Abstract
The attractive purple color of blueberries (Vaccinium spp.) is unstable and susceptible to degradation during food processing and storage. The effects of various factors on the color stability of fresh blueberry juice were investigated. Total soluble solid content, pH, and total anthocyanin content were measured. Heating at 30°C and 60°C for 300 min did not influence the color stability, but heating at 100°C drastically decreased it by 33.0%. Sugars decreased color in a concentration-dependent manner. However, glucose and galactose had significantly protective effects on the color disruption than fructose, maltose, and sucrose. Organic acids lowered the color intensity in the order of citric acid> tartaric acid> malic acid> formic acid> acetic acid during 10 days of storage. Color decreased faster during long-term light exposure than in the dark. The color in the dark was kept by 58.7% after 7 weeks, while 48.9% in the light. Color retention was significantly decreased to 93.5% and 93.8% at 4°C and −20°C, respectively, after 7 weeks, while 95.40% at −75°C. We suggest that blueberry juice color can be protected by keeping the extraction temperature below 60°C with the selective addition of glucose, galactose, or citric acid. For long-term storage, it is recommended to use a light-protected container and a deep freezer at −75°C.
Keywords: blueberry, color stability, anthocyanin, storage
INTRODUCTION
The blueberry (Vaccinium spp.) is a fruit native to North America and Europe, where it is widely cultivated and commercialized (1). As the consumption of imported frozen blueberry fruit has increased for processed juices and bakery foods, blueberry cultivation has also increased in Korea. Blueberries are known to have many nutritional health benefits (2). Blueberries are rich in vitamins C, E, and minerals, and blueberry juices contain phenolic substances such as flavonols, tannins, and anthocyanins with high antioxidant capacity (3).
Anthocyanins are natural pigments present in fruits and vegetables and widely distributed in nature. They can be found in the form of glucosides, hydrolyzed in sugars, and as aglycones, known as anthocyanidins (1). Anthocyanins are responsible for the taste and flavor of the fresh fruit and for the brilliant red color and its different hues in many fruits and berries. The attractive color is one of the main sensory characteristics of fruit and berry products (4). However, the anthocyanins are extremely unstable and easily degraded in the isolated form (5), thus restricting their use as food dyes (6). The intensity and stability of the anthocyanin pigments is dependent on factors including pH, chemical structure, temperature, light, oxygen, ascorbic acid, sulfur dioxide, and metal ions influence their stability during food processing and storage (7–9). Pigment recovery from the fresh materials involves limitations as variability and seasonal availability of raw materials, fresh material losses, and pigment degradation caused by storage and the extraction process (10).
Many studies have been conducted on the behavior of anthocyanins. Roobha et al. (6) reported from the Musa acuminata bract that increase in pH, temperature, or exposure to light is able to spoil the anthocyanin molecules. Anthocyanin extracts were more stable at pH 5.1 and 6.0, temperature at 20°C and 30°C both, in the presence and absence of light. They also suggested that the copigmentation of anthocyanins could affect the increase in both, hypochromic effects and bathochromic shifts.
Wrolstad et al. (10) reported that the protective effect of strawberry anthocyanins, and the prevention of browning by high concentrations of sugar are probably due to the inhibition of enzymatic reactions or the hindering of different condensation reactions by sucrose. They suggested that the effect of added sugar on the anthocyanin stability depends on its structure, concentration, and type of sugar.
An attractive red color is one of the most important quality characteristics of blueberry juice. However, the low stability of anthocyanin pigments causes a serious problem during storage. Moreover, the optimal storage conditions according to many variances in relation to their bioactive compounds could be different on such products. Therefore, the investigation of the color stability could be useful for blueberry juice processing in order to develop an effective production strategy for high retention of anthocyanins.
The overall goal of this study was to evaluate the color stability in whole blueberry juice and to investigate the changes occurring during domestic storage to avoid significant losses of the bioactive compounds and properties of these products. Our approach involved determining the effects of temperature, light, sugar, and organic acids on blueberry color stability through time.
MATERIALS AND METHODS
Materials and chemicals
The fully matured fresh fruits of wild blueberries were provided by WellRun B&F (Cheonan, Korea) just after being harvested in July 2015 in a mountain in Gimcheon, Korea. Buffers, sugars, acids, and chemical reagents were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Chemicals used were of analytical grade.
