Abstract
The anti-colitic effect of purple carrot (PC) on 2% dextran sulfate sodium (DSS)-induced colitis in C57BL6/J mice was compared with those of yellow carrot (YC), beet (BT), and red cabbage (RC). Component analysis showed that PC contained cyanidin-3-xyloglucoside, cyanidin-3-xylosyl(sinapoly-glucosyl)galactoside, cyanidin-3-xylosyl(feruloylglucosyl) galactoside, and cyanidin-3-O-(6-O-glycosyl-2-O-xylosylgalactoside). PC diet (5% in AIN 93G diet) strongly reduced DSS-induced colon shortening and inflammatory cell infiltration in mice, followed by RC, BT, and YC diets. Treatment with PC reduced serum levels of pro-inflammatory cytokines such as tumor necrosis factor-α and interleukin-6 as well as reduced mRNA expression in colon tissue of colitis mice in comparison with other treatments. In addition, PC treatment inhibited colonic mRNA expression of inflammatory factors such as inducible nitric oxide synthase and cyclooxygenase-2 in mice. These results suggest that PC can attenuate the inflammatory reaction in mice with DSS-induced colitis, probably due to the anthocyanins in PC.
Keywords: purple carrot, pro-inflammatory, anthocyanin, dextran sulfate sodium, colitis
INTRODUCTION
Purple carrots (PCs; Daucus carota L.) have been cultivated in central Asian countries such as Turkey for at least 3,000 years. They contain an abundance of anthocyanins depending on pH and temperature (1). It was reported over 80 years ago that consumption of PCs could reduce the occurrence of lung and breast cancers; nevertheless, a recent report showed that anthocyanins in PCs possess anti-inflammatory activity (2). In addition, PCs were shown to contain more phenolic compounds than other carrots, especially anthocyanins (3).
The efficacy of PCs is under investigation. Especially, the high antioxidant activity of PCs is due to high concentrations of polyphenols and anthocyanins compared to yellow carrots (4). PCs were shown to have anti-glycemic and preventive effects on metabolic syndrome (5) as well as anti-bacterial effects against food-poisoning bacteria such as Escherichia coli O157:H7 (6). Also, a clinical test showed the anti-obesity effect of PC, even if the main interest in PCs is the administration and adsorption of carrot-derived anthocyanins (7).
Yellow carrots (YCs), which contain an abundance of β-carotene (isoprenoid compounds), have been reported to improve enteritis and reduce serum lipids as well as to possess beneficial effects such as antioxidant activity and anti-inflammatory effects by reducing cyclooxygenase (COX)-2 levels (4,8–11). Other anthocyanin-containing vegetables include beet, red cabbage, red onion, and purple sweet potato. We selected beet and red cabbage in this study.
Beet roots (BT) are used to color pickled radish or are ground as juice. It was reported that 3% BT crisp administered to rats alleviated dyslipidemic conditions, and their antioxidative activity was found to suppress hyperoxidation and expression of inflammatory factors (12,13). The major compound in BT includes betalains, which have a molecular structure similar to those of anthocyanins (12). Red cabbage (RC), classified as a cruciferous vegetable, possesses antioxidative and anti-cholinesterase effects (14). The major anthocyanin in RC is cyanidin-3(sinapoyl)-diglucoside-5-glucoside (15). When comparing the anthocyanin contents and 1,1-diphenyl-2-picrylhydrazyl radical scavenging activities of BT and RC, RC was found to have higher anthocyanin contents and antioxidative activities (16).
Inflammatory bowel diseases (IBD), including ulcerative colitis (UC), are frequent in Koreans, and the incidence of UC in Koreans is approaching that in Western countries (17). Gastrointestinal inflammation due to abnormal immune reactions is likely to be the cause of UC (18). As reduction of enteric inflammation may have an effect on UC, we investigated the anti-inflammatory effect of PCs on UC, as previous research has reported the anti-inflammatory effect of PCs on lipopolysaccharides-driven inflammation (2).
In this study, PCs, carrots, beets, and RC were assessed for their preventative effects on a 2% DSS-induced colitis mouse model.
MATERIALS AND METHODS
Diet sample preparation
PCs were obtained from Boolim Agricultural Co. (Seoul, Korea) and other vegetables used as controls such as YC, BT, and RC were purchased from a local market (Busan, Korea). The vegetable samples were washed with water and freeze-dried into powder, mixed into AIN-93G base diet (Dooyeol Biotech Co., Seoul, Korea) at a concentration of 5%. Prepared feed was stored in a refrigerator at 4°C until feeding.
