Abstract
The Developmental Origins of Health and Disease (DOHaD) Hypothesis postulates that the in utero environment influences postnatal health and plays a role in disease etiology. Studies in both humans and animal models have shown that exposure to either under- or overnutrition in utero results in an increased risk of metabolic disease later in life. In addition, offspring born to overweight or obese mothers are more likely to be obese as children and into early adulthood and to have impaired glucose tolerance as adults. The Centers for Disease Control and Prevention estimates that over 70% of adults over the age of 20 are either overweight or obese and that nearly half of women are either overweight or obese at the time they become pregnant. Thus, the consequences of maternal overnutrition on the developing fetus are likely to be realized in greater numbers in the coming decades. This review will focus specifically on the effects of in utero overnutrition on pancreatic islet development and function and how the resulting morphological and functional changes influence the offspring’s risk of developing metabolic disease. We will discuss the advantages and challenges of different animal models, the effects of exposure to overnutrition during distinct periods of development, the similarities and differences between and within model systems, and potential mechanisms and future directions in understanding how developmental alterations due to maternal diet exposure influence islet health and function later in life.
Keywords: diabetes, high fat diet, maternal obesity, developmental origins, islet development, β cell
INTRODUCTION
The Developmental Origins of Health and Disease (DOHaD) Hypothesis postulates that the in utero environment influences postnatal health and plays a role in disease etiology. Early evidence for DOHaD was obtained from studies on humans born during the Dutch Hunger Winter, a period of famine in the Netherlands during World War II, and their siblings born outside this period of famine. Offspring born during this period of famine who were undernourished in utero during the first or second trimesters were more likely to develop obesity and metabolic disease in adulthood when compared with their siblings born before or after the famine [1]. In the subsequent decades following these observations, epidemiologist Dr. David Barker brought the DOHaD theory to prominence when he published a series of studies demonstrating that individuals born with low birthweight or in regions with high infant mortality (proxies for in utero undernutrition or stress) were at an increased risk of developing cardiovascular disease [2–4]. Interestingly, exposure to overnutrition in utero also results in an increased risk of metabolic disease later in life. Like offspring born with low birthweight, high birthweight is also associated with increased BMI in adulthood [5], and offspring born to overweight or obese mothers are more likely to be obese as children and into early adulthood [6]. Additionally, offspring of mothers who were obese before and during pregnancy developed insulin resistance and impaired glucose tolerance as adults at significantly higher rates independent of birth weight [7].
The impact of these findings is magnified when considering the prevalence of overweight and obesity in the United States. As of 2014, the Centers for Disease Control and Prevention (CDC) estimates that over 70% of adults over the age of 20 are either overweight or obese [8]. While the prevalence of overweight has remained relatively unchanged over the past few decades, rates of obesity and extreme obesity (defined as BMI greater than or equal to 40) are increasing rapidly in both men and women [9]. While obesity rates are often higher in older populations, the increasing prevalence of obesity affects women of reproductive age to a significant extent. The most recent data from the Pregnancy Risk Assessment Monitoring System, a surveillance system of the CDC, indicates that nearly half of women are either overweight or obese at the time they become pregnant [10]. Thus, the consequences of maternal overnutrition on the developing fetus are likely to be realized in greater numbers in the coming decades. This review will focus specifically on the effects of in utero overnutrition on pancreatic islet development and function and how the resulting morphological and functional changes influence the offspring’s risk of developing metabolic disease.
To this end, numerous studies using several different animal models have been published describing changes in islet structure and function due to in utero overnutrition. However, significant challenges exist when interpreting and comparing results due to differences in study design. Variables that must be taken into consideration include animal model, source of maternal overnutrition (e.g. high fat versus high sucrose diet, different sources and quantities of dietary fat, etc.), timing of overnutrition leading up to or during pregnancy, offspring post-weaning diet, and several other factors. Here we discuss the advantages and challenges of different animal models, the effects of exposure to overnutrition during distinct periods of development, the similarities and differences between and within model systems, and potential mechanisms and future directions in understanding how developmental alternations due to maternal diet exposure influence islet health and function later in life.
Pancreas development primer
In order to appreciate the in utero consequences of exposure to overnutrition, a brief overview of endocrine pancreas development is provided here. The following relies on a series of reviews [11–13] to which the reader is referred for a more in-depth analysis. Though the progression from progenitor to mature endocrine cells is very similar among different organisms, specific time points during mouse development are referred to, since most previous studies have utilized this model. For further reading on pancreas development in the human, two additional reviews on the topic may be of interest [14, 15].
At gastrulation, a migration inward of cells at or near the surface of the blastula forms a three-layered structure composed of ectoderm, mesoderm, and endoderm. In the mouse, the embryo rotates from a lordotic position to a fetal position, leading to internalization of the endoderm layer to form a gut tube. All endoderm-derived organs, including the pancreas, originate from this tube. Signals from the mesoderm lead to patterning of the epithelium and evagination of the pancreatic buds.
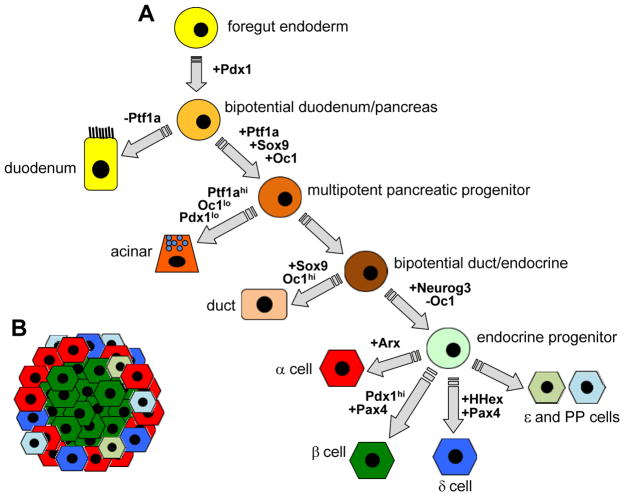
The basic cell lineage pathway and key developmental transcription factors leading to differentiated pancreatic endocrine cells are shown in Figure 1A. The transcription factor (TF) Pdx1 marks the pancreatic progenitor cells and is required for pancreas formation. Pdx1 is initially broadly expressed in the posterior foregut endoderm as early as embryonic day 8.5 (e8.5) in the mouse. Shortly thereafter, the dorsal pancreatic bud evaginates from the dorsal foregut endoderm that lies between the putative stomach and duodenum domains. A region of the ventral foregut endoderm becomes the ventral pancreatic bud. The dorsal bud first appears at e9.5. Co-expression of Pdx1 with the TF Ptf1a defines the pancreatic progenitor cells and allows pancreas development to proceed. The TFs Sox9 and Oc1 are also co-expressed with Ptf1a in a subset of Pdx1+ cells and are additional markers of pancreatic progenitor cells. Pdx1+/Ptf1a+/Sox9+/Oc1+ multipotent pancreatic progenitors (MPCs) are highly proliferative and give rise to all the different pancreatic cell types (acinar cells, ducts, endocrine cells). The MPCs expand and rearrange to form a multilayered stratified epithelium. By e11.5, the two pancreatic buds have expanded into the surrounding mesenchyme, leading to the formation of a highly branched tree-like structure embedded in a loose mesenchyme.