Preparation of blueberry juice
Blueberry samples were pre-selected with dark-purple color and size uniformity, and the berries with visible injuries and infections were discarded. After washing the selected fruits with water, the whole juice was prepared using a domestic food processor (HR-3653, Phillips Electronics, Amsterdam, The Netherlands) and filtered with gauzes (1).
Total soluble solid (TSS) content and pH
The pH of 10 times diluted blueberry juice was measured. TSS content was measured using an Atago PR-100 Palette (N-1E, Atago Co., Ltd., Tokyo, Japan) digital refractometer (0~32 Brix%). Measurements were made at ambient temperature, and TSS was expressed as oBrix. Prior to each set of measurements, the instrument was calibrated to 0oBrix using distilled water (12).
Determination of total anthocyanin content (TAC)
The TAC in blueberry juice was determined using the pH differential methods, with slight modifications (12,13). Briefly, 2 of 1 mL blueberry samples were taken, and one of them was adjusted to 10 mL with potassium chloride buffer, pH 1.0, and the other with sodium acetate buffer, pH 4.5. After equilibrium at 15 min, the absorbance was measured at 520 nm and 700 nm, respectively, using an UV/visible spectrophotometer (Shimadzu UV 1700, Shimazu Co., Kyoto, Japan). The TAC was calculated as milligrams of cyanidin-3-glucoside (C3G) equivalents (molar extinction coefficient 26,900).
A=(A520–A700) pH 1.0–(A520–A700) pH 4.5
ɛ=molar extinction coefficient for C3G (26,900)
MW=molecular weight of C3G (449.2 g/mol)
Effect of heating temperature
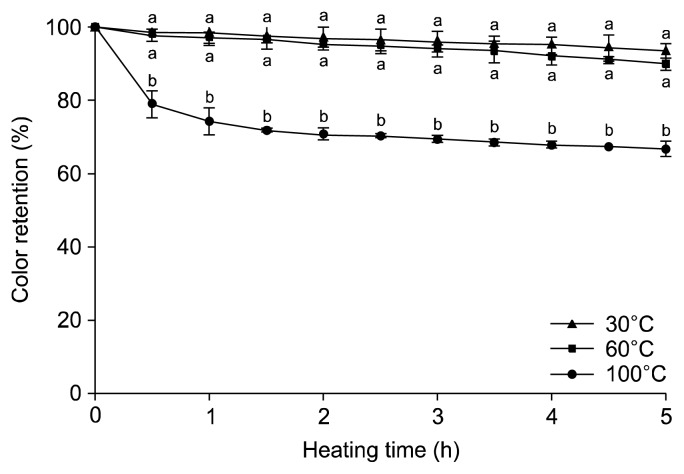
To determine the impact of temperature and thermal treatment time on the stability of color substances, 10 mL of 20% blueberry juices in McIlvaine buffer solutions (pH 3.0) were sealed in closed test tubes and heated at 30°C, 60°C, and 100°C for up to 300 min. Thermostability of pigments was assessed by measuring the absorption of the solutions every 30 min at 514 nm using an UV/visible spectrophotometer (Shimadzu UV 1700, Shimazu Co.) (14). Color retention (%) was calculated as the quotient of the absorbance value after storage divided by the initial reading.