Experimental animals
Male C57BL/6 mice were obtained from Samtaco Bio Korea (Osan, Gyeonggi, Korea) at 6~7 weeks of age and weighed 18.0±0.9 g. Mice were bred in a micro ventilation cage system (12/12-h light/dark cycle, 23±2°C, relative humidity 50±5%), and water and feed were given ad libitum. All animal experimental procedures were approved by the Pusan National University Institutional Animal Care and Use Committee (PNU-2014-0576). After a 1-week stabilization period, animals were divided into groups of seven mice each, and dextran sulfate sodium [DSS, molecular weight: 36,000~50,000, obtained from the MP Biomedical Co. (Solon, OH, USA)] was given to all mice except for the normal group via drinking water at a concentration of 2% for 7 days (n=7). Body weight and leftover feed was measured once every week. Stool condition and daily activity of experimental mice were checked to monitor induction of colitis.
Measurement of colon length and weight and serum separation
Experimental animals were given only water 12 h prior to sacrifice for inspection of colitis. Anesthesia was administered with diethyl ether, and blood collected from abdominal aortas underwent plasma separation by centrifugation at 12,000 g for 15 min and stored at −80°C until analysis. Separated colons were washed with saline water (0.9% NaCl) and their lengths and weights measured, followed by rapid freezing with liquid nitrogen and storage at −80°C until analysis.
Histological observation
Separated colons were immersed with 10% (v/v) neutral-buffered formalin for fixation and embedded into paraffin for preparation of paraffin sections (4 μm thick). The sections were subjected to hematoxylin and eosin staining and observed with an optic microscope (Olympus, Waltham, MA, USA).
Analyses of pro-inflammatory cytokines in serum
Separated sera were analyzed for pro-inflammatory cytokines, including tumor necrosis factor (TNF)-α and interleukin (IL)-6, using enzyme-linked immunosorbent assay (ELISA) kits (ELISA MAX, BioLegend, San Diego, CA, USA) following the instructions of the ELISA kit for each corresponding cytokine. The plate was analyzed by an ELISA reader to measure light absorbance at 450 nm within 30 min after the final reaction. The optical density value was calculated on the basis of the standard curve obtained from standard samples (20).
Reverse transcriptase-polymerase chain reaction (RT-PCR) analyses of colon tissue
Colon tissues were immersed in TriZol reagent (Invitrogen Co., Carlsbad, CA, USA) and subjected to homogenization on ice. mRNA extraction and cDNA constitution of the tissues were performed by the method of Bak et al. (21), with minor modifications. Constituted cDNA was mixed with one of the following primers, TNF-α (5′-GCG ACG TGG AAC TGG CAG AAG-3′, forward) 5′-GGT ACA ACC CAT CGG CTG GCA-3′, reverse), IL-6 (5′-CTG CAA GAG ACT TCC ATC CAG TT-3′, forward), 5′-AGG GAA GGC CGG TGG TTG T-3′, reverse), IL-1β (5′-CTC CAT GAG CTT TGT ACA AGG-3′, forward) 5′-TGC TGA TGT ACC AGT TGG GG-3′, reverse), inducible nitric oxide synthase (iNOS) (5′-GCA GCT GGG CTG TAC AAA-3′, forward), 5′-AGC GTT TCG GGA TCT GAA T-3′, reverse), COX-2 (5′-GAA TCA TTC ACC AGG CAA ATT G-3′, forward), 5′-TCT GTA CTG CGG GTG GAA CA-3′, (reverse), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal control, and placed in a 2720 Thermal Cycler (Applied Biosystems, Singapore) for RT-PCR. Amplification was performed with a denaturing phase at 94°C for 1 min, an annealing phase at 54°C for 1 min, and an extension phase at 72°C for 30 s as one cycle. After 40 amplification cycles, a finalization phase was carried out at 72°C for 7 min. The amplified PCR products were run on 1.0% agarose gels, stained with ethidium bromide, and visualized under UV light (19,20).
