Abstract
The purpose of this study was to investigate the effects of mixed fruit and vegetable juice on alcohol hangover in healthy adults in a randomized crossover trial. Angelica keiskei/green grape/pear juice (AGP juice) was a mixture of A. keiskei juice, green grape juice, and pear juice at 1:1:1 ratio. Alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH) activities of AGP juice were measured in vitro. Fifteen healthy adults consumed alcohol (1.25 g/kg weight), and either water (control group) or AGP juice (AGP juice group). Blood was collected and expiratory-air alcohol levels were measured at 0~360 min after drinking the alcohol. Compared with control, AGP juice had higher ADH and ALDH activity in vitro. The peak alcohol levels in expiratory-air and plasma after drinking AGP juice were significantly lower than those after drinking water. The area under the curves for expiratory-air and plasma alcohol of the AGP juice group were lower than those of the control group. Thirst and headache scores after intake of alcohol were significantly reduced by AGP juice consumption compared with the control group. These data demonstrated that AGP juice could contribute to eliminate alcohol toxicity and hangover symptoms by enhancing alcohol metabolizing enzyme activities.
Keywords: alcohol, hangover, alcohol metabolizing enzyme, healthy subjects, juice
INTRODUCTION
Alcohol is the main risk factor in liver disease, including fatty liver, alcoholic hepatitis, and cirrhosis (1). Excessive alcohol consumption is also associated with increased incidence of cancer and cardiovascular disease (2). It was reported that the alcohol-induced mortality rate for the total population was 9.3%. In particular, alcohol-related liver disease deaths increased from 3,600 per 100,000 in 2005 to 3,872 per 100,000 in 2015 (3).
The average annual alcohol consumption per capita in Korea is 9.3 L, which is relatively high compared with the 9.0 L per capita in Organization for Economic Cooperation and Development (OECD) countries (4). In the 2014 Korea National Health and Nutrition Examination Survey, 60.5% of adults consumed alcohol monthly (75.3% of men, 45.7% of women), and the rates of high-risk drinking were 19.7% in men and 5.4% in women (5).
More than 90% of alcohol is primarily metabolized in the liver, and the rest is eliminated via the kidneys and lungs (6). The three alcohol metabolic pathways in the body involve alcohol dehydrogenase (ADH), the microsomal ethanol oxidation system (MEOS), and catalase (7–9). Most alcohol is first metabolized by ADH into acetaldehyde, which is converted into acetate by aldehyde dehydrogenase (ALDH). Acetaldehyde, a major toxic metabolite, increases hepatic lipid peroxidation and oxidative stress, thereby aggravating liver damage (7,8). Under excessive and chronic alcohol intake, 10~20% of the absorbed alcohol is metabolized by the MEOS, which utilizes the cytochrome P450 2E1 (CYP2E1) enzyme. The CYP2E1 pathway increases reactive oxygen species, including hydrogen peroxide, hydroxyl radical, and superoxide, which can lead to liver damage by reacting with lipids, proteins, and DNA (7–9). Thus, improvement of antioxidant capacity is important to prevent liver injury against oxidative stress.
Alcohol hangover symptoms are characterized by diverse physical and psychological symptoms including drowsiness, difficulty concentrating, dry mouth, dizziness, gastrointestinal complaints, sweating, nausea, hyperexcitability, and anxiety (6,10,11). Intake of excessive alcohol can lead to hangover symptoms due to acetaldehyde that is generated by ADH (6,10). Therefore, the elimination of acetaldehyde by promoting the activity of alcohol-metabolizing enzymes can help relieve hangover symptoms.
Natural products containing fruit, vegetable, and herbal substances increased alcohol degrading enzyme activities and have been reported to alleviate alcohol hangover symptoms. Dandelion (12), pear (13), tomato (14), red ginseng (15), Hovenia dulcis (16), and Opuntia ficus indica (17) are reported to relieve hangovers by stimulating alcohol metabolism and have antioxidant effects on alcohol-induced oxidative stress.
In a previous study, we measured the effect of various fruit and vegetable juices on alcohol-degrading enzymes in vitro (18). Among them, Angelica keiskei juice and green grape juice strongly enhanced the activities of ADH and ALDH in vitro (18). However, the effects of A. keiskei juice and green grape juice on preventing hangover symptoms have not been reported. A. keiskei juice mixed with green grape juice is not palatable because of its bitterness and flavor. Korean pear (Pyrus pyrifolia cv. Shingo) is reported to promote the activities of ADH and ALDH in vitro (13) and has a sweet taste due to the high content of sugar. Therefore, we mixed A. keiskei/green grape juice with pear juice to improve its taste and flavor. A. keiskei/green grape/pear (AGP) juices were prepared by mixing A. keiskei juice, green grape juice, and pear juice in a ratio of 1:0.5:1, 1:1:0.5, and 1:1:1 and conducted sensory evaluation. AGP juice mixed at 1:1:1 ratio exhibited the highest scores of sensory acceptability for texture, flavor, and overall preference.