Effect of sugar
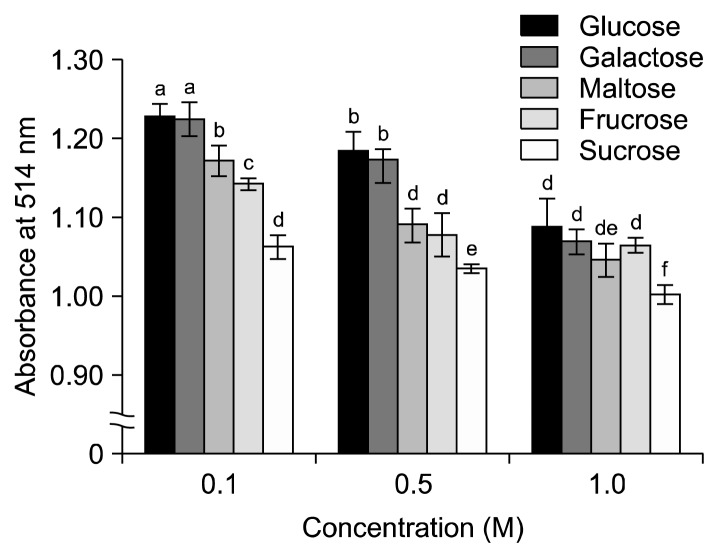
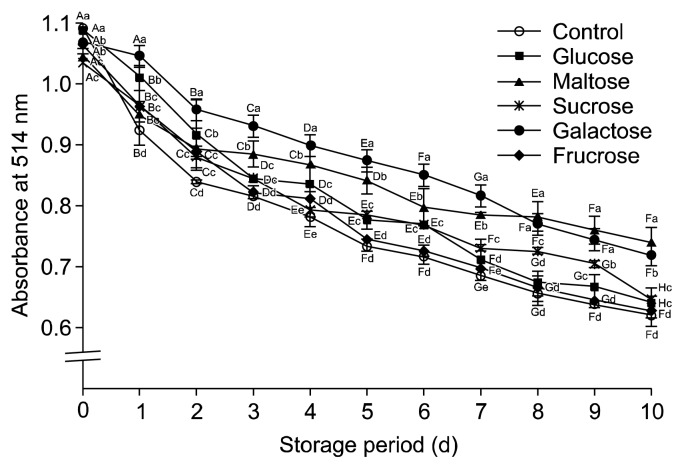
Five sugars, glucose, fructose, maltose, sucrose, and galactose, were selected. Different concentrations (0.1 M, 0.5 M, and 1.0 M) of each sugar were added to 40% of blueberry juice solution in McIlvaine buffer (pH 3.0, 0.1 M citric acid+0.2 M Na2HPO4). The color intensity was measured at 514 nm by UV/visible spectrophotometer (Shimadzu UV 1700, Shimazu Co.). The 1.0 M sugar-blueberry juice solutions, which showed the lowest color intensity, were selected for further examination of the effect on storage time. Sealed test tubes of 1.0 M sugar added blueberry juice were stored at 30°C during 10 days, and the absorbance changes were measured at 514 nm every 24 h (15).
Effect of organic acid
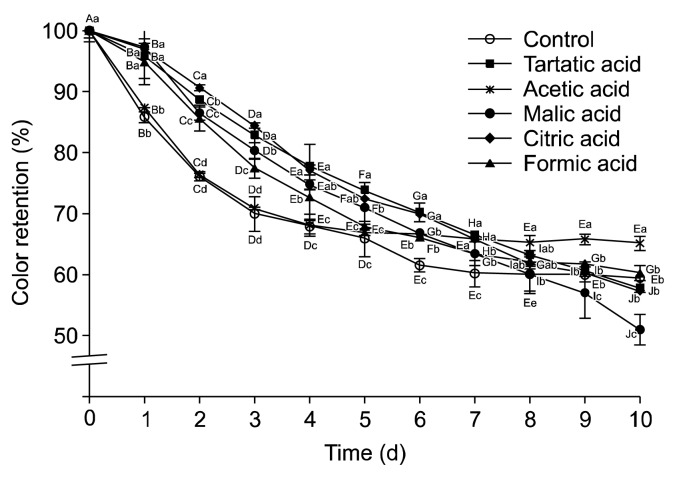
A 1.0 M concentration of five organic acids, acetic acid, citric acid, formic acid, malic acid, and tartaric acid, were added to 40% of blueberry juice solution in 1.0 M glycine buffer (pH 3.0). Sealed test tubes were stored at 30°C for 10 days and changes in absorbance measured every 24 h at 514 nm. Color retention (%) was calculated as the quotient of the absorbance value after storage divided by the initial reading (15).
Effect of long-term light exposure
Forty percent of blueberry juice solutions were prepared in McIlvaine buffer at pH 3.0. The light effect on color stability was performed with the samples inside capped glass test tubes sealed with parafilm under a white fluorescent lamp (8 W, 52.9 Lm/W) in a closed hardboard box for 24 h at room temperature. Dark samples were wrapped with aluminum foil and stored in a dark place. The absorbance was recorded at 514 nm every 24 h until the first 7 days and every 1 week until 7 weeks. Color retention (%) was calculated as the quotient of the absorbance value after storage divided by the initial reading (16,17).
