Summary
Emerging longitudinal research has highlighted poor sleep as a risk factor of a range of adverse health outcomes, including disabling pain conditions. In establishing the causal role of sleep in pain, it remains to be clarified whether sleep deterioration over time is a driver of pain and whether sleep improvement can mitigate pain-related outcomes. A systematic literature search was performed using PubMed MEDLINE, Ovid EMBASE, and Proquest PsycINFO, to identify 16 longitudinal studies involving 61,000 participants. The studies evaluated the effect of sleep changes (simulating sleep deterioration, sleep stability, and sleep improvement) on subsequent pain-related outcomes in the general population. A decline in sleep quality and sleep quantity was associated with a two- to three-fold increase in risk of developing a pain condition, small elevations in levels of inflammatory markers, and a decline in self-reported physical health status. An exploratory meta-analysis further revealed that deterioration in sleep was associated with worse self-reported physical functioning (medium effect size), whilst improvement in sleep was associated with better physical functioning (small effect size). The review consolidates evidence that changes in sleep are prospectively associated with pain-related outcomes and highlights the need for further longitudinal investigations on the long-term impact of sleep improvements.
Keywords: Sleep, Pain, Health, Public health, Prospective, Longitudinal, Systematic review, Meta-analysis
Abbreviations
- AHRQ
Agency for healthcare research and quality
- BMI
Body mass index
- CES-D
Center for epidemiological studies depression scale
- CI
Confidence interval
- CRP
C-reactive protein
- HR
Hazard ratio
- ICSD
International classification of sleep disorders
- IL-6
Interleukin-6
- MCS
Mental component summary of the short form health survey-36
- NKCA
Natural killer cell activity
- OR
Odds ratio
- PCS
Physical component summary of the short form health survey-36
- PGE2
Prostaglandin E2
- POMS
Profile of mood states
- PSG
Polysomnography
- PSQI
Pittsburgh sleep quality index
- RCT
Randomised controlled trial
- SF-36
Short form health survey-36
- SMD
Standardised mean difference
- STROBE
The strengthening the reporting of observational studies in epidemiology
- TNF-α
Tumour necrosis factor – alpha
Introduction
There is increasing epidemiological evidence highlighting sleep deficits and disturbances as risk factors of poor physical health [1], [2] and mental well-being [3], [4]. Sleep problems have been specifically linked to the development of chronic pain, which refers to pain that persists beyond the expected time of healing from injury, illness or tissue damage (3–6 mo) [5], [6]. Unlike acute pain that serves the important function of signalling harm to the body's integrity, chronic pain is in itself a disease with poor prognosis, featuring peripheral and central sensitisation to pain signals in the absence of clear underlying pathology [7], [8]. Despite its invisible nature, chronic pain – like insomnia – can considerably limit one's day-to-day functioning; from concentrating on a task, to walking, sleeping, maintaining social relationships, and holding down a job for independent living [9]. In primary care, chronic pain is ranked the top cause of quality-adjusted life-year loss, surpassing cardiovascular disease, high blood pressure, mood and anxiety disorders, diabetes, and common respiratory conditions [10]. Considering the high prevalence of both insomnia symptoms (10–30%; ∗[11], [12], [13]) and chronic pain (10–20%; [9], [14]) in the general population and their potency to impair well-being, the frequent co-occurrence of sleep and pain presents a serious public health challenge to our ageing society [15], [16], [17].
Evidence for the effect of sleep problems on pain from micro-longitudinal studies
Conventionally, sleep disturbance is thought to be a symptom secondary to pain and the two conditions are assumed to be broadly bi-directionally linked. However, recent research has been able to show that sleep problems may have a stronger contributory effect on pain than the effect of pain on sleep, shifting the research emphasis onto the temporal association from sleep to pain ∗[18], ∗[19]. Much of the evidence on the temporal impact of sleep on subsequent pain has come from longitudinal studies. Micro-longitudinal studies as described by Affleck et al. [20] use ‘time-series’ designs to examine day-to-day sleep and pain variations in individuals over a period of one to two weeks. These studies have shown that night-time sleep parameters more consistently predict next-day pain compared to pain predicting subsequent sleep. Edwards et al. [21] found evidence to support a close link between sleep and pain on a day-to-day basis. In their sample of 971 healthy adults assessed over the telephone for a week, self-reported sleep duration the previous night was a significant predictor of pain symptoms frequency the next day. Whilst pain symptoms did in turn predict subsequent sleep duration, the magnitude of the effect was only half as strong as the influence of sleep duration predicting pain.
Tang et al. [22] monitored sleep and pain reports over a week in a sample of 119 mixed chronic pain patients in their natural living and sleeping environments, using actigraphy and electronic daily diaries to assess sleep, pain, and mood reports at three time points over the course of the day. Results from multilevel modelling indicated that sleep quality was a significant and consistent predictor of next day pain at all assessment points. In contrast, whilst presleep pain was a predictor of poorer sleep efficiency calculated based on sleep diary entries, it was not a significant predictor of subsequent sleep efficiency as estimated by actigraphy. Compared to pain, presleep cognitive arousal and mood were better predictors of subsequent sleep.
Evidence for the effect of sleep problems on pain from macro-longitudinal studies
There are some macro-longitudinal (prospective) studies with less frequent assessments but longer timeframes that have examined the prevalence and incidence of insomnia and chronic pain at the population level. These studies have found evidence that poor sleep is a primary factor predicting aggravation of pain responses and determining longer-term risks of developing a pain condition. Mork and Nilsen [23] demonstrated in a sample of 12,350 healthy women that incidence of self-reported sleep problems tripled the risk of reporting physician-diagnosed fibromyalgia 11 y later. The analyses were adjusted for age, general physical health status, and psychological wellbeing, with the resultant risk increasing depending on the frequency and severity of sleep problems. Gupta et al. [24] and McBeth et al. [25] also reported that poor self-reported sleep quality strongly predicted the onset of widespread pain symptoms up to 3 y later, even when other psychological, lifestyle, and health factors were all controlled for. Similarly, Nitter et al. [26] found that self-reported disrupted and non-restorative sleep were significant predictors of chronic pain onset in pain-free individuals over the course of 17 y. The same sleep predictors also increased the risk of pain persistence and worsening among those who already had chronic pain at baseline. Despite not having intensive repeated assessments of sleep, findings from these prospective studies have shed light on the potential long-term impact of sleep on pain.
Focussing on the long-term impact of changes in sleep on pain outcomes
That said, the causality of the relationship between sleep and pain needs finer characterisation. Whilst it is understood that sleep patterns and sleep quality fluctuate over time, little is known about the effect of these sleep changes on pain and other health variables in the long run. This is in part due to the fact that many macro-longitudinal studies examined sleep statically at a certain time point rather than studying the dynamic changes in sleep across multiple assessment points. Further research using repeated measurements of sleep disturbances would help establish whether greater or lesser exposure to sleep problems over time leads to changes in incidence of pain and related symptoms ∗[27], [28].
Macro-longitudinal studies also often do not have suitable designs and assessment technologies to explore the processes underpinning sleep changes. In experimental studies, acute sleep restriction in healthy pain-free participants in the form of 88-h total sleep deprivation [29] and partial sleep deprivation of 6 h a night over a week [30] or 4 h a night over 10 d [31] were associated with impaired immunity, elevated inflammatory response and raised cytokines levels, namely, interleukin-6 (IL-6), C-reactive protein (CRP), cortisol, prostaglandin E2 (PGE2), and tumour necrosis factor alpha (TNF-α). These biomarkers are also believed to be related to greater self-reported pain, pain sensitivity, fatigue, and consequent decline in self-reported health status [32]. However, the use of measures that assess these biomarkers is sporadic in longitudinal studies and as such their roles in influencing the impact of sleep changes on long-term pain outcomes require confirmation. Finally, it should be mentioned that, of the few studies that examined change in sleep, the focus of analysis is primarily on the effects of negative rather than positive changes. It would be important to verify whether improvement in sleep – outside of clinical trials – is also associated with improvement in pain-related health outcomes.
Aims of the current systematic review
Given the above considerations, the present review examined prospective (macro-longitudinal) studies that have assessed improvement and deterioration in sleep over time and the influence of these changes on subsequent pain-related outcomes. The aims of this review were thus to: i) systematically summarise the state of the research, ii) critically assess the methodological quality of and consistency in findings across existing studies, and iii) carry out an exploratory meta-analysis to quantify the effect of changes in sleep on self-reported health outcome over time.
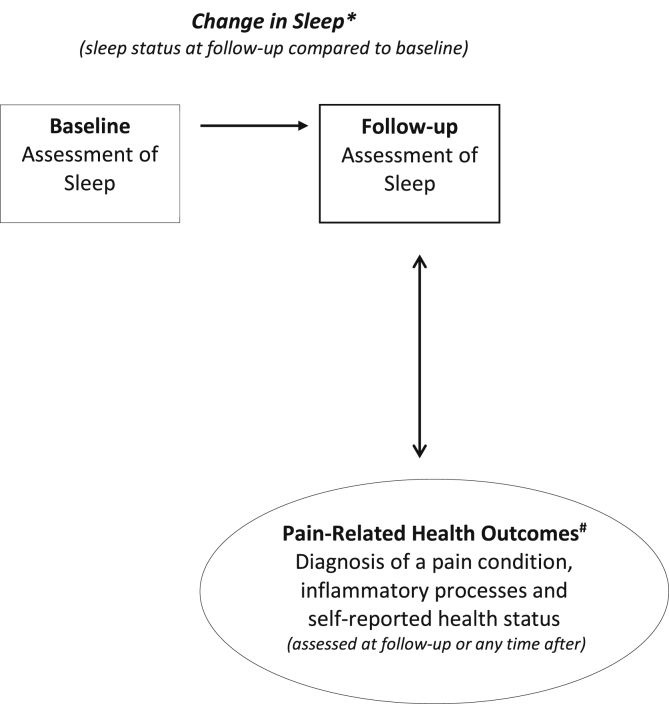
The predicting/exposure variable was defined as changes in sleep parameters e.g., insomnia symptoms, sleep quantity, and sleep quality. To gain a clearer idea of how different sleep measures influence pain, findings were separated by different pain-related health outcomes. These outcomes were not only limited to diagnosis of the pain condition itself, but also included pain-related physiological status such as inflammation/immune functions and self-reported health status (Refer to Fig. 1 for a schematic figure summarising this approach, and the methodologies of included studies). We acknowledge that factors such as anxiety and depression may play an important role in changes in sleep and subsequent health outcomes. However, since they were not the focus of this review, we refer readers to Alvaro et al. [33] and Lustberg and Reynolds [34] for comprehensive reviews on these topics.
Fig. 1.
