Abstract
Purpose
To compare longitudinal glaucoma progression detection using optical coherence tomography (OCT) and visual field (VF).
Design
Validity assessment
Method
We analyzed subjects with more than 5 follow-up visits (every 6 months) in the multi-center Advanced Imaging for Glaucoma Study. Fourier-domain optical coherence tomography (OCT) was used to map the thickness of the peripapillary retinal nerve fiber layer (NFL) and ganglion cell complex (GCC). OCT-based progression detection was defined as a significant negative trend for either NFL or GCC. VF progression was reached if either the event or trend analysis reached significance.
Result
The analysis included 417 glaucoma suspect/pre-perimetric glaucoma (GS/PPG) eyes and 377 perimetric glaucoma (PG) eyes. In the GS/PPG group, progression was detect in 38.9% of eyes by OCT, significantly more (P<0.001) than the detection rate of 18.7% by VF. In severity-stratified analysis of PG eyes, OCT had significantly higher detection rate in early PG (49.7% vs. 32.0%, p=0.02), but not significantly different in moderate and advanced PG. The rate of NFL thinning declined dramatically in advanced PG, but GCC thinning rate remained relatively steady and allowed good progression detection even in advanced disease. The rate of false positive progression detection in permutated series was over 10% for VF trend analysis in both GS/PPG and PG group, while under 7% for both GCC and NFL.
Conclusion
OCT is a more sensitive than VF for the detection of progression in early glaucoma. While the value of NFL declines in advanced glaucoma, GCC appears to be a useful progression detector from early to advanced stages.
Introduction
Glaucoma is the leading cause of irreversible blindness worldwide.1 Because glaucoma progression is insidious and its rate is unpredictable, it is important to monitor disease severity by periodic assessment. There is no gold standard test to evaluate progression. Visual field (VF) testing is essential in tracking functional loss but is subjective and has poor reproducibility, requiring a series of many tests to establish progression.2 Optic disc photography is also useful in the monitoring of glaucoma but is subjective, requires a high degree of expertise on the part of the clinician, and has a poor agreement between clinicians.3 Optical coherence tomography (OCT) is objective and precise,4 but is thought to be less useful in advanced glaucoma due to the “floor effect” of nerve fiber layer (NFL).5,6
The change in mean deviation (MD) in consecutive VF tests is commonly used to detect glaucoma progression. Another well accepted VF progression approach utilizes the Guided Progression Analysis (GPA) event analysis by Humphrey Field Analyzer (HFA). The event analysis program applies statistical criteria developed and tested in the Early Manifest Glaucoma Trial (EMGT)7 in which VF event progression showed excellent specificity. 8,9 In addition, the visual field index (VFI), which was introduced in 2008,10 has gained popularity in clinics for tracking glaucoma progression.11–13 Event and trend-based progression analyses showed similar sensitivity and specificity but moderate agreement.14 However, VF tests are difficult for some patients and are known to have increased variability, especially in patients with advanced disease.15 The between-visit reproducibility of VF mean deviation ranges from 0.6 to 1.2 dB (standard deviation) from early to advanced glaucoma, which translates to a coefficient of variation (CV) of approximately 13 to 28% when the dB (logarithmic) units are converted to linear sensitivity.16 In comparison, the between-visit reproducibility for OCT measurements in glaucoma patients is 2.7% for retinal nerve fiber layer (NFL) thickness17 and 2.2–3.5% for ganglion cell complex (GCC).18 Another problem with VF is the learning effect, i.e. a patient’s performance on VF tests can get better over time, reducing the sensitivity to detect glaucoma progression.
OCT has been widely used to measure the thickness of NFL and GCC, which have strong correlation with glaucoma disease stages. 19–21 OCT has demonstrated the ability to diagnose glaucoma with fair to good accuracy,22–30 and to improve the prediction of conversion from pre-perimetric glaucoma to perimetric glaucoma,31 and worsening of VF.32,33 It is currently believed that VF is more informative in established glaucoma and especially in moderate to advanced disease.34 OCT on the other hand is generally regarded as being more sensitive in detecting progression in the earlier stages of the disease.6,35–37 Since OCT measurements have good repeatability and reproducibility,17 it may be clinically advantageous to monitor glaucoma progression using objective OCT structural measurements, compared to VF, a subjective test that may have problems with reliability in some patients.
In this paper, we study the detection of progression using trend analysis of overall NFL and GCC thicknesses measured by OCT. The sensitivity and specificity of OCT-based progression detection is compared with standard VF-based methods in study participants with a range of glaucoma severity. We also considered the factors that may have impact on glaucoma detection, including the number of OCT scans used per visit, adjustment for normal age-related thinning, and the effect of OCT signal strength on thickness measurement. The primary goal of this paper is assess the potential for using OCT parameters to monitor glaucoma as a complement or alternative to VF methods, at various stages of glaucoma. A secondary goal of the paper is to determine whether the current clinical practice in OCT-based trend analysis is adequate for glaucoma monitoring, and recommend ways to improve it.
Methods
The data used for the study was taken from participants enrolled in the Advanced Imaging for Glaucoma Study 38, a multi-site bioengineering partnership and longitudinal prospective clinical study sponsored by the National Eye Institute (ClinicalTrials.gov identifier: NCT01314326). The study design and baseline participant characteristics have been previously described,39 and the Manual of Procedures is available online (www.AIGStudy.net). Clinical data for the AIG Study were collected from three clinical centers, including the Doheny Eye Institute at the University of Southern California, the University of Pittsburgh Medical Center, and Bascom Palmer Eye Institute at the University of Miami. The study procedures adhered to the Declaration of Helsinki that guides studies involving human subjects. Written consent was obtained from all of the participants and proper institutional review board approvals were obtained from all of the participating institutions.
The glaucomatous progression results were analyzed using data from 2 groups: the perimetric glaucoma (PG) eyes and the glaucoma suspect - pre-perimetric glaucoma (GS/PPG) eyes. The eyes categorized as glaucoma suspect (GS) do not have abnormal VF pattern standard deviation (PSD) or glaucoma hemifield test 40, and present with either ocular hypertension (IOP ≥ 22 mmHg) or with the fellow eye diagnosed with perimetric glaucoma. Pre-perimetric glaucoma (PPG) eyes do not have abnormal VF PSD or GHT, but have a glaucomatous appearance of the disc or NFL on dilated ophthalmoscopy defined as vertical cup-disc asymmetry greater than 0.2, notch or thinning of the neuroretinal rim, optic disc hemorrhage, or NFL defect. Eyes enrolled in the PG group have glaucomatous optic neuropathy as evidenced by diffuse or localized thinning of the neuroretinal rim or NFL defect on fundus examination, and corresponding repeatable abnormal standard automated perimetry (SAP) defects with glaucoma hemifield test 40 or pattern standard deviation (PSD, p < 0.05) outside normal limits. For PG eyes, we further defined glaucoma severity based on their average MD measurements over the entire follow-up using the Hodapp-Parrish-Anderson grading scale: 41 early glaucoma, defined as MD > -6db, moderate glaucoma, defined as MD between -12db and -6db, and late stage glaucoma, defined as MD < -12db.
The peripapillary nerve fiber layer (NFL), and macular ganglion cell complex (GCC) - were imaged and measured by FD-OCT (RTVue, Optovue, Inc., Fremont, CA, USA). During each visit, participants had three GCC and optic nerve head (ONH) scans. Only ONH scans with a signal strength index (SSI) above 37 and GCC scans above 42 were selected for analysis.42 Measurements of qualified scans in the same visit were averaged. The macular GCC scan covered a 7 by 7 mm square area in the macula. Scans were centered 0.75 mm temporal to the fovea to improve the coverage of the temporal macula. The macular GCC thickness was defined as the combination of NFL, GCL, and inner plexiform layer.21 The automated Optovue software derived a 6 mm diameter GCC thickness map centered 0.75 mm temporal to fovea. The ONH concentric (1.3–4.9 mm diameter) scans were centered on the optic disc and automatically registered with the 3D disc scan to provide the disc margin information. The NFL thickness profile at D=3.4 mm was resampled on the NFL map recentered according to detected optic disc center. The RTVue software (version 6.12) was used to provide the following OCT image-derived measurements: the overall GCC thickness map and the overall NFL thickness profiles.
