Abstract
Anorexia nervosa, a severe psychiatric illness, is associated with an intestinal microbial dysbiosis. Individual microbial signatures dominate in healthy samples, even over time and under controlled conditions, but whether microbial markers of the disorder overcome inter-individual variation during the acute stage of illness or renourishment is unknown. We characterized daily changes in the intestinal microbiota in three acutely ill patients with anorexia nervosa over the entire course of hospital-based renourishment and found significant, patient-specific changes in microbial composition and diversity. Even in a state of pathology, individual microbial signatures persist in accounting for the majority of intestinal microbial variation.
Keywords: anorexia nervosa, intestinal microbiota, gut-brain-microbiota axis, clinical refeeding
Introduction
Anorexia nervosa (AN) is a severe, often life-threatening illness associated with substantial medical comorbidity and mortality rates among the highest of any psychiatric disorder (Zipfel, Giel, Bulik, Hay, & Schmidt, 2015). Patients with AN often present with gastrointestinal (GI) symptoms, including abdominal distention and pain (Mehler & Brown, 2015), and GI-related effects of renourishment are uncomfortable and distressing—resulting in high treatment dropout. Moreover, the evidence base for therapeutic renourishment is weak, in part because of inadequate understanding of underlying biological mechanisms (Zipfel et al., 2015).
The intestinal microbiota, which influences gut motility, physiology, and energy regulation (Nieuwdorp, Gilijamse, Pai, & Kaplan, 2014), warrants investigation for improving AN treatment. AN is associated with intestinal microbial dysbiosis marked by lower microbial diversity, taxonomic differences compared with healthy controls, and associations with depression and eating disorder psychopathology (Kleiman et al., 2015). We have documented changes in the intestinal microbiota in patients with acute AN between hospital admission for renourishment and discharge (Kleiman et al., 2015) and now provide granular, longitudinal exploration of changes over the course of treatment.
Although individual microbial signatures dominate in healthy samples, even over time and under controlled conditions, intestinal microbial dysbiosis is associated with many pathologies (Marchesi et al., 2016). Whether effects of treatment (e.g., therapeutic renourishment in AN) generate consistent response in the intestinal microbiota, or inter-individual variation predominates in pathologic microbiotas, is unknown. During renourishment, patients with AN are prescribed increasing energy intake levels to achieve a target rate of weight gain and often traverse a hypermetabolic period (Schebendach, Golden, Jacobson, Hertz, & Shenker, 1997). We know little about changes to the intestinal microbiota during this metabolic transition. We therefore, (i) characterized daily changes in composition and diversity of the intestinal microbiota in three patients with acute AN during hospital-based renourishment; and (ii) identified enteric bacterial groups associated with metabolic changes during treatment.
Methods
The study was approved by the Biomedical Institutional Review Board at the University of North Carolina at Chapel Hill (UNC). All participants provided written informed consent before study participation, and parental permission forms and an age-appropriate assent forms were used for participants younger than 18 years.
Study Population
Females (n=3) admitted for inpatient treatment at the UNC Center of Excellence for Eating Disorders participated in the study. Patients ages 15 to 64 years meeting DSM-5 criteria for AN and presenting at <75% of ideal body weight were recruited from consecutive admissions between May and August 2015. Due to possible impact on the intestinal microbiota, potential participants were excluded for the following reasons: (i) history of gastrointestinal tract surgery (other than appendectomy or cholecystectomy); (ii) history of inflammatory bowel diseases, irritable bowel syndrome, celiac disease; (iii) treatment in the last two months with antibiotics or steroids; (iv) intentional use of probiotics during the last two months (via food or supplement); and/or (v) abuse of laxatives within the last month.
Body Composition and Assessments
Weight and height were assessed at hospital admission using a calibrated digital scale and stadiometer. Participants were weighed daily (before breakfast, in a gown) as part of standard treatment on the inpatient eating disorders unit and three times per week (before breakfast, without shoes) after stepping down to the partial hospitalization program. Eating disorders diagnosis and psychopathology were established via the Eating Disorder Examination (Cooper & Fairburn, 1987) and the Structured Clinical Interview for DSM-IV-TR Axis I Disorders (First, Spitzer, Gibbon, & Williams, 2002) conducted by credentialed members of the UNC Center of Excellence for Eating Disorders Assessment Core. Energy intake was prescribed by registered dietitians, confirmed via clinical consensus, and in line with each participant’s target weight gain trajectory.