Summary of the framework underlying this systematic review and methodological design of the included studies. Based on the experimental and epidemiological evidence, the figure illustrates the potential prospective relationship between changes in sleep and chronic pain experience. Changes in sleep from baseline to follow-up represents the variable predicting subsequent chronic pain experience. Change in sleep* refers to change in i) sleep duration, ii) sleep quality, and/ or iii) insomnia symptoms. Pain-related health outcomes# represents the factors that make up overall pain experience, namely, the risk of developing a pain condition, changes in physiological inflammatory and immune processes and changes in self-reported pain-related health and functioning status.
Methods
Data source and search strategy
The data source for this systematic review was original longitudinal studies that have evaluated the effect of prospective changes in sleep on pain-related health outcomes. Relevant articles were identified through electronic searches performed using PubMed MEDLINE, Ovid EMBASE, and ProQuest PsycINFO. Reference lists of included studies and relevant reviews were also hand-searched to ensure comprehensive coverage. The protocol of this systematic review has been reviewed and registered with PROSPERO, an international prospective register of systematic reviews. The PROSPERO registration number is 2015:CRD42015023943.
The initial search was carried out by EA in June 2015 and repeated just before the final analysis (April 2017) to provide an update. Searches were carried out on each database using both study subject (sleep*OR insomnia) AND study methodology (longitudinal OR prospective) search terms in the title and abstract fields. There was no restriction on publication year, but filters were set limiting the search to human studies and in the English language. Given that a broad array of measures can be used to index pain-related health outcomes, no restriction was set to limit the search by outcomes reported. This approach returned a large volume of potentially eligible articles. Whilst the screening process was laborious for the review team, it was considered a more comprehensive and inclusive method to capture all relevant studies.
Screening
Due to the large volume of hits returned, the first round of screening was a “title” screen carried out by EA to screen for unrelated articles, animal studies, studies in children populations and in critically ill/hospitalised patients. This resulted in a number of irrelevant articles being eliminated. The next step was an eligibility assessment of both titles and abstracts and involved four researchers (FR, SA, NK, and PC – a doctoral student and three undergraduate students) from the review team, all of whom had been given training and detailed guidance on the screening procedure. They did the screening collaboratively using an eligibility checklist to identify relevant studies. The screening checklist included six questions requiring a ‘yes’ or ‘no’ response. Each question reflects each of the inclusion criteria (see Table 1). Only studies with a ‘yes’ response to questions 1, 2, 4, 5 and 6 and a ‘no’ response to question 3 were included for full-text screening. The title and abstract screen was cross-checked by EA, differences in opinion among the reviewers were resolved through team discussion. The discussion erred on the side of caution to include studies for further full-text screening even if there were doubts on study eligibility based on response to the six questions. A total of 14 studies required further extensive discussion on eligibility. Interrater agreement rate (Cohen's Kappa) between reviewers and EA was 0.77, a value considered ‘good’ [35].
Table 1.
Screening criteria questions.
|
|
|
|
|
|
Note: *This excluded studies carried out on acutely ill and hospitalised medically ill or psychiatric patients but did not exclude populations within the general community with other pain or medical conditions.
Study selection
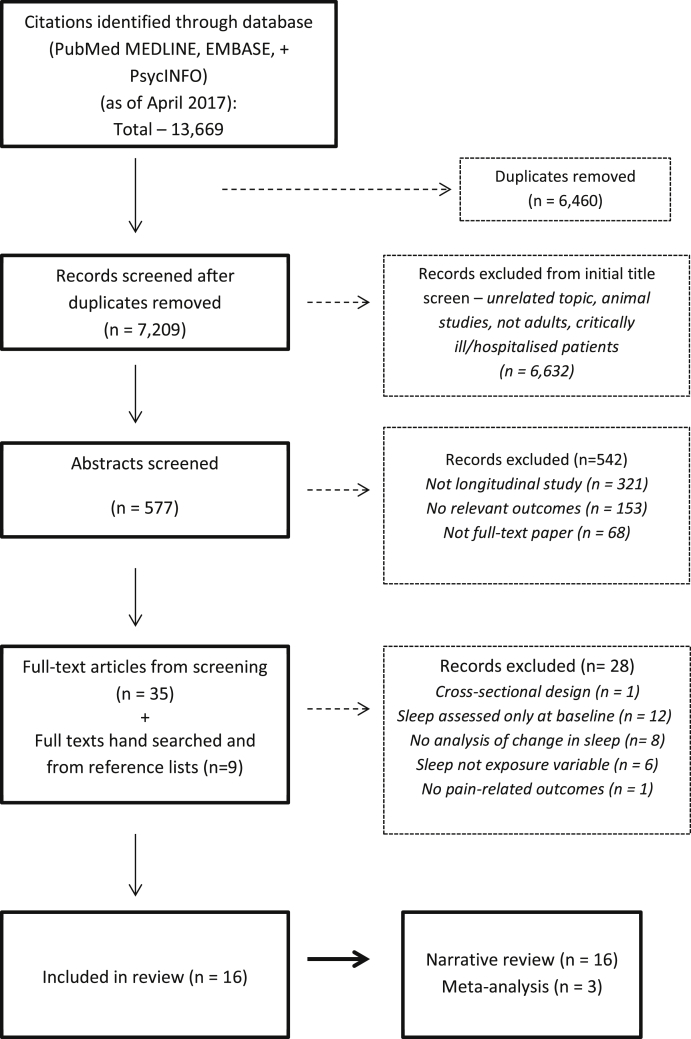
Fig. 2 (flow diagram) depicts the search and screening process. After the initial title and abstract screen, 44 articles were selected for full-text screening. The full texts of these studies were then assessed for eligibility by EA as per the inclusion criteria, using the same aforementioned screening checklist, but with further emphasis on the following qualities: i) the study had to have a prospective follow-up design, ii) that reported a change in sleep parameters using a measure of sleep, iii) on at least two occasions (baseline and follow-ups) and iv) that the association of change in sleep with a subsequent pain-related outcome was reported (See Fig. 1).
Fig. 2.
Flowchart of study selection.
Following the full-text screening, 28 studies were excluded because they did not meet all the inclusion criteria. Most were excluded as they only assessed sleep at baseline, did not report an analysis of change in sleep or sleep was not the main exposure or predicting variable. Sixteen studies met the full inclusion criteria and were selected for data extraction and data synthesis. All screened full-text articles and included studies were cross-checked for eligibility by a senior member of the review team (NT).
Predicting and outcome variables
The predicting variable was ‘change in sleep’ and outcome variable was ‘pain-related health outcomes’. ‘Change in sleep’ was defined as change in any sleep parameters assessed in the study between two time points, e.g., change in sleep quantity, sleep quality, and/or insomnia symptoms. Change in sleep was derived from the difference in sleep at follow-up compared to sleep at baseline. ‘Pain-related health outcomes’ was operationalised as measures indicative of any pain conditions and/or pain symptoms. This included incidence or presence of pain-related health conditions (back pain, fibromyalgia, arthritis, hip fractures etc.), inflammatory and immune system biomarkers, pain intensity, pain interference, fatigue, pain-related psychosocial functioning, and quality of life. This diverse definition of pain-related health outcome covered different dimensions of the chronic pain experience and can be grouped into those representing i) the diagnosis (given by health care professionals), ii) the physiological underlying factors (indicated by relevant biomarkers) and iii) individuals' self-reported health-related perceptions and judgements (reflected by responses to questionnaires).
Data extraction
Characteristics of the included studies are presented in Table 2 which summarises the extracted details on study methodology (i.e., final sample size at follow-up, participants' characteristics, sleep assessment measure, outcome assessment measure, number of follow-up assessments and duration of follow-up, adjusted variables, and main results on the effect of changes in sleep on outcome measure). For studies with multiple outcome measures, the main results included in this review were those related specifically to the effect of change in sleep on a pain-related health outcome. When the relevant information was missing or not reported in the preferred formats in the original paper, the corresponding author of the article was contacted by email, with another follow-up email sent after three weeks if no response. Requests were sent out requesting additional information for seven [36], [37], [38], [39], [40], [41], [42] of the included articles. Five authors responded and three [36], [40], [42] were able to provide the requested information.
Table 2.
Study characteristics.