Figure 1. Schematic of pancreatic cell lineages and key lineage transcription factors.
(A) Undifferentiated progenitor cell types are shown in circles. Differentiated cells are shown in other shapes. Key lineage-determining transcription factors up- or down-regulated at each stage are shown in bold. (B) Scheme of rodent islet showing insulin-producing β cells at the islet core and comprising the majority of the islet. All other endocrine cells are found at the islet periphery.
The majority of endocrine cells in the mouse differentiate between e13.5 and e18.5 (with a peak at e15.5) in what is referred to as the secondary transition of pancreas differentiation (the “first wave” of differentiation produces a relatively lower number of primarily glucagon-positive cells that do not contribute to the adult pancreas; it is unclear whether this first wave exists in humans). During this time, MPCs become lineage restricted to either Sox9+/Nkx6.1+ ductal-endocrine bipotent cells in the trunk of the tree-like structures, or Ptf1a+/Gata4+/Cpa+ cells in the tips that ultimately differentiate into acinar cells. Key TFs required for endocrine differentiation are expressed at this stage. Neurogenin3 is the definitive marker of the endocrine progenitor population. Bipotent endocrine/ductal cells are directed toward an endocrine fate by transient expression of this TF. Pdx1 becomes down-regulated as the endocrine pancreas develops, but is ultimately expressed at high levels specifically in the β cell. Dynamic changes in Pdx1 expression are necessary for proper endocrine differentiation.
Committed endocrine cells delaminate from the ductal epithelium and aggregate to form nascent islets around e18.5. Differentiation of single-hormone positive cells (insulin, glucagon, somatostatin, PP, ghrelin) depends on specific combinations of TF expression in gene regulatory networks that also repress alternate islet cell fates. The TF Pax4 plays a key role in the β cell gene regulatory network. Pax4 mRNA is first detected at e9.5 and is transiently expressed in all endocrine progenitor cells during development. It is downstream of Neurogenin3 and essential for appropriate initiation of β-cell differentiation. Loss of Pax4 prevents expression of Pdx1 (which becomes restricted to the β cell) and insulin mRNA in β-cell precursors. In the α cell, Arx plays a key role in differentiation. Arx expression begins at e9.5 and persists into mature α cells. Pax4 has an opposing role to Arx, and helps to suppress the α cell gene regulatory network in developing β cells. In all species examined, the majority of hormone-expressing endocrine cells are β cells; however, the proportion of β cells and their localization within the islet can differ in different species. In mice, islets are ultimately composed of a core of insulin-producing β cells, which account for about 80% of all endocrine cells, surrounded by a mantle of the other endocrine cell types: glucagon-producing α cells, somatostatin-producing δ cells, pancreatic polypeptide-producing PP cells, and ghrelin-producing ε cells (Figure 1B). During the second wave of endocrine differentiation, endocrine cells increase in number mainly due to neogenesis from bipotent progenitors. In both human and mouse, all the endocrine cell types are present at birth and mature endocrine cells aggregate into islet structures shortly after birth. Expansion of the endocrine population after birth is primarily due to proliferation rather than neogenesis.
β-cell mass, function, and type 2 diabetes
The insulin-producing β cell plays an essential role in maintaining glucose homeostasis in healthy individuals by sensing plasma glucose levels and secreting appropriate levels of insulin. Glucose enters the β cell through the facilitated glucose transporter GLUT2, and is then phosphorylated by the enzyme glucokinase. The resulting product, glucose-6-phosphate, is further metabolized ultimately producing ATP. An increase in intracellular ATP results in closure of plasma membrane ATP- sensitive potassium channels, causing membrane depolarization and insulin release.
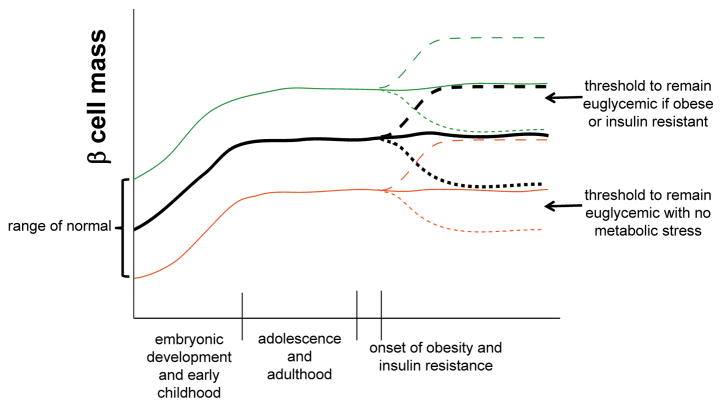
Human autopsy studies reveal that individuals with normal glucose homeostasis exhibit differences in total β-cell mass at birth and in adulthood (Figure 2). Thus, one can assume that there is a range of “normal” β-cell mass that can effectively maintain euglycemia under standard conditions. However, in both humans and mice in the face of additional metabolic demand, such as obesity-induced insulin resistance, a higher level of functional β-cell mass is required to maintain euglycemia. In the majority of individuals who are insulin resistant, β-cell compensation occurs, including an increase in insulin production and output per β cell, and an increase in β-cell mass via proliferation (Figure 2, dashed lines). However, in some individuals, often in the face of prolonged metabolic stress, β-cell compensation fails and there is a loss of functional β-cell mass leading to hyperglycemia and type 2 diabetes (Figure 2, dotted lines) [16].
Figure 2. Dyamics of β-cell mass under normal and impaired metabolic states.
β-cell mass is established via neogenesis during embryogenesis and proliferation in early postnatal life, plateauing in childhood. Individuals are born with a range of β-cell mass that is considered “normal” in that it is sufficient to maintain euglycemia in the absence of metabolic stress. Average β-cell mass is shown in the black solid line, upper and lower ranges of normal are shown in green and orange solid lines, respectively. With the onset of obesity and insulin resistance, the normal response is an expansion in β-cell mass (by proliferation) shown in the dashed lines. Individuals starting with a lower level of β-cell mass may not reach the threshold required to maintain euglycemia in the setting of insulin resistance, leading to increased susceptibility for type 2 diabetes. Alternatively, β-cell failure and/or death (dotted lines) can occur in the setting of obesity and insulin resistance, again leading to an increased susceptibility to type 2 diabetes for individuals starting with fewer β-cells.