Statistical analysis
All results are expressed as the mean±standard deviation (SD). Significances of intergroup differences were assessed using analysis of variance (ANOVA), followed by Duncan’s test using SPSS (version 18.0, SPSS inc., Chicago, IL, USA). P-values of <0.05 were considered significant.
RESULTS AND DISCUSSION
Body weight change of treated samples
As shown in Table 1, average body weights of the control group (19.94±1.45), DSS+YC (18.47±2.10), DSS+BT (19.76±1.49), and DSS+RC (18.92±0.87) groups were similar to each other. The average body weight of the DSS+PC (20.02±1.00) group, however, was similar to that of the normal group (20.25±2.14).
Table 1.
Changes in body weight of mice during the experimental period
| Treatment | Body weight (g) | ||
|---|---|---|---|
|
| |||
| Initial | 7th day | 14th day | |
| Normal | 21.58±1.08NS | 22.71±1.25NS | 20.25±2.14NS |
| DSS | 21.49±1.33 | 22.41±1.78 | 19.94±1.45 |
| DSS+PC | 21.79±1.26 | 23.10±1.88 | 20.02±1.00 |
| DSS+YC | 22.13±0.49 | 23.95±1.02 | 18.47±2.10 |
| DSS+BT | 21.87±1.60 | 22.95±1.97 | 19.76±1.49 |
| DSS+RC | 21.47±1.08 | 22.22±0.97 | 18.92±0.87 |
The present data were expressed the mean±SD.
NS, not significant; PC, purple carrot; YC, yellow carrot; BT, beet roots; RC, red cabbage.
Changes in mice colons by PC
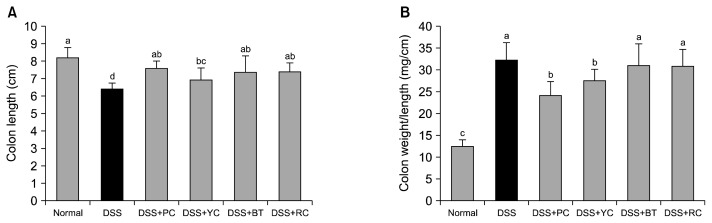
Inflammation of the colon leads to settlement and edema, followed by shortening of colon length, which is an indicator of colitis (22). Carrots have been shown to reduce the colon ulcer area and the colon weight-to-length ratio in acetic acid-induced colitis rats (23). In this experiment, the control group, which was given a pure AIN-93G diet and 2% DSS only, had the shortest colon length (6.4±0.3 cm) among the DSS-administered groups, whereas colon length of the PC group (7.6±0.4 cm) was close to that of the normal group (8.2±0.6 cm), followed by RC (7.4±0.5 cm), BT (7.4±0.9 cm), and YC groups (6.9±0.7 cm; Fig. 1A). Inflammatory progression is known to lead to over-activation of the immune system, eventually damaging colon tissue and shortening colon length (24). Phytochemicals in the PC, BT, and RC groups may have protected colon tissues from symptoms of colitis.
Fig. 1.
Effect of purple vegetable samples on colon length changes in DSS-treated colitis mice. (A) Colon length and (B) ratio of colon weight to length. Mean values with different letters (a–d) on the bars are significantly different (P<0.05) according to Duncan’s multiple range test. The present data were expressed as the mean±SD. PC, purple carrot; YC, yellow carrot; BT, beet roots; RC, red cabbage.
The weight-to-length ratio increases in response to inflammation, as edema following inflammation leads to shortening of colon length as well as increased colon weight. Thus, colon inflammation may be reduced or prevented when the colon weight-to-length ratio decreases to that of the normal group (24). The normal (12±1 mg/cm), PC (24±3 mg/cm), and YC groups (28±3 mg/cm) showed a significantly low weight-to-length ratio, whereas the RC (31±4 mg/cm) and BT groups (31±5 mg/cm) showed high weight-to-length ratios similar to that of the control group (32±4 mg/cm) (P<0.05, Fig. 1B).
