Abstract
Pain patients who are nicotine dependent report a significantly increased incidence and severity of pain intensity. The goal of this study was to determine the effects of prolonged nicotine administration on inflammatory proteins implicated in the development of peripheral and central sensitization of the trigeminal system. Behavioral, immunohistochemical, and microarray studies were utilized to investigate the effects of nicotine administered daily for 14 days via an Alzet®osmotic pump in Sprague Dawley rats. Systemic nicotine administration caused a significant increase in nocifensive withdrawals to mechanical stimulation of trigeminal neurons. Nicotine stimulated expression of the pro-inflammatory signal transduction proteins phosphorylated-extracellular signal-regulated kinase (p-ERK), phosphorylated-c-Jun N-terminal kinase (p-JNK), and protein kinase A (PKA) in the spinal trigeminal nucleus. Nicotine also promoted elevations in the expression of glial fibrillary acidic protein (GFAP), a biomarker of activated astrocytes, and the microglia biomarker ionized calcium-binding adapter molecule 1 (Iba1). Similarly, levels of eleven cytokines were significantly elevated with the largest increase in expression of TNF-α. Levels of PKA, p-ERK, and p-JNK in trigeminal ganglion neurons were increased by nicotine. Our findings demonstrate that prolonged systemic administration of nicotine promotes sustained behavioral and cellular changes in the expression of key proteins in the spinal trigeminal nucleus and trigeminal ganglion implicated in the development and maintenance of peripheral and central sensitization.
Keywords: central sensitization, cytokines, nicotine, spinal trigeminal nucleus, trigeminal ganglion
INTRODUCTION
Migraine is a painful neurological disorder that affects 18% of the general population, with prevalence highest in women of childbearing age (Buse et al., 2013). Migraineurs are thought to have a hyperexcitable nervous system characterized by a heightened sensitivity toward external stimuli that may be responsible for triggering a migraine attack (Dodick and Silberstein, 2006). Sensitization and activation of trigeminal nerves, which provide a nociceptive pathway from the peripheral tissues to the spinal trigeminal nucleus, is implicated in migraine pathology. A lowering of the activation threshold of trigeminal nociceptive neurons indicative of neuronal sensitization is associated with increased neuron-glia interactions and cellular changes in ion channels, receptors, gap junctions, and signal transduction pathways (Hucho and Levine, 2007). These cellular changes are mediated by increases in neuronal and glial expression of signal transduction proteins and elevated levels of cytokines in both the trigeminal ganglion and spinal trigeminal nucleus. In particular, members of the mitogen-activated protein kinase (MAPK) family and protein kinase A (PKA) are involved in intracellular signaling cascades that promote neuron–glia interactions and maintain a hyperexcitable state of nociceptive neurons (Ji et al., 2009; Seybold, 2009). Cytokines are a large family of proteins that modulate inflammatory responses and also play an important role in the development of peripheral and central sensitization, (Miller et al., 2009) and elevated cytokine levels have been reported in serum and the cerebrospinal fluid in migraine patients (Rozen and Swidan, 2007; Uzar et al., 2011).
Nicotine is a widely abused, alkaloid substance that accounts for about 0.6–2.9% of the dry weight of tobacco (Hoffmann and Hoffmann, 2012) and is reported to cause activation and hypersensitivity of neurons and to elicit pain (Liu and Simon, 1996). In support of this notion, pain patients who are chronic tobacco users and have severe nicotine dependency report significantly greater pain intensities than non-users (Weingarten et al., 2008; Zvolensky et al., 2009; Shi et al., 2010). In a study conducted by the Mayo clinic, pain patients diagnosed with a variety of pathologies and who were nicotine dependent also reported higher pain intensities that interfered with daily activities and mood (Weingarten et al., 2008). Similarly, findings from a later study showed a strong correlation existed between chronic pain and nicotine dependence (Zvolensky et al., 2009). This correlation likely extends to migraine sufferers since smokers are significantly more likely to experience autonomic symptoms during migraine attacks than non-smokers (Rozen, 2011) and heavy smoking is considered a risk factor for migraine (Le et al., 2011; Schramm et al., 2013). Nicotine exhibits high affinity for nicotinic acetylcholine (nAch) receptors, which are expressed on trigeminal neurons as well as astrocytes and microglia (Liu et al., 1998; De Simone et al., 2005; Oikawa et al., 2005). Nicotine activation of nAch receptors leads to an increase of intracellular calcium that facilitates increased neuronal and glial excitability (Delbono et al., 1997; Oikawa et al., 2005; Michel et al., 2011). Although nicotine dependency is associated with elevated pain states, the cellular mechanisms by which nicotine use affects the excitability state of trigeminal neurons are not well understood.
Sensitization of trigeminal nociceptive neurons is implicated in the underlying pathology of migraine (Pietrobon and Moskowitz, 2013). In our study, changes in nocifensive responses to mechanical stimuli over the eyebrow and masseter areas in response to prolonged nicotine administration were investigated. Immunohistochemistry was also utilized to determine changes in protein levels in neurons and glia implicated in the development of peripheral and central sensitization in response to prolonged nicotine administration. In addition, changes in the level of 29 cytokines in trigeminal ganglia and upper spinal cord tissue were investigated using cytokine protein profiling arrays. Results from our study provide evidence that prolonged nicotine administration equivalent to smoking >20 cigarettes per day increased sensitivity to mechanical stimuli in the facial area, which correlated with increased expression of proteins in the trigeminal ganglion and spinal cord that are known to promote peripheral and central sensitization of nociceptive neurons.
EXPERIMENTAL PROCEDURES
Animals
All animal studies were conducted in accordance with the protocols approved by the Institutional Animal Care and Use Committee at the Missouri State University and were in compliance with all guidelines established in the Animal Welfare Act of 2007 and National Institutes of Health. A mindful effort was made to minimize the number and suffering of the animals used during this study. Animals were housed in standard, clean plastic cages that allowed unrestricted access to food and water. Animals were acclimated to the environment that included a 12-h light/dark cycle upon arrival one week prior to use. All procedures were conducted at the same time of the day, between the hours of 7:00 a.m. and 12:00 p.m.
Administration of nicotine
Adult Sprague–Dawley male rats (200–250 g; Charles River Laboratories, Wilmington, MA, USA) were monitored until growth weights stabilized (400–500 g). Animals were anesthetized by inhalation of isoflurane (3–5%). Incision sites were prepared for pump implantation by trimming the hair located over the surgical area and disinfecting the site with 70% ethanol and Betadine scrub (Patterson Companies Inc, Devens, MA, USA). Breathing rates were monitored visually, and body temperature was maintained throughout the procedure through use of a Gaymar water blanket (Patterson Companies). Using the aseptic technique, an incision was made on the dorsal side inferior to the scapula using a sterile surgical scalpel. An Alzet®Osmotic Pump (Alzet, Cupertino, CA, USA) containing either 1× phosphate-buffered saline (PBS) or a PBS-buffered solution that systemically delivered nicotine hydrogen tartrate at 32-mg/kg/day (10.4-mg/kg/day as a free base; Sigma, St. Louis, MO, USA) when released at a rate of 5.22 μl/h for two weeks. Pumps were subcutaneously implanted and the incision was closed with Weblon nylon sutures (Patterson Companies). Animals were monitored during recovery and again 24 h after the procedure for normal grooming and feeding behaviors. Sutures were removed under 2% isoflurane seven days post-procedure and wounds were checked for infection. Nicotine or PBS was administered for two weeks, at which point animals were euthanized by CO2 asphyxiation and decapitation. Trigeminal ganglia and C1/C2 spinal cord tissue containing the dorsal medullary horn of the spinal trigeminal nucleus were acquired through cranial dissection. Spinal cord tissues were cut evenly from superior to inferior down the midline with a scalpel. The right side was used for immunohistochemistry and the left side used for cytokine analysis. If at any point during the study an animal showed signs of infection, excessive weight loss, abnormal behavior, or distress, the animal was removed from the study.