Effect of long-term cold storage
To determine the changes occurring during cold storage, 5 mL of 40% diluted blueberry juice solutions in McIlvaine buffer (pH 3.0) were stored at 4°C, −20°C, and −75°C in amber jars for 7 weeks. The absorbance was recorded at 514 nm every 24 h until the first 7 days and every 1 week until 7 weeks. Color retention (%) was calculated as the quotient of the absorbance value after storage divided by the initial reading (1).
Statistical analysis
The results are expressed as the mean±standard deviation (SD) of triplicate experiments. Statistical analysis was performed using SPSS software version 17.0 (SPSS Inc., Chicago, IL, USA). An analysis of variance (ANOVA) and Duncan’s multiple range tests were used to determine the significance of difference, and P<0.05 was considered statistically significant.
RESULTS AND DISCUSSION
TSS content, pH, and TAC
TSS content, an accepted measure of sweetness, is an important quality attribute for blueberries. It is usually determined from the juice extracted from fruit flesh using the refractometric method (18). The TSS was 15.35 oBrix (Table 1), which was much higher than that of the 20 commercial blueberry samples reported as 7~12oBrix (19). This high TSS was similar to 14.01~16.14oBrix of 9 black currant varieties (14). The pH of blueberry juice was 4.35, similar to the common range of 3.5~4.5 (20) (Table 1). Anthocyanins are normally stable at pH values between 1 and 4. They undergo reversible structural transformations with a change in pH. The red and purple colored oxonium form predominates at pH 1.0, whereas the blue quinoid bases predominate at pH 2~4. They are generally degraded above pH 7 (21). The TAC, expressed as an equivalent of C3G, was 22.0 mg/100 g fresh fruit (Table 1). The pH-differential method is based on this pH dependent reaction, and it permits accurate and rapid measurement of the total anthocyanins (22).
Table 1.
Total soluble solid (TSS) content, pH, and total anthocyanin content (TAC) of blueberry juice
| TSS (oBrix) | 15.35±0.12 |
| pH | 4.35±0.01 |
| TAC (mg/100 g) | 22.0±0.04 |
Values are mean±SD (n=3).
Effect of heating temperature
Thermal treatments are some of the most widely used methods to extend the shelf-life of liquid foods by the inactivation of microorganisms and enzymes. However, heat causes irreversible losses of nutritional compounds, undesirable changes in physicochemical properties, and alteration of their antioxidant properties (2). The effects of thermal treatment at different temperatures and the heating times were investigated at 30°C, 60°C, and 100°C (Fig. 1). It was found that 30°C and 60°C did not influence the color stability of blueberry juice. The color intensity at 30°C after 300 min decreased only by 6.1% from the beginning, while heating at 100°C drastically decreased color by 33.0%. The impact of heating time was different with heating temperature. The color decreased slowly at the lower temperatures of 30°C and 60°C; however, color deterioration was significantly fast within 30 min at 100°C. Rubinskiene et al. (13) reported that black currant anthocyanins were stable at 75°C, but 85°C and 95°C decreased the color intensity by 20~53%. Roobha et al. (6) suggested from the result of Musa acuminata bract that the speedy destruction of anthocyanins at higher temperatures could be due to hydrolyzation of the 3-glycoside structure, which has a protective effect in unstable anthocyanins.
Fig. 1.
Effect of heating temperature on the absorption of blueberry juice at different heating times. Results are mean±SD (n=3). Values with different letters (a,b) are significantly different (P<0.05) by Duncan’s multiple range test.
This result implies that hot water extraction for sever al hours, which is a common process for the preparation of plant extracts, could be a crucial factor in the loss of the characteristic blueberry color.