We used two OCT parameters to track glaucoma disease status: overall GCC thickness and NFL thickness. If either parameters exhibited a significant (p<0.05) trend for thinning over time, then the eye was classified as having OCT progression. At each visit, the series of OCT thickness parameters from baseline up to the current visit was fit over time (Figure 1). Progression was defined at the visit where a significant (P < 0.05) negative slope (thinning trend) was observed. The visit at which significant progression trend was first observed was recorded as the date of progression detection. Figure 1 is an example of a glaucoma patient with GCC and NFL progression detected.
Figure 1.
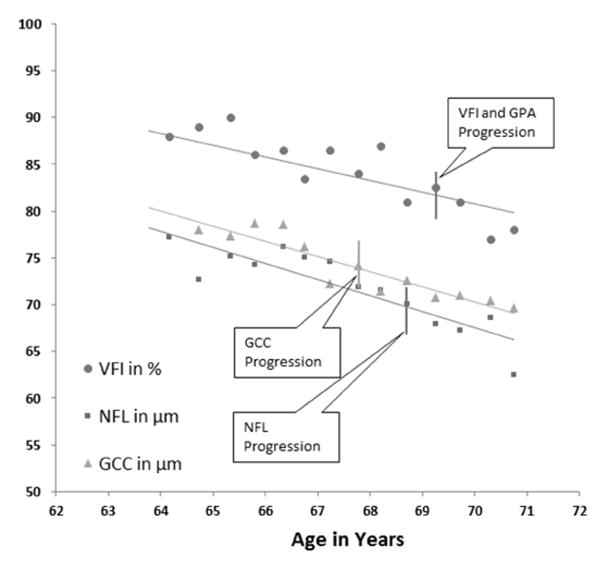
An example of glaucoma progression detection using trend analysis of visual field index (VFI), peripapillary retinal nerve fiber layer (NFL) thickness, and macular ganglion cell complex (GCC) thickness in a perimetric glaucoma (PG) eye. Progression is detect as a significant trend (negative linear regression slope with p<0.05) at an earliest visit by GCC, followed by NFL and then NFL.
The default method of GCC and NFL trend analysis averaged measurement from three scans, adjusted for normal age-related thinning (for GCC -0.2%/year, for NFL -0.14%/year).43 In case of NFL, the effect of the OCT signal strength index (SSI) on thickness measurement was also adjusted using a previously published linear regression analysis by 0.056 per SSI difference.42 A number of variations on the method of progression analysis were investigated: 1) only the first scan was used for the analysis; 2) aging adjustment was not used; and 3) SSI adjustment was not used. These variations were compared to the default method to determine whether the effects were significant.
We used the Guided Progression Analysis (GPA) software on the Humphrey Field Analyzer II to detect glaucoma progression. The analysis includes a VF trend analyses defined with either VFI or MD, and a pointwise event analysis. The event analysis defined progression as a significant change (compared to two baseline tests) detected in ≥3 test locations, and repeated at the same locations in 3 consecutive follow-up examinations, categorized by the software as “Likely Progression.” Trend-based progression was defined at the visit where significant VFI or MD negative slope was observed (Figure 1). In this paper, the VF progression endpoint is reach when either the event analysis or the trend analysis showed significant progression. All the trend progression analyses were programmed centrally with statistical software using parameters exported from the machines to the AIG central database.
The detection rate is the proportion of eyes which eventually experienced significant progression according to a certain disease metric. Due to the lack of a gold standard measure of progression, the detection rate is the best available measure of sensitivity. We used two methods to measure specificity, or false positive rate. The first is the improvement detection rate, defined as the proportion of eyes with a significant positive slope of change from the linear regression at any follow-up visit for the corresponding parameter. This is reasonable if we assume that the glaucomatous optic nerve does not regenerate, therefore the VF performance should not get better, and the GCC and NFL should not grow thicker. However, the improvement detection rate could be influenced by any systematic trends such as the learning effect in VF, aging changes, and disease progression. The second false positive measure is the progression detection rate on the permutated visits: for each patient, we performed a random permutation on all the visits (shuffling the order of the visits) for the patient, and then the linear regression was performed on the permutated series as previously described. The random permutation effectively eliminates any systematic trend and measures the chance of false positive detection due to test-retest variation.44,45
To ensure adequate numbers of data points for linear regression, only AIG study participants who had at least five study visits (one baseline visit and 4 follow-up visits) were analyzed in this paper. To ensure a fair comparison between OCT and VF detection rates, only visits with both VF and OCT data were used. Because Fourier-domain OCT technology was not available in the earliest years of the AIG study, this resulted in the truncation of VF data from early visits in many participants. To eliminate the interference of cataract on the visual field measurements, we also excluded eyes that experienced significant cataract progression any time during the follow-up. A significant cataract progression is defined as confirmed worsening of visual acuity scores by two or more lines at two or more follow-up visits, and confirmed clinical cataract progression assessment at two or more follow-up visits.
McNemar’s test was used to compare detection rate on the same group of patients. Kaplan-Meier survival curve analysis was used to compare time-to-event data. All statistical analyses were performed by SAS 9.4 (SAS Institute, Cary, NC, USA). When applicable, Generalized Estimating Equation (GEE) method46 was used to adjust between-eye correlation for two eyes from the same patient.
Results
The present analysis included 356 out of 663 total GS/PPG eyes and 153 out of 377 total PG eyes after applying the minimum complete follow-up visit requirement, and after excluding approximately 3% eyes showing significant cataract progression. Follow-up length was 54.1±16.2 months for GS/ PPG eyes and 56.7±16.0 for PG eyes. At the baseline, there was no significant difference between PG and GS/PPG subjects in age, gender, race, family history of glaucoma at the subject level and axial length at the eye level. PG eyes had significantly thinner central corneas compared to the GS/PPG eyes (Table 1). The PG patients were also significantly more likely to have systemic hypertension and diabetes Mellitus. The follow-up length was ranged 24-84 months for GS/PPG eyes with average follow-up of 54.1±16.2 months, the follow-up length was ranged 24–78 months for PG eyes with average 56.7±16 months. Averaged over the follow-up period (Table 2), the PG eyes had significantly worse VF measures (VFI, MD, PSD) and worse OCT parameters (overall GCC and NFL thicknesses).
Table 1.
Participant Baseline Characteristics
| Glaucoma Suspect/Pre- Perimetric Glaucoma (n=356 eyes) | Perimetric Glaucoma (n=153eyes) | p-Value | |
|---|---|---|---|
| Age (Years) | 60.8 ± 9.0 | 61.7 ± 9.6 | 0.13 |
| Gender - Female | 219 (61.5%) | 89 (58.2%) | 0.48 |
| Ethnicity - African American | 35(9.8%) | 15 (9.8%) | 0.99 |
| Family History of Glaucoma | 185 (52.0%) | 75 (50%) | 0.69 |
| Systemic Hypertension | 97 (27.3%) | 58 (37.9%)* | 0.017 |
| Diabetes Mellitus | 19 (5.3%) | 18 (11.8%)* | 0.01 |
| Axial Length (mm) | 24.17 ± 1.31 | 24.40 ± 1.36 | 0.9379 |
| Central Corneal Thickness (μm) | 556.12 ± 39.02 | 542.54 ± 36.57* | 0.0045 |
Indicates significant (p<0.05) difference between the glaucoma suspect/pre-perimetric glaucoma (GS/PPG) and perimetric glaucoma (PG) groups.
Table 2.