Measurement of cotinine levels
Serum levels of cotinine, which is the primary metabolite of nicotine, were measured at the end of the 2 week of administration to validate that the pumps were functioning properly. Blood samples were collected in BD Vacutainer®Plastic Serum Tubes with a serum separator (Becton Dickinson, Franklin Lakes, NJ, USA) upon completion of the study. Tubes were inverted 5 times and allowed to coagulate at room temperature for 2 h. Samples were centrifuged at 300g for 3 min and serum transferred to autoclaved tubes. Serum cotinine levels were determined using a selective Cotinine ELISA for rat serum (Calbiotech Inc, Spring Valley, CA, USA) according to manufacturer’s protocol. Absorbance was measured at a wavelength of 450 nm after the addition of the stopping solution to each well. Values are reported as the average level of serum cotinine ±standard error of the mean (standard error of the mean (SEM), ng/mL).
Behavioral testing using mechanical stimulation
All behavioral assessments were carried out essentially as described in a previously published study (Garrett et al., 2012) from our laboratory using the Ugo Basile Durham animal holding device (Ugo Basile, Gemonio, Varese, Italy). A series of calibrated von Frey filaments were applied in increasing force to the cutaneous area over the eyebrow and masseter muscle. Withdrawal reactions observed prior to the bending of the filament were verified by two other scientists, who were blinded to the experimental conditions. Each filament was applied 5 times over both the right and left eyebrow or masseter area. Right and left responses for each area were averaged separately to obtain an average number of reactions out of five tests. Baseline measurements were established prior to pump implantation and additional measurements were also taken 14 days post-surgery. Nocifensive reactions were reported as the combined average number of withdrawals ±SEM. Each condition was repeated in six independent experiments.
Immunohistochemistry
Trigeminal ganglia and upper spinal cord tissues used for immunohistochemistry studies were placed in 4% paraformaldehyde overnight at 4 °C after which they were cryoprotected by placing tissues in 12.5% sucrose for 1 h at 4 °C, and 25% sucrose for 24 h at 4 °C. Tissues were mounted using Optimal Cutting Temperature media (OCT, Sakura Finetek Inc, Torrance, CA, USA) and serial 14-μm sections from the −5-mm region from the obex which encompasses mostly Vc, were prepared at −25 °C and placed on SuperFrost Plus microscope slides (Fisher Scientific, Pittsburgh, PA, USA). Sections were blocked and permeabilized by incubating tissues with 5% normal donkey serum/0.1% Triton/PBS for 20 min. The following primary polyclonal antibodies were used: rabbit anti-Protein Kinase A (PKA,1:500, Epitomics, Burlingame, CA, USA), rabbit anti-phosphorylated Extracellular Signal-Regulated Kinase (p-ERK,1:200, Bioworld, St. Louis Park, MN, USA), rabbit anti-phosphorylated c-Jun N-terminal Kinase (p-JNK, 1:200 in STN or 1:100 in TG; Cell Signaling, Beverly, MA, USA), rabbit anti-Glial Fibrillary Acidic Protein (GFAP, 1:000, Dako, Carpinteria, CA, USA), and rabbit anti-Ionized Calcium-binding Adapter Molecule 1 (Iba1, 1:1000, Wako, Richmond, VA, USA). Working dilutions of antibodies directed against PKA, p-JNK, GFAP, and Iba1 were made in 5% normal donkey serum/PBS, while p-ERK antibodies were diluted in 1× PBS. Sections were incubated with primary antibodies either overnight at 4 °C (p-ERK, p-JNK), 3 h at room temperature (PKA, Iba1), or 30 min at room temperature (GFAP). Immunoreactivity was detected by incubating sections with fluorescently labeled anti-rabbit secondary antibodies (AlexaFlour 488, 1:200, Invitrogen, Grand Island, NY, USA) diluted in 1× PBS at room temperature for 1 h. Slides were mounted with Vectashield Mounting Media for Fluorescence with DAPI (Vector Laboratories, Burlingame, CA, USA) and covered with Fisherbrand glass coverslips (Fisher Scientific). A Ziess Z1 imager (Carl Ziess Microscopy, LLC, Thornwood, NY, USA) with apatome was used to acquire 10× images of the V1/V2 area of the trigeminal ganglion and medullary horn of the spinal trigeminal nucleus. Axiovision software (Carl Ziess) was utilized to evenly balance the background of each image prior to analysis, which was performed essentially as described in previous studies (Cady et al., 2010, 2013). Briefly, grayscale 10× JPEG images of the entire medullary horn (spinal trigeminal nucleus) or V1/V2 area (trigeminal ganglion) with similar number of cells as identified by DAPI were opened in ImageJ software (NIH). For spinal cord tissues, integrated densities were acquired in three independent experiments by measuring pixel densities in 10 non-overlapping, circular regions of interest (ROI) in areas along the entire medullary horn encompassing laminas I–III totaling 30 integrated intensity measurements for each experimental condition. A similar approach was used to measure integrated densities in bands of neuronal cell bodies and satellite glial cells in the V1/V2 regions of trigeminal ganglia. To normalize intensity measurements within each image, background intensity values were obtained from five non-overlapping regions in either the acellular area of the outer lamina or areas containing only Schwann cells and neural fibers in the ganglion, as determined by DAPI, and average values subtracted from ROI staining intensity values. Relative average means were determined for each condition and data reported as the average fold change±SEM relative to the average mean level for control animals that was set equal to one.
Cytokine protein array analysis
Trigeminal ganglia and upper spinal cord tissues (from zero to −5 mm from the obex) were used for protein array analysis after being immediately snap frozen in liquid nitrogen. Tissues were placed in 500 μl of 1× RayBio Cell Lysis Buffer (RayBiotech, Inc., Norcross, GA, USA) and sonicated using a Sonic Dismembrator Model 100 (Fisher Scientific). Whole tissue lysate samples were spun at 3200 rpm at 4 °C for 20 min. The resultant supernatant was collected and cytokine analysis was performed using the R&D System Proteome Profiler Rat Cytokine Array Kit (R&D Systems, ARY008, Minneapolis, MN, USA). Microarray profiling was conducted according to manufacturer’s protocol without deviation. The total amount of protein in each sample was determined by the Bradford Assay (BioRad, Hercules, CA, USA). A total of six trigeminal ganglia samples and six upper spinal cord tissue samples (n=3 for each condition) containing 200 μg of sample protein, as determined by the Bradford Assay, were used to determine the level of expression of 29 different cytokines. Detection was accomplished by placing 500 μl of diluted (40:1) Pierce ECL Plus detection system (Thermo Scientific, Southfield, MI, USA) on each array for 5 min. The arrays were placed in contact with X-ray film for several different lengths of time (30 s, 1, 4, and 5 min) to optimize exposure times. Immunoreactive spots were visualized using Kodak Developing and Fixing solutions (Eastman Kodak Company, Rochester, NY, USA). Integrated density measurements were acquired from scanned grayscale JPEG images of the films using Image J software with rolling ball background subtraction (NIH) and dot blot analysis plugins. Integrated density values were normalized to levels of positive control protein spots. The relative levels of each cytokine were determined in duplicate and were based on analysis of samples obtained from three independent experiments, totaling six integrated intensity measurements for each experimental condition for each cytokine arrayed. Integrated densities for control animals were averaged and were used to calculate the individual fold change from control for each RIO measurement for each cytokine arrayed. Relative average means were determined for each condition and data reported as the average fold change±SEM relative to the average mean level for control animals that was set equal to one.
Statistical analysis
Sample sizes for analysis were based on our previously published studies using these methods (Garrett et al., 2012; Vause and Durham, 2012; Cady et al., 2013). Nocifensive responses were assessed using the independent t-test, while statistical analysis for immunohistochemistry was performed using a one-way ANOVA. Cytokine array data were analyzed using a non-parametric Mann–Whitney U test because these data acquired did not exhibit equal variance and therefore the data were not considered as a normal distribution as determined by a Levene’s test. All statistical analysis was conducted in SPSS software (release 16; Chicago, IL, USA). Differences were considered significant at P ≤ 0.05.
RESULTS
Measurement of cotinine serum levels
Levels of serum cotinine, which is the primary metabolite of nicotine, was determined for each animal receiving a two-week administration of 32-mg/kg/day nicotine hydrogen tartrate via the Alzet pump. Only animals with cotinine levels within the reported range in active heavy smokers (>20 cigarettes/day) were used in this study (Hukkanen et al., 2005). The mean cotinine levels in the animals used in our molecular study were 798.5±93.6 ng/mL (n=3), while the mean levels for those used in the behavior studies were 1068.8±96.4 ng/mL (n=6).