Changes in colon morphology by PCs
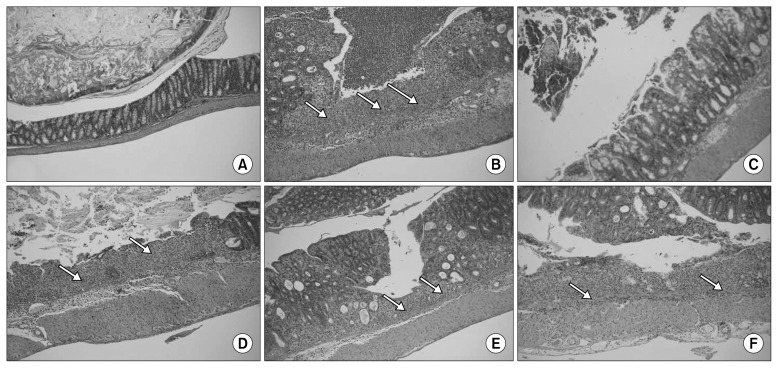
Colon tissue from the normal group showed no damage, whereas tissue from the control group showed severe inflammation and ulceration of the inner mucosa as well as proliferation and infiltration of lymphocytes. Tissues from the PC group showed partial ulcers and sites of inflammation, but most ulcers showed restoration of epithelial cells. The YC group also showed points of inflammation around blood vessels, but recovery was detected in comparison with those of the control group. The BT group showed a smaller range of inflammation, whereas inflammatory lymphocytes were condensed by over-proliferation. The RC group did not show invasion of ulcers on the inner mucosa, whereas the restoration range was smaller than others, and infiltration of lymphocytes was still detected (Fig. 2). Comprehensively, the PC group showed the best effects on colitis, followed by the BT, YC, and RC groups. Especially, anthocyanins from PCs were reported to have an antioxidative effect on the colon mucosa by suppression of reactive oxygen species (25). Thus, the efficacy of PCs to ameliorate inflammation might be due to such mechanism.
Fig. 2.
Histological observations of dextran sulfate sodium (DSS)-induced colitis in mice treated with different purple vegetable samples. Colon samples were obtained 7 days after DSS administration, sectioned, stained with hematoxylin and eosin, and observed with a light microscope (original magnification, 100×). Arrows on the picture stand for ulceration on the colon tissues. (A) Normal, (B) DSS, (C) DSS+purple carrot, (D) DSS+yellow carrot, (E) DSS+beet roots, and (F) DSS+red cabbage.
One gram of freeze-dried RC and BT was reported to have 8.5 mg and 0.1 mg of anthocyanins, respectively (16). According to our anthocyanin analysis, however, 1 gram of freeze-dried PC was found to have 147 mg of anthocyanins. Actually, 100 g of AIN-93G diet mixed with the freeze-dried powder PC sample at a concentration of 5% may contain 735 mg of anthocyanins, while those with freeze-dried powder RC and BT at the same concentration can be expected to have just 42.5 mg and 0.5 mg, respectively, which may partially support the unsurpassed anti-colitis efficacy of PC.
Previous high-performance liquid chromatography (HPLC)-mass spectrometry (MS) analyses of PCs have shown the presence of cyanidin-3-xylosyl(glucosyl)galactoside, cyanidin-3-xylosylgalactoside, cyanidin-3-xylosyl (sinapolyglucosyl)galactoside, and cyanidin-3-xylosyl(feruloylglucosyl) galactoside in PCs. Especially, cyanidin-3-xylosyl(feruloylglucosyl) galactoside was found to be predominant (26). Our analysis of PC anthocyanins by HPLC-LC showed that PCs mainly contained cyanidin-3-xyloglucoside, cyanidin-3-xylosyl(sinapoly-glucosyl)galactoside, cyanidin-3-xylosyl(feruloylglucosyl)galactoside, and cyanidin-3-O-(6-O-glycosyl-2-O-xylosylgalactoside). Especially, cyanidin-3-xyloglucoside was predominant. YC contains β-carotene, BT contains betalains, and RC contains cyanidin-3(sinapoyl)-diglucoside-5-glucoside (19). Especially, carrot roots ameliorated progress of IBD, as reflected in the colon ulcer index, colonic myeloperoxide, and nitric oxide (NO) in the acetic acid-induced colitis mice (23).
Polyphenols such as anthocyanins are involved in the inflammatory mechanism and manage intestinal barrier function (27). YCs and PCs differ in their color and compounds. YCs contain high concentrations of β-carotene while PCs contain high concentrations of anthocyanins as well as polyphenols in comparison with YCs. An in vivo experiment with β-carotene and PCs revealed their preventive effects on fatty liver disease by reduction of portal inflammation and fat deposition (4,5).