| Citation Country |
Final sample Gender Mean age (final sample) Ethnicity |
Predicting variable | Pain-related health outcome | Follow-up duration | Timing of assessments | Adjusted variables | Results: sleep deterioration | Results: sleep improvement |
|---|---|---|---|---|---|---|---|---|
| Agmon & Armon (2014) [51] Israel |
N = 2131 66% male 46.20 y not stated |
Change in self-reported insomnia symptoms from Athens insomnia scale. | Diagnosis of back pain (confirmed through medical records and medical interview with physician) | 3.7 y | Predicting variable and pain-related health outcome assessed at three time points spread over a period of 3.7 y. | Age, gender, education, physical activity, self-rated health, smoking, BMI, levels of high-sensitivity C-reactive protein. | Increase in insomnia symptoms from time 1 to time 2 was associated with increased risk of diagnosis of back pain at Time 3 (OR = 1.40 94% CI 1.10–1.71). | None reported. |
| Campbell et al. (2013) [52] UK |
N = 2622 42.1% male Not stated range 50–80+ y not stated |
Change in self-reported sleep quality (Jenkins sleep questionnaire). | Self-reported pain presence, persistence, interference and depressive symptoms. |
6 y | Predicting variable and pain-related health outcome assessed at three time points (baseline, 3 y and 6 y). | Age, gender, alcohol consumption, smoking, marital status, employment status, and BMI. | New onset of sleep problems associated with increased pain interference and increased risk of depression at follow-up. | None reported. |
| Ferrie et al. (2013) [36] UK |
N = 5003 71.8% male 49.3 y not stated |
Change in self-reported sleep quantity. | Immune marker – CRP and IL-6 levels. | 5 y | Predicting variable and pain-related health outcome assessed at two time points (baseline and follow-up). | Age, gender, occupation, systolic blood pressure, BMI, total cholesterol, and diabetes. | Decrease in sleep quantity significantly associated with higher IL-6 levels but not CRP at follow-up. | Increase in sleep quantity not significantly associated with CRP and Il-6 levels at follow-up. |
| Foley et al. (1999) [53] USA |
N = 6899 62% male Not stated aged 65+ y not stated |
Change in self-reported insomnia symptoms (difficulty falling asleep or early morning arousal). | Diagnosis of hip fracture by physician. | 3 y | Predicting variable and pain-related health outcome assessed at two time points (baseline and follow-up). | Age, gender, community (state of residence), income, and education. | New incidence and persistence of insomnia symptoms significantly associated with newly reported presence of hip fracture at follow-up (OR = 2.08 95% CI 1.18, 3.65). | None reported. |
| Irish et al. (2013) [37] USA |
N = 128 63% male 36.45 y 92% white |
Change in self-reported sleep quality (PSQI). | Self-report physical symptoms. Immune marker – natural killer cell number and cytotoxicity (n = 51). |
12 mo | Two time points. Predicting variable assessed at baseline and follow-up. Pain-related health outcome assessed only at follow-up. | None stated. | Deterioration of sleep quality not significantly correlated with pain-related health outcomes at follow-up. | Improvement in sleep quality not significantly correlated with pain-related health outcomes at follow-up. |
| Janson et al. (2001) [54] Sweden |
N = 2602 100% male Not stated range 30–69 y not stated |
Change in self-reported insomnia symptoms (difficulty falling asleep and difficulty maintaining sleep). | Diagnosis of a medical disorder, including joint or low back disorders by physician. | 10 y | Predicting variable and pain-related health outcome assessed at two time points (baseline and follow-up). | Age, BMI smoking, physical inactivity, alcohol dependence, and medical disorders. | Increase in insomnia symptoms associated with newly reported medical disorder at follow-up. | None reported. |
| Komada et al. (2012) [49] Japan |
N = 1577 43% male 58.6 y not stated |
Change in self-reported sleep quality (Japanese version of PSQI – cut-off score of 5.5 indicating insomnia). | SF36 – PCS | 2 y | Predicting variable and pain-related health outcome assessed at two time points (baseline and follow-up). | Age, gender, disease status, alcohol consumption, smoking habits, living status, sleep medication use, CES-D, MCS, PCS, and PSQI scores at baseline. | New incidence of insomnia symptom associated with a decline in PCS scores at follow-up. | Remission of insomnia symptoms not significantly associated with increase in PCS scores at follow-up. |
| Parthasarathy et al. (2015) [56] USA | N = 1409 45% male 47 y not stated |
Change in self-reported insomnia symptoms derived from ICSD insomnia diagnosis criteria. | Immune marker – CRP levels assessed in 722 participants. | 6 y | Two time points. Predicting variable assessed at baseline and follow-up. Pain-related health outcome assessed only at 6-y follow-up. | Age, gender, BMI, smoking, physical activity, use of alcohol and medications to get to sleep, marital status, habitual snoring, diabetes mellitus and hypertension. | Persistence of insomnia symptoms associated with an increase in and higher CRP levels at follow-up compared to those with intermittent or no insomnia. | None reported. |
| Quan et al. (2005) [55] USA |
N = 4667 40.9% male 72.3 y not stated |
Change in self-reported insomnia symptoms (trouble falling asleep, frequent awakenings and excessive daytime sleepiness). | Diagnosis of arthritis by physician. | 1–4 y (mean 3.55) | Predicting variable and pain-related health outcome assessed at two time points (baseline and follow-up). | Age, gender, race, time interval between baseline and follow-up examinations. | New incidence of insomnia symptoms associated with report of arthritis in women. | None reported. |
| Rueggeberg et al. (2012) [38] Canada |
N = 157 48.40% male 71.71 y not stated |
Change in self-reported sleep quantity using items from PSQI. | Immune marker – diurnal cortisol secretion. | 4 y | Predicting variable and pain-related health outcome assessed at three time points (baseline, 2 y and 4 y). | Age, gender, partnership status, education, chronic illness, cortisol-related medication usage, BMI and smoking. | Decrease in sleep quantity associated with significant increases in cortisol secretion level at follow-up. | Increase in sleep quantity not significantly associated with changes in cortisol level at follow-up. |
| Ropponen et al. (2013) [58] Finland |
N = 18,979 47% male 45 y not stated |
Change in self-reported sleep quality and sleep quantity. | Diagnosis of back pain by physician and included in national register database on disability pension due to low back pain diagnosis. | 23 y | Two time points. Predicting variable assessed at baseline and follow-up. Pain-related health outcome assessed only at follow-up. | Age, education, socioeconomic status, marital status, BMI, physical activity, musculoskeletal pain locations, smoking, alcohol, life satisfaction, use of hypnotic agents, diurnal type, and type of work. | Deterioration and persistent of poor sleep quality associated with higher risk of low back pain diagnosis at follow-up (HR = 1.84 95% CI 1.01–3.37). No association with decrease in sleep quantity. | Improvement in sleep quantity and quality not associated with risk of low back pain diagnosis at follow-up. |
| Shakhar et al. (2007) [39] USA |
N = 45 0% male 39.7 y 47% white 40% black |
Change in self-reported sleep quantity. | Immune marker – NKCA levels. | 1 mo | Two time points. Predicting variable and pain-related health outcome assessed at both baseline and follow-up. | POMS Depression and Tension subscales scores. | Decrease in sleep quantity not associated with NKCA levels at follow-up. | Increase in sleep quantity was significantly related to an increase in NKCA levels at follow-up. |
| Silva et al. (2009) [40] USA |
N = 3078 45% male 67.3 y 75% white |
Change in self-reported insomnia symptoms (difficulty initiating and maintaining sleep, daytime sleepiness). | SF36 – PCS. | 5 y | Predicting variable and pain-related health outcome assessed at two time points (baseline and follow-up). | Age, gender, BMI, smoking, sleeping pill use, PSG total sleep time, baseline coronary heart disease and respiratory disease. | Deterioration of insomnia symptoms was not associated with PCS scores. Increase in daytime sleepiness was associated with decline in PCS scores at follow-up. | Improvement of insomnia symptoms not significantly associated with PCS scores at follow-up. |
| Smagula et al. (2016) [57] Singapore |
N = 8265 41.05% male 64.59 y 98.6% asian |
Change in self-reported sleep quantity. | Diagnosis of arthritis by physician and diagnosis of hip fracture recorded on hospital database. | 12.7 y | Predicting variable and pain-related health outcome assessed at two time points (baseline and follow-up). | Age, gender, baseline sleep duration. | No association between change in sleep and arthritis. Increase in sleep quantity from 6 to 8 to >8 h was linked with greater risk of hip fracture at follow-up (OR = 1.52 95% CI 1.16–2.00). | None reported. |
| Suh et al. (2014) [41] Korea |
N = 1247 40.1% male 54.3 y not stated |
Change in self-reported insomnia symptoms (difficulty initiating and maintaining sleep, early morning awakenings and unrefreshed in the morning). | SF36 – PCS. | 2 y | Predicting variable and pain-related health outcome assessed at three time points spread over 2 y. | Age, gender, marital status, employment, smoking, alcohol, hypertension, diabetes, depression, PSQI and BMI score. | Deterioration and persistence of insomnia symptoms associated with significantly lower PCS scores at follow-up. | None reported. |
| Zhang et al. (2012) [42] Hong Kong |
N = 2291 50% male 46.3 y not stated |
Change in self-reported insomnia symptoms (non-restorative sleep). | Subjective physical health status. Diagnosis of arthritis and other chronic pain condition by physician. |
5 y | Predicting variable and pain-related health outcome assessed at two time points (baseline and follow-up). | Age, gender, education, family income, medication, and comorbid sleep problems (insomnia subtypes, habitual snoring, short sleep duration). | New incidence of insomnia symptoms significantly associated with higher risk of reporting a chronic pain condition at follow-up (OR = 2.47 95% CI 1.30–4.69) | Remission of insomnia symptoms associated with a relatively lowered risk of developing a chronic pain condition at follow-up (OR = 1.23, 95% CI 0.57–2.59). |
BMI: body mass index, CES-D: center for epidemiological studies depression scale, CI: confidence interval, CRP: creatinine reactive protein, HR: hazard ratio, ICSD: international classification of sleep disorders, IL-6: interleukin-6, MCS: mental component summary, NKCA: natural killer cell activities, OR: odds ratio, PCS: physical component summary, POMS: profile of mood states, PSG: polysomnography, PSQI: Pittsburgh sleep quality index.
Risk of bias assessment
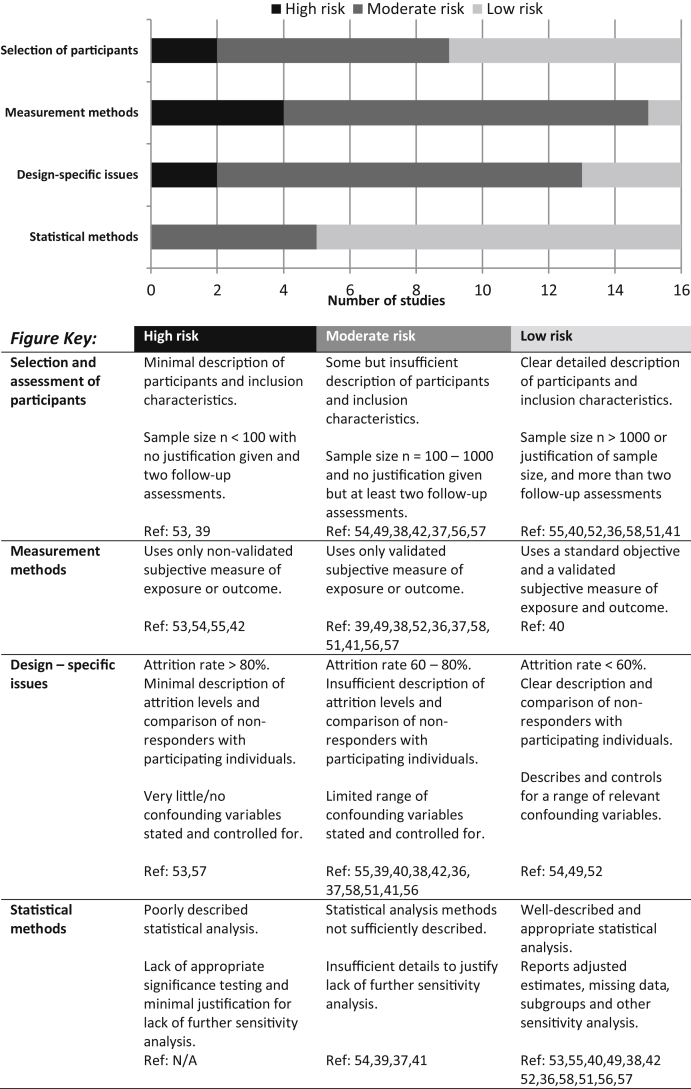
Risk of bias was assessed qualitatively using a checklist adapted from: the strengthening the reporting of observational studies in epidemiology (STROBE) guidelines for reporting observational epidemiological studies [43] and the modified agency for healthcare research and quality (AHRQ) quality assessment criteria for observational studies [44]. All included studies were assessed for risk of bias using four categories: i) methods of selecting and assessing study participants, ii) measurement methods, iii) design-specific issues (attrition and confounders), and iv) statistical analysis methods (see Fig. 3 for description of each category and the corresponding studies). This descriptive approach to risk of bias assessment provides details on the direction and magnitude of bias across the different methodological domains relevant to the study design and conduct. This approach has been used in other systematic reviews [45], [46] and follows the recommendation of the Cochrane collaboration [47], which advises against the use of summary scores and quality scales.
Fig. 3.
Risk of bias checklist and rating adapted from STROBE guidelines for reporting observational epidemiological studies and modified AHRQ quality assessment criteria for observational studies. AHRQ: agency for healthcare research and quality, STROBE: strengthening the reporting of observational studies in epidemiology.
