Abstract
Aims
To test the reliability and validity of a novel rat-holding device designed to be used in conjunction with the plantar test apparatus for studying nocifensive behavioral responses in an established model of temporomandibular joint (TMJ) pathology.
Methods
Thirty-five young adult male Sprague-Dawley rats were used. Withdrawal latencies in response to infrared 40 heat stimulation of the submandibular region in naïve animals (n = 4) and animals injected with saline or complete Freund’s adjuvant (CFA) in the TMJ (n > 9) were measured over a 2-week time period. Nocifensive responses to mechanical stimulation of the cutaneous tissue directly over the TMJ with von Frey filaments were investigated in animals injected with CFA in the TMJ (n = 6). The effect on nocifensive responses to heat and mechanical stimulation of subcutaneous administration of buprenorphine (0.05 mg/kg) into the hindquarter was assessed in CFA and cotreated animals (n = 6). Statistical analysis was performed using a nonparametric Mann-Whitney U test.
Results
Under basal conditions, withdrawal latencies to heat stimulation of the orofacial region remained consistently around 15 seconds over 14 days. Unilateral CFA injection in the TMJ significantly decreased heat-withdrawal latencies on days 1, 2, 7, and 14 in the ipsilateral side (P < .05), but not contralateral side, when compared with basal values. CFA also significantly decreased the nocifensive threshold to mechanical stimulation on days 1, 2, and 7 postinjection (P < .05). CFA-mediated changes in heat withdrawal and mechanical thresholds in the orofacial region were significantly suppressed by subcutaneous administration of buprenorphine into the hindquarter (P < .05).
Conclusion
Findings from this study provide evidence to validate the use of this holding device for studying nocifensive behaviors in the orofacial region of rats in response to heat or mechanical orofacial stimulation.
Keywords: inflammation, plantar test, TMJ, trigeminal, von Frey
Traditionally, the plantar test, based on the Hargreaves method,1 is used to measure thermal sensitivity in the plantar surface of the hindpaw and is therefore useful for correlating behavioral changes with cellular events in the dorsal root ganglia (DRG) and the spinal dorsal horn. The plantar test uses radiant heat produced by an infrared (IR) generator to measure cutaneous hyperalgesia in unrestrained rodents. In both rodents and humans, heat stimuli via IR lasers elicits a stinging or burning sensation mediated through activation of peripheral endings of Aδ- and C-fiber nociceptors.2–5 The ability to study mechanisms involved in sensitization and activation of DRG nociceptors, and correlate these cellular changes with behavioral events, has enabled researchers to test new pharmaceutical therapies and gain a better overall understanding of somatic pain pathologies. However, the majority of research has been limited to models of somatic, inflammatory, and neuropathic pain mediated by the DRG and the spinal (lumbar) dorsal horn.
Although the head and face represent some of the most common sites of pain,6 behavioral studies on orofacial pain models have been limited. Trigeminal nerves provide sensory innervation to much of the head and face and function to relay nociceptive information from peripheral tissues to the trigeminal ganglia and the trigeminal brainstem nuclei.6,7 There are three main branches of the trigeminal nerve—the ophthalmic (V1), maxillary (V2), and mandibular (V3). Activation and periphereal sensitization of trigeminal nerves in response to noxious or inflammatory mediators, as well as trigeminal central sensitization, are implicated in the pathology of migraine, sinusitis, and temporomandibular disorders (TMD).6,8,9 As much as 15% of the adult population is affected by TMD, which is a chronic condition characterized by pain in the muscles and/or temporomandibular joint (TMJ) associated with mastication.10,11 TMD is more prevalent in women than men and is highest during the reproductive years.12–14 Given the significant health impact of these diseases, it would be beneficial to have a better understanding of their underlying pathophysiology. Studies in TMD patients of thermal and mechanical pain sensitivity, which can involve peripheral and central sensitization, have provided evidence of thermal hyperalgesia in orofacial areas, including the TMJ and masseter muscle.15–18 While much progress has been made in understanding cellular events associated with TMD, the study of pain-related behaviors following trigeminal nerve activation has been hampered by the lack of a simple standardized objective measurement of thermal and mechanical hyperalgesia in rodents. The ability to measure nocifensive thresholds in animal models of orofacial pain is an essential requirement in pain and pharmacology research since it is considered an indirect measure of nociception and allows for direct correlation with cellular and molecular events. However, studying thermal and mechanical sensitivity in the face poses a challenge since it is difficult to position the animals in a manner conducive for testing nocifensive responses.