Participant metabolic rate was measured at admission and weekly thereafter during hospitalized renourishment. Resting energy expenditure (kJ/day) was measured after overnight fast, before breakfast with the MedGem indirect calorimeter (Microlife Medical Home Solutions, Inc., Golden, CO). The same procedure was repeated one hour post-breakfast to measure post-prandial resting energy expenditure and calculate diet-induced thermogenesis (as percentage increase over resting energy expenditure). Daily physical activity expenditure (kJ) was measured using the BODYMEDIA SenseWear armband (BodyMedia, Inc., Pittsburgh, PA), worn on the back of the upper left arm for a 24-hour period each week (with the exception of bathing or any other continuous contact with water). The 24-hour period commenced at the time of resting energy expenditure measurement. Active energy expenditure (kJ/day), defined as ≥3.0 metabolic equivalents, was also captured by the armband.
Sample Collection, Processing, and Storage
Fecal samples were collected on a daily basis (or as frequently as possible, if less than daily) from all participants. Input and output are measured as part of routine treatment on the inpatient eating disorders unit, minimizing risk of missing samples, and all samples were collected by unit nurses and nursing assistants trained in collection protocols. After stepping down to the partial hospitalization program, participants received training in sample collection procedures and were provided with at-home collection kits for use on-site or at home. All samples were refrigerated after collection and transferred within 24 hours to the laboratory, where they were mechanically homogenized with a sterile spatula, aliquoted into sterile 2 ml cryotubes, and stored in a −80 °C freezer for future DNA isolation and molecular microbiological analysis.
DNA Isolation
Bacterial DNA was isolated from collected samples using a phenol/chloroform extraction method combined with physical disruption of bacterial cells and a DNA clean-up kit (QIAmp DNA Stool Mini Kit [Qiagen, Valencia, CA]), as previously described (Carroll et al., 2011; Carroll, Ringel-Kulka, Siddle, & Ringel, 2012).
Sequencing of 16S rRNA Genes
Bacterial community composition in isolated DNA samples was characterized by amplification of the V4 variable region of the 16S rRNA gene by polymerase chain reaction (PCR) (forward primer 515, 5'-GA GTG CCA GCM GCC GCG GTA A-3'; reverse primer 806, 5'-ACG GAC TAC HVG GGT WTC TAA T-3') as previously described (Kleiman et al., 2017). Briefly, generation of 16S rRNA sequences consisted of two separate amplifications: (1) 95°C for three minutes, then 10 cycles of 95°C for 30 seconds, 50°C for 30 seconds, and 72°C for 30 seconds, followed by one cycle of 72°C for five minutes using 120 ng of fecal DNA as template, 0.4 µM of each 16S V4 primer, and the KAPA2G Robust PCR kit (Kapa Biosystems, Wilmington, MA); and (2) 95°C for three minutes, then 22 cycles of 95°C for 30 seconds, 50°C for 30 seconds, and 72°C for 30 seconds, followed by one cycle of 72°C for five minutes using 5 µL of purified PCR product from the first amplification as template, 1.25 µM of forward and reverse primers that contain Illumina MiSeq adapter sequences with a 12-base error-correcting Golay barcode incorporated in the reverse primer, and the KAPA HiFi HotStart ReadyMix PCR kit (Werner, Zhou, Caporaso, Knight, & Angenent, 2012). Purification of PCR products was carried out after each amplification using the HighPrep PCR clean-up kit (MagBio, Lausanne, Switzerland) with a DynaMag-96 side magnet (Life Technologies, Carlsbad, CA). 16S rRNA PCR products were then quantified and pooled for sequencing. Sequencing was performed on an Illumina MiSeq desktop sequencer (Illumina, San Diego, CA) by the High-Throughput Sequencing Facility in the Carolina Center for Genome Sciences at the UNC School of Medicine. The BioProject for this study (PRJNA382889) is available at https://www.ncbi.nlm.nih.gov/bioproject/PRJNA382889 with a direct link to the data at the SRA at https://www.ncbi.nlm.nih.gov/sra/?term=SRP103900 (Wheeler et al., 2007).
