Abstract
The current study investigated the antidiabetic property of Lactobacillus fermentum HP3–mediated fermented Hericium erinaceus juice (FHJ) using male Wistar rats with streptozotocin-induced diabetes mellitus (DM). FHJ was prepared using boiled mushroom juice and L. fermentum HP3. Amino acid and γ-aminobutyric acid (GABA) content of FHJ was analyzed. Streptozotocin-induced DM rats were supplemented with FHJ in a pre- and posttreatment method. The changes in plasma insulin, plasma glucose level, glycated hemoglobin (HbA1c), representative cytokines, and the antioxidant system were assessed in experimental rats using spectrophotometric methods and enzyme-linked immunosorbent assay. The supplementation of FHJ improved the body mass, insulin level, and recovery progress of hyperglycemia. HbA1c level was altered by the FHJ intervention. The inflammatory cytokines level was suppressed in FHJ supplemented group compared with control. Intervention of FHJ and insulin improved the production of interleukin-10 and transforming growth factor-–β1 in DM rat. The study suggested that fermented H erinaceus juice may be used as one of the food-based health-promoting supplement to manage DM along with medication.
Keywords: Hericium erinaceus juice, diabetes mellitus, fermentation, Lactobacillus fermentum HP3, functional food
Insulin is a primary metabolic hormone secreted by the pancreas.1,2 The secretion of insulin multiplies rapidly after a meal. Insulin permeates the blood-brain barrier via a saturable transport.3 The insulin secretion and functionality is influenced by several nutritional compounds mainly amino acids.4,5 γ-Aminobutyric acid (GABA) is a broadly distributed amino acid with a lot of notable functionality in human health particularly as antidiabetic agent.6 Adeghate and Ponery7 examined the localization and function of GABA in the pancreas of normal and streptozotocin (STZ)-induced diabetes mellitus (DM) rat and revealed that the secretion of insulin was induced by GABA in normal and DM male Wistar rat.7
Researchers studied the role of glucose concentration in the effect of GABA (1-1000 µmol/L) on insulin secretion from the beta cell and reported that the membrane depolarization was induced by GABA in a dose-depended manner at a low glucose concentration, whereas hyperpolarization was induced by GABA at high glucose concentration.8 At different glucose levels, GABA regulated the insulin secretion in beta cells by the bi-directional mode, which indicated that GABA plays an important role in modulating the islet hormone secretion.8 The daily supplementation of GABA (20 µmol/mouse) reversed the type 1 DM (T1DM) and supported the regenerative and protective effects on islet beta cells of mice.6 The GABA intervention improved the insulin sensitivity and glucose tolerance via hindering the inflammation and significantly decreased the starving blood glucose level in high-fat diet–fed mice.9
Hericium erinaceus is a widely used edible mushroom species in Asian countries.10 The methanolic extract of H. erinaceus has been reported to reduce the blood glucose level in STZ-induced DM rats.11 H. erinaceus is a suitable natural substrate to produce GABA-rich fermented juice12,13 because of its high protein content (8.01%) and free amino acid.14 Recently, the lactic acid bacteria (LAB), a well-known probiotic bacteria, have been reported for the antidiabetic nature.15 In our previous studies, we have reported Lactobacillus fermentum, and Enterococcus faecalis–mediated fermentation of H. erinaceus and also demonstrated that the LAB-mediated fermented H. erinaceus juice contains a high concentration of GABA.12,13 Recently, several studies have reported regarding the antidiabetic property of parts (leaf, root) of the terrestrial plants.16,17 The information about the impact of fermented juice with high GABA content on DM, however, is limited. Thus, in the current study, we report on the health-promoting property of L. fermentum HP3–mediated fermented H. erinaceus juice using male Wistar rats with STZ-induced DM.
Materials and Methods
Preparation of Lactic Acid Bacteria and Boiled Mushroom Juice
The dried H. erinaceus (10 g) was boiled in 90 mL of potable water for 5 minutes, and the boiled mushroom juice (BMJ) was filtered through a filter cloth to remove the matrix of mushroom.12 The clear boiled mushroom juice was used for the experiment. L. fermentum HP3 was propagated in the mixture of MRS (de Man, Rogosa, and Sharpe) medium and 10% cane sugar in a ratio of 1:1, and 5 g/L of peptone (Himedia, Cat No M369) at 37°C for 18 to 24 hours; then fresh cells were harvested by centrifugation at 5000 rpm for 10 minutes. L. fermentum HP3 culture was diluted with sterile water to the concentration of 109 colony-forming units/mL before use.