Effects on serum pro-inflammatory cytokines (TNF-α and IL-6)
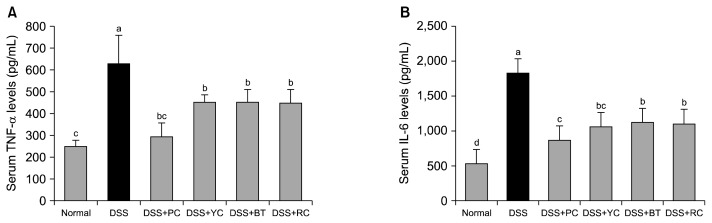
Overproduction of TNF-α in serum is known to occur via infiltration of inflammatory cells on the enteric mucosa, which may eventually lead to chronic colitis (24). While administration of DSS promoted the TNF-α expression to 629.7±129.0 pg/mL, the PC group showed suppression of TNF-α expression (296.4±61.0 pg/mL), which was similar to the normal group (254.3±24.8 pg/mL). Further, YC, BT, and RC were found to suppress TNF-α expression to 454.3±32.5 pg/mL, 454.3±57.2 pg/mL, and 450.7±59.6 pg/mL, respectively, and the expression levels were similar to each other (Fig. 3A).
Fig. 3.
Effect of purple vegetable samples on serum levels of (A) tumor necrosis factor (TNF)-α and (B) interleukin (IL)-6 in dextran sulfate sodium (DSS)-treated colitis mice. Mean values with different letters (a–d) on the bars are significantly different (P<0.05) according to Duncan’s multiple range test. The present data were expressed as the mean±SD. PC, purple carrot; YC, yellow carrot; BT, beet roots; RC, red cabbage.
IL-6 promotes differentiation of naive T cells and activation of innate immune cells such as dendritic cells or macrophages, which may promote inflammatory reactions (23). Each experimental group showed reduced expression of IL-6 in comparison with the normal group (535.1±50.2 pg/mL) and control group (1,833.3±199.6 pg/mL) in the DSS-induced colitis model. Especially, expression of IL-6 in the PC group was significantly low (871.8±68.8 pg/mL) and was similar to that of the normal group (P<0.05), followed by the YC (1,060.6±101.9 pg/mL), RC (1,109.0±43.9 pg/mL), and BT (1,126.5±59.5 pg/mL) groups (Fig. 3B). Thus, every experimental group suppressed the expression of pro-inflammatory cytokines. Especially, PCs contained the highest suppression of cytokines.
TNF-α, IL-6, and IL-1β: pro-inflammatory cytokines in colon tissue
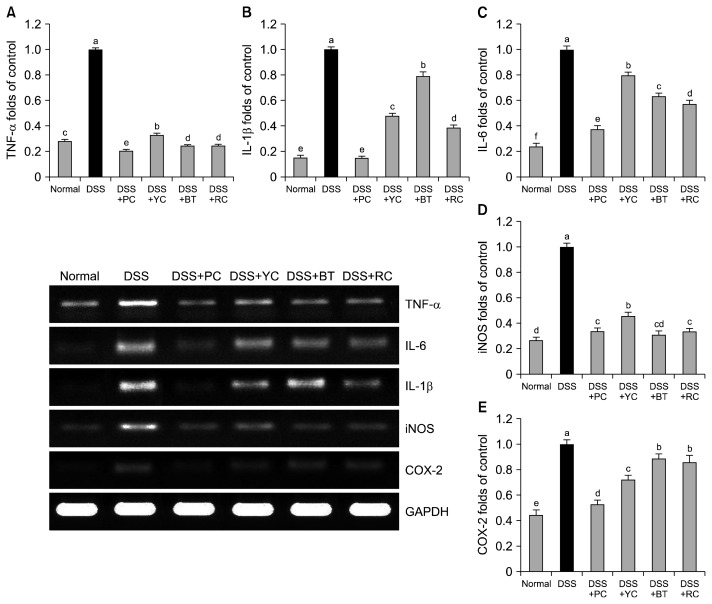
We also measured mRNA expression levels of inflammatory cytokines, including TNF-α, IL-6, and IL-1β in colon tissue as shown in Fig. 4. Expression of TNF-α and IL-6 was observed initially in serum and confirmed in colon tissues (23). IL-1β is known to promote permeability of epithelial and endothelial cells as well as boost activity of cytotoxic T cells, resulting in deterioration of inflammatory reactions, which was proven by a report that initiation and deterioration of colitis are proportional to IL-1β levels (24). One clinical report showed that IBS patients have a tendency to have significantly high serum concentrations of these inflammatory factors in comparison with the control (28). In our experiment, mRNA expression levels of TNF-α, IL-6, and IL-1β were lower in the PC-administered group than those of the other experimental groups, by which PC can partially suppress progression of enteric inflammation.