Analysis
Classification of 16S rRNA forward sequence reads was performed in duplicate with two pipelines: QIIME version 1.5.0, using previously described quality filtering, OTU clustering, and taxonomy assignment methods (Caporaso et al., 2010; Kleiman et al., 2017); and version 2.10.1 of the RDP classifier with a threshold of a 50% RDP score (Wang, Garrity, Tiedje, & Cole, 2007). Both pipelines yielded similar results, with strong separation between patients’ samples. Principal coordinates were generated with QIIME, using unweighted UniFrac distances (Lozupone & Knight, 2005). Sequences were log-normalized as previously described (McCafferty et al., 2013) and diversity of the intestinal microbiota was characterized by the Shannon diversity index (Haegeman et al., 2013; Hill, 1973).
Following sequencing, we had 141 samples with sufficient depth (> 10,000 sequences) for our downstream analysis and below this depth samples appeared as substantial outliers. The mean number of 16S rRNA sequence reads was 69,967 per sample (range: 12,606–198,025 sequence reads). The RDP classifier reported 185 non-rare taxa (7 phyla, 14 classes, 18 orders, 39 families, and 107 genera) that were present in at least 25% of our samples.
Linear models were constructed with bacterial taxa (or Shannon diversity index) regressed against interaction of time (i.e., length of hospital stay, in days) and patient, inclusive of their main effects. Taxa abundance and diversity of bacterial groups were considered at the phylum, class, order, family, and genus levels. Additional models that included patient metadata (e.g., BMI and energy intake), in addition to a time-patient interaction, were also explored. Lastly, linear models were constructed to incorporate metabolic metadata of interest (i.e., resting energy expenditure, diet-induced thermogenesis, and active energy expenditure) in addition to a time-patient interaction. FDR correction was applied to the number of comparisons per outcome and per taxonomic rank at a threshold of 0.05 (Benjamini, Drai, Elmer, Kafkafi, & Golani, 2001). P-values from ANOVA models were generated by leaving out each term. For example to evaluate the significance of time, an ANOVA comparing the full model (time + patient + time*patient) was compared with a reduced model of just patient. All analyses were conducted in R; R-scripts are available here: https://github.com/mcbtBINF/ANCaseSeries/ (R: A language and environment for statistical computing, 2015).
Results
Demographic and clinical characteristics of patients are presented in Table 1 and Figure 1. Patients A and B were treated in both inpatient and partial-hospitalization units, while Patient C only received inpatient treatment.
Table 1.
Demographic and clinical characteristics of patients
| Patient A | Patient B | Patient C | ||
|---|---|---|---|---|
| Age (years) | 25 | 29 | 16 | |
|
| ||||
| Race | Asian | Caucasian | Caucasian | |
|
| ||||
| BMI (kg/m2) | Admission | 15.6 | 17.6 | 13.7 |
| Discharge | 20.2 | 21.1 | 15.4 | |
|
| ||||
| Length of stay (days) | 73 | 58 | 34 | |
|
| ||||
| Number of fecal samples | 68 | 47 | 33 | |
|
| ||||
| Energy intake (kJ) | Admission | 4186 | 5860 | 5860 |
| Discharge | 13395 | 13395 | 13395 | |
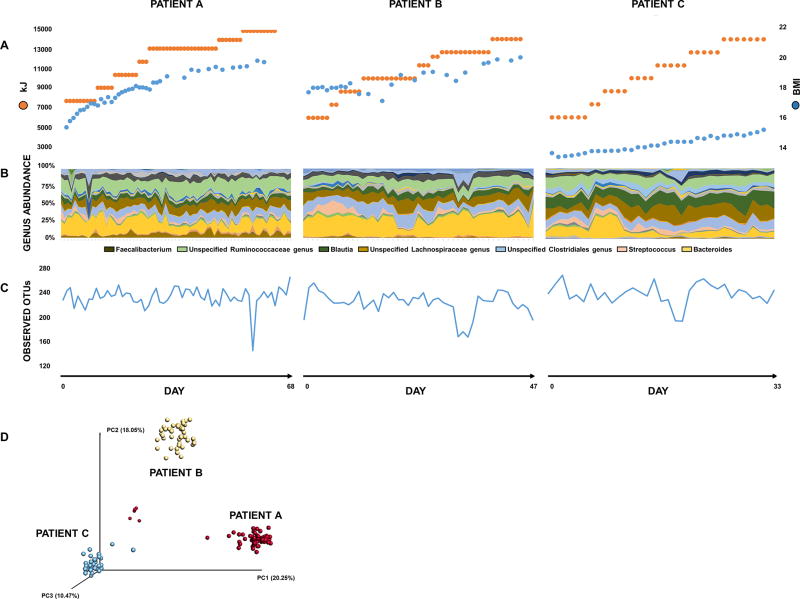
Figure 1. Intestinal microbiotas of three AN patients during renourishment.