In this study, we examined the effects of AGP juice on reducing hangovers in healthy subjects.
MATERIALS AND METHODS
Preparation of mixed fruit and vegetable juice
A. keiskei, green grape, and pear were purchased from a market in Gimhae, Korea and washed with tap water. Juices of A. keiskei, green grape, and pear were prepared using a low-speed masticating juicer (HH-SBF11, Hurom Co., Ltd., Gimhae, Korea). AGP juice was a mixture of A. keiskei juice, green grape juice, and pear juice at 1:1:1 ratio.
Determination of alcohol degrading enzyme activities in vitro
Alcohol dehydrogenase activity was measured using the method described by Blandino et al. (19). Briefly, the reaction mixture consisted of 1.4 mL distilled water, 750 μL 1.0 M Tris-HCl buffer (pH 8.8), 300 μL 20 mM nicotinamide adenine dinucleotide (NAD+), 300 μL ethanol, and 100 μL juice. Then, 150 μL ADH was added to the reaction mixture and incubated at 30°C for 5 min. After incubation, the absorbance was measured at 340 nm for 10 min at 30 s intervals using a microplate reader (model 550, Bio-Rad Laboratories Inc., Hercules, CA, USA). Aldehyde dehydrogenase activity was determined according to the method of Bostian and Betts (20). Briefly, the reaction mixture consisted of 2.1 mL distilled water, 300 μL 1.0 M Tris-HCl (pH 8.0), 100 μL 20 mM NAD+, 0.1 M acetaldehyde, 100 μL 3.0 M KCl, 100 μL 0.33 M 2-mercaptoethanol, and 100 μL juice. First, 100 μL ALDH was added to the reaction mixture, which was incubated at 30°C for 5 min. After incubation, the absorbance was measured at 340 nm for 10 min using a microplate reader. The increase in absorbance from time zero was measured, and the activities of ADH and ALDH were calculated as a percentage of the blank control. The measurements were performed in triplicate.
Determination of total polyphenol content and DPPH radical scavenging activity
The total polyphenol content was measured following the method of Folin and Denis (21). First, 1 mL AGP juice was added to 4 mL of methanol, incubated for 5 h at 4°C in the dark, and centrifuged at 850 g for 20 min. The supernatant (400 μL) was mixed with Folin-Ciocalteu reagent (200 μL), and then incubated for 3 min at room temperature. Then, 400 μL of 2% Na2CO3 was added to the mixture, and allowed to incubate for 60 min. The absorbance was recorded at a wavelength of 750 nm using a spectrophotometer (Libra 22, Biochrom, Cambridge, UK), and the total polyphenol content was quantified using tannic acid as a standard. The results were expressed as mg of tannic acid equivalents (TAE) per 100 mL of AGP juice. The 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity was measured using the method described by Blois (22). Briefly, 800 μL of DPPH solution (0.1 mM) prepared in 80% ethanol was added to an aliquot (200 μL) of AGP juice after centrifugation at 850 g for 10 min. The absorbance was measured at 517 nm at room temperature after 10 min. Analyses were performed in triplicate.
Clinical trials
Fifteen healthy adults (11 men and 4 women; 29.3±7.0 years old) participated in a randomized double-blind crossover trial. Informed consent was obtained from all subjects before participation. The exclusion criteria were pregnancy, breast-feeding, intestinal disorders, liver disease, diabetes mellitus, cardiovascular disease, cancer, or any other serious disorder requiring regular medical treatment. The study was conducted in a randomized crossover design. The subjects were asked to abstain from drinking for 3 days and to fast for 12 h before each study. During testing, the 15 subjects were divided into control and AGP juice groups. At the first visit, the subjects drank 240 mL of water or AGP juice 30 min before drinking alcohol. The subjects consumed alcohol (1.25g/kg weight) and 100 g of silken soy curd and drank water or AGP juice immediately afterwards. Blood samples were taken 0, 30, 60, 120, and 360 min after drinking the alcohol and water or AGP juice. Expiratory-air alcohol levels were measured 0, 30, 60, 120, 240, and 360 min after alcohol consumption using a breathalyzer (Sentech Korea Corp., Paju, Korea). At the second visit, the subjects followed the same protocol, and the control and AGP juice treatments were switched between the two groups. The Institutional Review Board of Inje University (No. 2-1041024-AB-N-01) approved the study protocol.