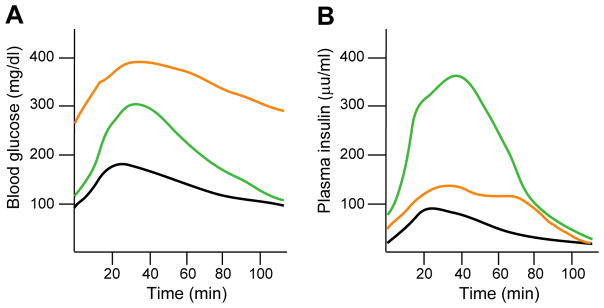
Glucose homeostasis is assessed using a glucose tolerance test (GTT) (Figure 3A) in which fasting plasma glucose is measured and then a bolus of glucose is administered followed by serial measurements of plasma glucose over a set time period. This test assesses the ability to clear glucose –a function of insulin secretion by the β cells and uptake by insulin-sensitive peripheral tissues (such as liver, muscle, and adipose). Plasma insulin levels can also be measured during the GTT (Figure 3B). Figure 3 depicts an illustrative GTT and plasma insulin profile showing typical results from a healthy individual (black line), someone with insulin resistance and impaired glucose tolerance (green line), and someone with diabetes (orange line). Obesity and insulin resistance are associated with increased glucose excursions and increased plasma insulin, with normal fasting and 2-hour post-prandial blood glucose levels (green lines). However, prolonged periods of insulin resistance place undue stress on the β cells [17], and in individuals with genetic susceptibility, can lead to fasting hyperglycemia and type 2 diabetes (orange lines).
Figure 3. Correlation of glucose homeostasis with plasma insulin levels under normal and impaired metabolic states.
(A) Representative intraperitoneal glucose tolerance test in which after an overnight fast, the subject is given a glucose bolus. Blood glucose is measured at fasting and at different time points after glucose administration over a two-hour period. Black line is the normal glucose excursion curve. Green line represents impaired glucose tolerance. Orange line represents diabetes. (B) Plasma insulin levels measured at the same time points as blood glucose in (A). Colored lines are same as in (A). Glucose intolerance is associated with hyperinsulinemia, while diabetes is associated with reduced plasma insulin in the face of elevated blood glucose.
The precise reasons why some insulin-resistant individuals develop diabetes while others are able to compensate remains unclear, and the etiology of diabetes can differ in different individuals. The final outcome is likely determined by a combination of the initial β-cell mass, the ability to increase insulin production and secretion, the extent of β-cell proliferation, and the susceptibility to β-cell dedifferentiation or death (Figure 2). An individual with β-cell mass at the upper end of normal (green line) has sufficient β-cell mass at baseline to maintain euglycemia even in the face of insulin resistance, provided there is no loss of β-cell mass. Conversely, an individual with β-cell mass at the lower end of normal (orange line) has sufficient β-cell mass to maintain euglycemia in the absence of metabolic stress, but in the face of insulin resistance the low initial β-cell mass is insufficient, even with a compensatory increase.
In summary, type 2 diabetes develops when the supply of functional β-cell mass is insufficient to meet the metabolic demand for insulin. This can occur due to an initially low level of β-cell mass, or an inability to compensate for insulin resistance. As discussed in the previous section, the development of the endocrine pancreas (and resulting baseline level of β-cell mass) relies on coordinated expression of specific TFs during critical windows. Many of these TFs are also expressed in the mature β-cell and have important roles in β-cell function. As a result, exposure to overnutrition in utero may increase type 2 diabetes susceptibility by affecting the initial mass of β cells produced during development, or altering the function of β cells such that they are unable to respond to metabolic stress.
Rodent models of maternal overnutrition
Rodent models are most frequently used to investigate the effects of in utero overnutrition on islet development and function. Rodents have several advantages that enable thorough, well-controlled experiments that would otherwise be difficult to conduct in large animal models. The advantages and disadvantages of both small and large animal models are discussed thoroughly in a 2009 review by McMullin and Mostyn [18], and the following is based on their review and the experience of the authors. Due to the short duration of gestation and lifespan, large litter size, and the relatively low cost of maintaining colonies, it is usually feasible to conduct rodent studies which are sufficiently powered. Additionally, it is easier to minimize genetic and environmental variability through inbred strains and uniform housing, it is feasible to monitor food intake, and multiple genetically modified models are available, enabling specific probing of pathways mediating the effects of maternal diet on the offspring and the ability to sort out the role of and effects on specific cell types. Furthermore, dietary components can be easily manipulated to test the effects of specific macronutrients on the health of the offspring.
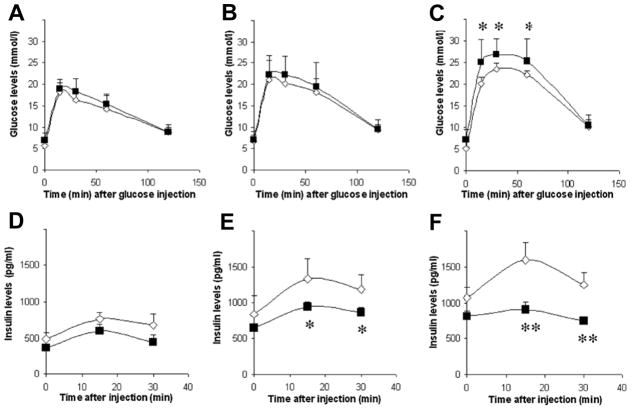
Multiple studies in rodents have investigated the effects of maternal high fat diet (HFD) or obesity during pregnancy and its effects on offspring islet development and function [19–29]. A summary of the effects of maternal overnutrition on offspring islets using rodent models is presented in Table 1. The majority of these studies were conducted in rats and completed before the year 2010. As previously mentioned, comparisons between studies are often difficult due to differences in animal model, diet, and study design. However, three themes emerge when considering the body of results as a whole. First, overwhelmingly, maternal HFD results in significant changes in islet architecture or function, many of which could be predicted to increase susceptibility to type 2 diabetes. For example, Graus-Nunes et al. reported that HFD-exposed males and their offspring had increased islet mass and fasting hyperinsulinemia [24] which can lead to insulin resistance. Meanwhile, Wistar rats exposed to HFD had reduced β-cell mass at birth [21], which may predispose these animals to develop diabetes later in life (Figure 2). Second, while changes in endocrine cell mass or islet architecture vary, in the majority of studies there is some evidence of impaired islet function. This manifests as whole body impairments in glucose tolerance in the offspring or decreased insulin secretion from islets isolated from HFD- or obesity-exposed offspring when treated with high glucose or other insulin secretagogues. Figure 4, adapted from Han et al., 2005 [26], demonstrates how offspring exposed to maternal obesity initially have normal glucose tolerance but demonstrate a progressive loss in β-cell function (i.e. insulin secretion) with age that ultimately results in impaired glucose tolerance in adulthood. Third, in the minority of studies that focused on sex differences, there were observable differences in islet function between males and females, although which sex was more severely affected depended on the study. There is some evidence in humans that adverse in utero exposures have differential effects on male and female offspring that can increase risk for metabolic disease. Specifically, male and female offspring born during the Dutch Hunger Winter had differential DNA methylation at metabolic gene loci [30]. Additionally, chemical exposure in utero results in differential levels of the satiety hormone leptin in males and females [31].
Table 1.