Nicotine increased nociceptive responses to mechanical stimulation of trigeminal neurons
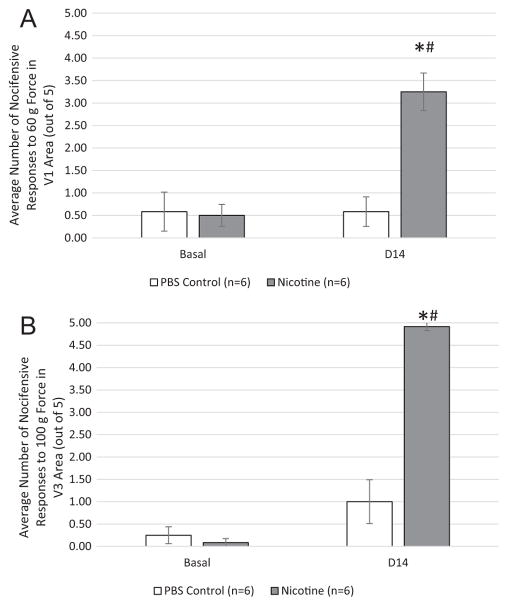
To examine the effect of prolonged nicotine administration on mechanical stimulation of trigeminal neurons, nocifensive responses were measured in the cutaneous regions over the eyebrow and masseter muscle. Animals administered nicotine for 14 days showed an increased nocifensive response to 60 g of force in the eyebrow region (Fig. 1A; 3.25±0.42, P=0.01, n=6) when compared to PBS control animal levels (0.58±0.33) or baseline levels (0.50±0.24). Similarly, nicotine administration for 14 days resulted in a significant increase in nocifensive responses to 100 grams of force in the masseter region (Fig. 1B; 4.92±0.09, P=0.01, n=6) when compared to PBS control levels (0.58±0.22) or baseline levels (0.08±0.09). No significant differences in the average number of nocifensive withdrawal responses were observed in the PBS control group in either the eyebrow or masseter regions when compared to their baseline values.
Fig. 1.
Nicotine increased nociceptive responses to mechanical stimulation of trigeminal neurons. Nicotine or PBS was delivered via an implanted osmotic for 14 days. (A) Bar graph depicting average nocifensive responses to 60 grams of force in the eyebrow region in both the PBS control and nicotine groups prior to implantation and 14 days later (n=6). (B) Bar graph depicting average nocifensive responses to 60 grams of force in the eyebrow region in both the PBS control and nicotine groups prior to implantation and 14 days post-surgery (n=6). *P ≤ 0.05 when compared to basal values, #P ≤ 0.01 when compared to control values.
Expression of PKA and MAP kinases p-ERK and p-JNK was increased in response to prolonged nicotine administration
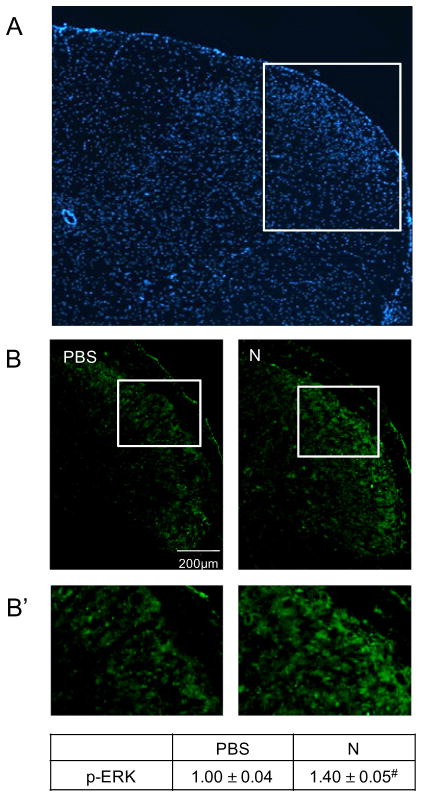
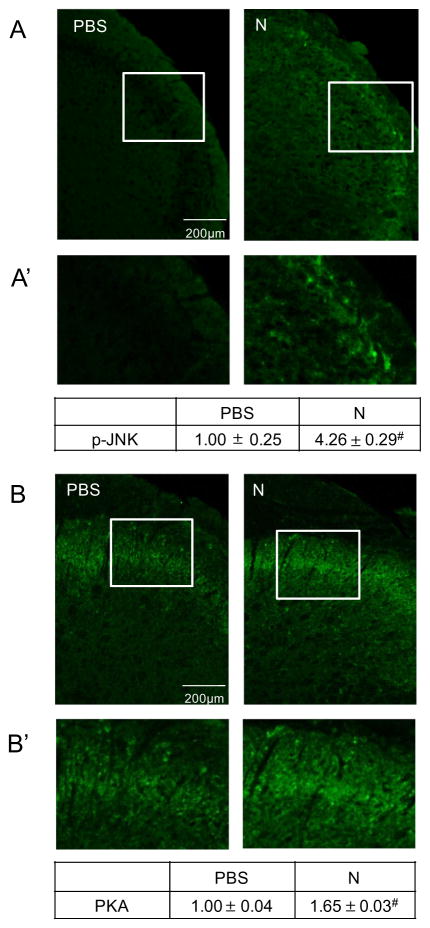
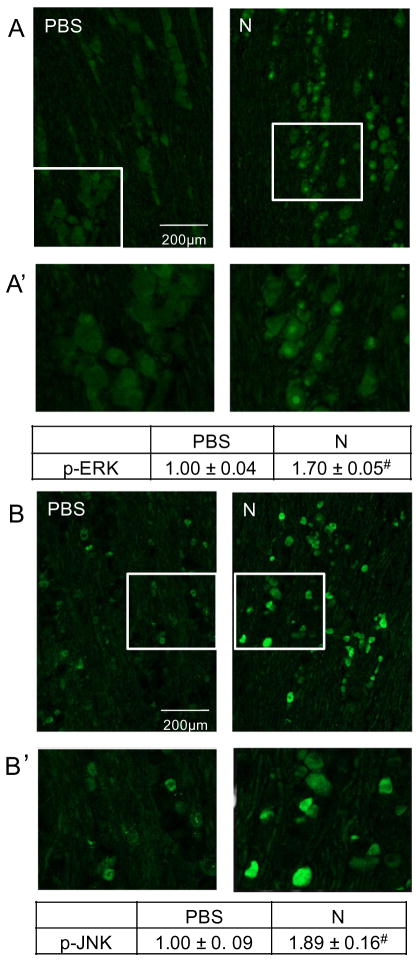
To determine the effects of prolonged nicotine on expression of p-ERK, p-JNK, and PKA in the medullary horn of the upper spinal cord (Fig. 2A), tissue sections containing the spinal trigeminal nucleus were obtained from treated animals, and immunostaining intensities were compared to those found in control animals. Levels of p-ERK expression in the outer lamina were found to be significantly increased (1.40±0.05, P=0.001, n=3, df=1) from levels observed in control animals (1.00±0.04, n=3) in response to nicotine administration for 14 days (Fig. 2B, B′). An even greater stimulatory effect of nicotine was observed on expression of p-JNK in the medullary horn (4.26±0.29, P=0.001, n=3, df=1) when compared to the relative intensity in control tissues (1.00±0.25, n=3, Fig. 3A, A′). The level of PKA in the medullary horn was also significantly increased (1.65±0.03, P=0.001, n=3, df=1) over staining intensity levels in control animals (1.00±0.04, n=3, Figs. 4B, 3B′).
Fig. 2.
Nicotine administration resulted in an increased p-ERK expression in the upper spinal cord tissue containing the spinal trigeminal nucleus. (A) Representative image of the upper spinal cord tissue stained with the nuclear dye DAPI. (B) Images acquired at 100× magnification representing spinal cord sections taken from vehicle control (PBS) and nicotine (N)-treated animals immunostained for p-ERK. (B′) Enlarged image of medullary horn delineated in panel B. The change in p-ERK staining intensities is reported as the average fold change±SEM as compared to mean levels in control samples that was set equal to 1 (n=3). #P ≤ 0.01 when compared to control values.