Anthropometric and biochemical measurements were performed in all subjects before the start of the study. Body weight and height of the subjects were measured in the morning. Body mass index was calculated as body weight in kilograms divided by height in meters squared. Body fat content was measured with a bioimpendance analyzer (HBF-300, Omron Corporation, Kyoto, Japan). Blood was centrifuged at 3,000 g for 10 min. Plasma alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities were measured enzymatically using commercial kits (Asan Pharmaceutical, Seoul, Korea). Plasma alcohol and acetaldehyde levels were determined using commercial enzyme-linked immunosorbent assay (ELISA) kits (Abcam, Cambridge, UK).
Hangover symptoms were determined by a survey from hangover assessment tools (23,24) at 360 min after alcohol consumption in each trial. The questionnaires consisted of 6 questions addressing thirst, sleepiness, headache, dizziness, nausea, and stomach pain. The scores ranged from 0 (symptoms absent) to 5 (extremely severe symptoms) and covered the 6 stages.
Statistical analysis
All data are expressed as the mean±standard error of the mean (SE). Differences among the groups were analyzed by a one-way analysis of variance (ANOVA) followed by Tukey’s test (P<0.05) using the SAS program (ver. 9.2, SAS Institute Inc., Cary, NC, USA). Student’s t-test was used to evaluate the significance (P<0.05) of differences between the mean values of the control and AGP groups.
RESULTS
Alcohol degrading enzyme activities in vitro
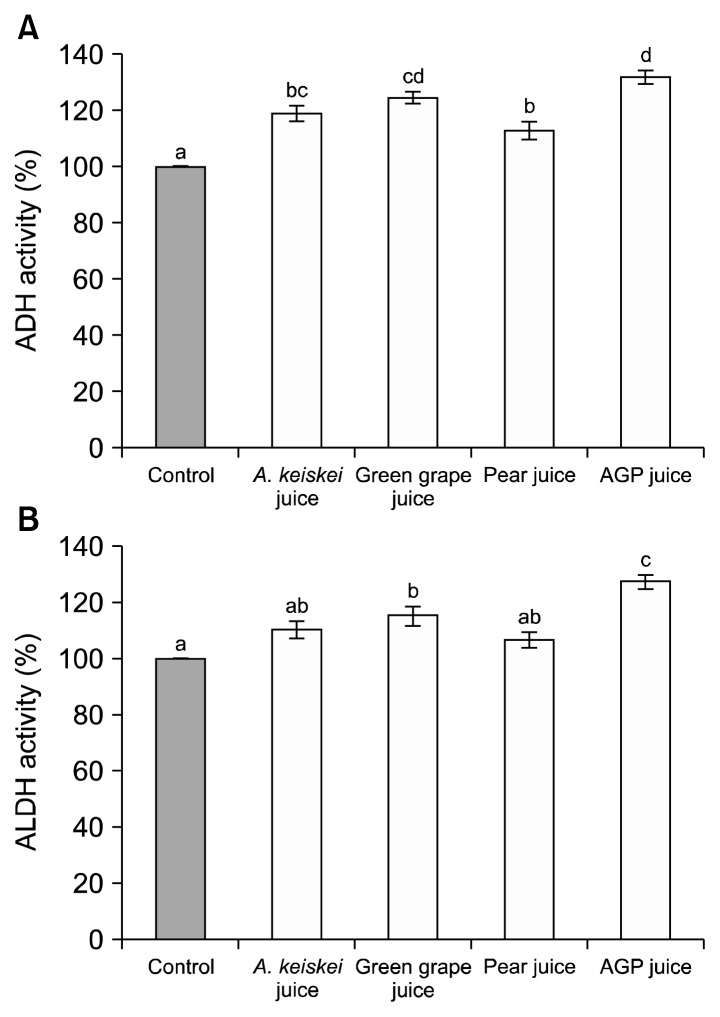
The influence of AGP juice on ADH and ALDH activities in vitro is shown in Fig. 1. ADH activities of A. keiskei juice, green grape juice, and pear juice were 119.2±2.8, 124.6±2.2, and 112.9±3.3%, respectively. ALDH activities of A. keiskei juice, green grape juice, and pear juice were 110.1±2.5, 115.2±2.8, and 106.7±2.3%, respectively. AGP juice increased ADH and ALDH activities by 132.0±2.4% and 127.4±2.1%, respectively. In this study, alcohol degrading enzyme activities of AGP juice may have a synergic effect rather than a single juice alone.
Fig. 1.
Activities of alcohol dehydrogenase (A) and aldehyde dehydrogenase (B) of AGP juice in vitro. Values represent mean±SE (n=3). Each bar with different letters (a–d) is significantly different at P<0.05.
Antioxidant effects
The total polyphenol content and DPPH radical scavenging activity of AGP juice are shown in Table 1. The total polyphenol content of the AGP juice was 20.3±0.1 mg TAE/100 mL juice. The DPPH radical scavenging activity of AGP juice was 83.4±0.2%.
Table 1.