Data synthesis and analysis
All included studies were synthesised in a narrative form, organised by outcome and presented under three subsections: i) change in sleep and risk of developing pain condition, ii) change in sleep and inflammatory or immune function biomarkers, and iii) change in sleep and self-reported pain-related health status. The primary aim of the systematic review was a narrative review but we were able to pool together a subset of the studies reporting the physical component summary (PCS) score from the short form health survey-36 (SF-36) as a measure of pain-related health status for an exploratory meta-analysis. The SF-36 [48] is a validated measure of health-related quality of life and gives two summary scores: mental component summary (MCS) and physical component summary (PCS). For this review, we were interested in the PCS score which provided a composite score that is a combination of four of the SF-36 subscales (physical functioning, physical role functioning, bodily pain, and general health). Data were available from the three studies which assessed PCS as an outcome measure [40], [41], [49] and these were included as part of the exploratory meta-analysis to compare differences between effects of sleep change over time on self-reported physical health status.
For the meta-analysis, means and standard deviations of relevant outcome (PCS scores) were extracted for the individuals with no change or with change in sleep over the follow-up period. Change in sleep was a change in the reporting of sleep status at follow-up compared to the reported sleep status at baseline. Standardised mean differences (SMD) between those with change and those with no change in sleep were estimated using a random effect model. A similar method was used for an additional analysis comparing the PCS scores between persistent poor sleepers (i.e., no change in poor sleep between two time points) and persistent good sleepers (i.e., no change in good sleep between two time points). Statistical heterogeneity among the studies was assessed using the I2 statistic along with visual inspection of the forest plot. Sensitivity analysis was carried out when a meta-analysis showed significant heterogeneity (I2 >50%) and involved omitting one study at a time to reveal the potential source of heterogeneity. All statistical analysis was performed using RevMan 5 [50].
Results
Demographic characteristics of included studies
The 16 prospective cohort studies included involved a total of 61,100 participants (female %: 50–100%; mean age: 30–80+ y) from 10 different countries (USA = 6, UK = 2, Israel, Sweden, Japan, Canada, Hong Kong, Finland, Korea and Singapore) and recruited from the community. The length across these longitudinal studies ranged from 1 mo to 23 y, with a median follow-up period of 4.5 y.
Measures of sleep changes
Most studies assessed sleep twice, once at baseline and once at follow-up, except for four studies [38], [41], [51], [52] that assessed sleep at three time points. Sleep was primarily assessed using self-report. Five of the studies [41], [42], [53], [54], [55] assessed self-reported insomnia symptoms (difficulty falling asleep, difficulty maintaining sleep, early morning awakenings, symptoms of impaired daytime functioning, concern about not getting enough sleep and daytime sleepiness) and other similar general indicators of non-restorative sleep. Parthasarathy et al. [56] assessed these insomnia symptoms based on definitions derived from the International classification of sleep disorders insomnia diagnostic criteria. Agmon and Armon [51] used a validated questionnaire (Athens insomnia scale) to assess insomnia symptoms. Three studies used validated questionnaires, e.g., the Pittsburgh sleep quality index (PSQI) [37], [49] and the Jenkins sleep questionnaire [52], to index sleep quality. Four studies [36], [38], [39], [57] assessed changes in sleep quantity by asking self-reported sleep duration at baseline and follow-up. One study [58] assessed sleep using both self-reported sleep quality and sleep quantity. Only one study [40] used both self-report and overnight polysomnography (PSG) to assess sleep. However, in this study, PSG was only used to index overnight respiratory disturbance and no other objective sleep parameters were reported.
Measures of pain-related health outcomes
Seven studies [42], [51], [53], [54], [55], [57], [58] focused on looking at change in sleep and risk of developing a pain-related health condition (namely; arthritis, back pain, general chronic pain, hip fractures) by means of self-report or using information from physician medical interviews, medical records, and linked national databases. Four studies [36], [38], [39], [56] assessed changes in sleep in relation to physiological health status. This included assessments of inflammatory and immune system biomarkers. There was no restriction placed on the diversity of biomarkers, as long as they were immune or inflammatory biomarkers with established connection to pain conditions [59]. Three studies [40], [41], [49] assessed the effect of changes in sleep on self-reported pain-related health status, mostly using the PCS and bodily pain scores derived from the SF-36. One study [52] used a general health assessment questionnaire to determine pain presence and pain interference. Only one study [37] assessed changes in both self-reported physical pain symptoms and immune biomarkers.
Risk of bias assessment results
The results of the risk of bias assessment are graphically presented in Fig. 3. Most of the reviewed studies were “low/medium risk” except for one [53], which was categorised as “high risk” in three out of the four risk categories. The main issues affecting the quality of the included studies were a heavy reliance on self-report and a lack of objective sleep/pain-related health outcome measures (i.e., polysomnography-determined sleep and quantitative sensory testing). In addition, some studies provided insufficient details on attrition, resulting in a lack of comparison with non-responding participants, which could affect the generalisability of association between variables and bias the interpretation of the results. Finally, other methodological issues included studies with small sample sizes (e.g., less than 50 in one study) and short follow-up period (i.e., 1 mo) for a longitudinal design. Small sample size in itself may not be an issue when combined with greater numbers of follow-up assessments as this would increase statistical power. However, statistical power to detect significant association will be limited in the case of both a small sample size and limited follow-up assessments.
Association of change in sleep with the risk of developing a pain condition
Increase in insomnia symptoms
The reviewed studies conveyed the relationship between a negative change in insomnia symptoms and risk of developing a pain condition in those with no pain condition at baseline. Foley et al. [53] reported that newly developed insomnia symptoms over a three-year period doubled the risk of the presence of self-reported hip fracture problems at follow-up (OR = 2.08 95% CI 1.18, 3.65). Zhang et al. [42] also reported that incidence of insomnia symptoms and non-restorative sleep was associated with over a two-fold increase in risk of reporting a chronic pain disorder at five-year follow-up (OR = 2.47 95% CI 1.30–4.69). Agmon and Armon [51] showed that increase in insomnia symptoms was associated with a 40% increased risk of new back pain diagnosis over a period of over 3 y (OR = 1.40 94% CI 1.10–1.71). With a much longer follow-up period of 23 y, Ropponen et al. [58] reported an association of persistent poor sleep (HR = 1.84 95% CI 1.01–3.37) with an 84% increased risk of being included on the national register for disability pension due to low back pain diagnosis at follow-up. However, reduction in sleep quality (HR = 1.17, 95% CI 0.77–1.77) was not significantly associated with an increased risk. Janson et al. [54] also found that an increase in insomnia symptoms over a ten-year period was associated with newly reported medical conditions including joint and low back pain disorder at follow-up, although the risk ratios were not specified in the report. In contrast to the other studies, Quan et al. [55] did not find a link between the development of insomnia symptoms over a four-year period and the presence of pain conditions at follow-up.
Decrease in insomnia symptoms
Notably, only Zhang et al. [42] reported the effect of a positive change in insomnia symptoms. They reported an association of remission of insomnia symptoms with a 23% lowered risk of developing a chronic pain condition at follow-up, but this association was not significant (OR = 1.23, 95% CI 0.57–2.59).
Increase in sleep quantity
Ropponen et al. [58] did not find an association between increased sleep quantity and risk of low back pain diagnosis over a 23-y follow-up period. Smagula et al. [57] similarly did not find a link between an increase in sleep quantity and developing arthritis at ten-year follow-up. However, these authors did report that an increase in nightly sleep quantity to >8h was associated with a 52% greater risk of hip fracture problems as registered on a national hospital database (OR = 1.52 95% CI 1.16–2.00). The causal order of the relationship was unclear; the authors suggested that recent hip fractures and consequent low physical activity might be key determinants of the lengthened sleep duration.
Summary – change in sleep and risk of developing a pain condition
In the studies reviewed, reporting a negative change in insomnia symptoms was associated with a greater risk of developing and reporting a pain-related medical condition. On the other hand, a remission or a positive change in insomnia symptoms did not necessarily neutralise or fully avert the risk of developing chronic pain. Moreover, an increase in sleep quantity might not be associated with a positive pain-related health outcome.
Association of change in sleep with inflammatory or immune biomarkers
Increase in sleep quantity and natural killer cells activities (NKCA)
Shakhar et al. and Irish et al. [37], [39] looked at the association between changes in sleep quantity and natural killer cells activities (NKCA). Natural killer cells play a physiologically protective role in activating immune responses to contain and clear viral infections. Low levels are linked to certain pain conditions such as fibromyalgia, and possibly contributing to exaggerated pain response in some individuals with chronic pain [59], [60]. Shakhar et al. [39] using a small sample (n = 45) with a short (1 mo) follow-up found that an increase in sleep quantity was associated with an increase in NKCA levels. On the other hand, Irish et al. [37] did not find a relationship between either an increase or decrease in sleep quantity and quality over 1-y with changes in natural killer cell levels. The analysis was carried out in a small subset (n = 51) of their sample and the standard deviation for mean NK cell number was quite large (Mean = 285.21, SD = 204.53).
Decrease in sleep quantity and cortisol levels
Cortisol is the body's primary stress hormone needed to activate the physiological ‘flight or fight’ response; however, high cortisol levels interfere with immune functions and are a risk factor for many illnesses [61], [62]. Chronic pain states have been linked to sustained stress response and consequently higher cortisol levels [63]. Rueggeberg et al. [38] found that a decrease in sleep quantity (by one standard deviation) over the first two years of their study was associated with an increase in diurnal cortisol secretion over the total four-year follow-up period. By contrast, an increase in sleep quantity was not significantly associated with cortisol levels.
Decrease in sleep quantity and interleukin-6 (IL-6) and C-reactive protein (CRP) levels
IL-6 and CRP are markers of systemic inflammation, activated to combat infections [64]. They also possess a pain facilitatory effect and can alter pain modulation and pain processing [32], [65], ∗[66]. Persistent elevated presence of these markers has been observed in several chronic illnesses and implicated in the generation, maintenance, and severity of chronic pain conditions [59], [67]. Ferrie et al. [36], analysing data from the UK Whitehall study, did not find an association between increases in sleep quantity and changes in levels of IL-6 and CRP. However, they revealed that a five-year decrease in sleep quantity was associated with higher levels of inflammatory markers. In the fully-adjusted analysis controlling for age, sex, occupation, blood pressure, BMI, cholesterol level and presence of diabetes, each 1-h decrease in sleep quantity was associated with a 2.7% higher level of IL-6. The reduction in sleep quantity was also associated with a 4.2% higher level of CRP, but this association was not statistically significant.
Summary – change in sleep and inflammatory or immune biomarkers
It appeared that a reduction in sleep quantity is temporally linked to elevated levels of inflammatory and immunological markers. However, a reverse association was not noted for an increase in sleep quantity. Only one study examined the effect of changes in insomnia symptoms and sleep quality on inflammatory and immunological markers indicative of pain. Parthasarathy et al. [56] found that CRP levels were higher and increased at a greater rate in those with persistent insomnia compared with those with intermittent or no insomnia. It is important to note that the biomarkers reviewed were not pain-specific. We cannot rule out other disease processes involving inflammation that might have affected the relationship of sleep changes with deterioration in health status.