Effect of sugar
The effect of glucose, fructose, maltose, sucrose, and galactose on the stability of blueberry juice was examined at different concentrations of 0.1 M, 0.5 M, and 1.0 M for each sugar (Fig. 2). Sugars decreased the color in a concentration-dependent manner. The higher the sugar concentration, the greater the decrease in color intensity. However, glucose and galactose had greater protective effects on color disruption than any other three sugars. This was similar to that of aronia anthocyanins, which have been shown to be negatively affected by maltose, fructose, and sucrose, while galactose and glucose did not affect color intensity (24). The presence of sugars is known to be an important factor on the quality of fruit and vegetable foods, such as soft drinks, fresh juices, and jams. De Rosso and Mercadante (22) showed that addition of sugars and salts had a negative effect on anthocyanin stability.
Fig. 2.
Effect of sugars on the color intensity of blueberry juice at different concentrations. Results are mean±SD (n=3). Values with different letters (a–f) are significantly different by Duncan’s multiple range test (P<0.05).
The effect of storage time with sugars was investigated for 10 days (Fig. 3). Although all the sugars decreased the color intensity over the storage time, they all kept above the control, which implies that sugars can be beneficial to protect color. Disaccharides, such as galactose and maltose protected color significantly more than monosaccharides like glucose and fructose for the whole period. Galactose was the most effective sugar to keep the color. Aronia anthocyanin showed similar results (24). The effect of added sugars on the anthocyanin stability depends on the structure, concentration, and type of sugar. Wrolstad et al. (10) reported that sugar contributed to not only the protective effect of anthocyanins but also the reduction of browning by polyphenoloxidase. They suggested that the protection of anthocyanins and prevention of browning by high concentrations of sugar is probably due to the inhibition of enzymatic reactions or the hindering of different condensation reactions by sucrose. Lowering of water activity by sugars can be protective against anthocyanin degradation (25). On the other hand, at low concentrations of sucrose, the degradation of anthocyanins from red cabbage, blackcurrant, and elderberry extracts was higher in soft drinks compared to buffer systems, both at pH 3 (26).
Fig. 3.
Effect of sugars (1.0 M) on the color intensity of blueberry juice during 10 days. Results are mean±SD (n=3). Different letters among the different days of same sugar (A–H) and the different sugars of same day (a–e) are significantly different at P<0.05 by Duncan’s multiple range test.
These results demonstrate that sugar addition to blueberry juices or beverages should be carefully considered in terms of the sugar types, concentrations, and storage period.
Effect of organic acid
The influence of organic acid addition on color stability is shown in Fig. 4. Organic acids lowered the color intensity in the order of citric acid> tartaric acid> malic acid> formic acid> acetic acid. Citric acid, which is commonly used in many processed food to improve the shelf life (24), was highly effective in protecting color. Acetic acid kept constant color retention for 4 days and was the most effective in the late stage of the storage. Overall, organic acids showed protective effects on color deterioration.
Fig. 4.
Effect of organic acid on the color intensity of blueberry juice during 10 days. Results are mean±SD (n=3). Different letters among the different days of same organic acid (A–J) and the different organic acids of same day (a–d) are significantly different at P<0.05 by Duncan’s multiple range test.
Hubbermann et al. (25) reported that citric acid and tartaric acid showed similar rate of color reduction on elderberry anthocyanin; moreover, acetic acid was the most protective. Interestingly, ascorbic acid was reported as the worst among five organic acids (acetic, adipinic, citric, tartaric, and ascorbic acid) to show a strong destabilizing effect on elderberry anthocyanins as almost 0% remained after 12 days of storage. Pedro et al. (15) observed the hyperchomic effect by glucose and organic acids, which is due to the interaction between the flavylium cation of the anthocyanin molecule and copigments within them.