Total polyphenol content and DPPH radical scavenging activity of Angelica keiskei/green grape/pear (AGP) juice
| Total polyphenol content (mg TAE/100 mL juice) | DPPH scavenging activity (%) | |
|---|---|---|
| AGP juice | 20.3±0.1 | 83.4±0.2 |
Values represent mean±SE of triplicate experiments.
DPPH, 1,1-diphenyl-2-picrylhydrazyl; TAE, tannic acid equivalents.
General characteristics
In this study, we evaluated the effects of AGP juice on alcohol metabolism and hangover symptoms in healthy subjects. Characteristics of the subjects are given in Table 2. The average age of the subjects in this study were 29.3±7.0 years. The ALT and AST activities of the subjects, biochemical markers of liver damage, were in the normal range. The average consumption of alcohol of subjects was 28.0±5.6 g/d.
Table 2.
General and biochemical characteristics of healthy subjects
| Parameters | |
|---|---|
| Height (cm) | 173.4±1.6 |
| Weight (kg) | 75.5±3.2 |
| Body mass index (kg/m2)1) | 25.1±0.9 |
| Body fat (%) | 25.9±1.4 |
| Alanine aminotransferase (U/L) | 13.9±2.4 |
| Aspartate aminotransferase (U/L) | 18.9±1.4 |
| Alcohol consumption (g/d) | 28.0±5.6 |
Values represent mean±SE (n=15).
Body mass index (kg/m2)=body weight (kg)/height (m)2.
Expiratory-air alcohol and plasma alcohol and acetaldehyde levels
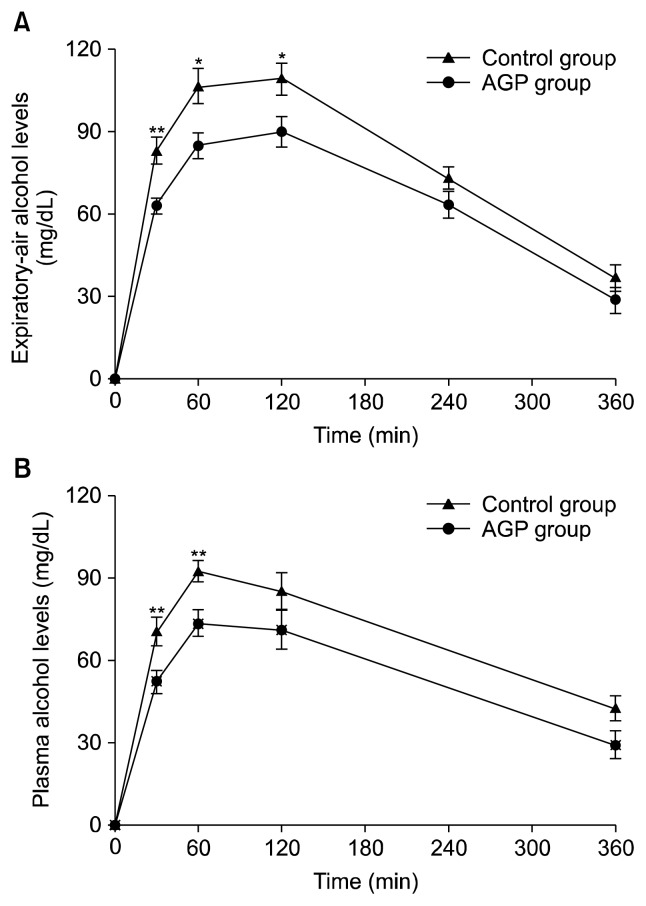
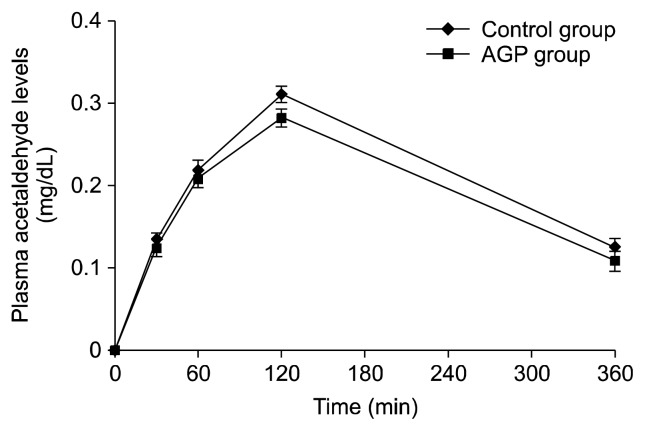
The increase of expiratory-air alcohol and plasma alcohol and acetaldehyde levels are shown in Fig. 2. The area under the alcohol and acetaldehyde response curves are shown in Table 3. The incremental expiratory-air alcohol levels of the control and AGP juice groups reached a maximum of 109.3±5.9 and 90.0±5.6 mg/dL at 120 min, respectively. The consumption of AGP juice significantly reduced the expiratory-air alcohol levels at 30 (P<0.01), 60, and 120 (P<0.05) min compared with the control group. The area under the curve (AUC) for the expiratory-air alcohol response of the AGP juice group (385.9±26.2 mg·h/dL) was lower than that of the control group (468.3±22.6 mg·h/dL, P<0.05). The incremental plasma alcohol levels of the control and AGP juice group reached a peak of 92.5±4.0 and 73.5±5.0 mg/dL at 60 min, respectively. AGP juice significantly alleviated the increase in plasma alcohol levels at 30 and 60 min compared with the control group (P<0.01). The AUC for the plasma alcohol response was significantly (P<0.05) lower in the AGP juice group (317.3±28.3 mg·h/dL) than in the control group (401.9±23.4 mg·h/dL). The plasma acetaldehyde levels of the control and AGP juice groups reached a maximum of 0.31±0.01 and 0.28±0.01 mg/dL at 120 min, respectively (Fig. 3). The incremental plasma acetaldehyde levels and the AUC for the acetaldehyde response of the two groups were not significantly different.