Eye Measurements Averaged over the Course of Follow-up
| Glaucoma Suspect/Pre- Perimetric Glaucoma (n=356 eyes) | Perimetric (n=153 eyes) | p-Value | |
|---|---|---|---|
| Mean Intraocular Pressure (mmHg) | 15.53 ± 3.27 | 13.67 ± 3.11 | 0.0056 |
| Median Deviation (db) | −0.38 ± 1.12 | −4.58 ± 4.33 | <.0001 |
| Pattern Standard Deviation (db) | 1.72 ± 0.49 | 5.67 ± 4.15 | <.0001 |
| Visual Field Index (%) | 98.71 ± 1.69 | 88.12 ± 12.85 | <.0001 |
| Overall Nerve Fiber Layer Thickness (μm) | 90.74 ± 7.99 | 82.09 ± 8.93 | <.0001 |
| Overall Ganglion Cell Complex Thickness (μm) | 91.67 ± 10.27 | 80.09 ± 11.37 | <.0001 |
We investigated various methods of linear regression trend analysis in NFL and GCC thicknesses and how they affected the detection of glaucoma progression (Table 3, 4). Our default method adjusted for the previously published rate of normal age-related thinning43 and used all 3 sets of OCT scans obtained at each visit. The NFL regression further took into account the known effect of OCT signal strength (measured by SSI) on thickness measurement.42 Taking out the aging adjustment increased the rate of detecting glaucoma progression by GCC and NFL thinning at the cost of decreased specificity. This increase in detection rate was statistically significant for both GCC and NFL in PG and GS/PPG group (P<0.001, P<0.001 for GCC, P=0.014, P < 0.001 for NFL). We also looked at the results from performing linear regression using only the first scan from each visit. As expected, using only 1 scan decreased the rate of progression detection compared to using all 3 scans, the difference was small but significant for GCC in PG and GS/PPG group (P = 0.042, P=0.034) but not statistically significant for NFL in either group. Removing signal strength compensation (SSI adjustment) from NFL moderately reduced the rate of progression detection rates, as would be expected from the addition measurement noise introduced by signal strength variation from visit to visit. This effect was significant (p=0.002) in the GS/PPG group but not significant in the PG group (P=0.32).
Table 3.
Progression Detection Performance in Perimetric Glaucoma Eyes
| visits >=5 153 eyes | Progression * Detection Rate | Improvement Detection Rate, in % (95% Confidence Interval) | False Positive Progression Detection Rate from series, in % | |
|---|---|---|---|---|
| Overall Ganglion Cell Complex Thickness Trend | Default | 45.1 (37.2, 53.0) | 8.5 (4.1, 12.9) | 3.3 (0.5, 6.1) |
| No Aging Effect | 58.2 (50.4, 66.0) | 5.2 (1.7, 8.8) | 5.9 (2.2, 9.6) | |
| Single Scan | 37.3 (29.6, 44.9) | 6.5 (2.6, 10.5) | 3.9 (0.9, 7.0) | |
| Overall Nerve Fiber Layer Thickness Trend | Default | 39.9 (32.1, 47.6) | 5.2 (1.7, 8.8) | 6.5 (2.6, 10.5) |
| No Aging Effect | 43.8 (35.9, 51.7) | 5.4 (0.0, 11.6) | 9.2 (4.6, 13.7) | |
| Single Scan | 36.0 (28.3, 43.6) | 7.8 (3.6, 12.1) | 5.2 (1.7, 8.8) | |
| No Signal Strength Index Adjustment | 37.3 (29.6, 44.9) | 6.5 (2.6, 10.5) | 5.9 (2.2, 9.6) | |
| Optical Coherence Tomography | Either Thickness | 62.1 (54.4, 69.8) | 11.1 (6.1, 16.1) | 8.5 (4.1, 12.9) |
| Visual Field Trend | Visual Field Index | 28.8 (21.6, 35.9) | 9.1 (4.6, 13.7) | 11.1 (6.1, 16.1) |
| Mean Deviation | 28.8 (21.6, 35.9) | 10.5 (5.6, 15.3) | 10.5 (5.6, 15.3) | |
| Visual Field Event | Guided Progression Analysis | 19.6 (13.3, 25.9) | n/a | n/a |
| Visual Field | Trend or Event | 41.8 (34.0, 49.7) | 16.3 (10.5, 22.2)** | 17.7 (11.6, 23.7)** |
In % (95% Confidence Interval).
False positives from the 2 visual field trend analyses were combined, but false positive rate from the event analysis was not available.
Table 4.
Progression Detection Performance in the Glaucoma Suspect and Pre-Perimetric Glaucoma Eyes
| visits >=5 356 eyes | Progression Detection Rate | Improvement Detection Rate | False Positive Progression Detection Rate from series | |
|---|---|---|---|---|
| Overall Ganglion Cell Complex Thickness Trend | Default | 43.3 (38.1, 48.4) | 7.6 (4.8, 10.3) | 6.5 (3.9, 9.0) |
| No Aging Effect | 53.7 (48.5, 58.8) | 5.3 (3.0, 7.7) | 9.3 (6.3, 12.3) | |
| Single Scan | 38.8 (33.7, 43.8) | 6.5 (3.9, 9.0) | 7.6 (4.8, 10.3) | |
| Overall Nerve Fiber Layer Thickness Trend | Default | 42.4 (37.3, 47.6) | 4.8 (2.6, 7.0) | 6.7 (4.1, 9.4) |
| No Aging Effect | 47.5 (42.3, 52.7) | 3.9 (1.9, 6.0) | 8.7 (5.8, 11.6) | |
| Single Scan | 39.3 (34.3, 44.4) | 3.9 (1.9, 6.0) | 5.3 (3.0, 7.7) | |
| No Signal Strength Index Adjustment | 37.1 (32.1, 42.1) | 6.7 (4.1, 9.4) | 7.6 (4.8, 10.3) | |
| Optical Coherence Tomography | Either Thickness | 59.8 (54.7, 64.9) | 11.8 (8.5, 15.2) | 11.5 (8.2, 14.8) |
| Visual Field Trend | Visual Field Index | 11.0 (7.7, 14.2) | 6.7 (4.1, 9.4) | 9.0 (6.0, 12.0) |
| Mean Deviation | 19.7 (15.5, 23.8) | 10.4 (7.2, 13.7) | 7.3 (4.6, 10.1) | |
| Visual Field Event | Guided Progression Analysis | 4.2 (2.1, 6.3) | n/a | n/a |
| Visual Field | Trend or Event | 27.3 (22.6, 31.9) | 13.8 (10.2, 17.3)** | 14.0 (10.4, 17.7)** |
In % (95% Confidence Interval).
False positives from the 2 visual field trend analyses were combined, but false positive rate from the event analysis was not available.
Using the default method, GCC and NFL detected progression at similar rates (Table 3, Table 4). Both had false positive rates near the nominal significance level of 5% in both PG and GS/PPG groups.
The three methods of VF progression analysis were compared. In the PG group (Tables 3), the detection rates from MD and VFI trend analyses were similar, and both were significantly higher than VF event analysis (P =0.016). In the GS/PPG group (Table 4), the MD trend analysis had significantly higher detection rate than VFI trend analysis (P <0.001), which in turn performed better than event analysis (P<0.001). The false positive progression detection rate using MD trend analysis and VFI trend analysis were10.5% and 11.1% in PG, which were more than twice that of the nominal significance level of 5% and significantly higher than that of GCC (3.3%) and NFL (6.5%) (P<0.05 in all four comparisons). The false positive rates of MD and VFI trend analyses in GS/ PPG were also higher than GCC and NFL but not significantly so. The rate of significant improvement, another indicator of false positive detection was also higher in the PG group.
The detection rates of OCT as a combination of both GCC and NFL were compared to combined VF event and trend analyses. OCT offered much higher detection rates in GS/PPG eyes (P<0.0001); the advantage was smaller in PG eyes but still highly significant (P=0.0001).
We assessed the overlap between various progression detection methods using Venn diagrams (Figures 1, 2). The 3 VF progression analysis method only overlapped moderately in both PG and GS/PPG groups. The 2 OCT parameters – GCC and NFL – also had moderate overlap and contributed approximately equally to progression detection in both PG and GS/PPG groups. In the PG group, OCT and VF had moderate overlap, with significantly more eyes with progression detected by OCT alone. OCT had further advantage over VF in the GS/PPG group, and progression was detected by VF alone in only 7% of eyes.
Figure 2.