Comparison of rodent models of maternal overnutrition.
| Animal model | Diet/Experimental groups | Glucose tolerance/Fasting glucose | Insulin tolerance/Fasting insulin | Endocrine cell mass/ratios | Notes | Reference |
|---|---|---|---|---|---|---|
| C57BL6/J mouse | CTR (11.5% kcal from fat) or HFD (62% kcal from fat) from conception to weaning. Offspring placed on CTR from 4–6 wk then CTR or HFD. | Impaired at 6 wk in HFD offspring. In males and females at 14 wk and 20 wk, HFD/HFD was worse than CTR/HFD. In males, HFD/CTR worse than CTR/CTR. | At 14 and 20 wk, HFD/HFD had worse insulin tolerance in both males and females. | Maternal HFD decreased islet area in males but increased islet area in HFD/HFD females (with higher proportion of large islets). | Greater body weight at birth and from 4–6 wk. From 6–20 wk HFD/HFD females (only) weighed more. Islet insulin content and Pdx1 mRNA was decreased in HFD/HFD males, but elevated in females. | [29] |
| C57BL/6 mouse | CTR (17% from fat-soybean oil); HFD (49%-soybean oil as in CTR plus additional fat in lard). CTR or HFD starting at 4 wk for 8 weeks before mating. Diet maintained through lactation; then offspring on CTR for 2 generations (F1 and F2). Male offspring evaluated at 3 months. | Glucose tolerance impaired (with higher AUC) in F1 and impaired at two time points in F2. F1 HFD had fasting hyperglycemia. | F1 and F2 had fasting hyperinsulinemia. | F1 and F2 HFD had higher islet mass, higher α-cell mass and much higher β-cell mass. Both F1 and F2 had reduced Pdx1 immunolabeling. HFD F1 and F2 had α cells in the core of islet. | F0 and F1 HFD groups were overweight before and during pregnancy. | [24] |
| C57BL/6 mouse with or without the agouti yellow mutation (obese but euglycemic) | Mothers (Obese = agouti yellow mutation; CON = wild type) were fed normal chow during pregnancy. Offspring fed normal chow until 15 wk, then CTR (22% from fat) or HFD (60% cal from fat) starting from 15 wk to make four groups: CON/CTR, Obese/CTR, CON/HFD, Obese/HFD. | 50 wk Obese/HFD females had impaired glucose tolerance relative to CON/HFD. | Obese-exposed females (Obese/HFD and Obese/CTR) had less insulin secretion during GTT at 50 wk. At 30 and 50 wk, female Obese/HFD offspring had reduced insulin secretion during GTT relative to CON/HFD. Isolated islets from Obese/HFD females had similar basal insulin secretion but impaired response to stimuli compared to CON/CTR. | Not analyzed. | Offspring of obese mothers had higher birth weight, but this normalized in adulthood. HFD increased islet insulin content in CON/HFD but not obese/HFD. Protein levels of select metabolic enzymes (Transketolase, GAPDH, PFK) were reduced in Obese/CTR and Obese/HFD islets. | [26] |
| Wistar rats | CTR (10/15/75% kcal from fat/protein/carbs) HFD (40/14/46) with fat from saturated animal fat. Groups: HFDG1, HFDG2, HFDG3 or HFDG. Examined at birth. | HFDG1 and HFDG2 were hypoglycemic. HFDG were hyperglycemic. | No differences in plasma insulin levels. | β-cell volume and numbers were reduced in HFDG neonates. α-cell volume, size, and number were increased in HFDG. | HFDG1 had low birth weight. | [21] |
| Wistar rats | CTR and HFD as above. Groups: HFDG, HFDGL1, HFDGL2, HFDGL3, HFDGL, and HFDL. Offspring examined at weaning. | Only CTR, HFDG and HFDGL were normoglycemic. | HFDG, HFDGL1, and HFDGL were hypoinsulinemic. | Not analyzed. | HFDG weighed less than controls, HFDL weighed more. Glucokinase mRNA and immunoreactivity reduced in HFDGL, HFDGL2 and HFDGL3. | [20] |
| Wistar rats | Same diet as above. Groups: HFDG neonates and adolescents, HFDL weanlings, HFDGL weanlings, HFDP adolescents, HFDG-P adolescents. | HFDP were hyperglycemic in adolescence but not HFDG. | HFDP and HFDG-P were hyperinsulinemic in adolescence. | No differences in β-cell mass among adolescents. No change in ratios. Increased islet cell proliferation in HFDP. Otherwise, no differences in proliferation. | HFDGL were the heaviest group in adolescence, then HFDL. HFDG were similar to CTR. HFDG neonates had reduced pancreas:body weight ratio. HFDG-P pancreas:body was increased at weaning but decreased at P90. | [19] |
| Sprague- Dawley rats | CTR (10.9/70% kcal from fat/carbs) and HFD (59.5/24.4% from fat/carbs). Diet from postnatal day 24 through gestation and lactation (mated at P120). Progeny weaned onto CTR or HSu diet. | At P90, HFD/CTR and HFD/HSu were glucose intolerant. At P120, HFD/CTR, CTR/HSu, and HFD/HSu had elevated plasma glucose. | Higher plasma insulin in HFD fetuses at term. Plasma insulin was higher in HFD/CTR compared to CTR/CTR from P40 onward. At P120, plasma insulin was high in HFD/CTR and HFD/HSu relative to respective controls. | Not analyzed. | Islets from HFD fetuses secreted more insulin at high glucose or in the presence of arginine or leucine (insulin secretagogues). HFD/CTR and HFD/HSu had increased body weight from P30 to P120. P120 islets exposed to HFD in utero or HSu diet after weaning had higher basal insulin and lower fold increase at high glucose. | [27] |
| Albino Wistar rats | CTR (22% protein, 5% fat, 31% polysaccharide, 31% simple sugars). Maternal obesity (MO) (23.5% protein, 20% animal lard, 5% corn oil, 21% polysaccharide, 21% simple sugars). Diets from P21 to P120, then through pregnancy and lactation. Offspring weaned to CTR. | No differences in plasma glucose levels. | In MO male offspring, plasma insulin was higher at P110. Otherwise, no differences. P110 islets secreted less insulin at basal (5 mM) glucose. | MO offspring had higher proportion of β cells (% of total islet cells) at P36, but fewer at P110. MO had higher proportion of α cells at both P36 and P110. | No effect on pup weight, but MO offspring had higher % body fat. | [23] |
| Wistar rats | CTR (45 g/kg fat); HFD (200 g/kg fat from vegetable oil) for one week before mating, then during pregnancy and lactation. Offspring examined at weaning. | HFD pups had lower plasma glucose at birth, but higher glucose at weaning. | No differences in plasma insulin. | Not analyzed. | Mothers had no difference in weight gain during pregnancy and CTR group ate more kcal/week. HFD offspring weighed more and had more body fat. | [25] |
| Sprague Dawley rats | CTR (5% fat by weight) or HFD (CTR supplemented with 20% lard by weight). Fed diet 10 days before pregnancy and during pregnancy and lactation. | Trend towards elevated fasting glucose at 6 months in HFD group. | 6-month-old HFD offspring had elevated fasting insulin, and 1-year-old offspring were insulin resistant (clamp studies). HFD islets secreted less insulin in response to 20 mM glucose and had lower islet insulin content. | Not analyzed. | On electron microscopy, HFD group β cells had a greater proportion of immature insulin granules. α cell granules did not differ between groups. No obvious differences in islet morphology on H&E. HFD offspring had increased abdominal adiposity. | [28] |
| Wistar rats | Mothers fed high saturated fat (SAFA) or high carb (CHO) diet from 9 to 12 wk, then through gestation and lactation. Offspring were fed either diet to make four groups, plus a fifth that was exposed to SAFA in utero, then nursed by CHO moms and maintained on CHO post-weaning. SAFA (60% energy from fat, 25% carbs, 15% protein). CHO (10/75/15). | No differences in plasma glucose levels at 14 wk. | No differences in plasma insulin levels at 14 wk. SAFA during gestation was negatively correlated with offspring islet insulin secretion in response to stimuli. SAFA during lactation was positively correlated. No correlation with post weaning diet. No differences in perifusion. | Not analyzed. | No difference in litter size or average weight at birth. No differences in weight and food intake throughout the study. Another study by the same group showed no differences in insulin sensitivity between groups. | [22] |
CTR = control diet; HFD = high fat diet; HFDG = HFD during gestation only; HFDG# = HFD during week X of gestation; HFDL = HFD during lactation only; HFDL# = HFD during week X of lactation; HFDGL = HFD during gestation and lactation; HFDGL# = HFD during gestation and week X of lactation; HFDP = HFD postnatally; HFDG-P = HFD during gestation and postnatally; HSu = high Sucrose diet; GTT = glucose tolerance test; AUC = area under the curve; wk = weeks of age; P = postnatal day.