Fig. 3.
Increased p-JNK and PKA expression in the medullary horn in response to prolonged nicotine administration. Representative image of upper spinal cord tissue from control (PBS) and nicotine (N)-treated animals stained with antibodies directed against p-JNK (A) or PKA (B). Enlarged images of medullary horn from either panel A (A′) or panel B (B′) are shown. The change in p-JNK or PKA staining intensities are reported as the average fold change±SEM as compared to mean levels in control samples that was set equal to 1 (n=3). #P ≤ 0.01 when compared to control values.
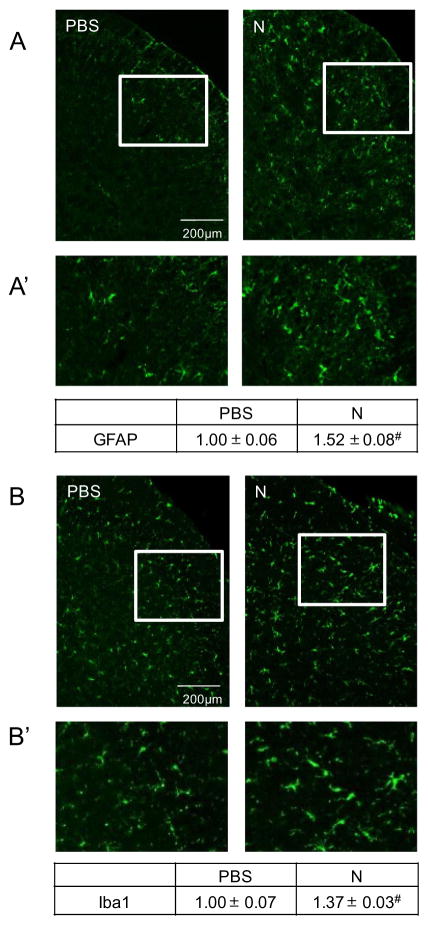
Fig. 4.
Nicotine promotes elevated immunoreactive levels of GFAP and Iba1 in the upper spinal cord. Representative images of tissue sections taken from control (PBS) and nicotine (N)-treated animals immunostained for proteins associated with astrocyte (GFAP, A) or microglial (Iba1, B) activation. Enlarged images of medullary horn from either panel A (A′) or panel B (B′) are shown. The change in GFAP or Iba1 staining intensities are reported as the average fold change±SEM as compared to mean levels in control samples that was set equal to 1 (n=3). #P ≤ 0.01 when compared to control values.
Prolonged nicotine administration promotes activation of astrocytes and microglia
Glial activation in the medullary horn of the upper spinal cord in response to prolonged nicotine administration was determined by measuring changes in the intensity of staining for the proteins GFAP and Iba1. GFAP expression was found to be significantly increased (1.52±0.08, P=0.001, n=3, df=1) above levels detected in control tissues (1.00±0.06, n=3, Fig. 4A, A′). Similarly, immunoreactivity levels of Iba1 were found to be significantly upregulated (1.37±0.03, P=0.001, n=3, df=1) when compared to levels observed in control tissues (1.00±0.07, n=3, Fig. 4B, B′).
Nicotine significantly increased several cytokines in upper spinal cord tissue
To investigate the effects of prolonged nicotine administration on cytokine levels in the upper spinal cord tissue containing the spinal trigeminal nucleus, profiles were acquired through microarray analysis of protein lysates obtained from nicotine-treated and control animals. Of the 29 different cytokines examined, 11 cytokines were found to be significantly increased in samples obtained from animals receiving systemic administration of 32-mg/kg/day nicotine hydrogen tartrate for 14 days when compared to control levels (Table 1, n=3 for all conditions). Interestingly, the expression of TNF-α(5.07±0.62, P=0.002), MIP-1α(2.54±0.50, P=0.015), and IP-10 (2.25±0.20, P=0.002) was increased two fold over immunoreactive levels reported in control samples. Similarly, the intensity levels of L-Selectin (1.87±0.14, P=0.009), IL-6 (1.85±0.14, P=0.002), VEGF (1.79±0.21, P=0.015), MIG (1.77±0.12, P=0.002), LIX (1.73±0.24, P=0.002), IL-4 (1.69±0.07, P=0.026), IL-17 (1.66±0.11, P=0.0109), and IL-2 (1.40±0.08, P=0.002) were significantly elevated over control levels observed in control samples. Cytokine levels and P values of the remaining 18 cytokines whose levels were not statistically different from control values are reported in Table 2.
Table 1.
Cytokines in the upper spinal cord significantly stimulated compared to control levels by systemic nicotine
| Cytokine | Fold change ± SEM | P |
|---|---|---|
| IL-2 | 1.40 ± 0.08 | 0.002 |
| IL-4 | 1.69 ± 0.07 | 0.026 |
| IL-6 | 1.85 ± 0.14 | 0.002 |
| IL-17 | 1.66 ± 0.11 | 0.009 |
| IP-10 | 2.25 ± 0.20 | 0.002 |
| LIX | 1.73 ± 0.24 | 0.002 |
| L-Selectin | 1.87 ± 0.14 | 0.009 |
| MIG | 1.77 ± 0.12 | 0.002 |
| MIP-1α | 2.54 ± 0.50 | 0.015 |
| TNF-α | 5.07 ± 0.62 | 0.002 |
| VEGF | 1.79 ± 0.21 | 0.015 |
IL-2=interleukin 2, IL-4=interleukin 4, IL-6=interleukin 6, IL-17=interleukin 17, IP-10=interferon gamma-induced protein 10, LIX=lipopolysaccharide-induced CXC chemokine, MIG=monokine-induced by gamma interferon, MIP-1α=macrophage-induced protein 1 alpha, TNF-α=tumor necrosis factor alpha, and VEGF=vascular endothelial growth factor.
Table 2.
Cytokines in the upper spinal cord not significantly increased compared to control levels in response to systemic nicotine
| Cytokine | Fold change ± SEM | P |
|---|---|---|
| CINC-1 | 0.94 ± 0.17 | 0.818 |
| CINC-2α | 1.56 ± 0.30 | 0.310 |
| CINC-3 | 1.42 ± 0.15 | 0.240 |
| CNTF | 0.90 ± 0.23 | 0.589 |
| Fractalkine | 1.10 ± 0.11 | 0.485 |
| GM-CSF | 1.56 ± 0.15 | 0.065 |
| sICAM-1 | 1.27 ± 0.07 | 0.394 |
| IFN-γ | 1.30 ± 0.13 | 0.180 |
| IL-1α | 1.31 ± 0.13 | 0.240 |
| IL-1β | 1.24 ± 0.17 | 0.394 |
| IL-1rα | 1.34 ± 0.18 | 0.132 |
| IL-3 | 1.52 ± 0.23 | 0.132 |
| IL-10 | 1.41 ± 0.30 | 0.310 |
| IL-13 | 1.32 ± 0.08 | 0.132 |
| RANTES | 1.21 ± 0.07 | 0.065 |
| Thymus chemokine | 0.97 ± 0.02 | 0.240 |
| TIMP-1 | 1.92 ± 0.44 | 0.180 |
CINC-1=cytokine-induced neutrophil chemoattractant 1, CINC-2α=cytokine-induced neutrophil chemoattractant 2 alpha, CINC3=cytokine-induced neutrophil chemoattractant 3, CNTF=ciliary neurotrophic factor, Fractalkine, GM-CSF= granulocyte macrophage-colony stimulating factor, sICAM-1=intercellular Adhesion Molecule 1, IFN-γ=interferon gamma, IL-1α=interleukin 1 alpha, IL-1β=interleukin 1 beta, IL-1rα=interleukin 1 receptor antagonist, IL-3= interleukin 3, IL-10=interleukin 10, IL-13=interleukin 13, RANTS=regulated on activation, normal T cell expressed and secreted, TIMP-1=tissue inhibitor of metalloproteinase 1.