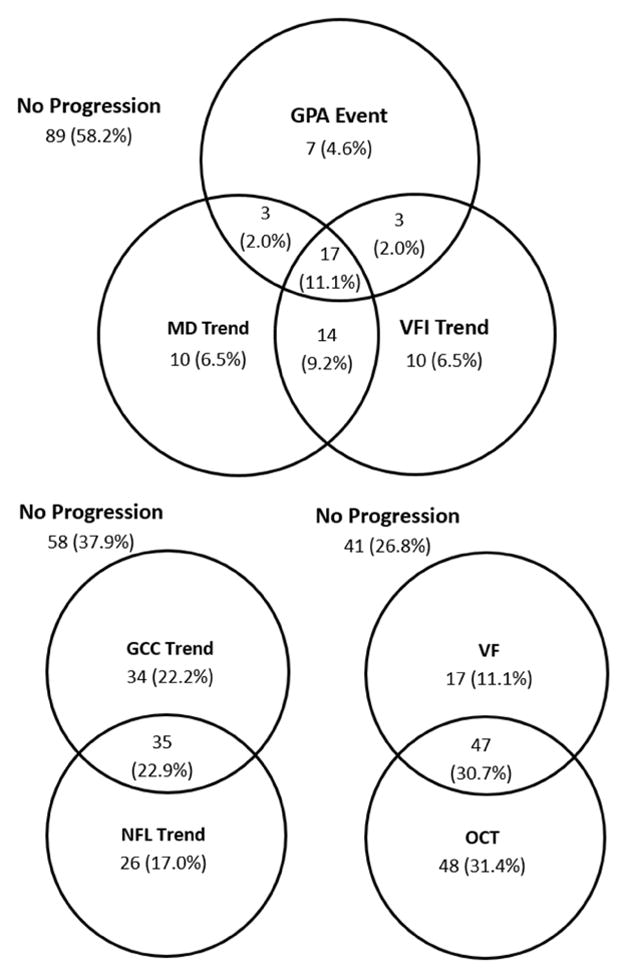
Venn diagram of glaucoma progression detection in perimetric glaucoma eyes using various methods. The visual field (VF) methods include the visual field event analysis by Guided Progression Analysis (GPA) and the visual field trend analysis. The optical coherence tomography (OCT) methods include analysis of thinning trend in macular ganglion cell complex (GCC) and peripapillary retinal nerve fiber layer (NFL).
We further divided the PG group into 3 subgroups to look at progression detection at various levels of disease severity (Table 5). In 101 eyes with mild glaucoma, OCT had significantly (p<0.001) higher detection rate than VF. In 42 eyes with moderate or advanced glaucoma, OCT had moderately higher detection rate than VF, but the difference was not statistically significant. It should be noted that the detection rate by NFL dropped from 43.2% in mild glaucoma to 26.7% in late glaucoma, while the detection rate by GCC remained constant at 44.1% and 46.7%. Using NFL alone, progression detection rate would be significantly (p=0.021) worse than VF in moderate /advanced glaucoma.
Table 5.
Progression Detection Rate Stratified by Severity of Perimetric Glaucoma
| Instrument | Parameter | Mild (N=111 ) | Moderate (N= 27) | Advanced (N= 15) |
|---|---|---|---|---|
| Optical Coherence Tomography | Ganglion Cell Complex | 44.1 | 48.2 | 46.7 |
| (34.9, 53.4) | (29.3, 67.0) | (21.4, 71.9) | ||
| Nerve Fiber Layer | 43.2 | 33.3 | 26.7 | |
| (34.0, 52.5) | (15.6, 51.1) | (4.3, 49.1) | ||
| Either Thickness | 63.1 | 55.6 | 66.7 | |
| (54.1, 72.0) | (36.8, 74.3) | (42.8, 90.5) | ||
| Visual Field | Visual Field Index Trend | 26.1 | 33.3 | 40 |
| (18.0, 34.3) | (15.6, 51.1) | (15.2, 64.8) | ||
| Mean Deviation Trend | 27.0 | 37.0 | 26.7 | |
| (18.8, 35.3) | (18.8, 55.3) | (4.3, 49.1) | ||
| Event Analysis | 15.3 | 29.6 | 33.3 | |
| (8.6, 22.0) | (12.4, 46.9) | (9.5, 57.2) | ||
| Any | 38.7 | 48.2 | 53.3 | |
| (29.7, 47.8) | (29.3, 67.0) | (28.1, 78.6) |
% progression detection rate (confidence interval)
We also looked at the rates of change of the OCT and VF perimeters used to track glaucoma at 4 stages of glaucoma severity (Table 6). The rate of GCC thinning was fairly constant at all stages of glaucoma. In contrast, NFL thinning dramatically slowed in advanced glaucoma (P = 0.028) to a rate not significantly different from zero. In contrast to the OCT parameters, the rate of VFI and MD decline accelerated as disease progressed (P<0.001, linear contrast test). Among the eyes with progression detection by OCT, the rate of change of GCC and NFL were both slightly more than 1 μm per year. We also analyzed the root-mean square residual of linear regression (Table 6), which is an indication of the variability of test results over time. Both OCT measurements – GCC and NFL – had low variability. The mean residual for GCC ranged between 1.13–1.58 μm over the stages of glaucoma. The mean residual for NFL ranged between 1.53–2.30 μm. These are roughly 1–3% of the mean thicknesses and comparable to the repeatability (coefficient of variation) of GCC and NFL. The mean residuals of VFI and VF-MD both grew with disease severity. In order to compare the variability of OCT and VF trend analyses, we divided the residuals by the mean slopes to obtain normalized ratios. These ratios have the unit of years indicating roughly the time scale over which change can be reasonably detected. For OCT parameters, this time scale was approximately 2–3 years, with the exception of NFL in advanced glaucoma due to the lack of progression (floor effect). For VF parameters, this time scale is greater than 5 years in GS/PPG and early PG, due to the lack of progression (lag effect).
Table 6.
Time Rate of Change of Diagnostic Parameters Stratified by Glaucoma Severity
| Glaucoma Suspect/Pre- Perimetric Glaucoma (N = 356) | Mild Perimetric Glaucoma (N=111 ) | Moderate Perimetric Glaucoma (N= 27) | Advanced Perimetric Glaucoma (N= 15) | Eyes with Progression of the type | ||
|---|---|---|---|---|---|---|
| Ganglion Cell Complex | Slope (μm/year) | −0.64 ± 1.03 | −0.59 ± 1.13 | −0.61 ± 1.05 | −0.54 ± 0.68 | −1.12 ± 0.69 |
| Residual (!m) | 1.21 | 1.38 | 1.58 | 1.13 | 1.07 | |
| Residual/slope (year) | 1.9 | 2.3 | 2.6 | 2.1 | 1.0 | |
|
| ||||||
| Nerve Fiber Layer | slope (μm/year) | −0.64 ± 1.03 | −0.63 ± 1.00 | −0.70 ± 1.17 | −0.03 ± 1.18 | −1.16 ± 0.97 |
| Residual (μm) | 1.70 | 1.80 | 1.53 | 2.30 | 1.47 | |
| Residual/slope (year) | 2.7 | 2.9 | 2.2 | 77 | 1.3 | |
|
| ||||||
| Visual Field Index | Slope (%/year) | −0.19 ± 0.97 | −0.25 ± 1.02 | −1.09 ± 2.21 | −1.28 ± 2.22 | −0.96 ± 1.64 |
| Residual (%) | 1.05 | 1.85 | 3.13 | 5.84 | 2.47 | |
| Residual/slope (year) | 5.5 | 7.4 | 2.9 | 4.6 | 2.6 | |
|
| ||||||
| Mean Deviation | Slope (db/year) | −0.09 ± 0.34 | −0.14 ± 0.33 | −0.49 ± 0.72 | −0.37 ± 0.57 | −0.46 ± 0.43 |
| Residual (db) | 0.65 | 0.81 | 1.13 | 1.68 | 0.78 | |
| Residual/slope (year) | 7.2 | 5.8 | 2.3 | 4.5 | 1.7 | |
The slopes of change over time are shown as mean ± standard deviation. The root-mean-square residual of linear regression was averaged for each subgroup to measure the noise in the rate measurements. The ratio of residual variation divided by the mean slope of change is an indicator of the precision with which progression can be measured. The ratio has a unit of years and roughly indicate the minimum duration of time over which progression can be detected.