Figure 4. High fat diet impairs islet function in offspring from obese female mice.
Glucose tolerance tests (A, B, C) and plasma insulin levels (D, E, F) from high fat diet-fed female offspring born to control (open diamonds) and obese (closed squares) dams. Offspring were analyzed at 20 (A, D), 30 (B, E), and 50 (C, F) weeks of age. Data are means ± SD. *p < 0.05, **p < 0.01 compared with control; n = 8–10. (Adapted from Han, J., Xu, J., Epstein, P. N. and Liu, Y. Q. 2005, Diabetologia, 48(9), 1810–8 with permission from Springer.)
Future studies are needed to help elucidate the mechanisms by which maternal overnutrition impairs offspring islet function. In rodent models, islets from offspring born to obese or HFD-fed mothers have decreased expression of genes involved in glucose metabolism and oxidative phosphorylation [20, 26]. Also, there is evidence of decreased insulin protein content in adult islets [26, 28, 29] and impaired insulin granule biosynthesis in β cells from adult offspring [28] exposed to maternal obesity during development. Changes in offspring gene expression due to in utero exposures are generally thought to be due to epigenetic mechanisms including DNA methylation (associated with gene repression) and histone marks associated with transcriptional activation (e.g. H3 Acetylation or H3K4 trimethylation) or repression (e.g. H3K9 dimethylation and H3K27 trimethylation). Support for this concept comes from mouse studies showing multi-generational effects of maternal HFD on offspring metabolism [24, 32] and mitochondrial function [33]. Additionally, paternal HFD often leads to impairments in offspring metabolism that can persist for multiple generations in mice [34]. In a rat model of maternal undernutrition, offspring born to protein-restricted dams had decreased activating and increased repressive histone marks at the locus for the transcriptional regulator Hnf4α, a Type 2 Diabetes susceptibility gene [35]. These epigenetic changes resulted in decreased expression of Hnf4α due to weaker promotor-enhancer interaction. Further studies are needed to elucidate additional islet-specific epigenetic alterations in response to maternal overnutrition. While the precise mechanisms by which in utero nutritional changes lead to epigenetic modifications are largely unknown, diet is thought to influence DNA methylation or histone modifications in three ways: providing the substrates necessary for DNA or histone methylation, providing metabolic co-factors for DNA- or histone-modifying enzymes, and altering enzymes in the methionine cycle (ultimately changing the bioavailability of methyl groups) [36].
While rodent models have been beneficial in studying effects of maternal overnutrition on the offspring due to the advantages described above, there are also several disadvantages to this model [18] that warrant the addition of studies in larger animal models. For example, the large litter size leads to an in utero environment significantly different from that in humans. Space sharing within the uterus results in differences in fetal access to nutrition depending on location within the uterus. Additionally, studies using multiple animals per litter per group when sample number is low may not be statistically sound. Most rodents are altricial – born at an underdeveloped state relatively to larger animals or man. Development of the pancreas continues into postnatal life in rodents; islet neogenesis and significant endocrine cell proliferation occur postnatally in the mouse. Mouse diets, especially purified diets high in specific nutrients, are not representative of human diets, though cafeteria-style diets partially mitigate this process.
The zebrafish model of overnutrition during development
Larger animal models often provide a more accurate representation of the human condition. However, before discussing the advantages and previous findings in larger animal models, we will briefly examine one small animal model that provides some unique insights. The zebrafish may seem an unlikely model to study the effects of overnutrition during development, but it has a few unique advantages over other animal models. Zebrafish hatch at 3–4 days post fertilization (dpf) but the pancreas does not reach its mature shape and position until 6 dpf [37]. Besides the low cost and feasibility of maintaining sufficient numbers of fish, this developmental scheme allows incubation of zebrafish larvae in solutions with specific macronutrient composition. Thus, precise probing of specific macronutrients can determine which aspects of the diet affect the development of β cells. Additionally, since larvae are transparent and development proceeds externally, islet development can be monitored longitudinally in the same individual using fluorescent reporter transgenes (see Figure 5). These benefits are in addition to many of the same benefits in other small animal models discussed above (ease of genetic manipulation, large litter size, etc.).
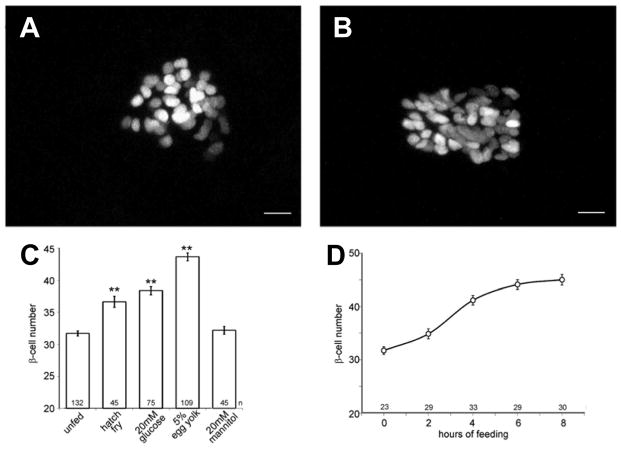
Figure 5. Exposure of zebrafish to overnutrition during islet development increases β-cell neogenesis.