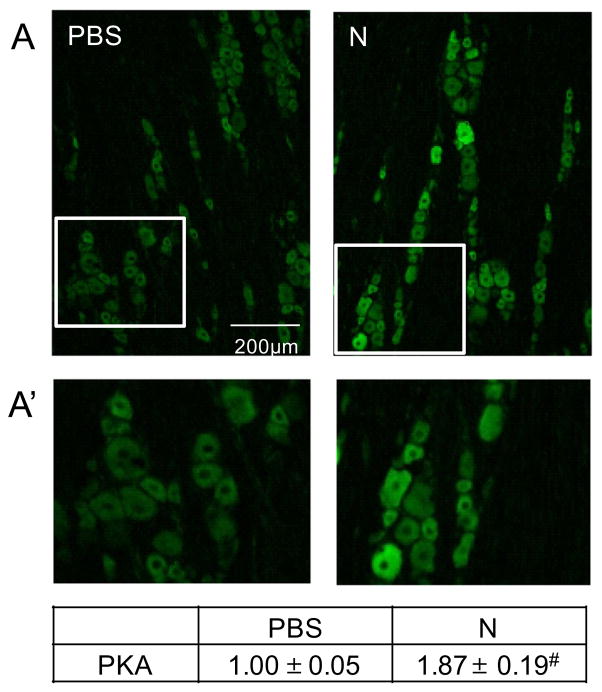
Nicotine increases PKA and MAP kinases p-ERK and p-JNK expression in trigeminal ganglion neurons
Changes in the expression of PKA and the phosphorylated active forms of the MAPKs p-ERK and p-JNK in response to a 14-day systemic nicotine administration were investigated using immunohistochemistry to detect changes in staining intensity as a measure of protein expression. As seen in Fig. 5A, prolonged nicotine administration significantly increased cytosolic PKA expression in the cell body of neurons in the V1/V2 region of the trigeminal ganglion (1.87±0.19, P=0.001, n=3, df=1) when compared to controls (1.00±0.05, n=3). Expression of p-ERK was significantly increased in the nucleus of neurons in animals treated with systemic nicotine for 14 days (1.70±0.05, P=0.001, n=3, df = 1, Fig. 6A, A′) when compared to control tissues (1.00±0.04, n=3). Additionally, expression of p-JNK was significantly increased in the cytosol of neuronal cell bodies in the V1/V2 region of the trigeminal ganglion (1.89±0.16, P=0.001, n=3, df = 1 Fig. 6B, B′) as compared to levels observed in control tissues (1.00±0.09, n=3). In contrast, elevated levels of PKA, p-ERK, or p-JNK immunoreactivity were not observed in satellite glial cells or Schwann cells within the trigeminal ganglion in response to systemic nicotine.
Fig. 5.
Nicotine administration increased expression of PKA in trigeminal ganglion neurons. (A) Representative images of sections from the V1/V2 region of trigeminal ganglia obtained from control (PBS) and nicotine (N)-treated animals immunostained using antibodies directed against PKA. Enlarged images of the ganglion that contained numerous neuronal cell bodies and associated satellite glial cells from panel A are shown (A′). The change in PKA staining intensities are reported as the average fold change±SEM as compared to mean levels in control samples that was set equal to 1 (n=3). #P ≤ 0.01 when compared to control values.
Fig. 6.
Nicotine administration increased expression of p-ERK and p-JNK in trigeminal ganglion neurons. (A) Representative images of sections from the V1/V2 region of trigeminal ganglia obtained from control (PBS) and nicotine (N)-treated animals immunostained using antibodies directed against p-ERK (A) or p-JNK (B). Enlarged images of the ganglion that contained numerous neuronal cell bodies and associated satellite glial cells from either panel A (A′) or panel B (B′) are shown. The change in p-ERK or p-JNK staining intensities are reported as the average fold change±SEM as compared to mean levels in control samples that was set equal to 1 (n=3). #P ≤ 0.01 when compared to control values.
Minimal changes in trigeminal ganglion cytokine levels in response to nicotine
To investigate the regulatory effects of prolonged nicotine administration on peripheral cytokine expression, protein lysates from trigeminal ganglia obtained from treated and control animals were used to determine changes in the levels of 29 cytokines by microarray analysis. Interestingly, prolonged nicotine administration had a significant effect on the immunoreactive level of only two of the 29 cytokines when compared to control levels. IP-10 was found to be significantly increased by 1.40±0.11-fold (P=0.015, n=3) over control levels (Table 3). In contrast, GM-CSF was found to be significantly decreased (0.58±0.03, P=0.009, n=3) when compared to control samples. The expression levels of the other 27 cytokines were not statistically different from control levels (Table 4).
Table 3.
Cytokines in the trigeminal ganglion significantly changed compared to control levels in response to systemic nicotine
| Cytokine | Fold change ± SEM | P |
|---|---|---|
| GM-CSF | 0.58 ± 0.03 | 0.009 |
| IP-10 | 1.40 ± 0.11 | 0.015 |
Table 4.
Cytokines in the trigeminal ganglion not significantly changed compared to control levels in response to systemic nicotine
| Cytokine | Fold change ± SEM | P |
|---|---|---|
| Cinc-1 | 0.78 ± 0.19 | 0.394 |
| Cinc-2α | 0.92 ± 0.19 | 0.937 |
| Cinc-3 | 0.72 ± 0.15 | 0.485 |
| CNTF | 1.11 ± 0.10 | 0.699 |
| Fractalkine | 0.85 ± 0.10 | 0.310 |
| sICAM-1 | 0.57 ± 0.07 | 0.394 |
| IFN-γ | 1.00 ± 0.16 | 1.000 |
| IL-1α | 0.75 ± 0.20 | 0.180 |
| IL-1β | 1.02 ± 0.13 | 0.818 |
| IL-1rα | 1.08 ± 0.09 | 0.310 |
| IL-2 | 0.89 ± 0.11 | 0.485 |
| IL-3 | 0.89 ± 0.06 | 0.132 |
| IL-4 | 0.75 ± 0.06 | 0.180 |
| IL-6 | 1.01 ± 0.07 | 1.000 |
| IL-10 | 1.12 ± 0.24 | 0.937 |
| IL-13 | 1.00 ± 0.20 | 0.937 |
| IL-17 | 0.93 ± 0.09 | 0.394 |
| LIX | 1.40 ± 0.11 | 0.093 |
| L-Selectin | 1.33 ± 0.10 | 0.310 |
| MIG | 1.00 ± 0.06 | 0.589 |
| MIP-1α | 1.11 ± 0.10 | 0.310 |
| MIP-3α | 1.29 ± 0.45 | 0.394 |
| RANTES | 1.03 ± 0.02 | 0.589 |
| Thymus chemokine | 1.08 ± 0.08 | 0.699 |
| TIMP-1 | 0.99 ± 0.79 | 0.937 |
| TNF-α | 1.60 ± 0.37 | 0.132 |
| VEGF | 1.26 ± 0.16 | 0.180 |
DISCUSSION
In this study, we found that prolonged nicotine administration increased nociception of V1 and V3 trigeminal neurons to mechanical stimulation. To our knowledge, this is the first evidence that nicotine can promote sustained sensitization of trigeminal neurons. In addition, results from our study demonstrate that systemic nicotine results in elevated level of expression of several signal transduction proteins and cytokines in the spinal trigeminal nucleus and trigeminal ganglion known to promote the development and maintenance of central and peripheral sensitization. Systemic nicotine for 14 days resulted in cellular changes in both primary and secondary nociceptive neurons, and glial cells associated with the peripheral and central nervous systems. Rationale for this study was based on reports that nicotinic receptors are present on sensory neurons and second-order nociceptive neurons, and that nicotine is known to promote neuronal excitability (Liu and Simon, 1996; Delbono et al., 1997; Liu et al., 1998; Michel et al., 2011). The amount of nicotine administered in our study as monitored by serum levels of cotinine, the metabolic product of nicotine, was in the range associated with nicotine dependence (Pentel et al., 2006; Cippitelli et al., 2011). Our finding that systemic nicotine can stimulate expression of proteins associated with neuronal excitability is in agreement with the reported stimulatory effect of nicotine on sensory neurons (Liu and Simon, 1996; Delbono et al., 1997; De Simone et al., 2005; Josiah and Vincler, 2006). Migraineurs are thought to have a hypersensitive nervous system to sensory stimuli including light intensity and patterns, loud or irregular sounds, pungent smells, and foods that can trigger a painful migraine (Dodick and Silberstein, 2006; Pietrobon and Moskowitz, 2013). Based on our findings, we propose that heavy nicotine use, which promotes sensitization of trigeminal neurons, should be considered a potential risk factor for migraine by lowering the activation threshold to other stimuli.