Association of change in sleep with pain-related health status
Three of the reviewed studies [40], [41], [49] assessed perceived physical health status using the PCS from the SF-36. PCS is a summary of the SF-36 subscales that assess physical functioning, physical role functioning, bodily pain and general health [48]. Lower scores on the PCS and the bodily pain subscale indicate greater physical health limitations and pain-related interference and disability. All three studies reviewed reported the PCS scores, but only the Silva et al. [40] study provided the subcomponent bodily pain score separately.
Increase in insomnia symptoms
All three studies revealed an association of lowered PCS scores over time with an increase in or maintenance of insomnia symptoms. Silva et al. [40] found that those who developed insomnia symptoms or whose insomnia symptoms persisted over a five-year follow-up period reported a decline in PCS scores and lower PCS scores at follow-up, compared with persistent good sleepers with no change in insomnia symptoms. Komada et al. [49] used PSQI scores to assess those with newly developed insomnia symptoms over a two-year follow-up period. These individuals who reported increased insomnia symptoms over time reported a decline in PCS scores from baseline to follow-up and worse PCS scores at follow-up compared with persistent good sleepers. Suh et al. [41] also reported a similar decrease in PCS score over a 2-y follow-up period for those with worsening and persistent insomnia symptoms.
Two studies used other forms of pain health assessment such as self-reported pain symptoms. Irish et al. [37] did not find a one-year increase or decrease in PSQI to be related to physical pain symptoms. In their sample of rescue workers who performed rescue and clean-up operations at the site of a major aeroplane crash, the findings might have been affected by life stressors and other symptoms of psychological distress following the crash. However, over 80% of the sample reported ‘good’ or ‘excellent’ physical health and had stable PSQI scores over the year. Campbell et al. [52] on the other hand found that new onset of sleep problems and insomnia symptoms in a chronic pain sample over three years significantly increased the risk of depression (relative risk 3.47) at six-year follow-up. Importantly, this risk was mediated by increased pain interference measured at three-year follow-up and the findings revealed a significant association between increased insomnia symptoms and increased pain interference.
Summary – change in sleep and pain-related health status
Compared to no change in good sleep, an increase in insomnia symptoms was both a predictor and an indicator of worse pain outcomes and physical functioning status over time.
Sleep change trajectories and PCS scores
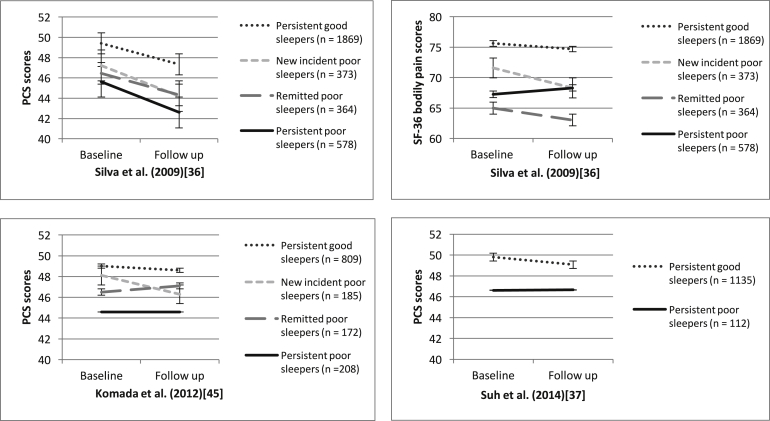
To further explore the observed trend of changes in sleep and physical functioning, we quantitatively and visually compared changes (Fig. 4) in PCS from baseline to follow-up by sleep change trajectories. Namely, the four trajectories are i) persistent good sleepers (no sleep disturbance at baseline and follow-up), ii) new incident poor sleepers (developed sleep disturbance from baseline to follow-up), iii) remitted poor sleeper (sleep disturbance resolved from baseline to follow-up), and iv) persistent poor sleepers (sleep disturbance at baseline and follow-up).
Fig. 4.
Comparison of PCS and SF-36 bodily pain scores from baseline to follow-up for different sleep change trajectories (Lower scores on the PCS and bodily pain subsection indicates greater physical health limitations and pain related interference and disability).
Across the three studies with this kind of data [40], [41], [49], persistent good sleepers fared the best and reported the highest PCS scores at both baseline and follow-up compared with the other trajectories. They also showed the greatest stability in PCS scores across both time points. New incident poor sleepers showed a decline in PCS scores. Whilst the PCS scores for remitted poor sleepers also showed fluctuations, the effect was not consistent across studies and the direction of the effect was unclear. Persistent poor sleepers fared the worst; they presented with the lowest PCS scores at both baseline and follow-up.
We could not extract subcomponent scores for bodily pain for all three studies, but using the data available from Silva et al. [40], we were able to compare the different sleep change trajectories using the bodily pain subscale of the PCS for this particular study. We noted that remitted poor sleepers had the highest bodily pain score at baseline and this worsened at follow-up. However, the other patterns observed for the bodily pain subscale were similar to overall PCS scores; persistent good sleepers fared the best whereas new incident poor sleepers showed an increase in pain scores over time.
Exploratory meta-analysis
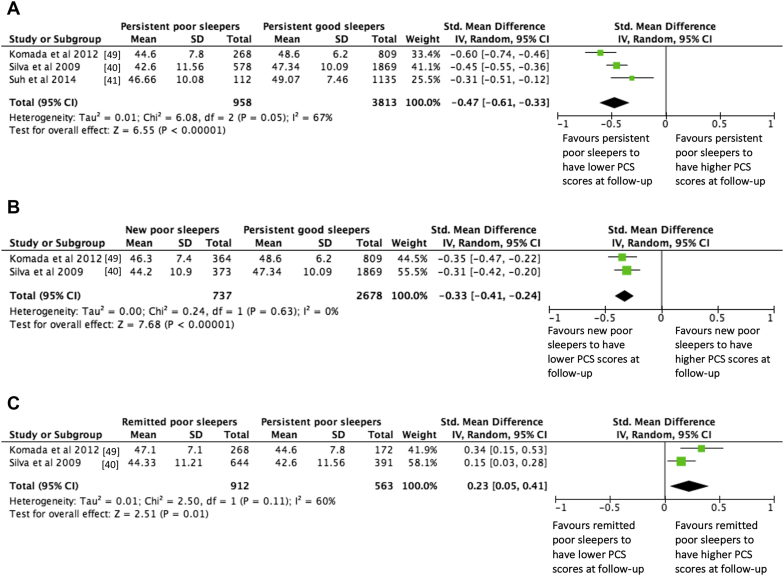
We carried out three exploratory meta-analyses to compare PCS score at follow-up to examine how different trajectories of sleep changes affected physical health. We used available data from the three studies [40], [41], [49] using PCS as an outcome measure. The total number of participants across the three studies was 5902 (female: 57.1%, mean age: 60 y) and the follow-up periods were 5 y and 2 y. We used an analytic approach that allowed us to compare the PCS scores at follow-up between persistence of poor sleep and good sleep, and then examine the separate effect of sleep deterioration and sleep improvement on PCS scores. Statistics of these analyses are summarised with forest plots in Fig. 5a–c.
Fig. 5.
Forest plots summarising the effects of changes in sleep on PCS scores at follow-up. Lower PCS scores represent poorer physical functioning. a) Compares individuals who were ‘persistent poor sleepers’ with those who were ‘persistent good sleepers’ over time. b) Compares individuals who developed sleep disturbances over time (‘new poor sleepers’) with those who were ‘persistent good sleepers’ (i.e. evaluating the effect of negative sleep deterioration). c) Compares individuals whose sleep disturbances remitted over time (‘remitted poor sleepers’) with those who were ‘persistent poor sleepers’ (i.e. evaluating the effect of positive sleep improvement).
Meta-analysis 1: Persistence of poor sleep and PCS scores
In comparing persistent poor sleep with persistent good sleep (Fig. 5a), persistent poor sleep was significantly associated with lower PCS scores at follow-up. This analysis showed significant heterogeneity (I2 = 67%). Sensitivity analysis identified Komada et al. [49] as the potential source, possibly due to the use of a Japanese version of the PSQI compared to individual questions for assessing insomnia symptoms as used in the other studies. Omitting this study reduced I2 from 67% to 39% and decreased the effect from −0.47 to −0.41 (95% CI −0.54, −0.25) Z = 6.23, p < 0.00001. This standardised mean difference indicates a medium effect size and that approximately 66% of those with persistent poor sleep had worse PCS scores at follow-up than those with persistent good sleep.
Meta-analysis 2: Sleep deterioration and PCS scores
New poor sleepers were compared with persistent good sleepers to assess the effect of sleep deterioration over time (Fig. 5b). Developing insomnia symptoms was associated with significantly lower PCS scores at follow-up compared with persistent good sleep. There was no significant heterogeneity across the studies. A standardised mean difference of −0.33 (95% CI −0.41, −0.24) Z = 7.68, p < 0.00001 is a medium effect size indicating that approximately 62% of those who developed sleep problems from baseline to follow-up had worse PCS scored at follow-up than those with persistent good sleep.
Meta-analysis 3: Sleep improvement and PCS scores
Finally, remitted poor sleepers were compared with persistent poor sleepers to assess the effect of sleep improvement over time (Fig. 5c). Remission of insomnia symptoms was significantly associated with higher PCS scores at follow-up compared with persistent poor sleepers whose sleep problems showed no improvement. However, the analysis did show heterogeneity across the two studies (I2 = 60%). Given that only two studies were included in the analysis, we were unable to conduct a sensitivity analysis to identify the source of heterogeneity. The standardised mean difference effect of 0.23 (95% CI 0.05, 0.41) Z = 2.51, p < 0.01, which if heterogeneity was not an issue, suggests that approximately 58% of those with improvement in sleep from baseline to follow-up had better PCS scores at follow-up than those with persistent sleep problems.
Summary – exploratory meta-analyses
Findings from the exploratory meta-analyses suggest that incidence and persistence of sleep problems may contribute to worse physical health over time. Remission of sleep disturbances was associated with better outcome but the effect was weak. PCS scores usually have high test-retest reliability in the general population, yet, we see a drop in PCS scores in those with persistent sleep problems and those who newly developed sleep problems. Such drop in PCS scores could be interpreted as signifying “some more” physical limitation among these individuals [48]. Moreover, the PCS scores of these individuals at follow-up were comparable to levels of PCS scores observed in population groups with minor medical conditions or serious physical illnesses [48], [68]. Together, these findings suggest a detrimental effect of deterioration in sleep quality and maintenance of sleep problems in contrast to persistent good sleep.