(A, B) Images of β cells expressing a nuclear mCherry transgene under control of the insulin promoter. Approximately 30 β cells are observed in unfed larvae (A). This number increases after 8 h of culture in chicken egg yolk (B). Scale bars, 10 μm. (C) Effects of overnutrition on β-cell numbers in 6-dpf larvae. Mannitol changes the osmolarity similar to glucose. Bars indicate mean with SE (**ANOVA versus unfed, P < 0.001, Tukey HSD). (D) Time course of the increase in β-cell number within the first 8 h of culturing in 5% chicken egg yolk. n indicates the number of individual larvae in each sample group. (Adapted from Maddison, L. A. and Chen, W. 2012, Diabetes, 61(10), 2517–24 with permission from American Diabetes Association)
A 2012 study by Maddison and Chen [38] investigated the effects of specific nutrient combinations on developing larvae at 5 dpf (the start of free feeding). At this stage, zebrafish posses a single islet with approximately 30 β cells, and are in the second wave of endocrine differentiation. At 5 dpf, unfed larvae had an average of 32 β cells in the islet. Experimental groups were provided hatchfry encapsulation (a zebrafish larval diet with 12% lipid), 20 mM (high) glucose, or 5% chicken egg yolk (a higher lipid diet at 26.5%). As shown in Figure 5C, HFD-exposed fish had an average of 43.1 β cells after eight hours of incubation compared with 36.7 in hatch fry. β cells were also larger in HFD-exposed fish (not shown). Interestingly, when specific components of this diet were administered individually (amino acids, intralipid, or glucose at low concentration), there was no increase in the number of β cells compared with unfed larvae, and only the three macronutrients combined resulted in β-cell numbers comparable with chicken egg yolk. Only with the administration of high concentrations of glucose were β-cell numbers increased, and still to a lesser extent than with chicken egg yolk, suggesting that overnutrition itself, rather than a specific macronutrient, was responsible for the phenotype observed. Another series of experiments demonstrated that the increase in β-cell number due to overnutrition was a result of increased differentiation from progenitors rather than proliferation of existing β cells, leading to the author’s conclusion that β-cell development was accelerated in this model. The long-term effects of these developmental alterations have not yet been examined in the fish.
Large animal models of maternal overnutrition
A series of studies in sheep also demonstrated a similar response to overnutrition during development as zebrafish larvae-fed HFD, which was then followed by impaired glucose metabolism later in life [39–41]. Ewes were fed either an obesogenic or control diet for 60 days before conception, then throughout pregnancy and lactation. Fetuses analyzed in mid-gestation (day 75) were larger in the obese group, with a disproportionate increase in pancreatic weight. These animals also had higher plasma glucose and insulin levels and increased β-cell mass, suggesting that maternal obesity accelerated β-cell development in this model. Unlike zebrafish, however, this increase in β-cell mass was primarily due to increased proliferation. In late gestation (day 135), fetuses in the obese group had similar weights to control animals, and pancreatic weight and β-cell mass were now reduced, with increased β-cell apoptosis. At birth, obese-exposed sheep had elevated blood glucose and reduced insulin levels. The relative loss of β-cell mass due to increased apoptosis was likely due to chronic stress on the developing β cells from at least mid-gestation to the time of birth. As adults, obese-exposed sheep were insulin resistant, glucose intolerant, and consumed more feed during a feeding challenge resulting in significant weight gain. This series of studies demonstrates that early increases in β-cell mass due to overnutrition during development, which would appear to be a beneficial compensation, may set the offspring up for failure later in life. It would be interesting to see if this were true for the zebrafish model as well, despite the different etiologies of the additional β cells (neogenesis vs. proliferation).
Large animal models like the sheep have several distinct advantages when modelling maternal overnutrition [18]. These include fewer offspring per pregnancy - sheep give birth to between one and three live offspring. These offspring are born at a similar birthweight to humans. Unlike rodents, which are atricial species, large animal models have a fully developed hypothalamic-pituitary-adrenal axis before birth. However, there are also disadvantages specific to the sheep model - sheep have a ruminant GI system, breaking down plant products via fermentation to produce volatile fatty acids, which serve as a major energy source. This leads to important differences in metabolism between sheep and humans. Additionally, glucose tolerance tests are less meaningful in sheep since they rely more on fatty acids than glucose. Another large animal model, the non-human primate (NHP) has notable similarities with human pregnancy in terms of placentation, longer infant-dependent state, metabolism, and milk composition. Furthermore, islet structure and function have been well characterized in the NHP and are very similar to that of humans, especially compared with the mouse.
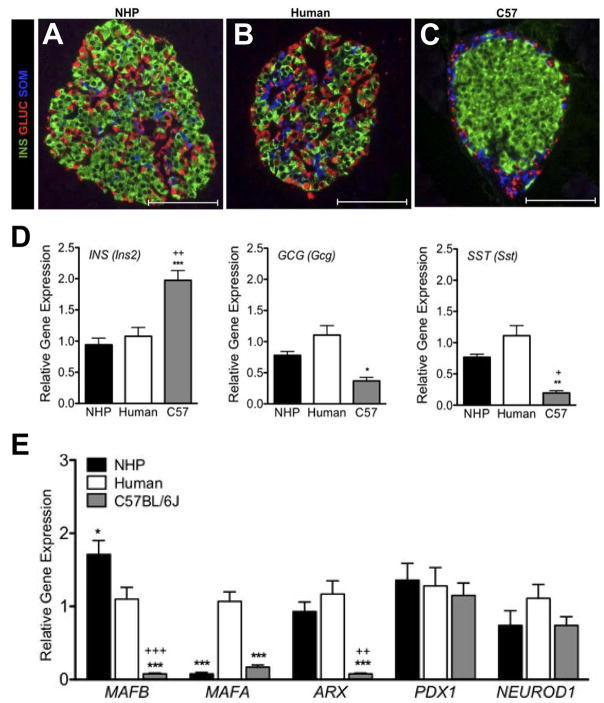
In humans, a phase of islet remodeling from late gestation through at least 4 years of age ultimately results in islets with a more complex structure, as mouse-like islets with β-cell cores coalesce to form larger islets that contain non-β cell types within the core of the islet. Additionally, the ratio of β:α cells in human islets is significantly lower than in mouse. Together, these differences increase the degree of cell-to-cell contact among the different endocrine cell types in humans compared with mouse. This is demonstrated in the immunohistochemically labelled islets in Figure 6A–C comparing NHP, human and mouse islets. Additionally, NHP and human islets have similar expression levels of key TF genes (Figure 6E) and insulin secretory profiles in response to various stimuli [42]. Together, these structural and functional similarities between NHP and human islets, as well as similarities in pregnancy and lactation, make the NHP a very attractive model. Unfortunately, the cost of maintaining colonies and length of gestation (in addition to time to reach sexual maturity, which is up to 4 years of age in this model) make widespread use of this animal prohibitive.
Figure 6. Differences in islet morphology and composition in different species.