MAP kinases are a family of signal transduction enzymes that mediate cellular activities in response to inflammatory stimuli implicated in central sensitization and the development and maintenance of nociception (Ji et al., 2009). Toward this end, we found that systemic nicotine stimulation of trigeminal neurons resulted in an increase in expression of the active, phosphorylated forms of ERK and JNK in the spinal trigeminal nucleus. Increased expression of p-ERK and p-JNK can mediate sensitization of second-order nociceptive neurons by increasing neuronal ion channel expression and activity, and expression of membrane receptors associated with nociception (Ji, 2004; Ji et al., 2009). In addition, p-ERK and p-JNK are known to stimulate the synthesis and secretion of cytokines from glial cells that facilitate a prolonged hyperexcitable state of neurons (Ji, 2004; Ji et al., 2009). In support of the important role of these signaling proteins in nociception, inhibitors of ERK and JNK are documented to alleviate hyperalgesia and allodynia in inflammatory and neuropathic pain models (Kaminska et al., 2009; Alter et al., 2010). Since these inhibitors only exhibit a minimal inhibitory effect on basal physiological pain perception, it has been suggested that these signaling proteins play a central role in the development of pain hypersensitivity characteristic of abnormal or pathological pain following tissue and nerve injury (Cheng and Ji, 2008; Ji et al., 2009).
In addition to MAP kinases, we observed significantly elevated levels of the signaling protein PKA in the spinal trigeminal nucleus in response to 14 days of systemic nicotine. Increased levels of PKA in the spinal cord are involved in the development of central sensitization by increasing the activity of glutamate receptors expressed on second-order neurons that facilitate pain transmission (Hucho and Levine, 2007; Latremoliere and Woolf, 2009). The stimulatory effects of calcitonin gene-related peptide (CGRP), a protein implicated in migraine pathology and known to promote central sensitization (Pietrobon and Moskowitz, 2013), on spinal cord neurons and glial cells are likely mediated in part via activation of PKA (Seybold, 2009). Binding of CGRP to its receptor couples to increase levels of the second messenger cyclic adenosine monophosphate (cAMP), which is a substrate known to stimulate PKA activity. Thus, increased activity of PKA in response to systemic nicotine can facilitate cellular changes in nociceptive neurons and glial cells as reported for CGRP. This notion is not too surprising since the stimulatory effects of CGRP on cAMP response element (CRE)-dependent gene expression are mediated primarily via activation of the PKA signaling pathway (Anderson and Seybold, 2004; Sun et al., 2004). Activation of intracellular signaling pathways involving PKA is reported to promote the induction and maintenance of central sensitization and persistent pain by phosphorylation of ion channels, and increasing the expression of pro-inflammatory and pro-nociceptive cytokine genes that contain CRE regulatory sites within their promoters (Kawasaki et al., 2004). PKA activity also leads to increased phosphorylation and hence activation of ERK in nociceptive neurons (Kohno et al., 2008). Furthermore, blocking PKA signaling results in reduction of inflammation-induced hyperalgesic behaviors (Malmberg et al., 1997; Aley and Levine, 1999). Taken together, nicotine-mediated increases in PKA levels in the spinal trigeminal nucleus are likely to facilitate neuron-glia communication and stimulate the synthesis and release of pro-inflammatory cytokines from glial cells within the spinal cord to promote central sensitization.
Activation of the glial cells, including astrocytes and microglia, within the spinal cord is known to contribute to prolonged sensitization of nociceptive neurons and development of chronic pain states (Gosselin et al., 2010). Toward this end, we observed increased levels of immunoreactivity of GFAP and Iba1 in spinal cord sections of nicotine-administered animals, indicating the activation of astrocytes and microglia, respectively. Our observed changes in glial cells are in agreement with prior studies, since systemic nicotine, which readily crosses the blood–brain barrier, is known to bind and activate nAch receptors expressed on astrocytes and microglia of the brain and spinal cord (Gahring et al., 2004; De Simone et al., 2005; Lockman et al., 2005). It is now generally accepted that hyperactivation of spinal glia, in particular astrocytes and microglia, contribute to the development and maintenance of inflammatory pain. These cells are thought to promote and sustain inflammatory pain by the release of cytokines that act directly to sensitize nociceptors and increase neuronal sensitivity to chemical, thermal, and mechanical stimuli by increasing the expression of receptors and ion channels involved in pain and analgesia (Ren and Torres, 2009; Uceyler et al., 2009). Based on our data, we propose that systemic nicotine functions to facilitate neuron–glia interactions and cytokine release and therefore stimulates key cellular events known to promote and maintain central sensitization.
Activation of the MAP kinase and PKA signaling pathways can promote synthesis and secretion of cytokines from activated glia cells (Miller et al., 2009). In this study, we observed a significant increase in several cytokine members including IP-10, LIX, L-Selectin, MIG, MIP-1α, TNF-α, and VEGF in spinal cord samples taken from animals exposed to prolonged high levels of nicotine. Cytokines are soluble proteins released from activated glial cells that directly sensitize nociceptors and increase neuronal sensitivity by increasing the expression of receptors and ion channels involved in pain and analgesia (Uceyler et al., 2009). The observed increased expression level of several cytokines would further suggest that prolonged nicotine administration leads to sensitization of the central nervous system by increasing neuron–glial cell interactions in the spinal cord. While we observed increased expression of cytokines in the trigeminal ganglia and upper spinal cord tissues, nicotine caused a more robust response in the spinal cord than seen in the ganglia, which provides further evidence of the ability of systemic nicotine to promote sensitization of second-order nociceptive neurons.
Nicotine caused the greatest increase in the expression of the pro-inflammatory cytokine TNF-α (Leung and Cahill, 2010). Elevated levels of TNF-α are associated with central sensitization and prolonged pain states (Kawasaki et al., 2008; Miller et al., 2009) and elevated cytokine levels have been reported in serum and cerebrospinal fluid in migraine patients (Rozen and Swidan, 2007; Uzar et al., 2011). TNF-α activation of its receptor leads to increased neuronal hyperexcitability and pain via stimulation of MAP kinase pathways (Kant et al., 2011). TNF-α promotes activation of p38 and JNK, but is reported to only cause minimal activation of ERK. In the central nervous system, activation of p38 in microglia due to TNF-α can lead to the microglia secreting the proinflammatory cytokines IL-1 and IL-6 as well as ATP (Leung and Cahill, 2010). Additionally, TNF-α stimulates its own production in astrocytes via the CXCR4 receptor. This self-regulation leads to production of IL-1, IL-6, ATP and nitric oxide, which are factors that contribute to enhanced neuronal activity and thus pathological pain. Taken together, elevated levels of TNF-α in the spinal trigeminal nucleus would facilitate sensitization of second-order neurons and promote an enhanced pain state.
In addition to changes in the spinal trigeminal nucleus, we found that prolonged administration to nicotine could mediate changes in neuronal expression of p-ERK, p-JNK, and PKA, which are signaling proteins implicated in peripheral sensitization in the trigeminal ganglion. Increased levels of p-ERK are associated with a sensitized state of primary nociceptive neurons by upregulating ion channel expression and activity of membrane receptor expression (Ji et al., 2009), while elevated levels of JNK are associated with pain hypersensitivity (Doya et al., 2005). Further evidence of the importance of MAP kinases in the induction of peripheral sensitization and persistent pain is provided by results from studies in which blocking MAP kinase activity with specific inhibitors was reported to suppress nociceptive responses and sensitization (Milligan et al., 2003; Tsuda et al., 2004; Ji et al., 2009). We also observed increased levels of PKA within trigeminal ganglia in response to systemic nicotine. Elevated levels of PKA in the ganglion would promote increased expression of cytokines and other inflammatory molecules that are likely to help sustain a hyperexcitable state. Toward this end, we found that nicotine stimulates expression of the chemokine IP-10, also referred to as CXCL10, in the ganglion. IP-10 is expressed at sites of inflammation where it functions to regulate immune responses via the activation and recruitment of leukocytes (Lee et al., 2009). Interestingly, we found that activation of trigeminal nociceptors increased the expression of Iba1, a protein used as a biomarker of activated macrophage cells (Nakamura et al., 2013), within the functional units of the trigeminal ganglion (Cady et al., 2014). Our results provide evidence that nicotine promotes changes in neurons within the trigeminal ganglia that are consistent with development and maintenance of peripheral sensitization of primary nociceptors.