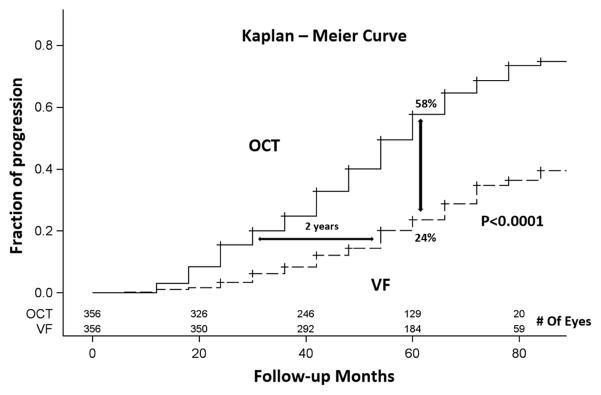
The time scale for VF progression detection is less than 3 years in moderate glaucoma, but worsens again in advanced glaucoma due to increased measurement variability. Overall, OCT had relatively lower measurement variability (residual/slope ratio) and was able to detect glaucoma over shorter time periods in most stages of glaucoma, with the exception of the moderate PG stage. The significant progressors experienced more rapid thinning of GCC and NFL of approximately 1.2% per year, and more rapid VFI (−1%/year) and MD (−0.5 dB/year) decline. The significant progressors tended to have smaller residuals as well. This is especially remarkable for the VF parameters, indicating that progression was more likely to be detected in patients able to perform VF testing reliably. The time-to-progression detection by OCT and VF were plotted in the Kaplan-Meier survival curves in GS/PPG and PG groups. Overall, OCT outperformed VF in term of progression detection rate in both PG and GS/PPG groups (log-rank P =0.0003 for PG and P<0.0001 for GS/PPG). In the PG group, the time to detect progression in 20% of eyes was 36 months for OCT and 48 months for VF – a 1 year difference (Figure 4). In the GS/PPG group, the time to detect progression in 20% of eyes was 36 months for OCT and 60 months for VF – a 2 year difference (Figure 5). At the 60 month follow-up, OCT detected 57% progression, significantly (P<0.001) higher than 36% detected by VF (Figure 4) in the PG group. The gap was larger in GS/PPG group, where OCT detected 56% progression vs. VF’s 24% (Figure 5, P<0.001).
Figure 4.
Kaplan-Meier plots of glaucoma progression detected by OCT and by visual field among pre-perimetric glaucoma eyes.
Figure 5.
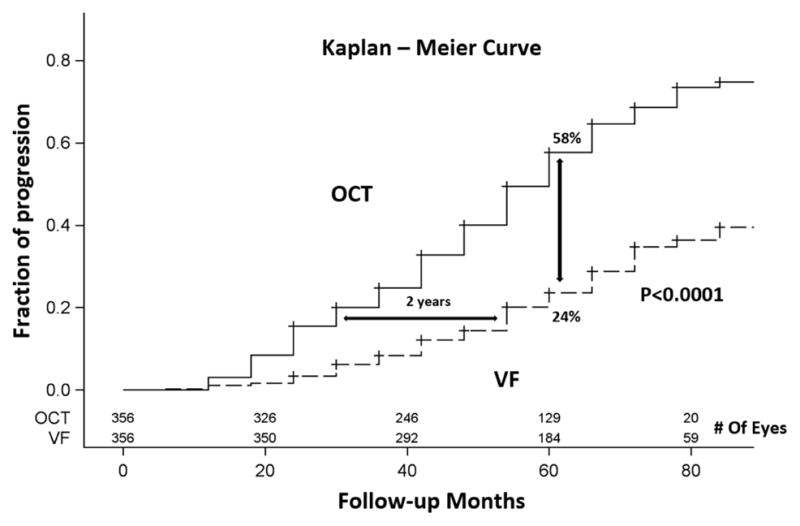
Kaplan-Meier plots of glaucoma progression detected by OCT and by visual field among glaucoma suspect/pre-perimetric glaucoma eyes.
Discussion
In glaucoma management, it is believed that structural tests can better identify progression in the early stages of the disease, while VF exams are more useful in the later stages.6,12,34,38,47 Recent studies have focused on the comparison between progression identified with NFL or optic disc OCT imaging and visual fields. 12,38,48 Abe et al suggested that in patients with different glaucoma stages, monitoring NFL with spectral-domain OCT gives a higher chance of detection of disease progression in early stages, while VF testing is more relevant in later stages 38. In another study, Banegas et al supported the role of progression detection using OCT NFL in pre-perimetric and early glaucoma, while they recommended the use of VF testing and optic disc photography for advanced glaucoma monitoring.12 The findings of the present study are largely in agreement with the above-mentioned studies, with NFL trend analysis showing good progression detection in early to moderate glaucoma, and VF being more useful in identifying progression in moderate to late stages. However, this study also provides novel evidence to support OCT imaging of the macula (specifically GCC) as a useful tool even in the later stages of glaucoma, with similar ability to detect progression as VF. This is important in that our study included the disease continuum from glaucoma suspects to advanced glaucoma patients and directly compared VF testing with both NFL and GCC trend analyses. The study supports the use of OCT imaging to monitor glaucoma from early to late stages.
A number of studies have already recognized that NFL reaches a minimum residual thickness plateau in late glaucoma.5,6,49 Compared to peripapillary NFL38, macular GCC appears to deplete later in the course of glaucoma. 13,38,50 The reason for this difference may be regional. The overall NFL cross-sectional area sampled by the peripapillary circular scan is dominated by the arcuate bundles which are damaged early in the course of glaucoma. In contrast, the macular region is relatively spared in most cases of early glaucoma with the papillomacular bundle usually depleted much later in the disease process. In our study, when the PG group was stratified according to disease severity, it became apparent that there was a significant difference between GCC and NFL in the moderate and advanced PG groups. In these later stages of glaucoma, GCC thinning continued while NFL thinning nearly halted (Table 6). As a consequence, GCC was more useful at detecting progression in these later stages (Table 5). This finding is in agreement with the study by Sung et al,13 where the authors studied overall macular thickness (without separate layer segmentation) in a cohort of advanced glaucoma eyes and recognized that it was superior in progression detection compared to optic disc or retinal NFL thickness. They concluded that it may be more reliably associated with VF progression than other OCT parameters. This was further investigated in another cohort of advanced glaucoma patients where the macular ganglion cell-inner plexiform layer (GCIPL) thickness was able to detect progression, further supporting the fact that structural change can be identified even in very advanced glaucoma eyes.50
By looking at GCC and NFL together, we found that OCT parameters detected progression at higher rates than VF in both the GS/PPG group and the PG group. Stratified analysis in the PG group showed that OCT also had higher detection rate in all severity groups, including advanced PG. Kaplan-Meier analysis showed that OCT is also able to detect progression in a shorter period of time (with the same visit frequency) in both the PG and GS/PPG groups. OCT parameters also had better specificity, with values close to the 5% cutoff for the significance of linear regression. In contrast, VF trend analyses detected a significantly higher number of false positives in the permutated series in the PG group. This may have been caused by the MD and VFI distribution deviating from the normal distribution assumed in ordinary least-square linear regression. The residual distribution of VF test error is known to be severely skewed toward the negative in areas of significant damage.51 VF event analysis has a well-characterized low false positive rate of 2.6%,8 but had relatively low detection rates in this study. Overall, OCT may be a more reliable test for glaucoma progression because it has good detection rate and acceptable specificity over a wide range of glaucoma severity. The reason that OCT outperformed VF in detecting progression in most disease stages stems from OCT’s greater measurement precision, as shown by our analysis of residuals (Table 6).
Many studies have already highlighted the predictive role of OCT NFL thinning on the future visual outcome. Faster rates of retinal NFL thinning were associated with increased risk of visual field loss in glaucoma suspects.52 In another prospective study, progressive retinal NFL thinning was again predictive of functional decline as measured with visual fields in glaucoma patients.53 In addition, previous reports from the AIG Study have already suggested that both NFL and GCC thinning can predict the development of glaucomatous VF loss in glaucoma suspects and preperimetric glaucoma36 and that focal GCC and NFL loss as measured by FD-OCT can strongly predict faster VF progression in established glaucoma.33 Therefore, there is evidence to support that thinning of the NFL or GCC on OCT can predict future vision loss.
