Abstract
This study aimed to compare the phenolic compounds and antioxidant activity of barley at different proportion (0, 5, 10, 15, and 20%), and using different cooking methods. The grains used in this experiment are barley (Hordeum vulgare L. cv. Huinchalssal) and Samkwang rice. The rice-barley mixture was cooked using general and high pressure cooking methods with and without fermented alcohol. The quality characteristics such as water binding capacity, pasting characteristic, water solubility, and swelling power of different proportions of barley were evaluated. The antioxidant characteristics evaluated are total polyphenol, flavonoid contents, 2,2-diphenyl-1-picrylhydrazyl (DPPH), and 2,2-azinobis(3-ethylbenothiazoline-6-sulphonic acid) (ABTS) diammonium salt radical scavenging activities. Results showed that peak [195.0~184.0 rapid visco units (RVU)], trough (130.0~116.2 RVU), final (252.0~221.8 RVU), and setback viscosity (57.0~37.5 RVU) decreased correspondingly with the increase in the amount of barley. Water binding capacity (187.31~136.01%) and swelling power (162.37~127.58%) decreased as amounts of barley increases, however the water solubility (5.35~6.89%) increased. Moreover, the total polyphenol and flavonoid, and the DPPH and ABTS radical scavenging activities contents increased as the amounts of barley in the mixture increases. This study generally aims to provide useful information for the manufacturing of processed products.
Keywords: barley (Hordeum vulgare L.), cooking characteristics, polyphenol, antioxidant activity
INTRODUCTION
Barley (Hordeum vulgare L.) is an essential cereal crop, ranking fifth in the world production, and plays an important role in human nutrition (1). Barley is considered as a nutraceutical grain because it contains bioactive compounds like β-glucan, phenolic compounds, B-complex vitamins, tocotrienols, and tocopherols (2–5). It has higher antioxidant activity as compared to the more widely consumed cereals such as wheat and rice. The risk imposed by the consumption of free radicals and oxidation products towards various forms of cancer and cardiovascular diseases could be lowered by the intake of dietary phenolics (6). Barley contains many phenolic compounds in the free and bound forms; these compounds include benzoic acid and cinnamic acid derivatives, proanthocyanidins, quinines, flavonols, chalcones, flavones, flavanones, and amino phenolic compounds (7,8).
Oxidative stress is manifested by the excessive production of reactive oxygen species and an insufficient or defective antioxidant defense system (9). Meanwhile, oxidative stress causes profound alterations in various biological structures, including cellular membranes, lipids, proteins, and nucleic acids (10). Interest in identifying biomarkers for disease, in which oxidative stress is always involved, is increasingly growing. Oxidative stress is involved in aging and in various diseases, including diabetes mellitus (11), atherosclerosis (12), Alzheimer’s disease (13), Parkinson’s disease (14), and some cancers (15). Therefore, the antioxidant activity of various grains has been intensively studied during recent years. Also, loss of nutritional components, generation of health deteriorating compounds, and non-ecofriendly and economic considerations are major setbacks for the processing industry. Due to these considerations minimally processed foods are gaining importance in day to day life (1). It is well documented that the minimally processed foods have more health benefits (8).
In Korea, barley is consumed in large amounts for mixed rice. Therefore, the purpose of this study was to determine the quality and physicochemical characteristics of cooked-rice with different mixing ratio of barley, and identify the appropriate cooking method to increase the palatability and functional component content of cooked-rice with barley. The results of the will be used to determine the optimum mixing ratio of rice and barley. In addition, the effect of fermented alcohol on heat treatment of cereals was investigated in order to improve the functionality of cooked-rice with barley (16).
MATERIALS AND METHODS
Chemicals and reagents
Folin-Ciocalteu reagent, sodium carbonate, gallic acid, sodium nitrite (NaNO2), aluminum chloride hexahydrate (AlCl3·6H2O), (+)-catechin, sodium carbonate (Na2CO3), 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2-azinobis(3-ethylbenothiazoline-6-sulphonic acid) diammonium salt (ABTS), potassium persulfate, and Trolox were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). High-performance liquid chromatography-grade water, acetonitrile, and methanol were purchased from J.T. Baker Inc. (Phillipsburg, NJ, USA). Reagents were the highest grade quality.
Sample preparation and extraction
Barley cultivars, Hordeum vulgare L. cv. Huinchalssal and rice cultivar, Oryza sativa cv. Samkwang were used in this experiment. The barley cultivars were grown at the National Institute of Crop Science, Rural Development Administration, Wanju, Korea during the 2015 growing season. The white rice was prepared by using rice huller (Model SY88-TH, Ssangyong Ltd., Incheon, Korea) and milling machine (MC-90A, Satake, Hiroshima, Japan). The barley was prepared by using barley milling machine (DK-108, Daedong AgriMachine Co., Ltd., Daegu, Korea). The samples were stored in a refrigerator at 4°C. The raw materials were pulverized using a micro hammer-cutter mill (Type 3, Culatti AG., Zürich, Switzerland) for qualitative analysis.