The goal of this study was to test the reliability and validity of a novel holding device for rats, which the authors’ laboratory originally designed, that situates the animal so there is no need for physical restraint by the investigator when investigating nocifensive responses to heat or mechanical stimuli in the orofacial region. In initial experiments to test reliability, withdrawal latencies were measured in the submandibular region in response to IR stimulation of naïve animals. Next, changes in heat sensitivity were investigated in an established inflammatory model of TMJ following injection of complete Freund’s adjuvant (CFA) or vehicle in the TMJ for 2 weeks postinjection. To test the feasibility of using the device for evaluating sensitivity in the orofacial region to mechanical stimuli, nocifensive responses to pressure applied with von Frey filaments to the cutaneous tissue directly over the TMJ was investigated in animals injected with CFA in the TMJ. To determine whether the device alters an expected physiologic response to an opioid, behavioral responses to heat and mechanical stimuli were determined in animals that were injected with CFA or that received a subcutaneous injection of buprenorphine 1 hour prior to testing.
Materials and Methods
Animals
Thirty-five adult male Sprague-Dawley rats initially weighing between 175 and 200 g were used. Animals were housed in clean plastic cages on a 12-hour light/dark cycle (8 am to 8 pm) with unrestricted access to food and water. All animals were acclimated to the facility for 1 week prior to start of experiments. The housing conditions and experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Missouri State University, Springfield, Missouri, USA. Every effort was made to minimize suffering and reduce the number of animals used in the study.
Inflammatory Agent and Drugs
CFA (Sigma-Aldrich) was prepared as a 1:1 CFA/0.9% sodium chloride (NaCl) emulsion. Buprenorphine hydrochloride (0.3 mg/mL; Webster Veterinary) was administered at a dose of 0.05 mg/kg.
Instrumentation
The rat-holding devices, manufactured in accordance with the authors’ original design and dimensions, (cat. no. 37100, Ugo Basile), were molded using red plastic to minimize incoming light and reduce stress to the animals. The anterior end of the device was contoured to limit head movement and facilitate direct contact of the skin covering the submandibular region with the glass platform of the Ugo Basile Plantar Test apparatus (cat. no. 37370, Ugo Basile). The posterior end contains a series of slits at the top of the device in which a restraining block can be placed to secure the animal in the optimal position and minimize movement. To facilitate mechanical testing, a 1.0 × 0.3-cm slit was cut into both sides of the device to allow access to the cutaneous tissue overlying the TMJ and masseter muscle. To accommodate different sized animals, small and large holding devices were used during the 14-day testing period. Throughout the acclimation period and the first 7 days of testing, the smaller unit was used to hold animals ranging from 270 to 375 g. Occasionally, the larger device was required for holding animals weighing more than 375 g at the 14-day time point.
Acclimation to Holding Device
Following the initial 1-week acclimation period, each animal was placed in the holding device for 5 minutes each day for 3 consecutive days. During this study, approximately 5% of the animals placed in the holding device failed to readily and reliably rest their submandibular region on the glass surface and were not included in further studies. Testing took place at the same time each day (8 am to 10 am) in a quiet room designated only for behavioral studies.
Behavioral Assessment
Heat Nocifensive Behavior
Following the third day of acclimation, the animals used for testing withdrawal latencies in response to heat stimulation of the submandibular region were anesthetized by inhalation of 3% isoflurane. The hair in the submandibular region was trimmed using clippers (PG-250, Remington) to reduce refraction of the Hargreaves IR beam of the heating apparatus.1 No skin irritation was noted. Animals were trimmed on the same day each week for the duration of the testing. Baseline readings were taken 48 hours after the initial shaving. The animals were placed in the device, and once quiescent, the movable IR source was positioned at the midline of the submandibular region for naïve unstimulated animals or under the left (ipsilateral) or right (contralateral) mandible for animals injected unilaterally with CFA in the TMJ capsule. The IR intensity for all facial testing was set at a numerical value of 40 (145 to 150 mW/cm2). Two researchers were required for taking behavioral readings; one individual was responsible for operating the plantar test control unit and recording withdrawal latency, while the other researcher, who was blinded to the experimental condition, positioned the movable IR source and noted avoidance behaviors. The IR source was turned off and the withdrawal latency recorded as soon as an avoidance behavior, which was characterized by a sudden movement of the head, was detected. Typically, the animal either pulled its head directly back or quickly turned its head to one side to avoid the thermal stimulation. A total of five readings, with 30 seconds between each exposure, were taken at the midline for the naïve studies. Likewise, five readings were taken under the mandible, alternating right and left, for the unilateral studies.