However, we are not advocating reliance on OCT alone or ignoring VF in the monitoring of glaucoma. Poor agreement between structural and functional measurements for tracking glaucoma has been noted in many previous publications, but this may relate to the variation of how different patients progress.54 In the current study, if VF was not used, progression would go undetected in many cases of PG – approximately 6% in moderate PG and 11% in advanced PG. Therefore, OCT and VF were both found to be necessary for tracking progression in perimetric glaucoma and it is crucial that both are used in clinical practice.
Between the two VF trend progression detection methods, MD trend detected more progression than VFI trend in GS/PPG, but they had exactly the same detection rates in PG, and surprisingly they only overlapped moderately (Figure 2). VFI has been shown to exhibit a ceiling effect in early glaucoma, and overestimation of remaining visual field.15 While our results showed MD trend to be more sensitive than VFI trend in GS/PPG, we also showed that VFI trend detected progression in a significant number of eyes missed by MD in the PG group. Therefore the two VF trend analyses may be complementary. Between the VF trend progression detection methods and event progression method, trend analyses had greater detection rates in all stages of glaucoma by either MD or VFI. However, they only moderately overlap and both contributed independently towards progression detection. Therefore the use of both trend and event analyses is recommended, in addition to OCT parameters, in the monitoring of glaucoma progression.
We investigated several variations in the methods used to detect progression with OCT parameters. The default analysis in this paper averaged measurements from 3 sets of OCT scans at each visit, accounted for the rate of normal age-related thinning in NFL and GCC,43 and compensated for the effect of OCT signal strength variation on NFL measurements.42 This differs from the standard clinical practice, where one OCT scan is made at each visit, and no compensation for signal strength or aging changes is made in the linear regression analysis software on the commercial RTVue OCT system software. Our analysis found that accounting for the change over time in NFL and GCC due to normal aging had significant effects on detecting progression; therefore it is desirable to adjust for aging effect when using the trend of NFL and GCC to define progression. This finding agreed with a previous investigation by Medeiros et al.38 Adjusting for signal strength index (SSI) slightly improved detection. Using results from only one OCT scan per visit (instead of the default of averaging results from 3 scans) slightly decreased the rate of detecting significant progression, but still gave better results than VF in GS/PPG and early PG groups. We conclude that the commercial OCT software provides good progression detection, when analyzing time series of only one OCT scan per visit, with better performance than VF monitoring in the early glaucoma patients and glaucoma suspects. However, it is worthwhile to add signal strength compensation and aging adjustment to the commercial OCT glaucoma progression analysis software.
Certain limitations should be considered when looking at the results of the present study. First, we can only use surrogate methods to indirectly determine false positive rate for progression detection. The ideal method to assess specificity is to acquire many measurements over a short period of time. 8,55 Unfortunately, this type of data was not available in the AIG Study. Another limitation is that there were very few patients with end stage glaucoma in the study. Our advanced glaucoma group had only 15 eyes and the MD was −14.4 ± 1.9 dB (range −12db to −19db). Thus the performance of GCC in monitoring end-stage glaucoma was not adequately studied. It is possible that GCC also reaches a floor thickness and poorly reflects disease severity in very advanced glaucoma stage. The floor effect is a consideration in all methods of detecting glaucoma progression, including VF. In very advanced glaucoma, standard 24–2 VF becomes insensitive to further progression, and changes to more central test pattern (i.e. to 10–2) and large stimulus sizes become advisable. Thus the monitoring of glaucoma progression in very advanced stages remains a challenge that could benefit from new solutions.56 In this study we did not require that changes were confirmed on subsequent testing, in order to increase sensitivity.57 This does not affect our conclusions, since the same methodology was applied to both VF and OCT trend analysis. However in clinical practice, it is still advisable to confirm findings before making significant clinical decisions concerning disease management.
In summary, OCT has higher sensitivity for progression detection than VF in both perimetric glaucoma eyes, and pre-perimetric glaucoma and early perimetric glaucoma eyes. OCT is able to detect progression within a shorter follow-up time in early glaucoma. Therefore clinicians could rely more heavily on OCT to monitor progression in the early stages of the disease. However, a number of patients seem to progress by either functional or structural tests or some by both in all glaucoma stages. Using OCT and VF together for disease monitoring is advisable, as this can track disease progression more frequently than using either method alone. Interestingly, in moderate and advanced glaucoma (with good evidence down to MD of –15 dB), OCT continues to be useful in progression monitoring, with GCC trend analysis being more useful than NFL trend analysis. This can also be especially useful in clinical practice, to overcome difficulties that some advanced glaucoma patients encounter when undertaking VF, since OCT is an objective test and does not depend on patient response. Obtaining one OCT scan per visit appears to be adequate for progression monitoring, though averaging more scans per visit could modestly improve the rate of progression detection. Adjusting for aging and signal strength effects could also improve the accuracy of progression rate calculations, and we recommend these improvements to the commercial software for OCT trend analysis.
Supplementary Material
Figure 3.
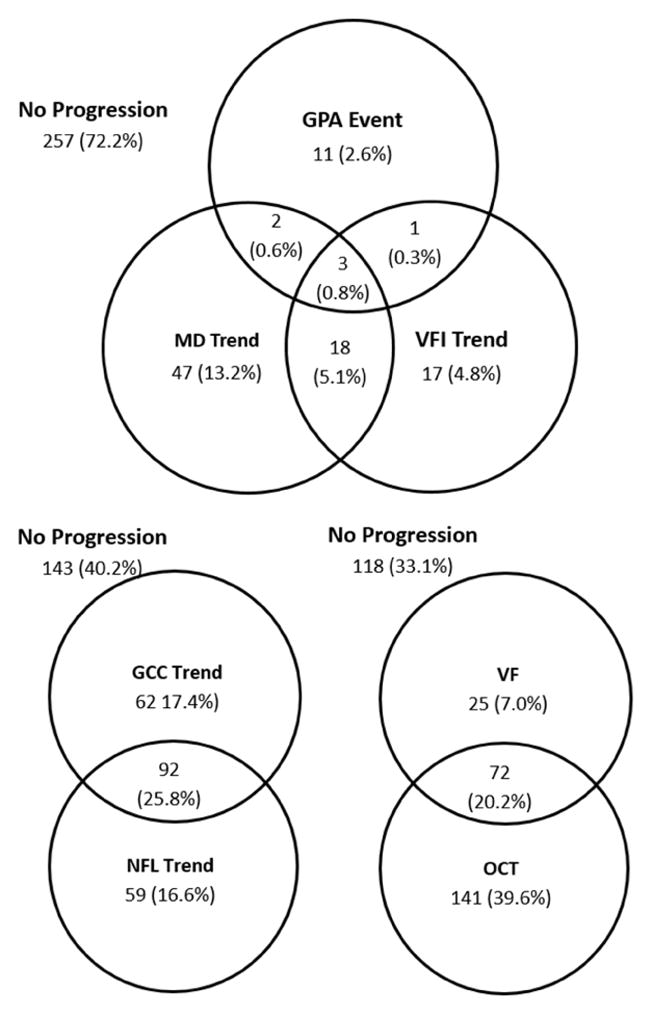
Venn diagram of glaucoma progression detection in the glaucoma suspect & pre-perimetric glaucoma group. The visual field (VF) methods include the visual field event analysis by Guided Progression Analysis (GPA) and the visual field trend analysis. Optical coherence tomography (OCT) progression was established by significant thinning trend in either macular ganglion cell complex (GCC) or peripapillary nerve fiber layer (NFL).