Analysis of pasting characteristics
The pasting characteristics of rice with different mixing ratio of barley (0, 5, 10, 15, 20, and 100%) were measured according to the methods in Kim et al. (17) by using a rapid viscosity analyzer (Model RVA-3D, Newport Scientific PTY Ltd., Warriewood, Australia). The rice-barley mixtures were pulverized to 60 mesh or more, then weighed into 3 g samples. Each sample was placed in aluminum can container and dispersed in 25 mL of distilled water. It was kept at 50°C for 1 min and then raised from 50°C to 95°C for 3.48 min, and maintained at 95°C for 2.05 min. Thereafter, it was cooled to 50°C for 3.48 min, and viscosity characteristics were assessed. The total experiment time is about 13 min. After the experiment, the peak, trough, break down, final, and set back viscosity were measured and compared.
Analysis of water binding capacity, swelling power and water solubility
The water binding capacity of rice with different mixing ratio of barley (0, 5, 10, 15, 20, and 100%) was measured by mixing 1 g of pulverized sample with 40 mL of distilled water and stirring for 1 h (18). The supernatant was removed by centrifugation at 3,000 rpm for 10 min, and then the weight of the precipitated powder was measured. The water binding force was calculated by subtracting the initial sample weight (g) from the weight (g) of the precipitated sample and as a percentage of the initial sample weight (g). The swelling power and water solubility index were measured by dispersing 1 g of the pulverized sample in 30 mL of distilled water and heating it in a constant temperature water bath at 90±1°C for 30 min. After centrifugation at 3,000 rpm for 20 min, the supernatant was dried at 105°C for 12 h and weighed, and then the precipitate was weighed (19).
Determination of palatability characteristics
The palatability characteristics of cooked-rice with different mixing ratio of barley (0, 5, 10, 15, 20, and 100%) were determined using a cooked-rice taste analyzer (SATA1B, Satake) (20). The 10 g of the sample was placed in a measuring dish, covered with a lap surface, subjected to constant pressure for 3 s, and left at room temperature for 2 min. Immediately prior to the measurement, pressure was applied for 1 s with constant force, and then the lap was removed. Its appearance, hardness, viscosity, balance, and palatability were measured three times.
Cooking methods of cooked-rice added barley
The rice-barley mix was prepared by adding 0, 5, 10, 15, and 20% of barley to white rice. The mix was washed three times and soaked in water at 25°C for 30 min and then drained. 120 mL water for cooking was then added. Also, based on research, the functionality of cereals is improved by the addition of fermented alcohol during heat treatment. This study also aims to determine the effect of adding fermented alcohol on the rice-barley mix (16). So instead of adding 120 mL pure water, a mixture of 100 mL of water and 20 mL of fermented alcohol was used as a treatment. This ratio is based on the results of a preliminary study. The mix was cooked using a general rice cooker (CR-0671V, Cuckoo, Seoul, Korea) and high pressure rice cooker (EHS035FW, Cuckoo). After being automatically boiled, and steamed for 15 min, the samples were analyzed.
Determination of phenolic compounds
In order to analyze the phenolic compounds and radical scavenging activity of the cooked-rice with different mixing ratio of barley, the cooked sample was blended with 80% ethanol using a homogenizer (HG-15A, Daihan Scientific Co., Ltd., Seoul, Korea). The samples for analysis was extracted through shaking (WiseCube WIS-RL010, Daihan Scientific Co.) at room temperature for 24 h, filtered (Advantec, Toyo Roshi Kaisha, Ltd., Tokyo, Japan), and stored at −20°C in a freezer. The total polyphenolic contents of the cooked rice mixed with different ratio of barley were measured using the Folin-Ciocalteu method (21). The standards or extracts (10 μL) were mixed with 200 μL of a sodium carbonate solution (2%, w/v) and 10 μL of Folin-Ciocalteu reagent (50%, v/v). The mixtures were incubated for 30 min at room temperature and the contents were measured at 750 nm. The data are expressed as μg of gallic acid equivalents (GAE) per g of sample. For total flavonoid contents, standards or extracts (50 μL) were mixed with 200 μL of water and 15 μL of NaNO2 (5%, w/v). After 5 min, 30 μL of AlCl3·6H2O (10%, w/v) was added and the solutions were incubated for another 6 min. The reaction was terminated by the addition of 1 M NaOH (100 μL) and the absorbance was measured at 510 nm (21). The data were converted to μg catechin equivalents (CE) per g of sample. All extracts were analyzed in triplicate.
Measurement of DPPH and ABTS radical scavenging activities
Scavenging activity of the samples for the DPPH and ABTS radical was measured according to the methods of Woo et al. (21), with some modifications. An 800 μL aliquot of 0.2 mM DPPH methanolic solution was mixed with 200 μL of the sample. The mixture was shaken vigorously and left to stand for 30 min under low light. The absorbance was measured at 515 nm. The ABTS cation radical was generated by adding 7 mM ABTS to 2.45 mM potassium persulfate solution and storing the mixture overnight in the dark at room temperature. The ABTS cation radical solution was diluted with methanol to obtain an absorbance of 1.4~1.5 at 735 nm (molar extinction coefficient, ɛ=3.6×104 mol−1·cm−1). A diluted ABTS cation radical solution (1 mL) was added to 50 mL of the extract, Trolox standard solution, or distilled water. After 30 min, the absorbance was measured at 735 nm using a spectrophotometer (MultiskanTM GO Microplate Spectrophotometer, Thermo Fisher Scientific, Waltham, MA, USA). The DPPH radical and ABTS cation radical scavenging activity are expressed in terms of Trolox equivalent antioxidant capacity, as milligrams of Trolox equivalents (TE) per 100 g of sample.