In summary, we found that sustained elevated systemic nicotine levels similar to those reported in heavy smokers increased sensitivity of trigeminal nociceptive neurons to mechanical stimulation and mediated cellular changes in the trigeminal ganglion and spinal trigeminal nucleus that are implicated in peripheral and central sensitization. Furthermore, our findings provide evidence to support the notion that smoking (nicotine dependency) should be considered a risk factor for migraine (Le et al., 2011; Schramm et al., 2013) by promoting a hyperexcitable state of trigeminal nociceptive neurons.
Acknowledgments
This study was funded by a grant from the National Headache Foundation.
Abbreviations
- cAMP
cyclic adenosine monophosphate
- CGRP
calcitonin gene-related peptide
- CRE
cAMP response element
- GFAP
glial fibrillary acidic protein
- Iba1
ionized calcium-binding adapter molecule 1
- MAPKs
mitogen-activated protein kinases
- nAch
nicotinic acetylcholine
- PBS
phosphate-buffered saline
- p-ERK
phosphorylated-extracellular signal-regulated kinase
- p-JNK
phosphorylated-c-Jun N-terminal kinase
- PKA
protein kinase A
- ROI
regions of interest
- SEM
standard error of the mean
Footnotes
CONFLICT OF INTEREST
The authors of this paper do not have any conflict of interests to report.
References
- Aley KO, Levine JD. Role of protein kinase A in the maintenance of inflammatory pain. J Neurosci. 1999;19:2181–2186. doi: 10.1523/JNEUROSCI.19-06-02181.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter BJ, Zhao C, Karim F, Landreth GE, Gereau RW. Genetic targeting of ERK1 suggests a predominant role for ERK2 in murine pain models. J Neurosci. 2010;30:11537–11547. doi: 10.1523/JNEUROSCI.6103-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson LE, Seybold VS. Calcitonin gene-related peptide regulates gene transcription in primary afferent neurons. J Neurochem. 2004;91:1417–1429. doi: 10.1111/j.1471-4159.2004.02833.x. [DOI] [PubMed] [Google Scholar]
- Buse DC, Loder EW, Gorman JA, Stewart WF, Reed ML, Fanning KM, Serrano D, Lipton RB. Sex differences in the prevalence, symptoms, and associated features of migraine, probable migraine and other severe headache: results of the American Migraine Prevalence and Prevention (AMPP) Study. Headache. 2013;53:1278–1299. doi: 10.1111/head.12150. [DOI] [PubMed] [Google Scholar]
- Cady RJ, Denson JE, Durham PL. Inclusion of cocoa as a dietary supplement represses expression of inflammatory proteins in spinal trigeminal nucleus in response to chronic trigeminal nerve stimulation. Mol Nutr Food Res. 2013;57:996–1006. doi: 10.1002/mnfr.201200630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cady RJ, Denson JE, Sullivan LQ, Durham PL. Dual orexin receptor antagonist 12 inhibits expression of proteins in neurons and glia implicated in peripheral and central sensitization. Neuroscience. 2014;269:79–92. doi: 10.1016/j.neuroscience.2014.03.043. [DOI] [PubMed] [Google Scholar]
- Cady RJ, Hirst JJ, Durham PL. Dietary grape seed polyphenols repress neuron and glia activation in trigeminal ganglion and trigeminal nucleus caudalis. Mol Pain. 2010;6:91. doi: 10.1186/1744-8069-6-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JK, Ji RR. Intracellular signaling in primary sensory neurons and persistent pain. Neurochem Res. 2008;33:1970–1978. doi: 10.1007/s11064-008-9711-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli A, Astarita G, Duranti A, Caprioli G, Ubaldi M, Stopponi S, Kallupi M, Sagratini G, Rodriguez de Fonseca F, Piomelli D, Ciccocioppo R. Endocannabinoid regulation of acute and protracted nicotine withdrawal: effect of FAAH inhibition. PLoS One. 2011;6:e28142. doi: 10.1371/journal.pone.0028142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Simone R, Ajmone-Cat MA, Carnevale D, Minghetti L. Activation of alpha7 nicotinic acetylcholine receptor by nicotine selectively up-regulates cyclooxygenase-2 and prostaglandin E2 in rat microglial cultures. J Neuroinflammation. 2005;2:4. doi: 10.1186/1742-2094-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbono O, Gopalakrishnan M, Renganathan M, Monteggia LM, Messi ML, Sullivan JP. Activation of the recombinant human alpha 7 nicotinic acetylcholine receptor significantly raises intracellular free calcium. J Pharmacol Exp Ther. 1997;280:428–438. [PubMed] [Google Scholar]
- Dodick D, Silberstein S. Central sensitization theory of migraine: clinical implications. Headache. 2006;46(Suppl 4):S182–S191. doi: 10.1111/j.1526-4610.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- Doya H, Ohtori S, Fujitani M, Saito T, Hata K, Ino H, Takahashi K, Moriya H, Yamashita T. C-Jun N-terminal kinase activation in dorsal root ganglion contributes to pain hypersensitivity. Biochem Biophys Res Commun. 2005;335:132–138. doi: 10.1016/j.bbrc.2005.07.055. [DOI] [PubMed] [Google Scholar]
- Gahring LC, Persiyanov K, Rogers SW. Neuronal and astrocyte expression of nicotinic receptor subunit beta4 in the adult mouse brain. J Comp Neurol. 2004;468:322–333. doi: 10.1002/cne.10942. [DOI] [PubMed] [Google Scholar]
- Garrett FG, Hawkins JL, Overmyer AE, Hayden JB, Durham PL. Validation of a novel rat-holding device for studying heat-and mechanical-evoked trigeminal nocifensive behavioral responses. J Orofac Pain. 2012;26:337–344. [PMC free article] [PubMed] [Google Scholar]
- Gosselin RD, Suter MR, Ji RR, Decosterd I. Glial cells and chronic pain. Neuroscientist. 2010;16:519–531. doi: 10.1177/1073858409360822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann D, Hoffmann I. Chemistry and toxicology. In: Burns DM, editor. Smoking and tobacco control monograph no 9. National Cancer Institute; 2012. [Google Scholar]
- Hucho T, Levine JD. Signaling pathways in sensitization: toward a nociceptor cell biology. Neuron. 2007;55:365–376. doi: 10.1016/j.neuron.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Hukkanen J, Jacob P, 3rd, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- Ji RR. Peripheral and central mechanisms of inflammatory pain, with emphasis on MAP kinases. Curr Drug Targets Inflamm Allergy. 2004;3:299–303. doi: 10.2174/1568010043343804. [DOI] [PubMed] [Google Scholar]
- Ji RR, Gereau RW, Malcangio M, Strichartz GR. MAP kinase and pain. Brain Res Rev. 2009;60:135–148. doi: 10.1016/j.brainresrev.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josiah DT, Vincler MA. Impact of chronic nicotine on the development and maintenance of neuropathic hypersensitivity in the rat. Psychopharmacology. 2006;188:152–161. doi: 10.1007/s00213-006-0481-5. [DOI] [PubMed] [Google Scholar]
- Kaminska B, Gozdz A, Zawadzka M, Ellert-Miklaszewska A, Lipko M. MAPK signal transduction underlying brain inflammation and gliosis as therapeutic target. Anat Rec. 2009;292:1902–1913. doi: 10.1002/ar.21047. [DOI] [PubMed] [Google Scholar]