Fig. 2.
Increase of expiratory-air alcohol (A) and plasma alcohol (B) after drinking of alcohol in healthy subjects. The control and AGP juice groups received 240 mL of water (control group) or AGP juice (AGP juice group) 30 min before and immediately after drinking alcohol (1.25 g/kg weight). Values represent mean±SE (n=15). Significantly different at *P<0.05, **P<0.01.
Table 3.
Area under the curves for alcohol and acetaldehyde response in healthy subjects
| Group1) | Control | AGP juice |
|---|---|---|
| Expiratory-air alcohol (mg·h/dL) | 468.3±22.6* | 385.9±26.2 |
| Plasma alcohol (mg·h/dL) | 401.9±23.4* | 317.3±28.3 |
| Plasma acetaldehyde (mg·h/dL) | 1.26±0.03 | 1.14±0.05 |
Values represent mean±SE (n=15).
Significantly different at P<0.05.
The control and Angelica keiskei/green grape/pear (AGP) juice groups received 240 mL of water (control group) or AGP juice (AGP juice group) 30 min before and immediately after drinking alcohol (1.25 g/kg weight).
Fig. 3.
Increase of plasma acetaldehyde after drinking of alcohol in healthy subjects. The control and AGP juice groups received 240 mL of water (control group) or AGP juice (AGP juice group) 30 min before and immediately after drinking alcohol (1.25 g/kg weight). Values represent mean±SE (n=15).
Alcohol hangover symptoms
Scores of hangover conditions of the subjects are given in Table 4. Consumption of AGP juice significantly decreased thirst (2.1±0.2) and headache (2.0±0.2) scores compared with the control group (3.1±0.3 and 2.9±0.2, respectively, P<0.05). Sleepiness, dizziness, nausea, and stomach pain of the AGP group were not significantly different from the control group.
Table 4.
Scores of hangover conditions in healthy subjects
| Group1) | Control | AGP juice |
|---|---|---|
| Thirst | 3.1±0.3* | 2.1±0.2 |
| Sleepiness | 2.7±0.3 | 2.6±0.2 |
| Headache | 2.9±0.2* | 2.0±0.2 |
| Dizziness | 3.0±0.3 | 2.3±0.4 |
| Nausea | 1.6±0.3 | 1.5±0.2 |
| Stomach pain | 1.2±0.2 | 1.2±0.1 |
Values represent mean±SE (n=15).
Significantly different at P<0.05.
The control and Angelica keiskei/green grape/pear (AGP) juice groups received 240 mL of water (control group) or AGP juice (AGP juice group) 30 min before and immediately after drinking alcohol (1.25 g/kg weight).
The scores ranged from 0 (symptom absent) to 5 (extremely severe symptom).
DISCUSSION
Alcohol is converted into acetaldehyde by ADH, and acetaldehyde is metabolized into acetate by ALDH (8). Alcohol metabolites, especially acetaldehyde, contribute to alcohol hangover symptoms and alcoholic liver disease (1, 7,10). Accelerating the clearance of alcohol and acetaldehyde can decrease ‘alcohol toxicity’ (7,8).