Statistical analysis
All data are expressed as the mean±standard deviation (SD). The significance of differences among treatment means was determined by one-way analysis of variance (ANOVA) and Duncan’s multiple range test, using SAS version 9.2 (SAS Institute, Cary, NC, USA) with a significance level of 0.05. Relationships between parameters were also investigated by regression analysis.
RESULTS AND DISCUSSION
Pasting characteristics with different mixing ratio of barley
The pasting characteristics according to the addition rate of barley were determined by using a Rapid visco analyzer to determine peak, trough, breakdown, final, and setback viscosities. The results are shown in Table 1. As the barley addition ratio increased, the pasting viscosity tends to decrease except for the breakdown viscosity. The breakdown viscosity is the difference between the peak and trough viscosity. The amylose content has a negative correlation with breakdown viscosity. In addition, it shows a high correlation with heat and shear resistance during processing (22,23). The breakdown viscosity was 67.1~68.0 RVU, and had no significant difference between the different proportion of barley added. The final viscosity is a process in which heating is stopped and cooling takes place, in which starch particles such as amylose are recombined again to increase the viscosity. As the addition rate of barley increased, it decreased to 252.0~221.8 RVU. The setback viscosity, which reflects the aging tendency of starch, means that the higher the value, the faster the aging process (22,23). The value of setback viscosity is obtained by subtracting the peak viscosity from the final viscosity, which is related to the aging of starch. The higher the value, the faster the aging process, thus lower values indicate, slower aging rate which means the longer the desired taste is maintained. The results of this study show an inverse relationship between the percentages of barley added and the setback viscosity. This suggests that the higher the proportion of barley, the slower is the aging rate of cooked rice. The trough viscosity was positively correlated with peak viscosity (r=0.928; P<0.01) (Table 2). The breakdown viscosity was positively correlated with peak viscosity (r= 0.993; P<0.001), and trough viscosity (r=0.878; P<0.05). The final viscosity was negatively correlated with peak viscosity (r=−0.768; P<0.05) and breakdown viscosity (r=−0.835; P<0.05). The setback viscosity was negatively correlated with peak viscosity (r=−0.977; P<0.001), trough viscosity (r=−0.828; P<0.05), and breakdown viscosity (r=−0.994; P<0.001), but significant positive correlations among final viscosity (r=0.887; P<0.05).
Table 1.
Pasting characteristics of barley with different mixing ratio [unit: rapid visco units (RVU)]
| Mixing ratio of barley (%) | Peak viscosity | Trough viscosity | Break down | Final viscosity | Set back |
|---|---|---|---|---|---|
| 0 | 195.0±1.4b | 127.9±2.5b | 67.1±3.9b | 252.0±2.3a | 57.0±3.6a |
| 5 | 195.9±0.6b | 130.0±2.8b | 65.9±3.3b | 252.0±1.0a | 56.1±1.6a |
| 10 | 193.4±1.8bc | 128.0±3.9b | 65.4±3.3b | 245.0±1.9b | 51.6±2.2b |
| 15 | 190.9±1.3c | 122.9±2.4c | 68.0±1.4b | 233.6±3.1c | 42.7±2.1c |
| 20 | 184.0±1.9d | 116.2±1.8d | 67.7±0.3b | 221.8±1.7d | 37.8±0.3d |
| 100 | 300.2±3.5a | 148.6±0.4a | 151.6±3.1a | 199.2±0.3e | −101.0±3.2e |
Values are expressed as the mean±SD of triplicate determinations.
Means with different letters (a–e) within a column are significantly different at P<0.05 by a Duncan’s multiple range test.
Table 2.
Correlation coefficients among pasting characteristics, water characteristics, and palatability characteristics of barley with different mixing ratio
| Factor | Pasting characteristics | Water characteristics | Palatability characteristics | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||
| Trough | Break down | Final | Set back | SP | WSI | Appearance | Hardness | Stickiness | Balance | Palatability | |
| Peak | 0.928** | 0.993*** | −0.768* | −0.977*** | −0.738* | 0.861* | −0.976*** | 0.981*** | −0.952** | −0.974*** | −0.767* |
| Trough | 1.000 | 0.878* | −0.479NS | −0.828* | −0.447NS | 0.617NS | −0.852* | 0.874* | −0.810* | −0.855* | −0.557NS |
| Break down | – | 1.000 | −0.835* | −0.994*** | −0.807* | 0.911** | −0.987*** | 0.986*** | −0.968** | −0.982*** | −0.809* |
| Final | – | – | 1.000 | 0.887* | 0.996*** | −0.983*** | 0.855* | −0.823* | 0.881* | 0.845* | 0.905** |
| Set back | – | – | – | 1.000 | 0.865* | −0.949** | 0.989*** | −0.982*** | 0.980*** | 0.984*** | 0.855* |
| SP | – | – | – | – | 1.000 | −0.972*** | 0.836* | −0.801* | 0.869* | 0.827* | 0.920** |
| WSI | – | – | – | – | – | 1.000 | −0.932** | 0.909** | −0.949** | −0.925** | −0.932** |
| Appearance | – | – | – | – | – | – | 1.000 | −0.997*** | 0.995*** | 0.999*** | 0.884** |
| Hardness | – | – | – | – | – | – | – | 1.000 | −0.987*** | −0.998*** | −0.861* |
| Stickiness | – | – | – | – | – | – | – | – | 1.000 | 0.994*** | 0.926** |
| Balance | – | – | – | – | – | – | – | – | – | 1.000 | 0.887* |
Significant at *P<0.05, **P<0.01, and ***P<0.001.