Fig. 4.
Effect of purple vegetable samples on mRNA levels of (A) tumor necrosis factor (TNF)-α, (B) interleukin (IL)-1β, (C) IL-6, (D) inducible nitric oxide synthase (iNOS), and (E) cyclooxygenase (COX)-2 in colon tissues of dextran sulfate sodium (DSS)-treated colitis mice. Band intensity was measured with a densitometer and expressed as fold-rate of control. Fold ratio=gene expression/GAPDH×control numerical value (control fold ratio: 1). Mean values with different letters (a–f) on the bars are significantly different (P<0.05) according to Duncan’s multiple range test. The present data were expressed as the mean±SD. PC, purple carrot; YC, yellow carrot; BT, beet roots; RC, red cabbage.
iNOS and COX-2: inflammation-related cytokines in colon tissues
iNOS is known to cause symptoms such as mucosal damage, ulcers, and internal bleeding by overproduction of NO, which is subjected to reaction with superoxide anion (20). COX-2 was found to worsen inflammatory conditions, and its expression is promoted at inflammation sites of the colonic mucosa (11). Thus, we observed the efficacy of PC on enteric expression of iNOS and COX-2 as well. The control group showed high mRNA expression levels of iNOS and COX-2, whereas sample-treated groups showed reduced expression of the two factors. PCs suppressed expression of iNOS similar to BT, followed by BC and YCs. In the case of COX-2, PCs showed the strongest suppression of iNOS expression, followed by YCs, BC, and BT (Fig. 4).
Taken together, expression levels of TNF-α, IL-1β, IL-6, iNOS, and COX-2 were lowered by the administration of PCs, which implies that PCs suppress mRNA expression of inflammation-related factors.
Suppression of colon length shortening was strongest in the PC group, followed by the RC and BT groups. Especially, PC showed the lowest ratio of colon weight-to-length, which represents the extent of enteric damage. PCs suppressed colon shortening and inflammation as well as edema. PCs reduced serum concentrations of cytokines such as TNF-α, IL-6, and IL-1β as well as gene expression of iNOS and COX-2. These effects of PCs on serum inflammatory cytokines were found to coincide with the suppression of enteric cytokines such as TNF-α by polyphenols (29). PCs showed anti-colitic effects, which were superior to the other samples such as YCs, RCs, and BTs. In comparison, the effects of PCs were the strongest owing to cyanidin-3-xyloglucoside of purple carrots, followed by RCs, BTs, and YCs. Thus, cyanidin-3(sinapoyl)-diglucoside-5-glucoside from RC and betalain from BT are more effective than β-carotene from carrots. These results are due to RC containing more polyphenols than BT (411 mg equivalent galic acid/100 g fresh RCs vs. 366 mg/100 g for BT), more anthocyanins than BT (164 mg equivalent cyanin/100 g fresh plant of RCs vs. 23.9 mg/100 g for BT), and higher antioxidative activity than BT (16). In vitro experiments using anthocyanin extracts have shown that cyanidin-3-glucoside has a suppressive effect on HT-29 human colorectal adenocarcinoma (29).
Taken together, the anti-colitic activity of PCs was superior to that of the other sample-treated groups, including the YC, BT, and RC groups. PCs suppressed expression of pro-inflammatory cytokines such as IL-6, TNF-α, and IL-1β as well as inflammatory cytokines such as iNOS and COX-2. Such efficacies might be due to PC-derived anthocyanins, which presumably led to the anti-inflammatory activity in the colon. However, more research is necessary to elucidate the in vitro and in vivo effects of anthocyanins from PCs.
ACKNOWLEDGEMENTS
This work was supported by the GRRC program of Gyeonggi province (GRRC-CHA2017-B03, Development of Functional Kimchi and Taemyeongcheong Beverage as a Functional Food and Dietary Supplement).