Acknowledgments
Funding/Support
Supported by NIH grants R01 EY013516, R01 EY023285 (Bethesda, MD),
Footnotes
ClinicalTrials.gov information: Identifier: NCT01314326; Responsible party: David Huang, Oregon Health and Science University; Official title: Advanced Imaging for Glaucoma Study
Financial Disclosure
Oregon Health & Science University (OHSU, Portland, OR), Dr. Huang and Dr. Tan have significant financial interests in Optovue, Inc. (Fremont, CA), a company that may have a commercial interest in the results of this research and technology. These potential conflicts of interest have been reviewed and managed by OHSU. Dr. Greenfield receives research support from Optovue, Inc., Carl Zeiss Meditec, Inc., and Heidelberg Engineering (Carlsbad, CA). Dr. Varma has received research grants, honoraria and/or travel support from Carl Zeiss Meditec, Inc., Heidelberg Engineering, and Optovue, Inc. Dr. Zhang, Dr. Francis and Dr. Dastiridou have no financial interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chauhan BC, Garway-Heath DF, Goni FJ, et al. Practical recommendations for measuring rates of visual field change in glaucoma. Br J Ophthalmol. 2008;92(4):569–573. doi: 10.1136/bjo.2007.135012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jampel HD, Friedman D, Quigley H, et al. Agreement among glaucoma specialists in assessing progressive disc changes from photographs in open-angle glaucoma patients. Am J Ophthalmol. 2009;147(1):39–44. e31. doi: 10.1016/j.ajo.2008.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bussel, Wollstein G, Schuman JS. OCT for glaucoma diagnosis, screening and detection of glaucoma progression. Br J Ophthalmol. 2014;98(Suppl 2):ii15–19. doi: 10.1136/bjophthalmol-2013-304326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mwanza JC, Kim HY, Budenz DL, et al. Residual and Dynamic Range of Retinal Nerve Fiber Layer Thickness in Glaucoma: Comparison of Three OCT Platforms. Invest Ophthalmol Vis Sci. 2015;56(11):6344–6351. doi: 10.1167/iovs.15-17248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hood DC, Kardon RH. A framework for comparing structural and functional measures of glaucomatous damage. Prog Retin Eye Res. 2007;26(6):688–710. doi: 10.1016/j.preteyeres.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leske MC, Heijl A, Hyman L, Bengtsson B. Early Manifest Glaucoma Trial: design and baseline data. Ophthalmology. 1999;106(11):2144–2153. doi: 10.1016/s0161-6420(99)90497-9. [DOI] [PubMed] [Google Scholar]
- 8.Artes PH, O'Leary N, Nicolela MT, Chauhan BC, Crabb DP. Visual field progression in glaucoma: what is the specificity of the Guided Progression Analysis? Ophthalmology. 2014;121(10):2023–2027. doi: 10.1016/j.ophtha.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 9.Artes PH, Nicolela MT, LeBlanc RP, Chauhan BC. Visual field progression in glaucoma: total versus pattern deviation analyses. Invest Ophthalmol Vis Sci. 2005;46(12):4600–4606. doi: 10.1167/iovs.05-0827. [DOI] [PubMed] [Google Scholar]
- 10.Bengtsson B, Heijl A. A visual field index for calculation of glaucoma rate of progression. Am J Ophthalmol. 2008;145(2):343–353. doi: 10.1016/j.ajo.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 11.Aptel F, Aryal-Charles N, Giraud JM, et al. Progression of visual field in patients with primary open-angle glaucoma - ProgF study 1. Acta Ophthalmol. 2015;93(8):e615–620. doi: 10.1111/aos.12788. [DOI] [PubMed] [Google Scholar]
- 12.Banegas SA, Anton A, Morilla A, et al. Evaluation of the Retinal Nerve Fiber Layer Thickness, the Mean Deviation, and the Visual Field Index in Progressive Glaucoma. J Glaucoma. 2016;25(3):e229–235. doi: 10.1097/IJG.0000000000000280. [DOI] [PubMed] [Google Scholar]
- 13.Sung KR, Sun JH, Na JH, Lee JY, Lee Y. Progression detection capability of macular thickness in advanced glaucomatous eyes. Ophthalmology. 2012;119(2):308–313. doi: 10.1016/j.ophtha.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 14.Anton A, Pazos M, Martin B, et al. Glaucoma progression detection: agreement, sensitivity, and specificity of expert visual field evaluation, event analysis, and trend analysis. Eur J Ophthalmol. 2013;23(2):187–195. doi: 10.5301/ejo.5000193. [DOI] [PubMed] [Google Scholar]
- 15.Artes PH, O'Leary N, Hutchison DM, et al. Properties of the statpac visual field index. Invest Ophthalmol Vis Sci. 2011;52(7):4030–4038. doi: 10.1167/iovs.10-6905. [DOI] [PubMed] [Google Scholar]
- 16.Wall M, Doyle CK, Zamba KD, Artes P, Johnson CA. The repeatability of mean defect with size III and size V standard automated perimetry. Invest Ophthalmol Vis Sci. 2013;54(2):1345–1351. doi: 10.1167/iovs.12-10299. [DOI] [PubMed] [Google Scholar]
- 17.Mwanza JC, Chang RT, Budenz DL, et al. Reproducibility of peripapillary retinal nerve fiber layer thickness and optic nerve head parameters measured with cirrus HD-OCT in glaucomatous eyes. Invest Ophthalmol Vis Sci. 2010;51(11):5724–5730. doi: 10.1167/iovs.10-5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garvin MK, Lee K, Burns TL, Abramoff MD, Sonka M, Kwon YH. Reproducibility of SD-OCT-based ganglion cell-layer thickness in glaucoma using two different segmentation algorithms. Invest Ophthalmol Vis Sci. 2013;54(10):6998–7004. doi: 10.1167/iovs.13-12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254(5035):1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan O, Li G, Lu AT, Varma R, Huang D. Mapping of macular substructures with optical coherence tomography for glaucoma diagnosis. Ophthalmology. 2008;115(6):949–956. doi: 10.1016/j.ophtha.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan O, Chopra V, Lu AT, et al. Detection of macular ganglion cell loss in glaucoma by Fourier-domain optical coherence tomography. Ophthalmology. 2009;116(12):2305–2314. e2301–2302. doi: 10.1016/j.ophtha.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bowd C, Zangwill LM, Berry CC, et al. Detecting early glaucoma by assessment of retinal nerve fiber layer thickness and visual function. Invest Ophthalmol Vis Sci. 2001;42(9):1993–2003. [PubMed] [Google Scholar]
- 23.Zangwill LM, Bowd C, Berry CC, et al. Discriminating between normal and glaucomatous eyes using the Heidelberg Retina Tomograph, GDx Nerve Fiber Analyzer, and Optical Coherence Tomograph. Arch Ophthalmol. 2001;119(7):985–993. doi: 10.1001/archopht.119.7.985. [DOI] [PubMed] [Google Scholar]
- 24.Loewen NA, Zhang X, Tan O, et al. Combining measurements from three anatomical areas for glaucoma diagnosis using Fourier-domain optical coherence tomography. Br J Ophthalmol. 2015 doi: 10.1136/bjophthalmol-2014-305907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mwanza JC, Oakley JD, Budenz DL, Anderson DR. Ability of cirrus HD-OCT optic nerve head parameters to discriminate normal from glaucomatous eyes. Ophthalmology. 2011;118(2):241–248. e241. doi: 10.1016/j.ophtha.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang RT, Knight OJ, Feuer WJ, Budenz DL. Sensitivity and specificity of time-domain versus spectral-domain optical coherence tomography in diagnosing early to moderate glaucoma. Ophthalmology. 2009;116(12):2294–2299. doi: 10.1016/j.ophtha.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 27.Medeiros FA, Zangwill LM, Bowd C, Vessani RM, Susanna R, Jr, Weinreb RN. Evaluation of retinal nerve fiber layer, optic nerve head, and macular thickness measurements for glaucoma detection using optical coherence tomography. Am J Ophthalmol. 2005;139(1):44–55. doi: 10.1016/j.ajo.2004.08.069. [DOI] [PubMed] [Google Scholar]
- 28.Schuman JS, Hee MR, Arya AV, et al. Optical coherence tomography: a new tool for glaucoma diagnosis. Curr Opin Ophthalmol. 1995;6(2):89–95. doi: 10.1097/00055735-199504000-00014. [DOI] [PubMed] [Google Scholar]