SP, swelling power; WSI, water solubility index.
Not significant.
Water characteristics with different mixing ratio of barley
As shown in Table 3, the water binding capacity of the mix tended to decrease with increasing barley proportion (0, 5, 10, 15, 20, and 100%). Water binding capacity indicates the affinity between the sample and water. Its size is known to increase with the number of amorphous portions in the starch particles, which is related to the swelling index of the starch (24). The water binding force as it indicates the degree of binding of water to the grain powder, further means that the water penetrates into the amorphous part of the starch particles or adsorbs to the particle surface (25). The swelling power showed a significant decrease from 162.37 to 105.08% while the water solubility index showed significant increase from 5.35 to 8.79% with increasing barley proportion. The swelling power and water solubility index are indicators of the magnitude of the interaction between amorphous starch chains and crystalline domains of starch particles, which are affected by the properties of amylose-lipid complexes, amylose and amylopectin (17). Therefore, the decrease of swelling power is thought to be due to the difference in composition of the components of barley, which has a relatively higher lipid content and lower sugar content than that of white rice (26). On the other hand, the increase in solubility is thought to be due to the elongation of some amylose or soluble carbohydrates as the barley is swollen by heating and the lipids and fibers collapse (27). The swelling power was negatively correlated with peak viscosity (r=−0.738; P<0.05) and breakdown viscosity (r=−0.807; P<0.05), but has significant positive correlations with final viscosity (r=0.996; P<0.001) and setback viscosity (r=0.865; P<0.05) (Table 2). The water solubility index was positively correlated with peak viscosity (r=0.861; P<0.05) and breakdown viscosity (r=0.911; P<0.01), but has significant negative correlations with final viscosity (r= −0.983; P<0.001), setback viscosity (r=−0.949; P<0.01), and swelling power (r=−0.972; P<0.001).
Table 3.
Water binding capacity, swelling power and water solubility of barley with different mixing ratio
| Mixing ratio of barley (%) | Water binding capacity (%) | Swelling power (%) | Water solubility index (%) |
|---|---|---|---|
| 0 | 187.31±2.46a | 162.37±4.04a | 5.35±0.16e |
| 5 | 143.80±0.90b | 160.67±1.26a | 5.48±0.01e |
| 10 | 143.02±6.67b | 149.66±0.87b | 5.82±0.08d |
| 15 | 136.96±7.40b | 138.70±0.51c | 6.10±0.03c |
| 20 | 136.01±6.41b | 127.58±0.31d | 6.89±0.16b |
| 100 | 135.11±4.56b | 105.08±0.35e | 8.79±0.06a |
All values are expressed as the mean±SD of triplicate determinations.
Means with different letters (a–e) within a column are significantly different at P<0.05 by a Duncan’s multiple range test.
Palatability characteristics with different mixing ratio of barley
As shown in Table 4, the appearance, hardness, stickiness, balance, and palatability of plain cooked rice were 4.75, 7.18, 4.36, 4.59, and 45.39, respectively, and was evaluated as excellent. The appearance, hardness, stickiness, balance, and palatability rating of cooked rice-barley mix with 5, 10, 15, and 20% barley were 4.64~4.20, 7.13~7.32, 4.10~3.63, 4.50~3.87, and 43.10~36.90, respectively. The mixes with 5 to 15% barley had similar taste to that of plain rice. Kim and Lee (28) reported that preference for mixed grains with high milling rate was higher due to the lack of soft texture, and a decrease in sensorial properties such as taste, color and texture. However, this study proves that cooked-rice with 10~15% barley will not significantly affect its taste.
Table 4.
Palatability characteristics of barley with different mixing ratio using a rice taste analyzer
| Mixing ratio of barley (%) | Appearance | Hardness | Stickiness | Balance | Palatability |
|---|---|---|---|---|---|
| 0 | 4.75±0.07a | 7.18±0.05cd | 4.36±0.07a | 4.59±0.08a | 45.39±0.94a |
| 5 | 4.64±0.09a | 7.13±0.06cd | 4.10±0.12a | 4.50±0.12a | 43.10±0.66ab |
| 10 | 4.09±0.24b | 7.49±0.17b | 3.58±0.22b | 3.87±0.29b | 37.68±1.90c |
| 15 | 4.65±0.23a | 7.03±0.13d | 4.04±0.25a | 4.59±0.25a | 41.39±2.71b |
| 20 | 4.20±0.29b | 7.32±0.20bc | 3.63±0.29b | 4.08±0.34b | 36.90±2.56c |
| 100 | 0.20±0.00c | 9.80±0.00a | 1.03±0.09c | 0.20±0.00c | 30.00±0.00d |
Values are expressed as the mean±SD of triplicate determinations.