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Montilla EC, Arzaba MR, Hillebrand S, Winterhalter P. Anthocyanin composition of black carrot (Daucus carota ssp. sativus var. atrorubens Alef.) cultivars Antonina, Beta Sweet, Deep Purple, and Purple Haze. J Agric Food Chem. 2011;59:3385–3390. doi: 10.1021/jf104724k. [DOI] [PubMed] [Google Scholar]
- 2.Metzger BT, Barnes DM, Reed JD. Purple carrot (Daucus carota L.) polyacetylenes decrease lipopolysaccharide-induced expression of inflammatory proteins in macrophage and endothelial cells. J Agric Food Chem. 2008;56:3554–3560. doi: 10.1021/jf073494t. [DOI] [PubMed] [Google Scholar]
- 3.Arscott SA, Simon PW, Tanumihardjo SA. Anthocyanins in purple-orange carrots (Daucus carota L.) do not influence the bioavailability of β-carotene in young women. J Agric Food Chem. 2010;58:2877–2881. doi: 10.1021/jf9041326. [DOI] [PubMed] [Google Scholar]
- 4.Singh DP, Beloy J, McInerney JK, Day L. Impact of boron, calcium and genetic factors on vitamin C, carotenoids, phenolic acids, anthocyanins and antioxidant capacity of carrots (Daucus carota) Food Chem. 2012;132:1161–1170. doi: 10.1016/j.foodchem.2011.11.045. [DOI] [PubMed] [Google Scholar]
- 5.Poudyal H, Panchal S, Brown L. Comparison of purple carrot juice and β-carotene in a high-carbohydrate, high-fat diet-fed rat model of the metabolic syndrome. Br J Nutr. 2010;104:1322–1332. doi: 10.1017/S0007114510002308. [DOI] [PubMed] [Google Scholar]
- 6.Degirmenci H, Karapinar M, Karabiyikli S. The survival of E. coli O157:H7, S. Typhimurium and L. monocytogenes in black carrot (Daucus carota) juice. Int J Food Microbiol. 2012;153:212–215. doi: 10.1016/j.ijfoodmicro.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 7.Wright ORL, Netzel GA, Sakzewski AR. A randomized, double-blind, placebo-controlled trial of the effect of dried purple carrot on body mass, lipids, blood pressure, body composition, and inflammatory markers in overweight and obese adults: The QUENCH Trial. Can J Physiol Pharmacol. 2013;91:480–488. doi: 10.1139/cjpp-2012-0349. [DOI] [PubMed] [Google Scholar]
- 8.Banga O. Origin of the European cultivated carrot. Euphytica. 1957;6:54–76. [Google Scholar]
- 9.Padayachee A, Netzel G, Netzel M, Day L, Mikkelsen D, Gidley MJ. Lack of release of bound anthocyanins and phenolic acids from carrot plant cell walls and model composites during simulated gastric and small intestinal digestion. Food Funct. 2013;4:906–916. doi: 10.1039/c3fo60091b. [DOI] [PubMed] [Google Scholar]
- 10.Potter AS, Foroudi S, Stamatikos A, Patil BS, Deyhim F. Drinking carrot juice increases total antioxidant status and decreases lipid peroxidation in adults. Nutr J. 2011;10:96. doi: 10.1186/1475-2891-10-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Momin RA, De Witt DL, Nair MG. Inhibition of cyclooxygenase (COX) enzymes by compounds from Daucus carota L. seeds. Phytother Res. 2003;17:976–979. doi: 10.1002/ptr.1296. [DOI] [PubMed] [Google Scholar]
- 12.Wroblewska M, Juskiewicz J, Wiczkowski W. Physiological properties of beetroot crisps applied in standard and dyslipidaemic diets of rats. Lipids Health Dis. 2011;10:178. doi: 10.1186/1476-511X-10-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kujawska M, Ignatowicz E, Murias M, Ewertowska M, Mikołajczyk K, Jodynis-Liebert J. Protective effect of red beetroot against carbon tetrachloride- and N-nitrosodi-ethylamine-induced oxidative stress in rats. J Agric Food Chem. 2009;57:2570–2575. doi: 10.1021/jf803315d. [DOI] [PubMed] [Google Scholar]