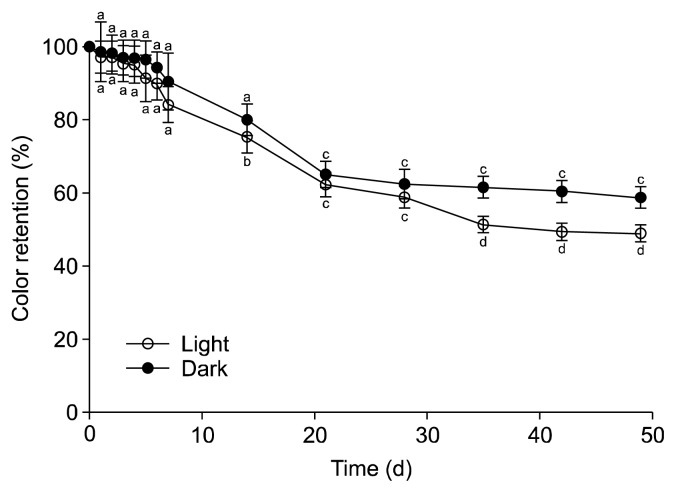
Effect of long-term light exposure
Light stability of anthocyanins is another important factor for their application in food products (28). Light and dark effects on color stability are shown in Fig. 5. The color decrease during light exposure was faster than that of the dark samples. The rate of reduction was drastically fast in the early 7 days, and the colors were continuously destabilized after 3 weeks. In the dark, the color still remained over 90% after 7 days. The color in the dark samples was kept by 58.7% after 7 weeks, while 48.9% in the light. Similar findings were reported that dried anthocyanin extracts from cranberry were considerably sensitive to natural light (29), and Ararnwit et al. (16) reported that after exposure of fluorescence light for 10 h at room temperature, the TAC in mulberry fruits significantly decreased.
Fig. 5.
Long-term light exposure stability on the color retention of blueberry juice for 7 weeks. Results are mean±SD (n=3). Different letters are significantly different among the different days of same condition at P<0.05.
Therefore, we suggest that blueberry juice should be kept in a light-protected bottle.
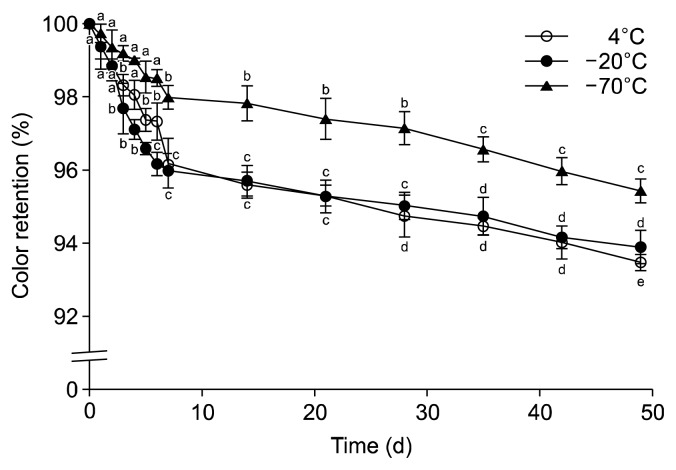
Effect of long-term cold storage
The color of blueberry juice was reduced during storage under refrigerated or frozen conditions for 7 weeks (Fig. 6.). Color retention was significantly dropped to 93.5 and 93.8% at 4°C and −20°C, respectively, after 7 weeks, while to 95.40% at −75°C. Interestingly, the result from freezing at −20°C was nearly the same as in the refrigerated conditions at 4°C. Freezing forms ice crystals, thus increasing the preservation time by reducing the water activity. Cooling in temperatures between −1°C and 8°C decreases enzymatic and microbial activities in the product (1). The gradual increase in the storage temperature is reported to accelerate the destruction of pigments in soft drinks (7). In long-term storage, Reque et al. (1) reported that in blueberry fruits stored at −18°C, an average of about 59% degradation of the anthocyanins was found after 6 months of storage. Considerable losses of total anthocyanins were reported for cherries stored at −23°C, and they found 88% degradation after 6 months of storage and 67% in the first 3 months (30,31). Requde et al. (1) reported that during refrigeration of blueberry juice for 10 days, cyanidin, pelargonidin, malvidin, and delphinidin glucoside were degraded in practically the same proportions (mean of about 83%), with C3G showing the greatest loss (1). They also reported that significant losses were observed at 2 days of refrigerated storage of blueberry juice.
Fig. 6.
Cold storage stability on the color retention of blueberry juice at 4°C, −20°C, and −75°C for 7 weeks. Results are mean±SD (n=3). Different letters are significantly different among the different days of same temperature at P<0.05.
In conclusion, we suggest that the blueberry juice color can be protected at lower extraction temperatures under 60°C with selective addition of glucose, galactose, or citric acid. For a long-term storage, it is recommended to use a light-protected container and a deep freezer at −75°C.