Means with different letters (a–d) within a column are significantly different at P<0.05 by a Duncan’s multiple range test.
The appearance was negatively correlated with peak viscosity (r=−0.976; P<0.001), trough viscosity (r= 0.852; P<0.05), breakdown viscosity (r=−0.987; P<0.001) and water solubility index (r=−0.972; P<0.001), but has significant positive correlations with final viscosity (r=0.855; P<0.05), setback viscosity (r=0.989; P<0.001), and swelling power (r=0.836; P<0.05) (Table 2). The hardness was positively correlated with peak viscosity (r=0.981; P<0.001), trough viscosity (r=0.874; P<0.05), breakdown viscosity (r=0.986; P<0.001), and water solubility index (r=0.909; P<0.01), but has significant negative correlations with final viscosity (r= −0.823; P<0.05), setback viscosity (r=−0.982; P<0.001), swelling power (r=−0.801; P<0.05), and appearance (r=−0.997; P<0.001). The stickiness was negatively correlated with peak viscosity (r=−0.952; P<0.01), trough viscosity (r=−0.810; P<0.05), breakdown viscosity (r=−0.968; P<0.01), water solubility index (r=−0.949; P<0.01) and hardness (r=−0.987; P<0.001), but has significant positive correlations with final viscosity (r=0.881; P<0.05), setback viscosity (r= 0.980; P<0.001), swelling power (r=0.869; P<0.05), and appearance (r=0.995; P<0.001). The balance was negatively correlated with peak viscosity (r=−0.974; P<0.001), trough viscosity (r=−0.855; P<0.05), breakdown viscosity (r=−0.982; P<0.001), water solubility index (r=−0.925; P<0.01) and hardness (r=−0.998; P<0.001), but has significant positive correlations with final viscosity (r=0.845; P<0.05), setback viscosity (r= 0.984; P<0.001), swelling power (r=0.827; P<0.05), appearance (r=0.999; P<0.001), and stickiness (r=0.994; P<0.001). The palatability was negatively correlated with peak viscosity (r=−0.767; P<0.05), breakdown viscosity (r=−0.809; P<0.05), water solubility index (r=−0.932; P<0.01) and hardness (r=−0.861; P<0.05), but has significant positive correlations with final viscosity (r=0.905; P<0.01), setback viscosity (r=0.855; P<0.05), swelling power (r=0.920; P<0.01), appearance (r=0.884; P<0.01), stickiness (r=0.926; P<0.01), and balance (r= 0.887; P<0.05).
Phenolic compounds of cooked-rice with different mixing ratio of barley
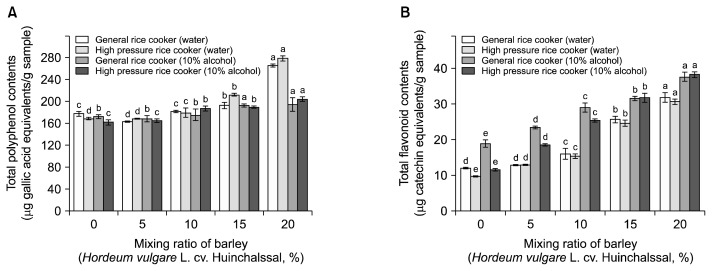
Phenolic compounds are some of the most effective antioxidative constituents in plant foods such as fruits, vegetables, and grains (29). Therefore, it is important to quantify polyphenolic contents and assess their contribution to antioxidant activity. The total polyphenol contents of the mixture at different barley proportion and the cooking method both showed significant differences as shown in Fig. 1A. The total polyphenol contents of plain rice according to the cooking method treatments ranges from 161.57~177.50 μg GAE/g sample. The total polyphenol contents of the mix with 20% barley has an average of 265.35±2.40 and 278.73±4.17 μg GAE/g sample in the general rice cooker and high pressure rice cooker, respectively. Among the antioxidants in cereals, polyphenolic compounds are known to have excellent antioxidant power. This has been reported to be due to the presence of a phenolic ring capable of stabilizing free radicals (30).
Fig. 1.
Total polyphenol (A) and flavonoid contents (B) of the ethanolic extracts with different mixing ratio of barley and cooking methods. Means in the same group with the different letters (a–e) are significantly (P<0.05) different by Duncan’s multiple range test.
The total flavonoid content of mix increased as the proportion of barley increased (Fig. 1B). The total flavonoid content of plain rice was 9.63~18.84 μg CE/g sample depending on the cooking method. The total flavonoid contents of rice with 20% barley cooked in plain water were 31.85±1.28 and 30.65±0.59 μg CE/g sample in the general rice cooker and high pressure rice cooker, respectively, while the same mix cooked in 10% fermented alcohol solution were 37.58±1.25 and 38.28±0.79 μg CE/g sample, respectively. Flavonoids are mainly composed of anthocyanidins, flavonols, flavones, cathechins, and flavanones. Depending on their structure, specific flavonoids have been reported to have various physiological activities such as antioxidation and antibacterial activities (30). Many antioxidant compounds are present in plant materials, mainly in a covalently bound form with insoluble polymers (31). Therefore, heat treatment might breakdown cell walls and liberate antioxidant compounds from insoluble portions of barley, increasing the pool of bioaccessible antioxidant compounds. The results of this study confirm that the addition of barley increases the antioxidant component of cooked rice. Though the concentration of antioxidants increases in proportion to barley addition, the cooking method should also be taken into consideration since it affects the texture acceptability.