The consumption of AGP juice effectively attenuated the increase of alcohol levels in plasma and expiratory-air after ingestion of alcohol and decreased the AUC in healthy subjects. Plasma acetaldehyde levels of the AGP juice group tended to be lower than the control group, although the differences were not significant. Acetaldehyde is responsible for hangover symptoms (10). A hangover involves various physical and psychological symptoms following excess alcohol intake. AGP juice improved headache and thirst symptoms after consumption of alcohol. Alcohol hangover symptoms were relieved by increasing the activities of alcohol degrading enzymes (13). It was reported that elevation of the activities of alcohol metabolizing enzymes could be beneficial in eliminating of alcohol metabolites (13,15,25). Several natural products have been reported to reduce the levels of blood alcohol metabolites by increasing the activities of alcohol degrading enzymes, including Korean pear juice (13), red ginseng (15), and herbal mixture (25). Lee et al. (13) demonstrated that Korean pear juice may improve alcohol detoxification by stimulating the activities of ADH and ALDH. In another study, a herbal mixture extract of Viscum album L., Lycium chinense L., Inonotus obliques, and Acanthopanax senticosus H. alleviated alcohol hangover symptoms by reducing plasma alcohol levels in healthy adult males (25). In the present study, AGP juice increased ADH and ALDH activities by 32.0% and 27.4% in vitro, indicating that alcohol and acetaldehyde are degraded rapidly after alcohol consumption, which prevents accumulation of alcohol metabolites. Thus, AGP juice may enhance alcohol metabolism by increasing ADH and ALDH activities. These data demonstrated that reduced plasma alcohol levels by consumption of AGP juice could contribute to eliminate alcohol toxicity.
AGP juice exhibited strong DPPH radical scavenging activity possibly because of its high polyphenol content. Polyphenols scavenge free radicals directly and thus exert strong antioxidant effects (26). This result is similar to previous research, which showed that the phenolic content had a positive correlation with the antioxidant capacity to scavenge the DPPH radical. Apple juice, grape juice, pineapple juice, and orange juice have high levels of total phenolic compounds and radical scavenging activities (27,28). Parsley juice has been known to be rich in phenolic contents and to exhibit strong antioxidant activity (29).
Alcohol abuse promotes reactive oxygen superoxide accumulation by depleting mitochondrial glutathione levels and, subsequently, increase oxidative stress (30,31). Increased oxidative stress can lead to suppressing ALDH activity, resulting in decreasing acetaldehyde clearance (20,32). Improvement of antioxidant status may be beneficial to prevent the reduction of ALDH activity and facilitate the metabolism of acetaldehyde, via reducing alcohol-induced oxidative stress. A. keiskei has long been used as a herbal medicine due to its antioxidant effects (33). A. keiskei contains bioactive substances, such as chalcone, coumarin, saponin, and flavonoids (34,35). Several studies have demonstrated that A. keiskei extract has beneficial effects on alcoholic liver diseases (34), antioxidant (33), anti-cancer (35), and anti-inflammatory effects (36). A. keiskei extract was reported to prevent D-galactosamine-induced hepatic failure in rats (37). Chronic consumption of A. keiskei extract improved liver function in habitual alcohol drinkers (34). It was suggested that antioxidant substances of A. keiskei could contribute to the hepatoprotective effects. Phenolic compounds in grape (38,39) and pear (40) have been reported to decrease oxidative stress by scavenging free radicals and inhibition of lipid peroxidation. Therefore, AGP juice may contribute to improve antioxidant status via reducing oxidative stress.
The limitation of this study may be a placebo effect by consumption of water instead of placebo juice in the control group. Since plasma ADH and ALDH activities were not measured, we could not elucidate the mechanisms for the beneficial effects in improving alcohol hangover in healthy subjects. Further study is necessary to understand the underlying mechanisms of action by measuring the activity or gene expression of alcohol metabolism enzymes.
In conclusion, AGP juice reduced plasma levels of alcohol metabolites and hangover symptoms in healthy adults by enhancing alcohol-metabolizing enzyme activities and exerting antioxidant effects. Thus, AGP juice may be helpful in alleviating alcohol hangover symptoms.
ACKNOWLEDGEMENTS
This research was supported by research funds (No.6) called The Anti-aging Bio R&D in the Gyeongsang Province.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Rusyn I, Bataller R. Alcohol and toxicity. J Hepatol. 2013;59:387–388. doi: 10.1016/j.jhep.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walsh K, Alexander G. Alcoholic liver disease. Postgrad Med J. 2000;76:280–286. doi: 10.1136/pmj.76.895.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Statistics Korea. [accessed Sep 2016];Causes of death statistics in 2015. 2016 http://kostat.go.kr/portal/eng/pressReleases/1/index.board?bmode=read&aSeq=357968.