(A) BMI (kg/m2, blue) and energy intake (kJ/day, orange). (B) Taxonomic composition: abundance of specific genera exhibit variability during refeeding. (C) Microbial richness: number of observed OTUs in fecal samples varies during refeeding. (D) Principal coordinate analysis of unweighted UniFrac distances: samples cluster by patient [p =0.001, analysis of similarity (ANOSIM)], suggesting disease state or renourishment is not sufficient to overpower unique enteric microbiota composition harbored by each individual.
Weekly metabolic indicators are presented in Table S1. In all patients, resting energy expenditure (REE) increased during treatment, in parallel with BMI and energy intake, and consistent with previous reports, diet-induced thermogenesis reached a peak of 158–181% of REE in week 2–3 (Schebendach et al., 1997). Total physical activity expenditure and active energy expenditure were stable during inpatient treatment but increased substantially in Patients A and B in partial-hospitalization, likely reflecting unsanctioned increases in physical activity during time off-unit.
In all patients, we observed significant changes in composition and diversity of the intestinal microbiota over time at the phylum (n=4), class (n=8), order (n=14), family (n=28) and genus (n=68) levels (Figure 1), after FDR correction (Table S2). Of taxa that changed over time, magnitude and direction of changes were largely patient-specific (Figure S1). These patient-specific patterns included fluctuations in the abundance of both consistently detectable taxa (for example, Figures S1 A, B, C) as well as intermittent periods of non-detection for certain taxa (for example, genus Lactonifactor, Figure S1 D).
As BMI and energy intake were strongly correlated with length of hospitalization and each other, similar results were generated by regressing bacterial taxa and diversity against these variables (Table S3). High correlation between variables, and reproduction of results across models, suggest that increasing model complexity would not yield additional significant findings. Moreover, the small number of significant changes in more complex models and strong correlation between terms makes meaningful interpretation of differences between energy intake, BMI increase, and time difficult. Weekly changes in REE, diet-induced thermogenesis, and active energy expenditure were also highly correlated with duration of hospitalization and did not generate significant associations with composition or diversity of the intestinal microbiota.
Discussion
These results support earlier assertions that individuals do not share a core microbiota, but rather house unique microbial communities that experience periods of both high variability and relative stability—due to intrinsic and extrinsic factors (David et al., 2014). The Human Microbiome Project underscores the diversity of “healthy” microbiotas, unexplained by phenotypic differences. Likewise, inter-individual differences were the predominant source of variation in the intestinal microbiota during a controlled feeding study (Wu et al., 2011). That this pattern of unique intestinal microbiotas as seen in healthy individuals also occurs and persists in a pathologic state associated with microbial dysbiosis is a novel outcome that warrants replication in other disorders associated with intestinal microbial dysbiosis. Moreover, although these results add to evidence of high inter-individual variability in composition of enteric microbial communities, different bacteria can serve similar metabolic functions. This could generate similar functional impact from different microbial compositions, which has led researchers to posit that individuals may share a “core microbiome ” of microbial genes that drive similar functional impact in the host (Turnbaugh et al., 2009).