ACKNOWLEDGEMENTS
This paper was supported by the Semyung University Research Grant of 2016.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Reque PM, Steffens RS, Jablonski A, Flôres SH, Rios ADO, de Jong EV. Cold storage of blueberry (Vaccinium spp.) fruits and juice: anthocyanin stability and antioxidant activity. J Food Compos Anal. 2014;33:111–116. doi: 10.1016/j.jfca.2013.11.007. [DOI] [Google Scholar]
- 2.Barba FJ, Jäger H, Meneses N, Esteve MJ, Frígola A, Knorr D. Evaluation of quality changes of blueberry juice during refrigerated storage after high-pressure and pulsed electric fields processing. Innovative Food Sci Emerging Technol. 2012;14:18–24. doi: 10.1016/j.ifset.2011.12.004. [DOI] [Google Scholar]
- 3.Atala E, Vásquez L, Speisky H, Lissi E, López-Alarcón C. Ascorbic acid contribution to ORAC values in berry extracts: an evaluation by the ORAC-pyrogallol red methodology. Food Chem. 2009;113:331–335. doi: 10.1016/j.foodchem.2008.07.063. [DOI] [Google Scholar]
- 4.Rein MJ, Heinonen M. Stability and enhancement of berry juice color. J Agric Food Chem. 2004;52:3106–3114. doi: 10.1021/jf035507i. [DOI] [PubMed] [Google Scholar]
- 5.Giusti MM, Wrolstad RE. Acylated anthocyanins from edible sources and their applications in food systems. Biochem Eng J. 2003;14:217–225. doi: 10.1016/S1369-703X(02)00221-8. [DOI] [Google Scholar]
- 6.Roobha JJ, Saravanakumar M, Aravindhan KM, Devi PS. The effect of light, temperature, pH on stability of anthocyanin pigments in Musa acuminata bract. Res Plant Biol. 2011;1:5–12. [Google Scholar]
- 7.Schwartz SJ, von Elbe JH, Giusti MM. Colorants. In: Damodaran S, Parkin KL, Fennema OR, editors. Fennema’s Food Chemistry. 4th ed. CRC Press; Boca Raton, FL, USA: 2008. pp. 571–638. [Google Scholar]
- 8.Mazza G, Miniati E. Anthocyanin in Fruits, Vegetables, and Grains. CRC Press; Boca Raton, FL, USA: 1993. Introduction; pp. 1–28. [Google Scholar]
- 9.Nakamura M, Takeuchi Y, Miyanaga K, Seki M, Furusaki S. High anthocyanin accumulation in the dark by strawberry (Fragaria ananassa) callus. Biotechnol Lett. 1999;21:695–699. doi: 10.1023/A:1005558325058. [DOI] [Google Scholar]
- 10.Wrolstad RE, Skrede G, Lea P, Enersen G. Influence of sugar on anthocyanin pigment stability in frozen strawberries. J Food Sci. 1990;55:1064–1065. doi: 10.1111/j.1365-2621.1990.tb01598.x. [DOI] [Google Scholar]
- 11.Song HN, Park MS, Youn HS, Park SJ, Hogstrand C. Nutritional compositions and antioxidative activities of two blueberry varieties cultivated in South Korea. Korean J Food Preserv. 2014;21:790–798. doi: 10.11002/kjfp.2014.21.6.790. [DOI] [Google Scholar]
- 12.Giusti MM, Wrolstad RE. Characterization and measurement of anthocyanins by UV-visible spectroscopy. In: Wrolstad RE, Acree TE, An H, Decker EA, Penner MH, Reid DS, Schwartz SJ, Shoemaker CF, Sporns P, editors. Current Protocols in Food Analytical Chemistry. John Wiley & Sons; New York, NY, USA: 2001. pp. F1.2.1–F1.2.13. [Google Scholar]
- 13.Rubinskiene M, Viskelis P, Jasutiene I, Viskeliene R, Bobinas C. Impact of various factors on the composition and stability of black currant anthocyanins. Food Res Int. 2005;38:867–871. doi: 10.1016/j.foodres.2005.02.027. [DOI] [Google Scholar]