Mechanical Nocifensive Behavior
Prior to mechanical testing, animals were conditioned for 5 minutes on 3 consecutive days to a mechanical stimulus by gently rubbing the hair follicles and epidermis in the TMJ region of the face with the tip of a pipette. Mechanical nocifensive thresholds were determined in response to a series of calibrated von Frey filaments (15, 26, 60, 100, 180, and 300 g) applied in increasing force to the skin over the TMJ. The researcher responsible for directly testing the response to each filament was blinded to the experimental conditions. A positive response, which was defined by head withdrawal prior to the bending of the filament, was recorded by a second researcher. Each filament was applied five times, and the data are reported as the mean number of responses obtained from five applications of each specific calibrated filament.
Inflammatory Hyperalgesia
To evaluate hyperaglesic responses to prolonged TMJ inflammation, rats were anesthetized by inhalation of 3% isoflurane and injected unilaterally in the left TMJ capsule with 50 μL of CFA. To serve as vehicle controls in some studies, a separate group of animals was injected in the TMJ with 0.9% saline (50 μL). Injections of either CFA or 0.9% saline were performed immediately following the basal readings. For the thermal behavioral studies, subsequent testing was performed at four time points postinjection (1, 2, 7, and 14 days). Similarly, baseline threshold nocifensive responses to mechanical stimulation were obtained prior to CFA injection into the TMJ in addition to four additional time points after CFA injection (1, 2, 7, and 14 days).
Pharmacologic Treatments
To test the antinociceptive effects of buprenorphine hydrochloride on thermal sensitivity in the submandibular region and mechanical sensitivity in the region over the TMJ, buprenorphine hydrochloride (0.05 mg/kg) was injected subcutaneously into the hindquarter 1 hour prior to thermal and mechanical testing.
Statistical Analysis
Each condition was repeated in a minimum of three independent experiments. For the thermal stimulation studies, the data are reported as a mean change in CFA-induced withdrawal latency (seconds) for each time point compared with the mean basal value, which was 0. For the mechanical stimulation studies, the data are reported as the mean number of withdrawal responses ± standard error of the mean (SEM) to 60 g of force at each time point. Due to unequal variances as determined by the Leven test, a nonparametric Mann-Whitney U test using SPSS 16.0 (IBM) was conducted to determine statistical differences. Differences were considered to be significant at P ≤ .05.
Results
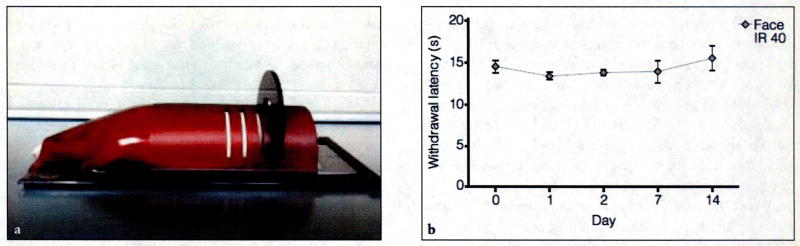
The design and dimensions of the holding device provided an environment conducive for the rats to naturally rest the submandibular region of their head on the glass surface (Fig 1a). In addition, the design of the device facilitated the rats going all the way to the front of the device with minimal physical contact.
Fig. 1.
(a) Side view of behavioral holding device with rat positioned inside. (b) Response to thermal IR stimulation of the V3 branch of the trigeminal nerve (IR 40) in unstimulated control animals. Withdrawal latency remained relatively unchanged throughout the 14-day testing period in the orofacial region.