Radical scavenging activity of cooked-rice with different mixing ratio of barley
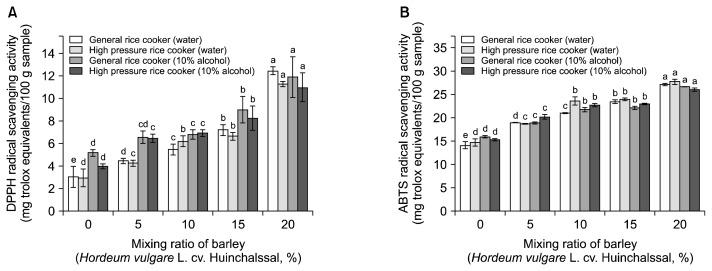
DPPH radical scavenging activity is a method that is used to measure the electron donating ability of antioxidants by reducing discoloration of dark purple color by reducing antioxidant components such as ascorbic acid, tocophenol, polyhydroxy aromatic compounds, and aromatic amines (32). The stable DPPH radical, which has maximum absorption at 515 nm, is widely used to evaluate the free radical-scavenging activity of hydrogen-donating antioxidants in many plant extracts (33). The DPPH radical scavenging activity of cooked-rice added barley was analyzed by comparing with trolox, which is a standard substance, as shown in Fig. 2A. As a result, it was increased as the addition rate of barley increased. The DPPH radical scavenging activity of plain white rice ranged from 2.97 to 5.19 mg TE/100 g sample, while the activity of the mixture with barley ranged from 3.97 to 12.45 mg TE/100 g sample depending on the cooking method.
Fig. 2.
DPPH (A) and ABTS (B) radicals scavenging activities of the ethanolic extracts with different mixing ratio of barley and cooking methods. Means in the same group with the different letters (a–e) are significantly (P<0.05) different by Duncan’s multiple range test.
ABTS radical scavenging activity was measured according to the method of Kim et al. (34). When ABTS with potassium persulfate is left in the dark place, ABTS+· is generated and the ABTS+· is cleared by the antioxidant component of the extract. Thus, the specific cyan color is discolored and is used as method of measuring the ABTS+· scavenging activity of the extract. The ABTS method is widely employed for measuring the relative radical scavenging activity of hydrogen-donating and chain-breaking antioxidants in many plant extracts (35). The ABTS radical scavenging activity of cooked-rice with barley was analyzed by comparing with Trolox, which is a standard substance, as shown in Fig. 2B. Results showed a corresponding increase of ABTS with the proportionate increase in barley. The ABTS radical scavenging activity of cooked plain rice ranges from 14.07 to 15.83 mg TE/100 g sample while the activity of cooked rice-barley mix ranges from 18.68 to 27.70 mg TE/100 g sample depending on the cooking method. The ABTS radical scavenging activity of the rice with 20% barley cooked in plain water were 7.08±0.25 and 27.70±0.52 mg TE/100 g sample using general rice cooker and high pressure rice cooker, respectively. Antioxidant activity hampers lipid oxidation in food and slows down aging due to active radicals in the body, thus playing an important role in inhibiting diseases and aging (36). Recently, a related study on tomato and coffee found that prolonged heat treatment enhances the antioxidant activity of these food items (37). Browning and antioxidant activities in tomato and coffee samples increased as a result of the increase in heating and roasting time. In the last decade, many studies have examined antioxidant activity after heat treatment and showed that heated products exhibit chain breaking and oxygen-scavenging activities (38). Therefore, when the rice-barley mix is cooked using a high pressure rice cooker, it undergoes a high radical scavenging activity. The use of high pressure rice cooker can be considered for industrial application.