Although a valid design for novel observations, a case series is limited by sample size, lack of controls, and in our case, a broad age range of participants. Nonetheless, our results contribute to understanding biological changes associated with renourishment in AN. We may have been underpowered to detect changes in more complex longitudinal models, but this does not rule out possible associations between changes in microbial composition and diversity and changes in BMI, dietary intake, or psychopathology. Although patients achieved significant weight gain, our results do not support findings of strong associations between the intestinal microbiota and BMI (Ley et al., 2005). In measuring metabolic changes on a weekly—rather than daily—basis, we may have also been underpowered to detect associations with microbial measures, despite evidence supporting a role for the intestinal microbiota in metabolic function and dietary energy extraction. As a case series, we were also unable to account for any differences across participants that may have been due to age. Although our three patients spanned a fairly broad age range, few data exist to support a large effect of age on the instestinal microbiota after age three (Yatsunenko et al., 2012). Lastly, our analysis focused on taxonomic and diversity measures, which describes microbial composition but does not account for functional impact.
In examining composition and diversity of the intestinal microbiota in three patients undergoing treatment for AN, we found significant, patient-specific changes during hospital-based renourishment. Even in a controlled environment, on a stringent refeeding protocol, with significant weight gain, and in a disease state marked by microbial dysbiosis, individual microbial signatures persisted in accounting for the majority of variation in microbial composition and diversity. Although all patients experienced peaks in hypermetabolism in treatment weeks 2–3, we were unable to detect specific associations between hypermetabolic state and the intestinal microbiota. Future work should aim to elucidate temporal changes to the intestinal microbiome during renourishment (and associated changes in metabolic activity), which will increase understanding of host-microbial dynamics during treatment and could help explain persistence of weight dysregulation and metabolic changes in recovered AN patients.
Supplementary Material
(A) Phylum Firmicutes;
(B) Order Bifidobacteriales;
(C) Family Clostridiaceae 1;
(D) Genus Lactonifactor.
Column (1) Taxonomic level; (2) Names (of bacterial taxa); (3) ANOVA->Day (p-value for effect of time); (4) ANOVA->patient (p-value for effect of patient); (5) ANOVA->Day:patient (p-value for effect of patient-time interaction); (6) adjANOVA->Day (p-value for effect of time, with FDR adjustment); (7) adjANOVA->patient (p-value for effect of patient, with FDR adjustment); (8) adjANOVA->Day:patient (p-value for effect of patient-time interaction, with FDR adjustment).
Column (1) Covariate (Day, BMI, or Energy Intake); (2) Taxonomic Level; (3) Names (of bacterial taxa); (4) ANOVA->Covariate (p-value for effect of time, BMI, or energy intake); (5) ANOVA->patient (p-value for effect of patient); (6) ANOVA->Covariate:patient (p-value for effect of interaction between patient and time, BMI, or energy intake); (7) Covariate (p-value for effect of time, BMI, or energy intake); (8) patientB (p-value for effect of Patient B vs. Patient A); (9) patient C (p-value for effect of Patient C vs. Patient A); (10) Covariate:patientB (p-value for effect of interaction of Patient B vs. Patient A and time, BMI, or energy intake); (11) Covariate:patientC (p-value for effect of interaction of Patient C vs. Patient A and time, BMI, or energy intake); (12) adjANOVA->Covariate (p-value for effect of time, BMI, or energy intake, with FDR adjustment); (13) adjANOVA->patient (p-value for effect of patient, with FDR adjustment); (14) adjANOVA->Covariate:patient (p-value for effect of interaction between patient and time, BMI, or energy intake, with FDR adjustment); (15) adjCovariate (p-value for effect of time, BMI, or energy intake, with FDR adjustment); (16) adjpatientB (p-value for effect of Patient B vs. Patient A, with FDR adjustment); (17) adjpatient C (p-value for effect of Patient C vs. Patient A, with FDR adjustment); (18) adjCovariate:patient (p-value for effect of interaction of Patient B vs. Patient A and time, BMI, or energy intake, with FDR adjustment); (19) adjCovariate:patientC (p-value for effect of interaction of Patient C vs. Patient A and time, BMI, or energy intake).
The following are provided for each patient on a weekly basis during renourishment: BMI (kg/m2), Resting Energy Expenditure (kJ/day; measured upon waking using MedGem indirect calorimeter), Post-Prandial Resting Energy Expenditure (kJ/day; measured one hour post-breakfast using MedGem indirect calorimeter), Diet-Induced Thermogenesis (%; ratio of post-prandial and resting energy expenditure), Physical Activity Expenditure (kJ/day; measured using BODYMEDIA SenseWear armband), and Active Energy Expenditure (kJ/day; measured using BODYMEDIA SenseWear armband and defined as ≥3.0 metabolic equivalents).