Reliability of Holding Device for Measuring Heat Sensitivity
To test the reliability of obtaining consistent thermal withdrawal thresholds in animals placed in the holding device, the investigators initially measured cutaneous thermal sensitivity in the submandibular region in naïve, unstimulated animals. The IR source was directed at the midline of the submandibular region, and withdrawal latency values were collected at five points (days 0, 1, 2, 7, and 14) over a 2-week period. In the holding device, animals subjected to heat stimulation of the submandibular region (n = 4) displayed consistent withdrawal latencies (seconds) throughout the 2-week testing period (Fig 1b). None of the values differed significantly from baseline levels (P > .05). These results demonstrate that heat sensitivity can be consistently measured in the submandibular region following activation of trigeminal afferents.
Heat Nocifensive Behavior in Response to CFA Injection into the TMJ
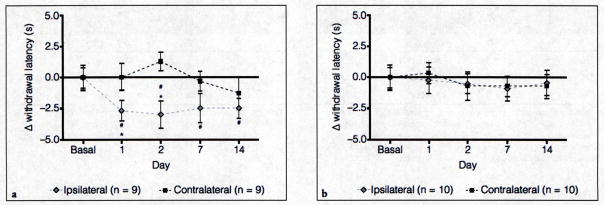
To determine whether the use of the holding device would yield similar results in a well-established chronic joint inflammation model, CFA was injected into the left TMJ (n = 9). In response to CFA-induced inflammation, there was a significant reduction in thermal withdrawal latency on the ipsilateral side at days 1, 2, 7, and 14 when compared with baseline values, the mean of which was set to 0 (Fig 2a). In contrast to the changes observed on the ipsilateral side, a significant change from baseline values was not seen at any of the time points (see Fig 2a) on the contralateral side. To serve as vehicle controls (n = 10), some animals were injected with 0.9% saline into the left TMJ capsule. Importantly, the withdrawal latency in these control animals did not differ significantly from baseline levels on the ipsilateral or contralateral sides throughout the 2-week testing period (Fig 2b).
Fig. 2.
(a) Response to thermal IR stimulation of the V3 region in animals injected with CFA into the left TMJ capsule. (b) Response to thermal IR stimulation in the submandibular region in animals injected unilaterally with 0.9% saline. #Indicates P < .05 when compared with basal values and *Indicates P < .05 when comparing ipsilateral with contralateral latencies.
Mechanical Nocifensive Behavior in Response to CFA Injection into the TMJ
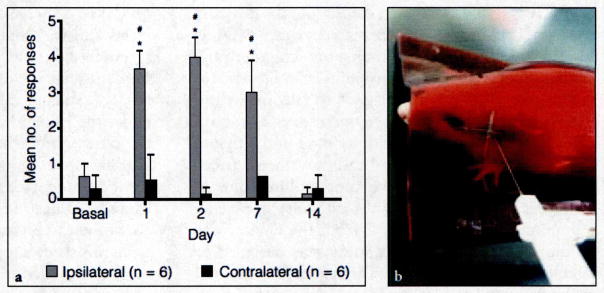
To determine if the holding device could also be used to measure mechanical sensitivity in response to CFA-induced TMJ inflammation, nocifensive thresholds were assessed with von Frey filaments applied to the cutaneous region directly over the joint. Initially, baseline withdrawal values were obtained for the full range of von Frey filaments (n = 6; data not shown). The 60 g force was chosen for all subsequent studies since at this force the number of withdrawal responses was less than 20% for both left and right TMJs (13.3% ± 7.3% and 6.7% ± 7.3%, respectively). Immediately after basal readings, CFA was injected into the left TMJ of each animal. A significant increase in the number of nocifensive responses was observed in the ipsilateral TMJ compared with the basal values and the contralateral side on days 1, 2, and 7 after the CFA injection (Fig 3). Resolution of mechanical sensitivity in the ipsilateral joint was seen on day 14 with the number of withdrawal responses returning to near baseline values. In contrast, the number of withdrawal responses to mechanical stimulation of the contralateral joint was not significantly greater than basal levels at any time point evaluated in this study (see Fig 3).
Fig. 3.
(a) Nocifensive withdrawal responses to a mechanical stimulus applied to the cutaneous tissue directly over the TMJ of animals tested. #Indicates P < .05 when compared with basal values and *indicates P < .05 when comparing ipsilateral with contralateral withdrawal responses. (b) Site of mechanical stimulation.