Non-human primate islets (A) are more similar in architecture to human islets (B). Both species have less defined mantle and core domains than mouse islets (C). In mice, β cells are restricted to the islet core while other cell types are found at the periphery. In non-human primates and humans, non-β cells are found at the periphery and internal to the islet. (D) β cells comprise a larger proportion of endocrine cells in mouse islets compared with the other two species. (E) Expression of islet transcription factors differs between species. *P < 0.05, **P < 0.01, and ***P < 0.001, mouse vs. human; + P < 0.05 and ++ P < 0.01, +++ P < 0.001 mouse vs. NHP. (Adapted from Conrad, E., Dai, C., Spaeth, J., Guo, M., Cyphert, H. A., Scoville, D., Carroll, J., Yu, W. M., Goodrich, L. V., Harlan, D. M., Grove, K. L., Roberts, C. T. Jr., Powers, A. C., Gu, G. and Stein, R. 2016, Am. J. Physiol. Endocrinol Metab., 310(1), E91–e102 with permission from American Physiological Society.)
Two studies by the Grove group [43, 44] on the Japanese Macaque have investigated the effects of maternal HFD on offspring islets. In both cases, control animals (CTR) were fed a diet containing 14.7% calories from fat, while high fat diet (HFD) animals were fed 31.8% of calories from fat for 4–7 years before pregnancy, during pregnancy, and during lactation. After weaning, offspring were placed onto either CTR diet or HFD to generate four experimental groups. In fetuses exposed to HFD, α-cell mass was reduced, as well as pancreatic Insulin expression and GLUT2 expression when normalized to β-cell area. Due to the decreased α-cell mass, there was also an increase in the β:α cell ratio. After weaning, which occurs at 8 months of age, both CTR/HFD and HFD/HFD offspring had increased islet mass (analyzed at 13 months of age). In CTR/HFD offspring this was due to an increase in islet number per unit area (suggesting increased neogenesis), whereas HFD/HFD offspring had larger islets (suggestive of β-cell proliferation). The latter group also had a persistently elevated β:α cell ratio, which was likely due to a decrease in α-cell area. In a similar study design, Pound et al. [44] found that fetuses exposed to HFD had reduced islet vascularization, indicating impaired vasculogenesis during development. Interestingly, if HFD-fed mothers were switched to CTR diet at the start of pregnancy, this developmental phenotype was reversed. The reduction in islet vasculature caused by in utero HFD-exposure could also be ameliorated if offspring were weaned to CTR diet. At 13-month of age, CTR/HFD offspring had increased islet vasculature, suggesting a normal compensatory response to increased metabolic demand. This increase in vasculature was absent in islets from HFD/HFD animals. Together, these studies suggest that defects in islet structure and function caused by maternal overnutrition may only manifest when animals consume an unhealthy diet after weaning. Additionally, the effects of maternal overnutrition and metabolic dysfunction due to HFD consumption may be prevented if mothers switch from a HFD to a CTR diet before pregnancy.
Conclusions
Each animal model reviewed has inherent advantages and disadvantages when studying exposure to overnutrition in utero. For small animal models, advantages include low cost to maintain large colonies, ease of genetic manipulation, and ability to minimize genetic variability, while disadvantages center around differences between rodent and human gestation, diets, and islet structure. Large animals provide a better approximation of human gestation and islet biology at the price of greater costs, ethical considerations, and increased variability.
While the severity of the islet phenotype due to in utero HFD exposure depends on the specific diet composition, duration, and animal model, overwhelmingly maternal overnutrition leads to alternations in islet structure and – in the majority of cases – measurable islet dysfunction in terms of impaired insulin secretion or glucose tolerance.
While several studies have quantified changes in islet structure, function, and gene expression, more studies are needed to determine the precise mechanisms by which maternal diet mediates these effects.
Because the majority of women who become pregnant are either overweight or obese at the start of pregnancy, future studies in the DOHaD area are needed and have significant implications for future generations. Not only will this work increase awareness of overnutrition in utero as a serious risk factor for future disease, but it will also allow the discovery of specific interventions that overweight or obese mothers can implement to decrease this risk.
Importantly, some of the studies reviewed here suggest that negative outcomes due to in utero overnutrition may be prevented by maternal diet modification at the start of pregnancy, or potentially mitigated when offspring consume a healthy diet after weaning.
Acknowledgments
We would like to thank Dr. Peter Kropp for help with figures and for helpful discussions. J. M. E. was supported in part by NIGMS of the National Institutes of Health under award number T32GM007347. M. G. was supported by NIDDK grant 2R24 DK090964-06 and a Merit award from the Department of Veterans Affairs 1I01 BX003744-01. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts to report.
References
- 1.Ravelli GP, Stein ZA, Susser MW. N Engl J Med. 1976;295(7):349–53. doi: 10.1056/NEJM197608122950701. [DOI] [PubMed] [Google Scholar]
- 2.Barker DJ. Nutr Health. 1993;9(2):99–106. doi: 10.1177/026010609300900206. [DOI] [PubMed] [Google Scholar]
- 3.Barker DJ, Osmond C. Lancet. 1986;1(8489):1077–81. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- 4.Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. BMJ. 1989;298(6673):564–7. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oken E, Gillman MW. Obes Res. 2003;11(4):496–506. doi: 10.1038/oby.2003.69. [DOI] [PubMed] [Google Scholar]
- 6.Drake AJ, Reynolds RM. Reproduction. 2010;140(3):387–98. doi: 10.1530/REP-10-0077. [DOI] [PubMed] [Google Scholar]
- 7.Mingrone G, Manco M, Mora ME, Guidone C, Iaconelli A, Gniuli D, Leccesi L, Chiellini C, Ghirlanda G. Diabetes Care. 2008;31(9):1872–6. doi: 10.2337/dc08-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CDC. Obesity Statistics. 2016. [Google Scholar]
- 9.NIDDK Overweight & Obesity Statistics.
- 10.Pregnancy Risk Assessment Monitoring System, CDC.