- 14.Hwang ES, Ki KN. Stability of the anthocyanin pigment extracted from aronia (Aronia melancocarpa) Korean J Food Sci Technol. 2013;45:416–421. doi: 10.9721/KJFST.2013.45.4.416. [DOI] [Google Scholar]
- 15.Pedro AC, Granato D, Rosso ND. Extraction of anthocyanins and polyphenols from black rice (Oryza sativa L.) by modeling and assessing their reversibility and stability. Food Chem. 2016;191:12–20. doi: 10.1016/j.foodchem.2015.02.045. [DOI] [PubMed] [Google Scholar]
- 16.Ararnwit P, Bang N, Srichana T. The properties and stability of anthocyanins in mulberry fruits. Food Res Int. 2010;43:1093–1097. doi: 10.1016/j.foodres.2010.01.022. [DOI] [Google Scholar]
- 17.Noh HK, Lu R. Hyperspectral laser-induced fluorescence imaging for assessing apple fruit quality. Postharvest Biol Technol. 2007;43:193–201. doi: 10.1016/j.postharvbio.2006.09.006. [DOI] [Google Scholar]
- 18.Cho WJ, Song BS, Lee JY, Kim JK, Kim JH, Yoon YH, Choi JI, Kim GS, Lee JW. Composition analysis of various blueberries produced in Korea and manufacture of blueberry jam by response surface methodology. J Korean Soc Food Sci Nutr. 2010;39:319–323. doi: 10.3746/jkfn.2010.39.2.319. [DOI] [Google Scholar]
- 19.Jo HJ, Kim JE, Yu MJ, Lee WH, Song KB, Kim HY, Hwang IG, Yoo SM, Han GJ, Park JT. Effect of freezing temperature on blueberry quality. J Korean Soc Food Sci Nutr. 2014;43:1906–1912. doi: 10.3746/jkfn.2014.43.12.1906. [DOI] [Google Scholar]
- 20.Castañeda-Ovando A, Pacheco-Hernández ML, Páez-Hernández ME, Rodríguez JA, Galán-Vidal CA. Chemical studies of anthocyanins: A review. Food Chem. 2009;113:859–871. doi: 10.1016/j.foodchem.2008.09.001. [DOI] [Google Scholar]
- 21.Sutharut J, Sudarat J. Total anthocyanin content and antioxidant activity of germinated colored rice. Int Food Res J. 2012;19:215–221. [Google Scholar]
- 22.De Rosso VV, Mercadante AZ. Evaluation of colour and stability of anthocyanins from tropical fruits in an isotonic soft drink system. Innovative Food Sci Emerging Technol. 2007;8:347–352. doi: 10.1016/j.ifset.2007.03.008. [DOI] [Google Scholar]
- 23.De Ancos B, Cano MP, Hernandez A, Monrea M. Effects of microwave heating on pigment composition and color of fruit purees. J Sci Food Agric. 1999;79:663–670. doi: 10.1002/(SICI)1097-0010(199904)79:5<663::AID-JSFA232>3.0.CO;2-L. [DOI] [Google Scholar]
- 24.Nikkhah E, Khayamy M, Heidari R, Jamee R. Effect of sugar treatment on stability of anthocyanin pigments in berries. J Biol Sci. 2007;7:1412–1417. doi: 10.3923/jbs.2007.1412.1417. [DOI] [Google Scholar]
- 25.Hubbermann EM, Heins A, Stöckmann H, Schwarz K. Influence of acids, salt, sugars and hydrocolloids on the colour stability of anthocyanin rich black currant and elderberry concentrates. Eur Food Res Technol. 2006;223:83–90. doi: 10.1007/s00217-005-0139-2. [DOI] [Google Scholar]
- 26.Xu H, Liu X, Yan Q, Yuan F, Gao Y. A novel copigment of quercetagetin for stabilization of grape skin anthocyanins. Food Chem. 2015;166:50–55. doi: 10.1016/j.foodchem.2014.05.125. [DOI] [PubMed] [Google Scholar]
- 27.Bononi M, Tateo F. Stabilization of cranberry anthocyanins in nutraceutical capsules. Int J Food Sci Nutr. 2007;58:142–149. doi: 10.1080/09637480601154061. [DOI] [PubMed] [Google Scholar]