- Kant S, Swat W, Zhang S, Zhang ZY, Neel BG, Flavell RA, Davis RJ. TNF-stimulated MAP kinase activation mediated by a Rho family GTPase signaling pathway. Genes Dev. 2011;25:2069–2078. doi: 10.1101/gad.17224711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki Y, Kohno T, Zhuang ZY, Brenner GJ, Wang H, Van Der Meer C, Befort K, Woolf CJ, Ji RR. Ionotropic and metabotropic receptors, protein kinase A, protein kinase C, and Src contribute to C-fiber-induced ERK activation and cAMP response element-binding protein phosphorylation in dorsal horn neurons, leading to central sensitization. J Neurosci. 2004;24:8310–8321. doi: 10.1523/JNEUROSCI.2396-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci. 2008;28:5189–5194. doi: 10.1523/JNEUROSCI.3338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno T, Wang H, Amaya F, Brenner GJ, Cheng JK, Ji RR, Woolf CJ. Bradykinin enhances AMPA and NMDA receptor activity in spinal cord dorsal horn neurons by activating multiple kinases to produce pain hypersensitivity. J Neurosci. 2008;28:4533–4540. doi: 10.1523/JNEUROSCI.5349-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le H, Tfelt-Hansen P, Skytthe A, Kyvik KO, Olesen J. Association between migraine, lifestyle and socioeconomic factors: a population-based cross-sectional study. J Headache Pain. 2011;12:157–172. doi: 10.1007/s10194-011-0321-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EY, Lee ZH, Song YW. CXCL10 and autoimmune diseases. Autoimmun Rev. 2009;8:379–383. doi: 10.1016/j.autrev.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Leung L, Cahill CM. TNF-alpha and neuropathic pain–a review. J Neuroinflammation. 2010;7:27. doi: 10.1186/1742-2094-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Chang GQ, Jiao YQ, Simon SA. Neuronal nicotinic acetylcholine receptors in rat trigeminal ganglia. Brain Res. 1998;809:238–245. doi: 10.1016/s0006-8993(98)00862-2. [DOI] [PubMed] [Google Scholar]
- Liu L, Simon SA. Capsaicin and nicotine both activate a subset of rat trigeminal ganglion neurons. Am J Physiol. 1996;270:C1807–C1814. doi: 10.1152/ajpcell.1996.270.6.C1807. [DOI] [PubMed] [Google Scholar]
- Lockman PR, McAfee G, Geldenhuys WJ, Van der Schyf CJ, Abbruscato TJ, Allen DD. Brain uptake kinetics of nicotine and cotinine after chronic nicotine exposure. J Pharmacol Exp Ther. 2005;314:636–642. doi: 10.1124/jpet.105.085381. [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Brandon EP, Idzerda RL, Liu H, McKnight GS, Basbaum AI. Diminished inflammation and nociceptive pain with preservation of neuropathic pain in mice with a targeted mutation of the type I regulatory subunit of cAMP-dependent protein kinase. J Neurosci. 1997;17:7462–7470. doi: 10.1523/JNEUROSCI.17-19-07462.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel K, Michaelis M, Mazzuoli G, Mueller K, Vanden Berghe P, Schemann M. Fast calcium and voltage-sensitive dye imaging in enteric neurones reveal calcium peaks associated with single action potential discharge. J Physiol. 2011;589:5941–5947. doi: 10.1113/jphysiol.2011.219550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RJ, Jung H, Bhangoo SK, White FA. Cytokine and chemokine regulation of sensory neuron function. Handb Exp Pharmacol. 2009;194:417–449. doi: 10.1007/978-3-540-79090-7_12. http://dx.doi.org/10.1007/978-3-540-79090-7_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan ED, Twining C, Chacur M, Biedenkapp J, O’Connor K, Poole S, Tracey K, Martin D, Maier SF, Watkins LR. Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain in rats. J Neurosci. 2003;23:1026–1040. doi: 10.1523/JNEUROSCI.23-03-01026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura R, Nishimura T, Ochiai T, Nakada S, Nagatani M, Ogasawara H. Availability of a microglia and macrophage marker, iba-1, for differential diagnosis of spontaneous malignant reticuloses from astrocytomas in rats. J Toxicol Pathol. 2013;26:55–60. doi: 10.1293/tox.26.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikawa H, Nakamichi N, Kambe Y, Ogura M, Yoneda Y. An increase in intracellular free calcium ions by nicotinic acetylcholine receptors in a single cultured rat cortical astrocyte. J Neurosci Res. 2005;79:535–544. doi: 10.1002/jnr.20398. [DOI] [PubMed] [Google Scholar]
- Pentel PR, Dufek MB, Roiko SA, Lesage MG, Keyler DE. Differential effects of passive immunization with nicotine-specific antibodies on the acute and chronic distribution of nicotine to brain in rats. J Pharmacol Exp Ther. 2006;317:660–666. doi: 10.1124/jpet.105.097873. [DOI] [PubMed] [Google Scholar]
- Pietrobon D, Moskowitz MA. Pathophysiology of migraine. Annu Rev Physiol. 2013;75:365–391. doi: 10.1146/annurev-physiol-030212-183717. [DOI] [PubMed] [Google Scholar]
- Ren K, Torres R. Role of interleukin-1beta during pain and inflammation. Brain Res Rev. 2009;60:57–64. doi: 10.1016/j.brainresrev.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozen T, Swidan SZ. Elevation of CSF tumor necrosis factor alpha levels in new daily persistent headache and treatment refractory chronic migraine. Headache. 2007;47:1050–1055. doi: 10.1111/j.1526-4610.2006.00722.x. [DOI] [PubMed] [Google Scholar]
- Rozen TD. A history of cigarette smoking is associated with the development of cranial autonomic symptoms with migraine headaches. Headache. 2011;51:85–91. doi: 10.1111/j.1526-4610.2010.01707.x. [DOI] [PubMed] [Google Scholar]
- Schramm SH, Obermann M, Katsarava Z, Diener HC, Moebus S, Yoon MS. Epidemiological profiles of patients with chronic migraine and chronic tension-type headache. J Headache Pain. 2013;14:40. doi: 10.1186/1129-2377-14-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seybold VS. The role of peptides in central sensitization. Handb Exp Pharmacol. 2009;194:451–491. doi: 10.1007/978-3-540-79090-7_13. [DOI] [PubMed] [Google Scholar]
- Shi Y, Weingarten TN, Mantilla CB, Hooten WM, Warner DO. Smoking and pain: pathophysiology and clinical implications. Anesthesiology. 2010;113:977–992. doi: 10.1097/ALN.0b013e3181ebdaf9. [DOI] [PubMed] [Google Scholar]
- Sun R, Tu Y, Lawand N, Yan J, Lin Q, Willis W. Calcitonin gene-related peptide receptor activation produces PKA- and PKC-dependent mechanical hyperalgesia and central sensitization. J Neurophysiol. 2004;92:2859–2866. doi: 10.1152/jn.00339.2004. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Mizokoshi A, Shigemoto-Mogami Y, Koizumi S, Inoue K. Activation of p38 mitogen-activated protein kinase in spinal hyperactive microglia contributes to pain hypersensitivity following peripheral nerve injury. Glia. 2004;45:89–95. doi: 10.1002/glia.10308. [DOI] [PubMed] [Google Scholar]
- Uceyler N, Schafers M, Sommer C. Mode of action of cytokines on nociceptive neurons. Exp Brain Res. 2009;196:67–78. doi: 10.1007/s00221-009-1755-z. [DOI] [PubMed] [Google Scholar]
- Uzar E, Evliyaoglu O, Yucel Y, Ugur Cevik M, Acar A, Guzel I, Islamoglu Y, Colpan L, Tasdemir N. Serum cytokine and pro-brain natriuretic peptide (BNP) levels in patients with migraine. Eur Rev Med Pharmacol Sci. 2011;15:1111–1116. [PubMed] [Google Scholar]
- Vause CV, Durham PL. Identification of cytokines and signaling proteins differentially regulated by sumatriptan/naproxen. Headache. 2012;52:80–89. doi: 10.1111/j.1526-4610.2011.02048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten TN, Moeschler SM, Ptaszynski AE, Hooten WM, Beebe TJ, Warner DO. An assessment of the association between smoking status, pain intensity, and functional interference in patients with chronic pain. Pain Physician. 2008;11:643–653. [PubMed] [Google Scholar]
- Zvolensky MJ, McMillan K, Gonzalez A, Asmundson GJ. Chronic pain and cigarette smoking and nicotine dependence among a representative sample of adults. Nicotine Tob Res. 2009;11:1407–1414. doi: 10.1093/ntr/ntp153. [DOI] [PMC free article] [PubMed] [Google Scholar]