- 29.Hood DC, Raza AS, Kay KY, et al. A comparison of retinal nerve fiber layer (RNFL) thickness obtained with frequency and time domain optical coherence tomography (OCT) Opt Express. 2009;17(5):3997–4003. doi: 10.1364/oe.17.003997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang JY, Pekmezci M, Mesiwala N, Kao A, Lin S. Diagnostic power of optic disc morphology, peripapillary retinal nerve fiber layer thickness, and macular inner retinal layer thickness in glaucoma diagnosis with fourier-domain optical coherence tomography. J Glaucoma. 2011;20(2):87–94. doi: 10.1097/IJG.0b013e3181d787b6. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X, Loewen N, Tan O, et al. Predicting Development of Glaucomatous Visual Field Conversion Using Baseline Fourier-Domain Optical Coherence Tomography. American Journal of Ophthalmology. 163:29–37. doi: 10.1016/j.ajo.2015.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sehi M, Bhardwaj N, Chung YS, Greenfield DS. Evaluation of baseline structural factors for predicting glaucomatous visual-field progression using optical coherence tomography, scanning laser polarimetry and confocal scanning laser ophthalmoscopy. Eye (Lond) 2012;26(12):1527–1535. doi: 10.1038/eye.2012.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang X, Dastiridou A, Francis BA, et al. Baseline Fourier-Domain Optical Coherence Tomography Structural Risk Factors for Visual Field Progression in the Advanced Imaging for Glaucoma Study. Am J Ophthalmol. 2016;172:94–103. doi: 10.1016/j.ajo.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sommer A, Katz J, Quigley HA, et al. Clinically detectable nerve fiber atrophy precedes the onset of glaucomatous field loss. Arch Ophthalmol. 1991;109(1):77–83. doi: 10.1001/archopht.1991.01080010079037. [DOI] [PubMed] [Google Scholar]
- 35.Wollstein G, Schuman JS, Price LL, et al. Optical coherence tomography longitudinal evaluation of retinal nerve fiber layer thickness in glaucoma. Arch Ophthalmol. 2005;123(4):464–470. doi: 10.1001/archopht.123.4.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang X, Loewen N, Tan O, et al. Predicting Development of Glaucomatous Visual Field Conversion Using Baseline Fourier-Domain Optical Coherence Tomography. Am J Ophthalmol. 2016;163:29–37. doi: 10.1016/j.ajo.2015.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuang TM, Zhang C, Zangwill LM, Weinreb RN, Medeiros FA. Estimating Lead Time Gained by Optical Coherence Tomography in Detecting Glaucoma before Development of Visual Field Defects. Ophthalmology. 2015;122(10):2002–2009. doi: 10.1016/j.ophtha.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abe RY, Diniz-Filho A, Zangwill LM, et al. The Relative Odds of Progressing by Structural and Functional Tests in Glaucoma. Invest Ophthalmol Vis Sci. 2016;57(9):OCT421–428. doi: 10.1167/iovs.15-18940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Le PV, Zhang X, Francis BA, et al. Advanced imaging for glaucoma study: design, baseline characteristics, and inter-site comparison. Am J Ophthalmol. 2015;159(2):393–403. e392. doi: 10.1016/j.ajo.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McNaught AI, Crabb DP, Fitzke FW, Hitchings RA. Visual field progression: comparison of Humphrey Statpac2 and pointwise linear regression analysis. Graefes Arch Clin Exp Ophthalmol. 1996;234(7):411–418. doi: 10.1007/BF02539406. [DOI] [PubMed] [Google Scholar]
- 41.Hodapp E, Parrish RI, Anderson D. Clinical decisions in glaucoma. St Louis: The CV Mosby Co; 1993. Clinical decisions in glaucoma; pp. 52–61. [Google Scholar]
- 42.Zhang X, Iverson SM, Tan O, Huang D. Effect of Signal Intensity on Measurement of Ganglion Cell Complex and Retinal Nerve Fiber Layer Scans in Fourier-Domain Optical Coherence Tomography. Transl Vis Sci Technol. 2015;4(5):7. doi: 10.1167/tvst.4.5.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang X, Francis BA, Dastiridou A, et al. Longitudinal and Cross-Sectional Analyses of Age Effects on Retinal Nerve Fiber Layer and Ganglion Cell Complex Thickness by Fourier-Domain OCT. Transl Vis Sci Technol. 2016;5(2):1. doi: 10.1167/tvst.5.2.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O'Leary N, Chauhan BC, Artes PH. Visual field progression in glaucoma: estimating the overall significance of deterioration with permutation analyses of pointwise linear regression (PoPLR) Invest Ophthalmol Vis Sci. 2012;53(11):6776–6784. doi: 10.1167/iovs.12-10049. [DOI] [PubMed] [Google Scholar]
- 45.Redmond T, O'Leary N, Hutchison DM, Nicolela MT, Artes PH, Chauhan BC. Visual field progression with frequency-doubling matrix perimetry and standard automated perimetry in patients with glaucoma and in healthy controls. JAMA Ophthalmol. 2013;131(12):1565–1572. doi: 10.1001/jamaophthalmol.2013.4382. [DOI] [PubMed] [Google Scholar]
- 46.Liang K-Y, Zeger Scott L. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 47.Ohnell H, Heijl A, Brenner L, Anderson H, Bengtsson B. Structural and Functional Progression in the Early Manifest Glaucoma Trial. Ophthalmology. 2016;123(6):1173–1180. doi: 10.1016/j.ophtha.2016.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Medeiros FA, Zangwill LM, Bowd C, Mansouri K, Weinreb RN. The structure and function relationship in glaucoma: implications for detection of progression and measurement of rates of change. Invest Ophthalmol Vis Sci. 2012;53(11):6939–6946. doi: 10.1167/iovs.12-10345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mwanza JC, Budenz DL, Warren JL, et al. Retinal nerve fibre layer thickness floor and corresponding functional loss in glaucoma. Br J Ophthalmol. 2015;99(6):732–737. doi: 10.1136/bjophthalmol-2014-305745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Belghith A, Medeiros FA, Bowd C, et al. Structural Change Can Be Detected in Advanced-Glaucoma Eyes. Invest Ophthalmol Vis Sci. 2016;57(9):OCT511–518. doi: 10.1167/iovs.15-18929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saunders LJ, Russell RA, Crabb DP. Measurement precision in a series of visual fields acquired by the standard and fast versions of the Swedish interactive thresholding algorithm: analysis of large-scale data from clinics. JAMA Ophthalmol. 2015;133(1):74–80. doi: 10.1001/jamaophthalmol.2014.4237. [DOI] [PubMed] [Google Scholar]
- 52.Miki A, Medeiros FA, Weinreb RN, et al. Rates of retinal nerve fiber layer thinning in glaucoma suspect eyes. Ophthalmology. 2014;121(7):1350–1358. doi: 10.1016/j.ophtha.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu M, Lin C, Weinreb RN, Lai G, Chiu V, Leung CK. Risk of Visual Field Progression in Glaucoma Patients with Progressive Retinal Nerve Fiber Layer Thinning: A 5-Year Prospective Study. Ophthalmology. 2016;123(6):1201–1210. doi: 10.1016/j.ophtha.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 54.Vizzeri G, Bowd C, Weinreb RN, et al. Determinants of agreement between the confocal scanning laser tomograph and standardized assessment of glaucomatous progression. Ophthalmology. 2010;117(10):1953–1959. doi: 10.1016/j.ophtha.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Medeiros FA, Weinreb RN, Moore G, Liebmann JM, Girkin CA, Zangwill LM. Integrating event- and trend-based analyses to improve detection of glaucomatous visual field progression. Ophthalmology. 2012;119(3):458–467. doi: 10.1016/j.ophtha.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rao HL, Begum VU, Khadka D, Mandal AK, Senthil S, Garudadri CS. Comparing glaucoma progression on 24-2 and 10-2 visual field examinations. PLoS One. 2015;10(5):e0127233. doi: 10.1371/journal.pone.0127233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu Z, Saunders LJ, Daga FB, Diniz-Filho A, Medeiros FA. Frequency of Testing to Detect Visual Field Progression Derived Using a Longitudinal Cohort of Glaucoma Patients. Ophthalmology. 2017;124(6):786–792. doi: 10.1016/j.ophtha.2017.01.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.