- 14.Melega S, Canistro D, De Nicola GR, Lazzeri L, Sapone A, Paolini M. Protective effect of Tuscan black cabbage sprout extract against serum lipid increase and perturbations of liver antioxidant and detoxifying enzymes in rats fed a high-fat diet. Br J Nutr. 2013;110:988–997. doi: 10.1017/S0007114513000068. [DOI] [PubMed] [Google Scholar]
- 15.McDougall GJ, Fyffe S, Dobson P, Stewart D. Anthocyanins from red cabbage–stability to simulated gastrointestinal digestion. Phytochemistry. 2007;68:1285–1294. doi: 10.1016/j.phytochem.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Lungu L, Popa CV, Savoiu M, Danet AF, Dinoiu V. Antioxidant activity of Brassica oleracea L., Allium cepa L. and Beta vulgaris L. extracts. Rev Chim (Bucharest) 2010;61:911–914. [Google Scholar]
- 17.Yang DH, Yang SK. Trends in the incidence of ulcerative colitis in Korea. Korean J Gastroenterol. 2009;76:637–642. [Google Scholar]
- 18.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 19.Kim YJ. MS Thesis. Pusan National University; Busan, Korea: 2015. Preventive effect of purple carrot on colitis, obesity and colorectal cancer in C57BL/6J mice. [Google Scholar]
- 20.Kim SJ, Kim SH, Lim YI, Kim YG, Park KY. Inhibitory effects of ginger and Beopje ginger on DSS-induced colitis in mice. J Korean Soc Food Sci Nutr. 2014;43:477–484. doi: 10.3746/jkfn.2014.43.4.477. [DOI] [Google Scholar]
- 21.Bak SS, Kong CS, Rhee SH, Rho CW, Kim NK, Choi KL, Park KY. Effect of sulfur enriched young radish kimchi on the induction of apoptosis in AGS human gastric adenocarcinoma cells. J Food Sci Nutr. 2007;12:79–83. [Google Scholar]
- 22.Melgar S, Karlsson L, Rehnström E, Karlsson A, Utkovic H, Jansson L, Michaëlsson E. Validation of murine dextran sulfate sodium-induced colitis using four therapeutic agents for human inflammatory bowel disease. Int immunopharmacol. 2008;8:836–844. doi: 10.1016/j.intimp.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 23.Patil MVK, Kandhare AD, Bhise SD. Anti-inflammatory effect of Daucus carota root on experimental colitis in rats. Int J Pharm Pharm Sci. 2012;4:337–343. [Google Scholar]
- 24.Reinecker HC, Steffen M, Witthoeft T, Pflueger I, Schreiber S, MacDermott RP, Raedler A. Enhanced secretion of tumour necrosis factor-alpha, IL-6, and IL-1β by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn’s disease. Clin Exp Immunol. 1993;94:174–181. doi: 10.1111/j.1365-2249.1993.tb05997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olejnik A, Rychlik J, Kidoń M, Czapski J, Kowalska K, Juzwa W, Olkowicz M, Dembczyński R, Moyer MP. Antioxidant effects of gastrointestinal digested purple carrot extract on the human cells of colonic mucosa. Food Chem. 2016;190:1069–1077. doi: 10.1016/j.foodchem.2015.06.080. [DOI] [PubMed] [Google Scholar]
- 26.Martin DA, Bolling BW. A review of the efficacy of dietary polyphenols in experimental models of inflammatory bowel diseases. Food Funct. 2015;6:1773–1786. doi: 10.1039/C5FO00202H. [DOI] [PubMed] [Google Scholar]
- 27.Romier B, Schneider YJ, Larondelle Y, During A. Dietary polyphenols can modulate the intestinal inflammatory response. Nutr Rev. 2009;67:363–378. doi: 10.1111/j.1753-4887.2009.00210.x. [DOI] [PubMed] [Google Scholar]
- 28.Liebregts T, Adam B, Bredack C, Röth A, Heinzel S, Lester S, Downie-Doyle S, Smith E, Drew P, Talley NJ, Holtmann G. Immune activation in patients with irritable bowel syndrome. Gastroenterology. 2007;132:913–920. doi: 10.1053/j.gastro.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 29.Jing P, Bomser JA, Schwartz SJ, He J, Magnuson BA, Giusti MM. Structure-function relationships of anthocyanins from various anthocyanin-rich extracts on the inhibition of colon cancer cell growth. J Agric Food Chem. 2008;56:9391–9398. doi: 10.1021/jf8005917. [DOI] [PubMed] [Google Scholar]