ACKNOWLEDGEMENTS
This work carried out with the support of “Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ01175403)” Rural Development Administration, Republic of Korea.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Baba WN, Rashid I, Shah A, Ahmad M, Gani A, Masoodi FA, Wani IA, Wani SM. Effect of microwave roasting on antioxidant and anticancerous activities of barley flour. J Saudi Soc Agric Sci. 2016;15:12–19. [Google Scholar]
- 2.Madhujith T, Izydorczyk M, Shahidi F. Antioxidant properties of pearled barley fractions. J Agric Food Chem. 2006;54:3283–3289. doi: 10.1021/jf0527504. [DOI] [PubMed] [Google Scholar]
- 3.Sharma P, Gujral HS. Milling behavior of hulled barley and its thermal and pasting properties. J Food Eng. 2010;97:329–334. doi: 10.1016/j.jfoodeng.2009.10.025. [DOI] [Google Scholar]
- 4.Sharma P, Gujral HS. Antioxidant and polyphenol oxidase activity of germinated barley and its milling fractions. Food Chem. 2010;120:673–678. doi: 10.1016/j.foodchem.2009.10.059. [DOI] [Google Scholar]
- 5.Sharma P, Gujral HS. Effect of sand roasting and microwave cooking on antioxidant activity of barley. Food Res Int. 2011;44:235–240. doi: 10.1016/j.foodres.2010.10.030. [DOI] [Google Scholar]
- 6.Sharma P, Gujral HS, Singh B. Antioxidant activity of barley as affected by extrusion cooking. Food Chem. 2012;131:1406–1413. doi: 10.1016/j.foodchem.2011.10.009. [DOI] [Google Scholar]
- 7.Goupy P, Hugues M, Boivin P, Amiot MJ. Antioxidant composition and activity of barley (Hordeum vulgare) and malt extracts and of isolated phenolic compounds. J Sci Food Agric. 1999;79:1625–1634. doi: 10.1002/(SICI)1097-0010(199909)79:12<1625::AID-JSFA411>3.0.CO;2-8. [DOI] [Google Scholar]
- 8.Shahidi F. Nutraceuticals and functional foods: whole versus processed foods. Trends Food Sci Technol. 2009;20:376–387. doi: 10.1016/j.tifs.2008.08.004. [DOI] [Google Scholar]
- 9.Shen Y, Zhang H, Cheng L, Wang L, Qian H, Qi X. In vitro and in vivo antioxidant activity of polyphenols extracted from black highland barley. Food Chem. 2016;194:1003–1012. doi: 10.1016/j.foodchem.2015.08.083. [DOI] [PubMed] [Google Scholar]
- 10.Zitka O, Skalickova S, Gumulec J, Masarik M, Adam V, Hubalek J, Trnkova L, Kruseova J, Eckschlager T, Kizek R. Redox status expressed as GSH:GSSG ratio as a marker for oxidative stress in paediatric tumour patients. Oncol Lett. 2012;4:1247–1253. doi: 10.3892/ol.2012.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu JF, Liu YH, Chen CM, Chang WH, Chen CY. The effect of almonds on inflammation and oxidative stress in Chinese patients with type 2 diabetes mellitus: a randomized crossover controlled feeding trial. Eur J Nutr. 2013;52:927–935. doi: 10.1007/s00394-012-0400-y. [DOI] [PubMed] [Google Scholar]
- 12.Nwose EU, Jelinek HF, Richards RS, Tinley P, Kerr PG. Atherothrombosis and oxidative stress: the connection and correlation in diabetes. Redox Rep. 2009;14:55–60. doi: 10.1179/135100009X392458. [DOI] [PubMed] [Google Scholar]
- 13.Shen S, Callaghan D, Juzwik C, Xiong H, Huang P, Zhang W. ABCG2 reduces ROS-mediated toxicity and inflammation: a potential role in Alzheimer’s disease. J Neurochem. 2010;114:1590–1604. doi: 10.1111/j.1471-4159.2010.06887.x. [DOI] [PubMed] [Google Scholar]
- 14.Kaur H, Chauhan S, Sandhir R. Protective effect of lycopene on oxidative stress and cognitive decline in rotenone induced model of Parkinson’s disease. Neurochem Res. 2011;36:1435–1443. doi: 10.1007/s11064-011-0469-3. [DOI] [PubMed] [Google Scholar]
- 15.Oueslati S, Ksouri R, Falleh H, Pichette A, Abdelly C, Legault J. Phenolic content, antioxidant, anti-inflammatory and anticancer activities of the edible halophyte Suaeda fruticosa Forssk. Food Chem. 2012;132:943–947. doi: 10.1016/j.foodchem.2011.11.072. [DOI] [Google Scholar]
- 16.Lee KH, Ham H, Kim MJ, Ko JY, Kim HJ, Oh SK, Jeong HS, Woo KS. Effects of heating condition and cultivar on phenolic compounds and their radical scavenging activity on sorghum. Acad J Biotechnol. 2016;4:347–352. [Google Scholar]
- 17.Kim JM, Yu M, Shin M. Effect of mixing ratio of white and germinated brown rice on the physicochemical properties of extruded rice flours. Korean J Food Cook Sci. 2012;28:813–820. doi: 10.9724/kfcs.2012.28.6.813. [DOI] [Google Scholar]
- 18.Kim MJ, Ko JY, Lee KH, Kim HJ, Lee SK, Park HY, Sim EY, Oh SK, Woo KS. Quality and antioxidant characteristics of commercially available mixed grains in Korea. Korean J Food Nutr. 2017;30:31–40. doi: 10.9799/ksfan.2017.30.1.031. [DOI] [Google Scholar]