- 4.OECD/WHO. Health at a Glance: Asia/Pacific 2016: Measuring Progress towards Universal Health Coverage. OECD Publishing; Paris, France: 2016. Alcohol; pp. 56–57. [Google Scholar]
- 5.KCDC. Korea Health Statistics 2014: Korea National Health and Nutrition Examination Survey (KNHANES VI-2) Korea Centers for Disease Control and Prevention; Sejong, Korea: 2015. [Google Scholar]
- 6.Wang F, Zhang YJ, Zhou Y, Li Y, Zhou T, Zheng J, Zhang JJ, Li S, Xu DP, Li HB. Effects of beverages on alcohol metabolism: potential health benefits and harmful impacts. Int J Mol Sci. 2016;17:354. doi: 10.3390/ijms17030354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lieber CS. Ethanol metabolism, cirrhosis and alcoholism. Clin Chim Acta. 1997;257:59–84. doi: 10.1016/S0009-8981(96)06434-0. [DOI] [PubMed] [Google Scholar]
- 8.Caballería J. Current concepts in alcohol metabolism. Ann Hepatol. 2003;2:60–68. [PubMed] [Google Scholar]
- 9.Mukherjee S. Alcohol metabolism and generation of free radicals: a deep insight. OA Alcohol. 2014;2:10. [Google Scholar]
- 10.Swift R, Davidson D. Alcohol hangover: mechanisms and mediators. Alcohol Health Res World. 1998;22:54–60. [PMC free article] [PubMed] [Google Scholar]
- 11.Min JA, Lee K, Ki DJ. The application of minerals in managing alcohol hangover: a preliminary review. Curr Drug Abuse Rev. 2010;3:110–115. doi: 10.2174/1874473711003020110. [DOI] [PubMed] [Google Scholar]
- 12.Noh KH, Jang JH, Kim JJ, Shin JH, Kim DK, Song YS. Effect of dandelion juice supplementation on alcohol-induced oxidative stress and hangover in healthy male college students. J Korean Soc Food Sci Nutr. 2009;38:683–693. doi: 10.3746/jkfn.2009.38.6.683. [DOI] [Google Scholar]
- 13.Lee HS, Isse T, Kawamoto T, Baik HW, Park JY, Yang M. Effect of Korean pear (Pyrus pyrifolia cv. Shingo) juice on hangover severity following alcohol consumption. Food Chem Toxicol. 2013;58:101–106. doi: 10.1016/j.fct.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Ushida Y, Oshima S, Aizawa K, Suganuma H, Nemoto A, Ishikiriyama H, Kitagawa Y. Aqueous components of tomato accelerate alcohol metabolism by increasing pyruvate level. Food Nutr Sci. 2014;5:870–879. doi: 10.4236/fns.2014.510096. [DOI] [Google Scholar]
- 15.Lee MH, Kwak JH, Jeon G, Lee JW, Seo JH, Lee HS, Lee JH. Red ginseng relieves the effects of alcohol consumption and hangover symptoms in healthy men: a randomized crossover study. Food Funct. 2014;5:528–534. doi: 10.1039/c3fo60481k. [DOI] [PubMed] [Google Scholar]
- 16.Jung SY, Lim JS, Song HS. Alcohol dehydrogenase activity and sensory evaluation of Hutgae (Hovenia dulcis Thunb) fruit soy sauce. Korean J Food Nutr. 2012;25:747–754. doi: 10.9799/ksfan.2012.25.4.747. [DOI] [Google Scholar]
- 17.Wiese J, McPherson S, Odden MC, Shlipak MG. Effect of Opuntia ficus indica on symptoms of the alcohol hangover. Arch Intern Med. 2004;164:1334–1340. doi: 10.1001/archinte.164.12.1334. [DOI] [PubMed] [Google Scholar]
- 18.Kim MJ, Lim SW, Ahn HJ, Jun JG, Kang MJ. Effect of fruit-vegetable juices containing Angelica keiskei on alcohol metabolizing enzyme activities in vitro. KSBB J. 2016;31:8–13. doi: 10.7841/ksbbj.2016.31.1.8. [DOI] [Google Scholar]
- 19.Blandino A, Caro I, Cantero D. Comparative study of alcohol dehydrogenase activity in flor yeast extracts. Biotechnol Lett. 1997;19:651–654. doi: 10.1023/A:1018386731116. [DOI] [Google Scholar]
- 20.Bostian KA, Betts GF. Rapid purification and properties of potassium-activated aldehyde dehydrogenase from Saccharomyces cerevisiae. Biochem J. 1978;173:773–786. doi: 10.1042/bj1730773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- 22.Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–2000. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- 23.Rohsenow DJ, Howland J, Minsky SJ, Greece J, Almeida A, Roehrs TA. The Acute Hangover Scale: a new measure of immediate hangover symptoms. Addict Behav. 2007;32:1314–1320. doi: 10.1016/j.addbeh.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slutske WS, Piasecki TM, Hunt-Carter EE. Development and initial validation of the hangover symptoms scale: prevalence and correlates of hangover symptoms in college students. Alcohol Clin Exp Res. 2003;27:1442–1450. doi: 10.1097/01.ALC.0000085585.81711.AE. [DOI] [PubMed] [Google Scholar]