Discussion
Summary of findings
Findings from this systematic review and meta-analysis indicate that sleep deterioration has a negative effect on pain-related health outcomes. There was, however, insufficient evidence to suggest a clear positive effect of sleep improvement on pain. Overall, the findings extend previous evidence highlighting poor sleep at baseline as a risk factor for developing a future pain condition ∗[24], [25], [69]. The review further consolidates evidence that changes in sleep are prospectively associated with the experience of pain, adding weight to the argument for a causal association.
Disentangling the effect of different sleep parameters
Sleep is a multidimensional construct and research has suggested that sleep quality and other aspects of sleep behaviours (e.g., use of sleep medications) may be more strongly associated with future health and well-being and should be considered alongside sleep quantity [70], [71]. Notably, it emerged from this review that changes in sleep quality but not sleep quantity were associated with the risk of developing a pain condition and worse self-reported health outcomes. Whereas, changes in sleep quantity were mostly reported to be contributing to altered levels of pain-related biomarkers. These differing patterns of association reflect potential specificity in the roles of sleep quality and quantity on pain.
That said, we note that there were considerable variations across studies in the way changes in insomnia symptoms, sleep quality and quantity were measured. Some studies reported changes in insomnia symptoms such as difficulty in initiating and maintaining sleep, some assessed only reports of non-restorative sleep, and others used questionnaires to assess sleep quality. For sleep quantity, some used predetermined sleep duration categories, e.g., short (<7 h), average (7–8 h), and long (>8 h), whilst others gathered single-item responses on average nightly sleep duration. In addition, these measures also vary in assessment of severity and chronicity of sleep problems, with studies assessing different sleep problems (e.g., mild vs. severe symptoms) across different time frames (e.g., currently, past month, past year, or in a lifetime). This further limits generalisation and meaningful comparisons.
Distinctions should also be made between insomnia symptoms and general dissatisfaction with sleep quality and quantity, as changes in insomnia symptoms and other sleep disturbance parameters may have differential effects on different health outcomes [11]. Cross-sectional studies have provided some evidence to support this; Yokohama et al. [72] compared three sub-symptoms of insomnia – difficulty initiating sleep, early morning awakenings and difficulty maintaining sleep, and they found that difficulty initiating sleep was more strongly associated with depression than the other insomnia symptoms. Consequently, future studies exploring the association of sleep changes should consider not only assessing different parameters of sleep disturbances but also using standardised measures. Similar recommendations for assessing core outcome measures have been made for trials of chronic pain treatment efficacy and effectiveness. The initiative on methods, measurement, and pain assessment in clinical trials (IMMPACT) proposed and encouraged the use of key standardised measures for assessing and reporting changes in pain intensity, physical functioning, emotional functioning and improvement and satisfaction with treatment [73]. Whilst it is appreciated that aspects of such proposal may be more applicable to clinical trials than longitudinal studies, the IMMPACT recommendations can be useful as an illustrative guideline on how core sleep outcomes could be measured, defined, and reported for improving comparability and interpretability across studies.
Mechanisms underlying the interaction between sleep and pain-related biomarkers
The current review shows that a negative change in sleep quantity may be a key predictor of elevated pro-inflammatory markers and deterioration of pain-related physical health over time. There is also some evidence suggesting that a negative change in insomnia symptoms is associated with raised inflammatory levels. These findings are consistent with longitudinal studies that have reported baseline sleep quality as a predictor for pathogenic levels of inflammatory markers at follow-up [74], [75]. That said, the observed effect on these biomarkers may not be pain-specific but reflecting a decline in physiological health status in general. Future development of a more comprehensive biopsychosocial model linking sleep quality, pain, and inflammation would enable more rigorous examination of the underpinning physiological mechanisms [76]. It is thought that the effects of sleep problems on pain responses are mediated by impaired immunity, elevated inflammatory responses and raised cytokines levels such as those assessed by studies considered in this review, namely IL-6, CRP, and cortisol [30], [31]. However, their meditational roles are yet to be verified in the general population whereby sleep disruptions and pain symptoms are assessed in a more naturalist way with ecological validity.
Clarifying the effect of sleep improvement on pain outcomes
Findings from this review suggest that deterioration in sleep and persistent poor sleep are key risk factors of poor health. However, the findings do not provide sufficient evidence that an improvement in sleep quality or an increase in sleep quantity has a protective function of mitigating disease risk, as many clinicians and researchers would assume. The meta-analysis revealed that the development of sleep problems over time has a negative effect on self-reported physical health [48], [68]. The meta-analysis also showed that remission of sleep problems over time was associated with higher PCS scores at follow-up, but the effect size was small and was only significant when compared with those who reported persistent poor sleep. However, the amount of evidence available for the current review was limited due to the small number of studies examining positive sleep changes over time outside of the context of a clinical trial.
There is some evidence emerging to suggest that naturally occurring good sleep is a potential predictor of chronic pain remission in the general population. Aili et al. [77] reported in their prospective analysis that out of 883 participants from the general population, for the 53 individuals who reported multi-site pain at baseline but not at follow-up, a lack of or minimal report of sleep disturbance at baseline was a significant predictor of the ‘resolution’ of their multi-site pain (adjusted OR 3.96 95% CI 1.69–9.31), after controlling for age, gender, smoking, BMI, physical occupational risks, and psychosocial activities. However, the small size of the group limits the statistical power, risk estimation, and generalisability of these findings. Davies et al. [78] similarly showed that self-reported restorative sleep at baseline was a predictive factor for the ‘resolution’ of chronic widespread pain (adjusted OR 2.0 95% CI 1.02–3.8) in 300 of the 679 participants presenting with pain at baseline but not at follow-up. However, the participants in this study who reported resolved widespread pain at follow-up still reported some regional pain. As such, the impact of restorative sleep on pain experience may have been overstated. What these studies are not able to say is whether resolution in pain are preceded by positive changes in sleep and to what extent improvement in pain is sustained. The current review has highlighted that there is room for further longitudinal studies with longer follow-up, to strengthen the evidence for the impact of sleep improvement on long-term pain outcomes.
Methodological considerations and recommendations
Although the included studies were mostly of low and medium risk of bias, there were some recurrent methodological issues that could affect the rigour and generalisability of the findings and conclusions drawn. Based on the findings of the risk of bias assessment in the current review, future studies could improve methodological rigour by clarifying the rate of participation and attrition and by ensuring sufficient statistical power for detecting significant (and meaningful) results over a sufficiently long follow-up period. Two further specific recommendations are offered below:
-
I.
Improving research designs to substantiate the impact of changes in sleep on pain outcomes
One of the main methodological drawbacks noted in the included studies was a reliance on self-report and a lack of objective sleep and quantitative pain outcome measures. Objective sleep assessments beyond self-report are less vulnerable to reporting biases. It is important for future prospective studies to strive to include assessment of both self-reported and objective changes in sleep using polysomnography or actigraphy. This can then be used in combination with quantitative sensory testing that assesses normal and abnormal psychophysical pain responses and physiological pain sensitivity. Whilst these methods would increase research costs, they are often utilised in experimental studies and could have an equally important role to play in sleep epidemiology research that combines experimental laboratory studies with longitudinal follow-up assessments. This would provide clarity in our understanding of the physiological factors underlying sleep and pain disturbances.
In addition, the evidence from the meta-analysis was also restricted to a broad evaluation of general physical health and well-being scores derived from the SF-36. For example, only one of the included studies [40] provided data specifically on PCS bodily pain subcomponent scores over time, whereas none of the other studies with PCS scores as an outcome measure allowed for this level of detail for comparison. Future studies should also consider using other pain measures to better demonstrate the long-term temporal relationship between sleep and pain intensity. Additional repeated ratings of pain [79] can mark the trajectory of pain intensity over time and clarify the influence of pain intensity, rather than pain interference, on health outcomes.
-
II.
Improving longitudinal assessments of sleep and pain
Most of the reviewed studies have just two assessment points. Two observations are the bare minimum needed to provide information about change over time, but this information is usually insufficient for a thorough understanding of the processes responsible for these changes. If our criteria only allowed for the inclusion of studies with more than two assessment time-points, only four studies would have met this requirement. This highlights the need for additional assessments for investigating the temporal relationship between changes in sleep and pain outcomes and for revealing the trajectories of health status over time within groups of individuals with different patterns of sleep. Cross-lagged analysis could be applied to these multi-wave data to establish directions of causality [80].
Finally, the findings from these longitudinal studies were mostly drawn from analyses at the general population level to maximise generalisability. Future studies may benefit from incorporating subgroup analyses to dissect the sleep and pain relationship, for example, through stratification by age, by gender, by those with malignant and non-malignant chronic pain conditions, and by those with chronic pain but no sleep problems. This would help reveal the context in which a change in sleep is a contributing factor to the development, perpetuation, or alleviation of these conditions. It would also provide new insights into the potential of sleep as an amenable treatment target in the management of these conditions across different spectra of the population.
Limitations of the review
Some limitations in the present review and meta-analysis should be acknowledged. First, the number of studies included in the review and the meta-analysis was limited due to a lack of access to required data and the stringent inclusion criteria. The results of the meta-analysis should thus be considered as exploratory. That said, the stringent inclusion criteria were necessary to capture only studies with an appropriate longitudinal design that addresses and analyses the effect of change in sleep on pain-related outcomes. Second, the high level of heterogeneity observed in the analysis was possibly due to variations in research methodologies, but the small number of studies eligible for meta-analysis has made it impossible to pin down the source of heterogeneity at this stage. Also, not all studies assessed sleep in the same way despite having similar outcome measures and there was consequently no consistent definition of what denotes sleep stability, sleep deterioration and sleep improvements.
Finally, as inherent in most systematic reviews, there is a risk of publication bias although we found no obvious evidence of publication bias. The studies reviewed were limited to texts in English even though studies included in the review involved cities/countries that are not English-speaking. There appeared to be no indication that cultural differences in pain reports distort the sleep-pain association being examined. Nevertheless, future studies should consider the possible influence of culture on the perception of sleep and pain.
Conclusion
The current evidence provides moderate support that negative changes in sleep have detrimental health effects and that consistently good sleep favours better pain-related health outcomes. Although there is emerging evidence for the relationship between changes in sleep status and pain-related health outcomes, full understanding of the mechanisms underlying the causal relationship between sleep and pain remains incomplete. In this review, improvements in sleep quantity and sleep quality were not consistently associated with better health outcomes. The jury is out regarding whether positive changes in sleep could lead to a reduction of, or even full recovery from, pain symptoms.
Practice points.
-
1)This systematic review of longitudinal studies illuminates the prospective associations between i) changes in sleep and subsequent risk of developing pain conditions, and ii) changes in sleep and subsequent self-report of pain-related health status. There is also some preliminary evidence for iii) changes in sleep and subsequent inflammatory or immune function biomarkers.
-
•A general decline in sleep quality and sleep quantity was associated with a greater risk of developing a pain condition, small elevations of inflammatory markers, and a decline in self-reported physical health status.