Acknowledgments
Sponsors: This study was funded by the Foundation of Hope, the UNC Center of Excellence for Eating Disorders Board of Visitors Fund, and TJ's Fund for Eating Disorders Research (Academy for Eating Disorders; Bulik: Principal Investigator) and partially supported by a K01 DK 092330, an R01 MH 105684, and a CGIBD pilot feasibility grant P30DK03498 awarded to Dr. Carroll. Dr. Bulik acknowledges funding from the Swedish Research Council (VR Dnr: 538-2013-8864).
References
- Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behavioural Brain Research. 2001;125(1–2):279–284. doi: 10.1016/S0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Knight R. QIIME allows analysis of high-throughput community sequencing data. Nature Methods. 2010;7(5):335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll IM, Ringel-Kulka T, Keku TO, Chang YH, Packey CD, Sartor RB, Ringel Y. Molecular analysis of the luminal- and mucosal-associated intestinal microbiota in diarrhea-predominant irritable bowel syndrome. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2011;301(5):G799–807. doi: 10.1152/ajpgi.00154.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll IM, Ringel-Kulka T, Siddle JP, Ringel Y. Alterations in composition and diversity of the intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterology and Motility. 2012;24(6):521–530. e248. doi: 10.1111/j.1365-2982.2012.01891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper Z, Fairburn C. The eating disorders examination: a semi-structured interview for the assessment of the specific psychopathology of eating disorders. The International Journal of Eating Disorders. 1987;6(1):1–8. doi: 10.1002/1098-108X(198701)6:1<1::AID-EAT2260060102>3.0.CO;2-9. [DOI] [Google Scholar]
- David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. New York: New York State Psychiatric Institute; 2002. [Google Scholar]
- Haegeman B, Hamelin J, Moriarty J, Neal P, Dushoff J, Weitz JS. Robust estimation of microbial diversity in theory and in practice. The ISME Journal. 2013;7(6):1092–1101. doi: 10.1038/ismej.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MO. Diversity and evenness: A unifying notation and its consequences. Ecology. 1973;54(2):427–432. doi: 10.2307/1934352. [DOI] [Google Scholar]
- Kleiman SC, Bulik-Sullivan EC, Glenny EM, Zerwas SC, Huh EY, Tsilimigras MCB, Carroll IM. The gut-brain axis in healthy females: Lack of significant association between microbial composition and diversity with psychiatric measures. Plos One. 2017;12(1):e0170208. doi: 10.1371/journal.pone.0170208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiman SC, Watson HJ, Bulik-Sullivan EC, Huh EY, Tarantino LM, Bulik CM, Carroll IM. The intestinal microbiota in acute anorexia nervosa and during renourishment: Relationship to depression, anxiety, and eating disorder psycopathology. Psychosomatic Medicine. 2015;77(9):969–981. doi: 10.1097/PSY.0000000000000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(31):11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone C, Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Applied and Environmental Microbiology. 2005;71(12):8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi JR, Adams DH, Fava F, Hermes GD, Hirschfield GM, Hold G, Hart A. The gut microbiota and host health: A new clinical frontier. Gut. 2016;65(2):330–339. doi: 10.1136/gutjnl-2015-309990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCafferty J, Muhlbauer M, Gharaibeh RZ, Arthur JC, Perez-Chanona E, Sha W, Fodor AA. Stochastic changes over time and not founder effects drive cage effects in microbial community assembly in a mouse model. The ISME Journal. 2013;7(11):2116–2125. doi: 10.1038/ismej.2013.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehler PS, Brown C. Anorexia nervosa - medical complications. Journal of Eating Disorders. 2015;3:11. doi: 10.1186/s40337-015-0040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwdorp M, Gilijamse PW, Pai N, Kaplan LM. Role of the microbiome in energy regulation and metabolism. Gastroenterology. 2014;146(6):1525–1533. doi: 10.1053/j.gastro.2014.02.008. [DOI] [PubMed] [Google Scholar]