- 25.Hong YH. Effects of the herb mixture, DTS20, on oxidative stress and plasma alcoholic metabolites after alcohol consumption in healthy young men. Integr Med Res. 2016;5:309–316. doi: 10.1016/j.imr.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stevenson DE, Hurst RD. Polyphenolic phytochemicals –just antioxidants or much more? Cell Mol Life Sci. 2007;64:2900–2916. doi: 10.1007/s00018-007-7237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khaw HW, Haron H, Chan BK. Comparison of total phenolic contents (TPC) and antioxidant activities of fresh fruit juices, commercial 100% fruit juices and fruit drinks. Sains Malays. 2016;45:1319–1327. [Google Scholar]
- 28.Maragò E, Iacopini P, Camangi F, Scattino C, Ranieri A, Stefani A, Sebastiani L. Phenolic profile and antioxidant activity in apple juice and pomace: effects of different storage conditions. Fruits. 2015;70:213–223. doi: 10.1051/fruits/2015015. [DOI] [Google Scholar]
- 29.Papuc C, Predescu C, Nicorescu V, Stefan G, Nicorescu I. Antioxidant properties of a parsley (Petroselinum crispum) juice rich in polyphenols and nitrites. Curr Res Nutr Food Sci. 2016;4:114–118. doi: 10.12944/CRNFSJ.4.Special-Issue-October.15. [DOI] [Google Scholar]
- 30.Singh S, Brocker C, Koppaka V, Chen Y, Jackson BC, Matsumoto A, Thompson DC, Vasiliou V. Aldehyde dehydrogenases in cellular responses to oxidative/electrophilic stress. Free Radic Biol Med. 2013;56:89–101. doi: 10.1016/j.freeradbiomed.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wheeler MD, Nakagami M, Bradford BU, Uesugi T, Mason RP, Connor HD, Dikalova A, Kadiiska M, Thurman RG. Overexpression of manganese superoxide dismutase prevents alcohol-induced liver injury in the rat. J Biol Chem. 2001;276:36664–36672. doi: 10.1074/jbc.M105352200. [DOI] [PubMed] [Google Scholar]
- 32.Lyon I, Kaplowitz N. Acetaldehyde-dependent oxidation of glutathione catalyzed by rat liver cytosol. Biochem Biophys Res Commun. 1985;129:949–957. doi: 10.1016/0006-291X(85)91983-7. [DOI] [PubMed] [Google Scholar]
- 33.Luo L, Wang R, Wang X, Ma Z, Li N. Compounds from Angelica keiskei with NQO1 induction, DPPH· scavenging and α-glucosidase inhibitory activities. Food Chem. 2012;131:992–998. doi: 10.1016/j.foodchem.2011.09.099. [DOI] [Google Scholar]
- 34.Noh HM, Ahn EM, Yun JM, Cho BL, Paek YJ. Angelica keiskei Koidzumi extracts improve some markers of liver function in habitual alcohol drinkers: a randomized double-blind clinical trial. J Med Food. 2015;18:166–172. doi: 10.1089/jmf.2014.3222. [DOI] [PubMed] [Google Scholar]
- 35.Akihisa T, Tokuda H, Ukiya M, Iizuka M, Schneider S, Ogasawara K, Mukainaka T, Iwatsuki K, Suzuki T, Nishino H. Chalcones, coumarins, and flavanones from the exudate of Angelica keiskei and their chemopreventive effects. Cancer Lett. 2003;201:133–137. doi: 10.1016/S0304-3835(03)00466-X. [DOI] [PubMed] [Google Scholar]
- 36.Sugii M, Ohkita M, Taniguchi M, Baba K, Kawai Y, Tahara C, Takaoka M, Matsumura Y. Xanthoangelol D isolated from the roots of Angelica keiskei inhibits endothelin-1 production through the suppression of nuclear factor-κB. Biol Pharm Bull. 2005;28:607–610. doi: 10.1248/bpb.28.607. [DOI] [PubMed] [Google Scholar]
- 37.Choi SH, Park KH. Protective effects of Angelica keiskei extracts against D-galactosamine (GalN)-induced hepatotoxicity in rats. J Fd Hyg Safety. 2011;26:235–241. [Google Scholar]
- 38.Xia EQ, Deng GF, Guo YJ, Li HB. Biological activities of polyphenols from grapes. Int J Mol Sci. 2010;11:622–646. doi: 10.3390/ijms11020622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pakhale SS, Karibasappa GS, Ramchandani AG, Bhushan B, Sharma A. Scavenging effect of Indian grape polyphenols on 2,2′-diphenyl-1-picrylhydrazyl (DPPH) radical by electron spin resonance spectrometry. Indian J Exp Biol. 2007;45:968–973. [PubMed] [Google Scholar]
- 40.Tanrıöven D, Ekşi A. Phenolic compounds in pear juice from different cultivars. Food Chem. 2005;93:89–93. doi: 10.1016/j.foodchem.2004.09.009. [DOI] [Google Scholar]