-
•
-
2)An exploratory meta-analysis was carried out to quantitatively estimate the magnitude of the effect of sleep changes on self-reported pain-related physical health status.
-
•New incidence and persistence of sleep problems may contribute to worse perceived physical functioning over time (medium effect size). Compared with consistently good sleepers, those with new and persistent sleep problems reported ‘some more’ physical limitation than usual as indicated by a decrease in PCS scores.
-
•Remission of sleep disturbances was associated with better physical functioning (small effect size). Compared with persistent poor sleepers, those whose sleep improved had ‘some less’ physical limitation than usual as indicated by an increase in PCS scores.
-
•
-
3)
Integrated management and treatment of chronic pain and insomnia may lead to better patient outcomes and improvements not only in sleep quality and psychological health status but also pain-related symptoms.
Research agenda.
-
1)
Experimental and longitudinal studies are needed to verify the specific causal links between sleep, inflammatory processes, and the experience of pain.
-
2)
Longitudinal studies with more than two follow-up assessments and cross-lagged analysis are needed to substantiate the temporal relationship between changes in sleep and pain outcomes and to provide a framework to examine the trajectories of health status over time across individuals with different patterns of sleep changes.
-
3)
It would be desirable to further investigate the effectiveness of interventions, e.g., cognitive behavioural therapies, exercise programmes, medications, as possible tools to enhance pain-related health outcomes and quality of life via promoting sleep. This can serve as further tests of the causal association between sleep, pain, and wellbeing.
-
4)
Subgroup analyses by phenotype (e.g., those in the general population with chronic pain conditions, with or without sleep problems) would help isolate factors linked to the development, perpetuation, or alleviation of pain experience. Knowledge of how sleep influences an individual's pain experience may promote personalised integrated interventions and management by sleep phenotype.
Conflicts of interest
The authors do not have any conflicts of interest to declare.
Funding
NT's research is supported by the National Institute for Health Research, UK (Grant reference number: PB-PG-0213-30121). This paper presents independent research funded by the NIHR under its Research for Patient Benefit (RfPB) Programme. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Acknowledgments
The authors thank Sarina Afzalishamsabad, Natasha Kwok, and Preeya Chibbra for their assistance with screening and eligibility assessment.
Footnotes
The most important references are denoted by an asterisk.
Contributor Information
Esther F. Afolalu, Email: e.f.afolalu@warwick.ac.uk.
Nicole K.Y. Tang, Email: n.tang@warwick.ac.uk.
References∗
- 1.Cappuccio F.P., Cooper D., D'Elia L., Strazzullo P., Miller M.A. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32(12):1484–1492. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- 2.Leng Y., Cappuccio F.P., Wainwright N.W.J., Surtees P.G., Luben R., Brayne C. Sleep duration and risk of fatal and nonfatal stroke: a prospective study and meta-analysis. Neurology. 2015;84(11):1072–1079. doi: 10.1212/WNL.0000000000001371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riemann D., Voderholzer U. Primary insomnia: a risk factor to develop depression? J Affect Disord. 2003;76(1–3):255–259. doi: 10.1016/s0165-0327(02)00072-1. [DOI] [PubMed] [Google Scholar]
- 4.Bernert R.A., Turvey C.L., Conwell Y., Joiner T.E., Jr. Association of poor subjective sleep quality with risk for death by suicide during a 10-year period: a longitudinal, population-based study of late life. JAMA Psychiatry. 2014;71(10):1129–1137. doi: 10.1001/jamapsychiatry.2014.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merskey H., Bogduk N. IASP Press; Seattle: 1994. Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain states. [Google Scholar]
- 6.Treede R.D., Rief W., Barke A., Aziz Q., Bennett M.I., Benoliel R. A classification of chronic pain for icd-11. Pain. 2015;156(6):1003–1007. doi: 10.1097/j.pain.0000000000000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tracey I., Bushnell M.C. How neuroimaging studies have challenged us to rethink: is chronic pain a disease? J Pain. 2009;10(11):1113–1120. doi: 10.1016/j.jpain.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Siddall P.J., Cousins M.J. Persistent pain as a disease entity: implications for clinical management. Anesth Analg. 2004;99(2):510–520. doi: 10.1213/01.ANE.0000133383.17666.3A. [DOI] [PubMed] [Google Scholar]
- 9.Breivik H., Collett B., Ventafidda V., Cohen R., Gallacher D. Survey of chronic pain in europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10(4):287–333. doi: 10.1016/j.ejpain.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Fernandez A., Saameno J.A., Pinto-Meza A., Luciano J.V., Autonell J., Palao D. Burden of chronic physical conditions and mental disorders in primary care. Br J Psychiatry. 2010;196(4):302–309. doi: 10.1192/bjp.bp.109.074211. [DOI] [PubMed] [Google Scholar]
- Ohayon M.M. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6(2):97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 12.Roth T. Insomnia: definition, prevalence, etiology, and consequences. J Clin Sleep Med. 2007;3(Suppl. 5):S7–S10. [PMC free article] [PubMed] [Google Scholar]
- 13.LeBlanc M., Merette C., Savard J., Ivers H., Baillargeon L., Morin C.M. Incidence and risk factors of insomnia in a population-based sample. Sleep. 2009;32(8):1027–1037. doi: 10.1093/sleep/32.8.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldberg D.S., McGee S.J. Pain as a global public health priority. BMC Public Health. 2011;11(1):770. doi: 10.1186/1471-2458-11-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simon G.E., Von Korff M. Prevalence, burden, and treatment of insomnia in primary care. Am J Psychiatry. 1997;154(10):1417–1423. doi: 10.1176/ajp.154.10.1417. [DOI] [PubMed] [Google Scholar]
- 16.Gureje O., Von Korff M., Simon G.E., Gater R. Persistent pain and well-being. A world health organization study in primary care. JAMA. 1998;280(2):147–151. doi: 10.1001/jama.280.2.147. [DOI] [PubMed] [Google Scholar]
- 17.Tang N.K.Y., Afolalu E.F., Ramlee F. Sleep and pain. In: Cappuccio F.P., Miller M.A., Lockley S.W., editors. Sleep health and society – from aetiology to public health. 2nd ed. Oxford University Press; Oxford: 2018. (in press) [Google Scholar]
- Smith M.T., Haythornthwaite J.A. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev. 2004;8(2):119–132. doi: 10.1016/S1087-0792(03)00044-3. [DOI] [PubMed] [Google Scholar]
- Finan P.H., Goodin B.R., Smith M.T. The association of sleep and pain: an update and a path forward. J Pain. 2013;14(12):1539–1552. doi: 10.1016/j.jpain.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Affleck G., Zautra A., Tennen H., Armeli S. Multilevel daily process designs for consulting and clinical psychology: a preface for the perplexed. J Consult Clin Psychol. 1999;67(5):746–754. doi: 10.1037//0022-006x.67.5.746. [DOI] [PubMed] [Google Scholar]
- 21.Edwards R.R., Almeida D.M., Klick B., Haythornthwaite J.A., Smith M.T. Duration of sleep contributes to next-day pain report in the general population. Pain. 2008;137(1):202–207. doi: 10.1016/j.pain.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N.K.Y., Goodchild C.E., Sanborn A.N., Howard J., Salkovskis P.M. Deciphering the temporal link between pain and sleep in a heterogeneous chronic pain patient sample: a multilevel daily process study. Sleep. 2012;35(5) doi: 10.5665/sleep.1830. 675-87A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mork P.J., Nilsen T.I. Sleep problems and risk of fibromyalgia: longitudinal data on an adult female population in Norway. Arthritis Rheum. 2012;64(1):281–284. doi: 10.1002/art.33346. [DOI] [PubMed] [Google Scholar]
- Gupta A., Silman A.J., Ray D., Morriss R., Dickens C., MacFarlane G.J. The role of psychosocial factors in predicting the onset of chronic widespread pain: results from a prospective population-based study. Rheumatology. 2007;46(4):666–671. doi: 10.1093/rheumatology/kel363. [DOI] [PubMed] [Google Scholar]
- 25.McBeth J., Lacey R.J., Wilkie R. Predictors of new-onset widespread pain in older adults: results from a population-based prospective cohort study in the UK. Arthritis Rheumatol. 2014;66(3):757–767. doi: 10.1002/art.38284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nitter A.K., Pripp A.H., Forseth K.Ø. Are sleep problems and non-specific health complaints risk factors for chronic pain? A prospective population-based study with 17 year follow-up. Scand J Pain. 2012;3(4):210–217. doi: 10.1016/j.sjpain.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Ferrie J.E., Kumari M., Salo P., Singh-Manoux A., Kivimaki M. Sleep epidemiology–a rapidly growing field. Int J Epidemiol. 2011;40(6):1431–1437. doi: 10.1093/ije/dyr203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bradford-Hill A. The environment and disease: association or causation? Proc R Soc Med. 1965;58(5):295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haack M., Lee E., Cohen D.A., Mullington J.M. Activation of the prostaglandin system in response to sleep loss in healthy humans: potential mediator of increased spontaneous pain. Pain. 2009;145(1–2):136–141. doi: 10.1016/j.pain.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vgontzas A.N., Zoumakis E., Bixler E.O., Lin H.M., Follett H., Kales A. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89(5):2119–2126. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- 31.Haack M., Sanchez E., Mullington J.M. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep. 2007;30(9):1145–1152. doi: 10.1093/sleep/30.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Irwin M.R. Inflammation at the intersection of behavior and somatic symptoms. Psychiatr Clin North Am. 2011;34(3):605–620. doi: 10.1016/j.psc.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alvaro P.K., Roberts R.M., Harris J.K. A systematic review assessing bidirectionality between sleep disturbances, anxiety, and depression. Sleep. 2013;36(7):1059–1068. doi: 10.5665/sleep.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lustberg L., Reynolds C.F. Depression and insomnia: questions of cause and effect. Sleep Med Rev. 2000;4(3):253–262. doi: 10.1053/smrv.1999.0075. [DOI] [PubMed] [Google Scholar]
- 35.Altman D.G. Chapman and Hall; London: 1991. Practical statistics for medical research. [Google Scholar]
- 36.Ferrie J.E., Kivimaki M., Akbaraly T.N., Singh-Manoux A., Miller M.A., Gimeno D. Associations between change in sleep duration and inflammation: findings on c-reactive protein and interleukin 6 in the whitehall ii study. Am J Epidemiol. 2013;178(6):956–961. doi: 10.1093/aje/kwt072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Irish L.A., Dougall A.L., Delahanty D.L., Hall M.H. The impact of sleep complaints on physical health and immune outcomes in rescue workers: a 1-year prospective study. Psychosom Med. 2013;75(2):196–201. doi: 10.1097/PSY.0b013e31827d85ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rueggeberg R., Wrosch C., Miller G.E. Sleep duration buffers diurnal cortisol increases in older adulthood. Psychoneuroendocrinology. 2012;37(7):1029–1038. doi: 10.1016/j.psyneuen.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 39.Shakhar K., Valdimarsdottir H.B., Guevarra J.S., Bovbjerg D.H. Sleep, fatigue, and nk cell activity in healthy volunteers: significant relationships revealed by within subject analyses. Brain Behav Immun. 2007;21(2):180–184. doi: 10.1016/j.bbi.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 40.Silva G.E., Ming-Wen A., Goodwin J.L., Shahar E., Redline S., Resnick H. Longitudinal evaluation of sleep-disordered breathing and sleep symptoms with change in quality of life: the sleep heart health study (shhs) Sleep. 2009;32(8):1049–1057. doi: 10.1093/sleep/32.8.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suh S., Yang H.C., Fairholme C.P., Kim H., Manber R., Shin C. Who is at risk for having persistent insomnia symptoms? A longitudinal study in the general population in korea. Sleep Med. 2014;15(2):180–186. doi: 10.1016/j.sleep.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 42.Zhang J., Lam S.P., Li S.X., Li A.M., Wing Y.K. The longitudinal course and impact of non-restorative sleep: a five-year community-based follow-up study. Sleep Med. 2012;13(6):570–576. doi: 10.1016/j.sleep.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 43.Sanderson S., Tatt I.D., Higgins J.P. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. Int J Epidemiol. 2007;36(3):666–676. doi: 10.1093/ije/dym018. [DOI] [PubMed] [Google Scholar]
- 44.Manchikanti L., Singh V., Smith H.S., Hirsch J.A. Evidence-based medicine, systematic reviews, and guidelines in interventional pain management: part 4: observational studies. Pain Physician. 2009;12(1):73–108. [PubMed] [Google Scholar]
- 45.Hertenstein E., Tang N.K.Y., Bernstein C.J., Nissen C., Underwood M.R., Sandhu H.K. Sleep in patients with primary dystonia: a systematic review on the state of research and perspectives. Sleep Med Rev. 2016;26:95–107. doi: 10.1016/j.smrv.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 46.Bernstein C.J., Ellard D.R., Davies G., Hertenstein E., Tang N.K.Y., Underwood M. Behavioural interventions for people living with adult-onset primary dystonia: a systematic review. BMC Neurol. 2016;16:40. doi: 10.1186/s12883-016-0562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Higgins J.P.T., Green S. 2011. Cochrane handbook for systematic reviews of interventions version 5.1.0: the Cochrane Collaboration.http://handbook.cochrane.org/ Available from: [Google Scholar]
- 48.Ware J.E. The Health Institute, New England Medical Center; Boston: 1993. Sf-36 health survey. Manual & interpretation guide. [Google Scholar]
- 49.Komada Y., Nomura T., Kusumi M., Nakashima K., Okajima I., Sasai T. A two-year follow-up study on the symptoms of sleep disturbances/insomnia and their effects on daytime functioning. Sleep Med. 2012;13(9):1115–1121. doi: 10.1016/j.sleep.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 50.The Cochrane Collaboration. Review manager (Rev man). 5.3 ed. Copenhagen: The Nordic Cochrane centre.