Effect of Buprenorphrine on Heat and Mechanical Nocifensive Behaviors
To assess the effect of systemic opioid administration on heat and mechanical thresholds, animals were injected with buprenorphine 1 hour prior to testing 1 and 2 days after CFA injection into the TMJ (n = 3 for each experimental condition). Pre-treatment with buprenorphine effectively blocked CFA-induced heat withdrawal latencies in the submandibular region (Table 1, n = 3) on days 1 and 2 (P < .05). In contrast, none of the animals injected with buprenorphine exhibited a positive withdrawal response at even 30 seconds, which was set as the cutoff value to avoid causing injury to the animal. Similarly, buprenorphine completely repressed the number of CFA-induced nocifensive responses in the TMJ in response to mechanical stimuli of 60 g over the joint capsule (Table 2, n = 3) 1 and 2 days (P < .05) postinjection.
Table 1.
Buprenorphine Blocks CFA-induced Heat Withdrawal Latencies in Submandibular Area
Data are represented at the average withdrawal latency (seconds) ± SEM. n ≥ 3 for all conditions.
The cutoff value used for these studies was 30 seconds. Animals that did not respond within this time were assigned a withdrawl latency value of 30.0 seconds. CFA, complete Freund’s adjuvant; BUP, buprenorphine.
Table 2.
Buprenorphine Blocks CFA-induced Hyperalgesia in Response to Mechanical Stimuli of 60 g over the TMJ
| CFA | CFA + BUP | P | |
|---|---|---|---|
| Basal | 0.7 ± 0.4 | 1.3 ± 0.8 | .334 |
| D1 | 3.7 ± 0.5 | 0 ± 0 | .017 |
| D2 | 4.0 ± 0.6 | 0 ± 0 | .016 |
Data are presented as the mean number of nocifensive responses ± SEM out of five stimulations, n ≥ 3 for all conditions. SEM, standard error of the mean; CFA, complete Freund’s adjuvant; BUP, buprenorphine.
Discussion
The reliability and validity of a novel, commercially available holding device that can be used in conjunction with the plantar test apparatus to study changes in behavioral hyperalgesia in the orofacial region of rats was tested. The overall design of the device is based on cylinder-type restraining devices.19,20 While several plastic tube restrainers are commercially available for performing physiologic studies on rodents, none of them are designed to adequately immobilize the head. Therefore, these devices cannot be used to obtain heat sensitivity data in the tissues of the head and face, since head movements occur randomly and sporadically. Another limitation of other restraining devices is the difficulty in placing the animal in or removing it from the device. The holding device used in this study was designed to circumvent these problems. The device is contoured at the front end to limit freedom, such that the head of the rat is naturally positioned in close proximity (in contact) with the glass surface of the apparatus. To facilitate placing the animal in the device, a small opening in the front allows the nose of the rat to slightly protrude. The inclusion of slits in the posterior end allows a plastic sheet to be inserted to minimize the animal’s movements. Typically, the rats would willingly move all the way to the front of the device with minimal physical contact. Another important feature of the device was the use of a durable red plastic material that selectively filters ambient light from entering the device, thereby minimizing stress by creating an opaque environment. Taken together, this holding device provides an enclosure that the animals readily enter with minimal handling, quickly become quiescent, and naturally rest their head on the glass surface, a prerequisite for reliably measuring thermal sensitivity in the orofacial region when using the plantar test apparatus. In this study, withdrawal responses to heat stimulation of the submandibular region of naïve rats remained relatively constant over 2 weeks. This finding provides evidence of the reliability of the holding device to obtain consistent heat sensitivity measurements in the orofacial region of rats.
Unilateral injection of CFA, which is an adjuvant used to cause prolonged inflammation of the TMJ,21,22 was shown to cause a significant decrease in heat withdrawal latencies on the ipsilateral side on days 1, 2, 7, and 14 when compared with basal levels and was significantly different from the contralateral side on days 1 and 2 postinjection. In addition, the injection of saline, which served as a vehicle for CFA, did not cause a change in heat sensitivity in either the ipsilateral or contralateral sides at any of the time points when compared to baseline values. Findings from this study are in agreement with heat responses reported in other studies of orofacial pain caused by injection of CFA either into the TMJ capsule or into the masseter muscle.23,24 Thus, results from this study support the utility of the holding device in conjunction with the plantar test apparatus as a nonintrusive method for studying nocifensive responses to heat activation of trigeminal afferents.