- R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. [Google Scholar]
- Schebendach JE, Golden NH, Jacobson MS, Hertz S, Shenker IR. The metabolic responses to starvation and refeeding in adolescents with anorexia nervosa. Annals of the New York Academy of Sciences. 1997;817:110–119. doi: 10.1111/j.1749-6632.1997.tb48200.x. [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Gordon JI. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Applied and Environmental Microbiology. 2007;73(16):5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner JJ, Zhou D, Caporaso JG, Knight R, Angenent LT. Comparison of Illumina paired-end and single-direction sequencing for microbial 16S rRNA gene amplicon surveys. The ISME Journal. 2012;6(7):1273–1276. doi: 10.1038/ismej.2011.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DL, Barrett T, Benson DA, Bryant SH, Canese K, Chetvernin V, Yaschenko E. Database resources of the National Center for Biotechnology Information. Nucleic Acids Research. 2007;36:D13–21. doi: 10.1093/nar/gkm1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Lewis JD. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Gordon JI. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel S, Giel KE, Bulik CM, Hay P, Schmidt U. Anorexia nervosa: Aetiology, assessment, and treatment. Lancet Psychiatry. 2015;2(12):1099–1111. doi: 10.1016/S2215-0366(15)00356-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Phylum Firmicutes;
(B) Order Bifidobacteriales;
(C) Family Clostridiaceae 1;
(D) Genus Lactonifactor.
Column (1) Taxonomic level; (2) Names (of bacterial taxa); (3) ANOVA->Day (p-value for effect of time); (4) ANOVA->patient (p-value for effect of patient); (5) ANOVA->Day:patient (p-value for effect of patient-time interaction); (6) adjANOVA->Day (p-value for effect of time, with FDR adjustment); (7) adjANOVA->patient (p-value for effect of patient, with FDR adjustment); (8) adjANOVA->Day:patient (p-value for effect of patient-time interaction, with FDR adjustment).
Column (1) Covariate (Day, BMI, or Energy Intake); (2) Taxonomic Level; (3) Names (of bacterial taxa); (4) ANOVA->Covariate (p-value for effect of time, BMI, or energy intake); (5) ANOVA->patient (p-value for effect of patient); (6) ANOVA->Covariate:patient (p-value for effect of interaction between patient and time, BMI, or energy intake); (7) Covariate (p-value for effect of time, BMI, or energy intake); (8) patientB (p-value for effect of Patient B vs. Patient A); (9) patient C (p-value for effect of Patient C vs. Patient A); (10) Covariate:patientB (p-value for effect of interaction of Patient B vs. Patient A and time, BMI, or energy intake); (11) Covariate:patientC (p-value for effect of interaction of Patient C vs. Patient A and time, BMI, or energy intake); (12) adjANOVA->Covariate (p-value for effect of time, BMI, or energy intake, with FDR adjustment); (13) adjANOVA->patient (p-value for effect of patient, with FDR adjustment); (14) adjANOVA->Covariate:patient (p-value for effect of interaction between patient and time, BMI, or energy intake, with FDR adjustment); (15) adjCovariate (p-value for effect of time, BMI, or energy intake, with FDR adjustment); (16) adjpatientB (p-value for effect of Patient B vs. Patient A, with FDR adjustment); (17) adjpatient C (p-value for effect of Patient C vs. Patient A, with FDR adjustment); (18) adjCovariate:patient (p-value for effect of interaction of Patient B vs. Patient A and time, BMI, or energy intake, with FDR adjustment); (19) adjCovariate:patientC (p-value for effect of interaction of Patient C vs. Patient A and time, BMI, or energy intake).
The following are provided for each patient on a weekly basis during renourishment: BMI (kg/m2), Resting Energy Expenditure (kJ/day; measured upon waking using MedGem indirect calorimeter), Post-Prandial Resting Energy Expenditure (kJ/day; measured one hour post-breakfast using MedGem indirect calorimeter), Diet-Induced Thermogenesis (%; ratio of post-prandial and resting energy expenditure), Physical Activity Expenditure (kJ/day; measured using BODYMEDIA SenseWear armband), and Active Energy Expenditure (kJ/day; measured using BODYMEDIA SenseWear armband and defined as ≥3.0 metabolic equivalents).