- 11.Mastracci TL, Sussel L. Wiley Interdiscip Rev Membr Transp Signal. 2012;1(5):609–28. [Google Scholar]
- 12.O’Dowd JF, Stocker CJ. Front Physiol. 2013;4:170. doi: 10.3389/fphys.2013.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romer AI, Sussel L. Curr Opin Endocrinol Diabetes Obes. 2015;22(4):255–64. doi: 10.1097/MED.0000000000000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jennings RE, Berry AA, Kirkwood-Wilson R, Roberts NA, Hearn T, Salisbury RJ, Blaylock J, Piper Hanley K, Hanley NA. Diabetes. 2013;62(10):3514–22. doi: 10.2337/db12-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piper KBS, Turnpenny LW, Cameron IT, Ball SC, Wilson DI, Hanley NA. J Endocinrology. 2004;181(1):11–23. doi: 10.1677/joe.0.1810011. [DOI] [PubMed] [Google Scholar]
- 16.Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. Diabetes Obes Metab. 2008;10(Suppl 4):32–42. doi: 10.1111/j.1463-1326.2008.00969.x. [DOI] [PubMed] [Google Scholar]
- 17.Fonseca SG, Gromada J, Urano F. Trends Endocrinol Metab. 2011;22(7):266–74. doi: 10.1016/j.tem.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McMullen S, Mostyn A. Proc Nutr Soc. 2009;68(3):306–20. doi: 10.1017/S0029665109001396. [DOI] [PubMed] [Google Scholar]
- 19.Cerf ME, Louw J. Jop. 2014;15(3):228–36. doi: 10.6092/1590-8577/1534. [DOI] [PubMed] [Google Scholar]
- 20.Cerf ME, Muller CJ, Du Toit DF, Louw J, Wolfe-Coote SA. Br J Nutr. 2006;95(2):391–6. doi: 10.1079/bjn20051632. [DOI] [PubMed] [Google Scholar]
- 21.Cerf ME, Williams K, Nkomo XI, Muller CJ, Du Toit DF, Louw J, Wolfe-Coote SA. Am J Physiol Regul Integr Comp Physiol. 2005;288(5):R1122–8. doi: 10.1152/ajpregu.00335.2004. [DOI] [PubMed] [Google Scholar]
- 22.Dyrskog SE, Gregersen S, Hermansen K. Rev Diabet Stud. 2005;2(3):136–45. doi: 10.1900/RDS.2005.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elena Zambrano TSL, Calzada Lizbeth, Ibanez Carlos A, Mendoza-Rodriguez Carmen A, Morales Angelica, Morimoto Sumiko. Journal of Endocrinology. 2016;231:49–57. doi: 10.1530/JOE-16-0321. [DOI] [PubMed] [Google Scholar]
- 24.Graus-Nunes F, Dalla Corte Frantz E, Lannes WR, da Silva Menezes MC, Mandarim-de-Lacerda CA, Souza-Mello V. Nutrition. 2015;31(2):380–7. doi: 10.1016/j.nut.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Guo F, Jen KL. Physiol Behav. 1995;57(4):681–6. doi: 10.1016/0031-9384(94)00342-4. [DOI] [PubMed] [Google Scholar]
- 26.Han J, Xu J, Epstein PN, Liu YQ. Diabetologia. 2005;48(9):1810–8. doi: 10.1007/s00125-005-1854-8. [DOI] [PubMed] [Google Scholar]
- 27.Srinivasan M, Katewa SD, Palaniyappan A, Pandya JD, Patel MS. Am J Physiol Endocrinol Metab. 2006;291(4):E792–9. doi: 10.1152/ajpendo.00078.2006. [DOI] [PubMed] [Google Scholar]
- 28.Taylor PD, McConnell J, Khan IY, Holemans K, Lawrence KM, Asare-Anane H, Persaud SJ, Jones PM, Petrie L, Hanson MA, Poston L. Am J Physiol Regul Integr Comp Physiol. 2005;288(1):R134–9. doi: 10.1152/ajpregu.00355.2004. [DOI] [PubMed] [Google Scholar]
- 29.Yokomizo HIT, Sonoda N, Sakaki Y, Maeda Y, Inoue T, Hirata E, Takei R, Ikeda N, Fujii M, Fukuda K, Sasaki H, Takayanagi R. Am J Physiol Endocrinol Metab. 2014;306(10):E1163–65. doi: 10.1152/ajpendo.00688.2013. [DOI] [PubMed] [Google Scholar]
- 30.Tobi EW, Lumey L, Talens RP, Kremer D, Putter H, Stein AD, Slagboom PE, Heijmans BT. Hum Mol Genet. 2009;18(21):4046–53. doi: 10.1093/hmg/ddp353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kautzky-Willer A, Harreiter J, Pacini G. Endocr Rev. 2016;37(3):278–316. doi: 10.1210/er.2015-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang YH, Ye TT, Liu CX, Wang L, Chen YW, Dong Y. Nutr Metab(Lond) 2017;14:67. doi: 10.1186/s12986-017-0222-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saben JL, Boudoures AL, Asghar Z, Thompson A, Drury A, Zhang W, Chi M, Cusumano A, Scheaffer S, Moley KH. Cell Reports. 2016;16(1):1–8. doi: 10.1016/j.celrep.2016.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cropley JE, Eaton SA, Aiken A, Young PE, Giannoulatou E, Ho JWK, Buckland ME, Keam SP, Hutvagner G, Humphreys DT, Langley KG, Henstridge DC, Martin DIK, Febbraio MA, Suter CM. Mol Metab. 2016;5(8):699–708. doi: 10.1016/j.molmet.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sandovici I, Smith NH, Nitert MD, Ackers-Johnson M, Uribe-Lewis S, Ito Y, Jones RH, Marquez VE, Cairns W, Tadayyon M, O’Neill LP, Murrell A, Ling C, Constância M, Ozanne SE. Proceedings of the National Academy of Sciences. 2011;108(13):5449–5454. doi: 10.1073/pnas.1019007108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jimenez-Chillaron JC, Diaz R, Martinez D, Pentinat T, Ramon-Krauel M, Ribo S, Plosch T. Biochimie. 2012;94(11):2242–63. doi: 10.1016/j.biochi.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 37.Gnugge L, Meyer D, Driever W. Methods Cell Biol. 2004;76:531–51. doi: 10.1016/s0091-679x(04)76024-0. [DOI] [PubMed] [Google Scholar]
- 38.Maddison LA, Chen W. Diabetes. 2012;61(10):2517–24. doi: 10.2337/db11-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ford SP, Zhang L, Zhu M, Miller MM, Smith DT, Hess BW, Moss GE, Nathanielsz PW, Nijland MJ. Am J Physiol Regul Integr Comp Physiol. 2009;297(3):R835–43. doi: 10.1152/ajpregu.00072.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Long NM, George LA, Uthlaut AB, Smith DT, Nijland MJ, Nathanielsz PW, Ford SP. Journal of Animal Science. 2010;88(11):3546–3553. doi: 10.2527/jas.2010-3083. [DOI] [PubMed] [Google Scholar]
- 41.Zhang L, Long NM, Hein SM, Ma Y, Nathanielsz PW, Ford SP. Domest Anim Endocrinol. 2011;40(1):30–9. doi: 10.1016/j.domaniend.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Conrad E, Dai C, Spaeth J, Guo M, Cyphert HA, Scoville D, Carroll J, Yu WM, Goodrich LV, Harlan DM, Grove KL, Roberts CT, Jr, Powers AC, Gu G, Stein R. Am J Physiol Endocrinol Metab. 2016;310(1):E91–e102. doi: 10.1152/ajpendo.00285.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Comstock SM, Pound LD, Bishop JM, Takahashi DL, Kostrba AM, Smith MS, Grove KL. Mol Metab. 2012;2(1):10–22. doi: 10.1016/j.molmet.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pound LD, Comstock SM, Grove KL. Am J Physiol Endocrinol Metab. 2014;307(1):E115–23. doi: 10.1152/ajpendo.00131.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]