A simple modification of the holding device allowed measurement of mechanical sensitivity in the cutaneous area directly over the TMJ. CFA injection into the left TMJ resulted in a significant increase in the number of nocifensive withdrawal responses on days 1, 2, and 7, a behavior that was no longer observed on day 14. Results from this study are similar to those reported by other investigators who showed that CFA injection into the TMJ mediated prolonged mechanical allodynia in the ipsilateral but not contralateral joint.25,26 However, increased Fos expression, which is used as a marker of nociceptive neuronal activation, has been reported on the contralateral side in the trigeminal brainstem nuclei following unilateral injection of CFA.27 In that study, behavioral studies were not performed so it is not known if increased Fos expression on the contralateral side would have correlated with nocifensive responses. An advantage of using the holding device is that physically holding the animal is not required as reported in other mechanical sensitivity studies in the orofacial region of rats.24,28 In this study, animals quickly became acclimated to the device and no additional conditioning or training of the animals was required, as described by others26 in which animals had limited access to water prior to behavioral testing. In that study, animals were trained to continue drinking even during noxious mechanical stimulation of the lateral face. In addition, although the use of a restraining device to facilitate mechanical sensitivity in the orofacial region has been referred to in the literature, details of the acrylic holder29 were not provided to facilitate replicating these studies. Another potential advantage of the holding device used in the present study, when compared to other methods, is that restraint of animals in their device allows both heat and mechanical nocifensive responses to be measured in the same animal. Data from this study provided evidence of the feasibility for studying nocifensive behaviors following activation of trigeminal afferents innervating the TMJ capsule. Although the holding device was used to measure changes in sensitivity evoked by stimulation of areas innervated by the V3 branch of the trigeminal nerve, mechanical sensitivity could also be determined in cutaneous tissue covering other orofacial areas innervated by V1 or V2 nerves such as the eyebrow or whisker pad, respectively.
To further validate the use of the holding device, it was demonstrated that CFA-induced decreases in heat and mechanical sensitivity in the orofacial region were effectively blocked by pretreatment with the opioid receptor agonist buprenorphine. This finding is in agreement with a previously published study in which buprenorphine was shown to block the effects of CFA on trigeminal neurons.30 Opioid analgesics such as buprenorphine are known to suppress pain by blocking pain transmission from peripheral tissues to the central nervous system and activating neurons in the descending pain-inhibitory pathway.31 In support of the antinociceptive effects of buprenorphine seen in this study, results from other TMJ models that involve activation of trigeminal nociceptors have demonstrated that treatment with other opioids can also suppress nocifensive responses and inflammatory cellular events in the TMJ.30–35
Conclusions
Data from this study validate the use of a novel holding device for measuring responses to heat and mechanical stimulation in a rat model of orofacial (TMJ) inflammation. Furthermore, the holding device used in this study provides a simple reliable method for measuring heat and mechanical behavioral changes, which will facilitate studies aimed at providing a better understanding of pain mechanisms associated with orofacial diseases.
Acknowledgments
The authors would like to thank Thomas Lopez for technical advice and Larry Vause for technical advice and construction of the prototype holding device.
Contributor Information
Filip G. Garrett, Senior Research Scientist, Center for Biomedical and Life Sciences, Missouri State University, Springfield, Missouri, USA.
Jordan L. Hawkins, Research Technician II, Center for Biomedical and Life Sciences, Missouri State University, Springfield, Missouri, USA.
Allison E. Overmyer, Animal Facilities Manager, Center for Biomedical and Life Sciences, Missouri State University, Springfield, Missouri, USA.
Joshua B. Hayden, Research Assistant, Center for Biomedical and Life Sciences, Missouri State University, Springfield, Missouri, USA.
Paul L. Durham, Professor, Department of Biology, Center for Biomedical and Life Sciences, Missouri State University, Springfield, Missouri, USA.