- 51.Agmon M., Armon G. Increased insomnia symptoms predict the onset of back pain among employed adults. PLoS One. 2014;9(8) doi: 10.1371/journal.pone.0103591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Campbell P., Tang N.K.Y., McBeth J., Lewis M., Main C.J., Croft P.R. The role of sleep problems in the development of depression in those with persistent pain: a prospective cohort study. Sleep. 2013;36(11):1693–1698. doi: 10.5665/sleep.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Foley D.J., Monjan A., Simonsick E.M., Wallace R.B., Blazer D.G. Incidence and remission of insomnia among elderly adults: an epidemiologic study of 6,800 persons over three years. Sleep. 1999;22(Suppl. 2):S366–S372. [PubMed] [Google Scholar]
- 54.Janson C., Lindberg E., Gislason T., Elmasry A., Borman G. Insomnia in men—a 10-year prospective population based study. Sleep. 2001;24(4):425–430. doi: 10.1093/sleep/24.4.425. [DOI] [PubMed] [Google Scholar]
- 55.Quan S.F., Katz N.P., Olson J., Bonekat W., Enright P.L., Young T. Factors associated with incidence and persistence of symptoms of disturbed sleep in an elderly cohort: the cardiovascular health study. Am J Med Sci. 2005;329(4):163–172. doi: 10.1097/00000441-200504000-00001. [DOI] [PubMed] [Google Scholar]
- 56.Parthasarathy S., Vasquez M., Halonen M., Bootzin R.R., Quan S.F., Martinez F.D. Persistent insomnia is associated with mortality risk. Am J Med. 2015;128(3):268–275. doi: 10.1016/j.amjmed.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smagula S.F., Koh W.P., Wang R., Yuan J.M. Chronic disease and lifestyle factors associated with change in sleep duration among older adults in the Singapore Chinese health study. J Sleep Res. 2016;25(1):57–61. doi: 10.1111/jsr.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ropponen A., Silventoinen K., Hublin C., Svedberg P., Koskenvuo M., Kaprio J. Sleep patterns as predictors for disability pension due to low back diagnoses: a 23-year longitudinal study of Finnish twins. Sleep. 2013;36(6):891–897. doi: 10.5665/sleep.2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marchand F., Perretti M., McMahon S.B. Role of the immune system in chronic pain. Nat Rev Neurosci. 2005;6(7):521–532. doi: 10.1038/nrn1700. [DOI] [PubMed] [Google Scholar]
- 60.Landis C.A., Lentz M.J., Tsuji J., Buchwald D., Shaver J.L. Pain, psychological variables, sleep quality, and natural killer cell activity in midlife women with and without fibromyalgia. Brain Behav Immun. 2004;18(4):304–313. doi: 10.1016/j.bbi.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 61.McEwen B.S. Stress, adaptation, and disease: allostasis and allostatic load. Ann N Y Acad Sci. 1998;840(1):33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 62.McEwen B.S., Kalia M. The role of corticosteroids and stress in chronic pain conditions. Metabolism. 2010;59(Suppl. 1):S9–S15. doi: 10.1016/j.metabol.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 63.Vachon-Presseau E., Roy M., Martel M.O., Caron E., Marin M.F., Chen J. The stress model of chronic pain: evidence from basal cortisol and hippocampal structure and function in humans. Brain. 2013;136(3):815–827. doi: 10.1093/brain/aws371. [DOI] [PubMed] [Google Scholar]
- 64.Watkins L.R., Maier S.F. The pain of being sick: implications of immune-to-brain communication for understanding pain. Annu Rev Psychol. 2000;51(1):29–57. doi: 10.1146/annurev.psych.51.1.29. [DOI] [PubMed] [Google Scholar]
- 65.De Jongh R.F., Vissers K.C., Meert T.F., Booij L.H.D.J., De Deyne C.S., Heylen R.J. The role of interleukin-6 in nociception and pain. Anesth Analg. 2003:1096–1103. doi: 10.1213/01.ANE.0000055362.56604.78. [DOI] [PubMed] [Google Scholar]
- Irwin M.R., Olmstead R., Carroll J.E. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry. 2016;80(1):40–52. doi: 10.1016/j.biopsych.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lund Haheim L., Nafstad P., Olsen I., Schwarze P., Ronningen K.S. C-reactive protein variations for different chronic somatic disorders. Scand J Public Health. 2009;37(6):640–646. doi: 10.1177/1403494809104358. [DOI] [PubMed] [Google Scholar]
- 68.Ware J.E., Kosinski M., Keller S.D. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(4):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 69.Mundal I., Gräwe R.W., Bjørngaard J.H., LInaker O.M., Fors E.A. Prevalence and long-term predictors of persistent chronic widespread pain in the general population in an 11-year prospective study: the hunt study. BMC Musculoskelet Disord. 2014;15(1):213. doi: 10.1186/1471-2474-15-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pilcher J.J., Ginter D.R., Sadowsky B. Sleep quality versus sleep quantity: relationships between sleep and measures of health, well-being, and sleepiness in college students. J Psychosom Res. 1997;42(6):583–596. doi: 10.1016/s0022-3999(97)00004-4. [DOI] [PubMed] [Google Scholar]
- 71.Tang N.K.Y., Fiecas M., Afolalu E.F., Wolke D. Changes in sleep duration, quality, and medication use are prospectively associated with health and well-being: analysis of the UK household longitudinal study. Sleep. 2017;40(3) doi: 10.1093/sleep/zsw079. [DOI] [PubMed] [Google Scholar]
- 72.Yokoyama E., Kaneita Y., Yasuhiko S., Uchiyama M., Matsuzaki Y., Tamaki T. Association between depression and insomnia subtypes: a longitudinal study on the elderly in Japan. Sleep. 2010;33(12):1693–1702. doi: 10.1093/sleep/33.12.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dworkin R.H., Turk D.C., Farrar J.T., Haythornthwaite J.A., Jensen M.P., Katz N.P. Core outcome measures for chronic pain clinical trials: immpact recommendations. Pain. 2005;113(1–2):9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 74.Prather A.A., Epel E.S., Cohen B.E., Neylan T.C., Whooley M.A. Gender differences in the prospective associations of self-reported sleep quality with biomarkers of systemic inflammation and coagulation: findings from the heart and soul study. J Psychiatr Res. 2013;47(9):1228–1235. doi: 10.1016/j.jpsychires.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nakamura K., Sakurai M., Miura K., Morikawa Y., Nagasawa S.Y., Ishizaki M. Overall sleep status and high sensitivity c-reactive protein: a prospective study in Japanese factory workers. J Sleep Res. 2014;23(6):717–727. doi: 10.1111/jsr.12182. [DOI] [PubMed] [Google Scholar]
- Smith M.T., Quartana P.J., Okonkwo R.M., Nasir A. Mechanisms by which sleep disturbance contributes to osteoarthritis pain: a conceptual model. Curr Pain Headache Rep. 2009;13(6):447–454. doi: 10.1007/s11916-009-0073-2. [DOI] [PubMed] [Google Scholar]
- 77.Aili K., Nyman T., Svartengren M., Hillert L. Sleep as a predictive factor for the onset and resolution of multi-site pain: a 5-year prospective study. Eur J Pain. 2015;19(3):341–349. doi: 10.1002/ejp.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies K.A., Macfarlane G.J., Nicholl B.I., Dickens C., Morriss R., Ray D. Restorative sleep predicts the resolution of chronic widespread pain: results from the epifund study. Rheumatology. 2008;47(12):1809–1813. doi: 10.1093/rheumatology/ken389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jensen M.P., Karoly P. Self-report scales and procedures for assessment pain in adults. In: Turk D.C., Melzack R., editors. Handbook of pain assessment. The Guilford Press; New York: 2011. pp. 19–44. [Google Scholar]
- 80.Kenny D.A. Cross-lagged panel correlation: a test for spurious. Psychol Bull. 1975;82(6):887–903. [Google Scholar]