- 19.Woo KS, Song SB, Ko JY, Kim YB, Kim WH, Jeong HS. Antioxidant properties of adzuki beans, and quality characteristics of sediment according to cultivated methods. Korean J Food Nutr. 2016;29:134–143. doi: 10.9799/ksfan.2016.29.1.134. [DOI] [Google Scholar]
- 20.Lee KH, Kim HJ, Lee SK, Park HY, Sim EY, Cho DH, Oh SK, Lee JH, Ahn EK, Woo KS. Functional components and radical scavenging activity of brown rice according to addition rate and cooker. J Korean Soc Food Sci Nutr. 2017;46:350–357. doi: 10.3746/jkfn.2017.46.3.350. [DOI] [Google Scholar]
- 21.Woo KS, Ko JY, Jeong HS. Effect of milling time on antioxidant compounds and activities of methanol extracts of sorghum [Sorghum bicolor (L.) Moench] Food Sci Biotechnol. 2014;23:1741–1746. doi: 10.1007/s10068-014-0238-6. [DOI] [Google Scholar]
- 22.Lee JS, Woo KS, Chun A, Na JY, Kim KJ. Waxy rice variety-dependent variations in physicochemical characteristics of sogokju, a Korean traditional rice wine. Korean J Crop Sci. 2009;54:172–180. [Google Scholar]
- 23.Woo KS, Ko JY, Kim JI, Lee JS, Song SB, Cho JM, Jung TW, Kim KY, Oh IS. Cooking properties and antioxidant activity of cooked rice according to the addition of glutinous and non-glutinous sorghum. Korean J Crop Sci. 2013;58:399–407. doi: 10.7740/kjcs.2013.58.4.399. [DOI] [Google Scholar]
- 24.Konik-Rose CM, Moss R, Rahman S, Appels R, Stoddard F, McMaster G. Evaluation of the 40 mg swelling test for measuring starch functionality. Starch. 2001;53:14–20. doi: 10.1002/1521-379X(200101)53:1<14::AID-STAR14>3.0.CO;2-4. [DOI] [Google Scholar]
- 25.Wi E, Park J, Shin M. Comparison of physicochemical properties and cooking quality of Korean organic rice varieties. Korean J Food Cookery Sci. 2013;29:785–794. doi: 10.9724/kfcs.2013.29.6.785. [DOI] [Google Scholar]
- 26.Jung HN, Choi OJ. The physicochemical characteristics of rice flour with different milling degree of rice cultivar “Deuraechan”. Korean J Food Cook Sci. 2014;30:139–145. doi: 10.9724/kfcs.2014.30.2.139. [DOI] [Google Scholar]
- 27.Yun HR. MS Thesis. Chonnam National University; Gwangju, Korea: 2007. Properties of milled, brown and germinated brown rice flours preparation of poundcake using them. [Google Scholar]
- 28.Kim YS, Lee GC. A survey on the consumption and satisfaction degree of the cooked rice mixed with multi-grain in Seoul·Kyeonggi and Kangwon area. Korean J Food Culture. 2006;21:661–669. [Google Scholar]
- 29.Velioglu YS, Mazza G, Gao L, Oomah BD. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J Agric Food Chem. 1998;46:4113–4117. doi: 10.1021/jf9801973. [DOI] [Google Scholar]
- 30.Middleton E, Kandaswami C. Potential health-promoting properties of citrus flavonoids. Food Technol. 1994;48:115–119. [Google Scholar]
- 31.Peleg H, Naim M, Rouseff RL, Zehavi U. Distribution of bound and free phenolic acids in oranges (Citrus sinensis) and grapefruits (Citrus paradisi) J Sci Food Agric. 1991;57:417–426. doi: 10.1002/jsfa.2740570312. [DOI] [Google Scholar]
- 32.Moreno MI, Isla MI, Sampietro AR, Vattuone MA. Comparison of the free radical-scavenging activity of propolis from several regions of Argentina. J Ethnopharmacol. 2000;71:109–114. doi: 10.1016/S0378-8741(99)00189-0. [DOI] [PubMed] [Google Scholar]
- 33.Wettasinghe M, Shahidi F. Scavenging of reactive-oxygen species and DPPH free radicals by extracts of borage and evening primrose meals. Food Chem. 2000;70:17–26. doi: 10.1016/S0308-8146(99)00269-1. [DOI] [Google Scholar]
- 34.Kim JE, Joo SI, Seo JH, Lee SP. Antioxidant and α-glucosidase inhibitory effect of tartary buckwheat extract obtained by the treatment of different solvents and enzymes. J Korean Soc Food Sci Nutr. 2009;38:989–995. doi: 10.3746/jkfn.2009.38.8.989. [DOI] [Google Scholar]
- 35.Netzel M, Strass G, Bitsch I, Könitz R, Christmann M, Bitsch R. Effect of grape processing on selected antioxidant phenolics in red wine. J Food Eng. 2003;56:223–228. doi: 10.1016/S0260-8774(02)00256-X. [DOI] [Google Scholar]
- 36.Kim SM, Cho YS, Sung SK. The antioxidant ability and nitrite scavenging ability of plant extracts. Korean J Food Sci Technol. 2001;33:626–632. [Google Scholar]
- 37.Baublis AJ, Lu C, Clydesdale FM, Decker EA. Potential of wheat-based breakfast cereals as a source of dietary antioxidants. J Am Coll Nutr. 2000;19:308S–311S. doi: 10.1080/07315724.2000.10718965. [DOI] [PubMed] [Google Scholar]
- 38.Jung HA, Lee HJ, Kim YA, Park KE, Ahn JW, Lee BJ, Moon SG, Seo Y. Antioxidant activity of Artemisia capillaris Thunberg. Food Sci Biotechnol. 2004;13:328–331. [Google Scholar]