References
- 1.Hargreaves K, Dubner R, Brown F, et al. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 2.Bromm B, Treede RD. Nerve fibre discharges, cerebral potentials, and sensations induced by CO2 laser stimulation. Hum Neurobiol. 1984;3:33–40. [PubMed] [Google Scholar]
- 3.Treede RD. Peripheral acute pain mechanisms. Ann Med. 1995;27:213–216. doi: 10.3109/07853899509031961. [DOI] [PubMed] [Google Scholar]
- 4.Magerl W, Ali Z, Ellrich J, et al. C- and A delta-fiber components of heat-evoked cerebral potentials in healthy human subjects. Pain. 1999;82:127–137. doi: 10.1016/S0304-3959(99)00061-5. [DOI] [PubMed] [Google Scholar]
- 5.Tzabazis A, Klyukinov M, Manering N, et al. Differential activation of trigeminal C or Adelta nociceptors by infrared diode laser in rats: Behavioral evidence. Brain Res. 2005;1037:148–156. doi: 10.1016/j.brainres.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 6.Sessle B. Acute and chronic craniofacial pain: Brainstem mechanisms of nociceptive transmission and neuroplasticity and their clinical correlates. Crit Rev Oral Biol Med. 2000;11:57–91. doi: 10.1177/10454411000110010401. [DOI] [PubMed] [Google Scholar]
- 7.Shankland WE., II The trigeminal nerve. Part I: An overview. Cranio. 2000;18:238–248. doi: 10.1080/08869634.2000.11746137. [DOI] [PubMed] [Google Scholar]
- 8.Bereiter DA, Okamoto K, Bereiter DF. Effect of persistent monoarthritis of the temporomandibular joint region on acute mustard oil-induced excitation of trigeminal subnucleus caudalis neurons in male and female rats. Pain. 2005;117:58–67. doi: 10.1016/j.pain.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Shimizu K, Guo W, Wang H, et al. Differential involvement of trigeminal transition zone and laminated subnucleus caudalis in orofacial deep and cutaneous hyperalgesia: The effects of interleukin-10 and glial inhibitors. Mol Pain. 2009;5:75. doi: 10.1186/1744-8069-5-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Jundi MA, John MT, Setz JM, et al. Meta-analysis of treatment need for temporomandibular disorders in adult nonpatients. J Orofac Pain. 2008;22:97–107. [PubMed] [Google Scholar]
- 11.Herb K, Cho S, Stiles M. Temporomandibular joint pain and dysfunctioning. Curr Pain Headache Rep. 2006;10:408–414. doi: 10.1007/s11916-006-0070-7. [DOI] [PubMed] [Google Scholar]
- 12.Dao TT, LeResche L. Gender differences in pain. J Orofac Pain. 2000;14:169–184. [PubMed] [Google Scholar]
- 13.Riley JL, III, Gilbert GH. Orofacial pain symptoms: An interaction between age and sex. Pain. 2001;90:245–256. doi: 10.1016/S0304-3959(00)00408-5. [DOI] [PubMed] [Google Scholar]
- 14.Shinal RM, Fillingim RB. Overview of orofacial pain: Epidemiology and gender differences in orofacial pain. Dent Clin North Am. 2007;51:1–18. doi: 10.1016/j.cden.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Raphael KG, Janal MN, Anathan S, et al. Temporal summation of heat pain in temporomandibular disorder patients. J Orofac Pain. 2009;23:54–64. [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandez-de-las-Penas C, Galan-del-Rio F, Ortega-Santiago R, et al. Bilateral thermal hyperalgesia in trigeminal and extra-trigeminal regions in patients with myofascial temporomandibular disorders. Exp Brain Res. 2010;202:171–179. doi: 10.1007/s00221-009-2121-x. [DOI] [PubMed] [Google Scholar]
- 17.Park JW, Clark GT, Kim YK, et al. Analysis of thermal pain sensitivity and psychological profiles in different subgroups of TMD patients. Int J Oral Maxillofac Surg. 2010;39:968–974. doi: 10.1016/j.ijom.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Svensson P, Cairns BE, Wang K, et al. Glutamate-evoked pain and mechanical allodynia in the human masseter muscle. Pain. 2003;101:221–227. doi: 10.1016/S0304-3959(02)00079-9. [DOI] [PubMed] [Google Scholar]
