowledge, although the CHADS2 score has already been tested in a previous study, the prognostic role of the R2CHADS2 score in long-term composite outcome in patients with CAD has not been addressed before. This study suggests that the R2CHADS2 score may predict risk with reasonable efficacy for patients with stable CAD and ACS.
Limitations
This study has several limitations. First, detailed information about the complexity of coronary artery lesions, such as the SYNTAX score, total or non-total arterial revascularization, was not evaluated. These factors are associated with long- term CV outcomes. In addition, the results need to be further validated in multicenter trials.
Conclusion
The CHADS2 and R2CHADS2 scores can be used to estimate the risk of clinical adverse events in patients with CAD. These scoring systems could lead to optimization of therapy, which might reduce the risks of subsequent adverse events.
Acknowledgments
This study was supported by grants from the National Key Research Program of China (2017YFC0840100 and 2017YFC0840103) and the Central Health Care Fund (W2015ZD02).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Townsend N, Wilson L, Bhatnagar P, Wickramasinghe K, Rayner M, Nichols M. Cardiovascular disease in Europe: epidemiological update 2016. Eur Heart J. 2016;37(42):3232–3245. doi: 10.1093/eurheartj/ehw334. [DOI] [PubMed] [Google Scholar]
- 2.Tang EW, Wong CK, Herbison P. Global registry of acute coronary events (GRACE) hospital discharge risk score accurately predicts long-term mortality post acute coronary syndrome. Am Heart J. 2007;153(1):29–35. doi: 10.1016/j.ahj.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA. 2000;284(7):835–842. doi: 10.1001/jama.284.7.835. [DOI] [PubMed] [Google Scholar]
- 4.Rietbrock S, Heeley E, Plumb J, van Staa T. Chronic atrial fibrillation: Incidence, prevalence, and prediction of stroke using the Congestive heart failure, Hypertension, Age >75, Diabetes mellitus, and prior Stroke or transient ischemic attack (CHADS2) risk stratification scheme. Am Heart J. 2008;156(1):57–64. doi: 10.1016/j.ahj.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Huang SS, Chen YH, Chan WL, Huang PH, Chen JW, Lin SJ. Usefulness of the CHADS2 score for prognostic stratification of patients with acute myocardial infarction. Am J Cardiol. 2014;114(9):1309–1314. doi: 10.1016/j.amjcard.2014.07.063. [DOI] [PubMed] [Google Scholar]
- 6.Piccini JP, Stevens SR, Chang Y, et al. ROCKET AF Steering Committee and Investigators Renal dysfunction as a predictor of stroke and systemic embolism in patients with nonvalvular atrial fibrillation: validation of the R(2)CHADS(2) index in the ROCKET AF (Rivaroxaban Once-daily, oral, direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation) and ATRIA (AnTicoagulation and risk factors in atrial fibrillation) study cohorts. Circulation. 2013;127(2):224–232. doi: 10.1161/CIRCULATIONAHA.112.107128. [DOI] [PubMed] [Google Scholar]
- 7.Yiu KH, de Graaf FR, Schuijf JD, et al. Prognostic value of renal dysfunction for the prediction of outcome versus results of computed tomographic coronary angiography. Am J Cardiol. 2011;108(7):968–972. doi: 10.1016/j.amjcard.2011.05.031. [DOI] [PubMed] [Google Scholar]
- 8.Du X, Hu B, Jiang L, et al. Implication of CKD-EPI equation to estimate glomerular filtration rate in Chinese patients with chronic kidney disease. Ren Fail. 2011;33(9):859–865. doi: 10.3109/0886022X.2011.605533. [DOI] [PubMed] [Google Scholar]
- 9.Lau KK, Chan PH, Yiu KH, et al. Roles of the CHADS2 and CHA2DS2- VASc scores in post-myocardial infarction patients: risk of new occurrence of atrial fibrillation and ischemic stroke. Cardiol J. 2014;21(5):474–483. doi: 10.5603/CJ.a2014.0034. [DOI] [PubMed] [Google Scholar]
- 10.Tasolar H, Cetin M, Balli M, et al. CHA2DS2-VASc-HS score in non-ST elevation acute coronary syndrome patients: assessment of coronary artery disease severity and complexity and comparison to other scoring systems in the prediction of in-hospital major adverse cardiovascular events. Anatol J Cardiol. 2016;16(10):742–748. doi: 10.14744/AnatolJCardiol.2015.6593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welles CC, Whooley MA, Na B, Ganz P, Schiller NB, Turakhia MP. The CHADS2 score predicts ischemic stroke in the absence of atrial fibrillation among subjects with coronary heart disease: data from the Heart and Soul Study. Am Heart J. 2011;162(3):555–561. doi: 10.1016/j.ahj.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poci D, Hartford M, Karlsson T, Herlitz J, Edvardsson N, Caidahl K. Role of the CHADS2 score in acute coronary syndromes: risk of subsequent death or stroke in patients with and without atrial fibrillation. Chest. 2012;141(6):1431–1440. doi: 10.1378/chest.11-0435. [DOI] [PubMed] [Google Scholar]
- 13.Morrow DA, Antman EM, Charlesworth A, et al. TIMI risk score for ST-elevation myocardial infarction: a convenient, bedside, clinical score for risk assessment at presentation: an intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation. 2000;102(17):2031–2037. doi: 10.1161/01.cir.102.17.2031. [DOI] [PubMed] [Google Scholar]
- 14.Chen SL, Han YL, Zhang YJ, et al. The anatomic- and clinical-based NERS (new risk stratification) score II to predict clinical outcomes after stenting unprotected left main coronary artery disease: results from a multicenter, prospective, registry study. JACC Cardiovasc Interv. 2013;6(12):1233–1241. doi: 10.1016/j.jcin.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Fu S, Zhou S, Luo L, Ye P. R2(GFR)CHADS2 and R2(GFR) CHA2DS2VASc schemes improved the performance of CHADS2 and CHA2DS2VASc scores in death risk stratification of Chinese older patients with atrial fibrillation. Clin Interv Aging. 2017;12:1233–1238. doi: 10.2147/CIA.S138405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang FY, Huang BT, Pu XB, et al. CHADS2, CHA2DS2-VASc and R2CHADS2 scores predict mortality in patients with coronary artery disease. Intern Emerg Med. 2017;12(4):479–486. doi: 10.1007/s11739-017-1608-x. [DOI] [PubMed] [Google Scholar]
- 17.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32(5 Suppl 3):S112–S119. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 18.Wada M, Ueda Y, Higo T, et al. Chronic kidney disease and coronary artery vulnerable plaques. Clin J Am Soc Nephrol. 2011;6(12):2792–2798. doi: 10.2215/CJN.06780711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kockx MM, Herman AG. Apoptosis in atherogenesis: implications for plaque destabilization. Eur Heart J. 1998;19(Suppl G):G23–G28. [PubMed] [Google Scholar]