- 19.Pitts WG. Rodent restrainer for biomicroscopy. Am J Optom Physiol Opt. 1976;53:154–155. doi: 10.1097/00006324-197603000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Warden SJ, Bennell KL, McMeeken JM, et al. A technique for restraining rodents during hindlimb interventions. Contemp Top Lab Anim Sci. 2000;39:24–27. [PubMed] [Google Scholar]
- 21.Sato T, Kitagawa J, Ren K, et al. Activation of trigeminal intranuclear pathway in rats with temporomandibular joint inflammation. J Oral Sci. 2005;47:65–69. doi: 10.2334/josnusd.47.65. [DOI] [PubMed] [Google Scholar]
- 22.Takeda M, Tanimoto T, Ikeda M, et al. Temporomandibular joint inflammation potentiates the excitability of trigeminal root ganglion neurons innervating the facial skin in rats. J Neurophysiol. 2005;93:2723–2738. doi: 10.1152/jn.00631.2004. [DOI] [PubMed] [Google Scholar]
- 23.Neubert JK, Widmer CG, Malphurs W, et al. Use of a novel thermal operant behavioral assay for characterization of orofacial pain sensitivity. Pain. 2005;116:386–395. doi: 10.1016/j.pain.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 24.Ambalavanar R, Moritani M, Moutanni A, et al. Deep tissue inflammation upregulates neuropeptides and evokes nociceptive behaviors which are modulated by a neuropeptide antagonist. Pain. 2006;120:53–68. doi: 10.1016/j.pain.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Villa G, Ceruti S, Zanardelli M, et al. Temporomandibular joint inflammation activates glial and immune cells in both the trigeminal ganglia and in the spinal trigeminal nucleus. Mol Pain. 2010;6:89. doi: 10.1186/1744-8069-6-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamazaki Y, Ren K, Shimada M, et al. Modulation of paratrigeminal nociceptive neurons following temporomandibular joint inflammation in rats. Exp Neurol. 2008;214:209–218. doi: 10.1016/j.expneurol.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Q, Imbe H, Dubner R, et al. Persistent Fos protein expression after orofacial deep or cutaneous tissue inflammation in rats: Implications for persistent orofacial pain. J Comp Neurol. 1999;412:276–291. doi: 10.1002/(sici)1096-9861(19990920)412:2<276::aid-cne7>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 28.Ren K. An improved method for assessing mechanical allodynia in the rat. Physiol Behav. 1999;67:711–716. doi: 10.1016/s0031-9384(99)00136-5. [DOI] [PubMed] [Google Scholar]
- 29.Green PG, Alvarez P, Gear RW, et al. Further validation of a model of fibromyalgia syndrome in the rat. J Pain. 2011;12:811–818. doi: 10.1016/j.jpain.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho HJ, Staikopoulos V, Furness JB, et al. Inflammation-induced increase in hyperpolarization-activated, cyclic nucleotide-gated channel protein in trigeminal ganglion neurons and the effect of buprenorphine. Neuroscience. 2009;162:453–461. doi: 10.1016/j.neuroscience.2009.04.063. [DOI] [PubMed] [Google Scholar]
- 31.Vadivelu N, Hines RL. Buprenorphine: A unique opioid with broad clinical applications. J Opioid Manag. 2007;3:49–58. doi: 10.5055/jom.2007.0038. [DOI] [PubMed] [Google Scholar]
- 32.Bakke M, Hu JW, Sessle BJ. Morphine application to peripheral tissues modulates nociceptive jaw reflex. Neuroreport. 1998;9:3315–3319. doi: 10.1097/00001756-199810050-00030. [DOI] [PubMed] [Google Scholar]
- 33.Fischer L, Arthuri MT, Torres-Chavez KE, et al. Contribution of endogenous opioids to gonadal hormones-induced temporomandibular joint antinociception. Behav Neurosci. 2009;123:1129–1140. doi: 10.1037/a0017063. [DOI] [PubMed] [Google Scholar]
- 34.Morgan JR, Gebhart GF. Characterization of a model of chronic orofacial hyperalgesia in the rat: Contribution of NA(V) 1. 8. J Pain. 2008;9:522–531. doi: 10.1016/j.jpain.2008.01.326. [DOI] [PubMed] [Google Scholar]
- 35.Chicre-Alcantara TC, Torres-Chavez KE, Fischer L, et al. Local kappa opioid receptor activation decreases temporomandibular joint inflammation. Inflammation. 2012;35:371–376. doi: 10.1007/s10753-011-9329-1. [DOI] [PubMed] [Google Scholar]