Preparation of GABA-Rich Fermented H Erinaceus Juice
The fermented H. Erinaceus Juice (FHJ) was prepared as detailed in our previous study and 10% of H. erinaceus was used for the preparation of FHJ.13 Then, the GABA-containing FHJ was subjected to vacuum evaporation (Rotavapor R-210, BUCHI) at 55°C for 5 minutes and the concentration of GABA was analyzed by high-performance liquid chromatography. FHJ was diluted with sterile potable water to achieve the net GABA concentration of 450 mg/mL.
Analysis of Amino Acid Composition
Amino acid profile of the dry mushroom and FHJ was determined according to Association of Analytica Chemists official method 994.12 and 988.15 by Central Laboratory (Thailand) Co Ltd.18
Animal Groups and Induction of Diabetes Mellitus
About 6 weeks old male Wistar rats were obtained from the National Laboratory Animal Centre, Mahidol University, divided into 13 groups, each group consisting of 10 rats (weight: 150-170 g/rat) and coded as detailed in Table 1. LAB, FHJ (containing GABA and LAB), and BMJ are the test supplements that were administered to the respective rat along with normal standard diet (Commercial Food No. C.P. 082, Perfect Companion Group Co, Ltd, Bangkok, Thailand) as described in Table 1.
Table 1.
Grouping of Wistar Rats and Dosage of Test Supplements.
| Groups | Codes | Treatmentsa | Dosage (per Day) |
|---|---|---|---|
| Normal rat (control) | NC | Water | 1.27 mL/kg |
| LabC | LAB | 109 CFU/mL | |
| GabaC | FHJ containing GABA and LAB | GABAb 1.27 g/kg | |
| BmjC | BMJ | 1.27 mL/kg | |
| DM rat (control) | DMC | Water | 1.27 mL/kg |
| DM rat (pretreatment) | LabPre | LAB | 109 CFU/mL |
| GabaPre | FHJ containing GABA and LAB | GABAb 1.27 g/kg | |
| BmjPre | BMJ | 1.27 mL/kg | |
| InPre | Insulin (Humurin N) | 6 U/kg | |
| DM rat (posttreatment) | LabPost | LAB | 109 CFU/mL |
| GabaPost | FHJ containing GABA and LAB | GABAb 1.27 g/kg | |
| BmjPost | BMJ | 1.27 mL/kg | |
| InPost | Insulin (Humurin N) | 6 U/kg |
Abbreviations: LAB, lactic acid bacteria; FHJ, fermented Hericium erinaceus juice; BMJ, boiled mushroom juice; GABA, γ-aminobutyric acid; DM, diabetes mellitus; NC, normal control; LabC, LAB supplemented to naive rat; GabaC, FHJ supplemented to naive rat; BmjC, BMJ supplemented to naive rat; DMC, DM rat control; LabPre, LAB supplemented to naive rat and induced DM; GabaPre, FHJ supplemented to naive rat and induced DM; BmjPre, BMJ supplemented to naive rat and induced DM; InPre, insulin supplemented to naive rat and induced DM; LabPost, LAB supplemented to DM rat; GabaPost, FHJ supplemented to DM rat; BmjPost, BMJ supplemented to DM rat; InPost, insulin supplemented to DM rat.
aThe test supplements were administered to the rats along with the normal standard diet and normal drinking water.
bThe dose of GABA supplement was calculated based on the previous study.9
Single intraperitoneal injection of STZ (55 mg/kg) was used for the induction of diabetic condition in rats.19 After 14 days, the rats with the fasting plasma glucose level of ≥250 mg/dL (hyperglycemia) was considered as a DM rat and used in the experiment. Normal control rats were injected with citrate buffer pH 4.7. The body weight of the animals was measured once a week throughout the experimental period.20 Rats in the pretreatment group and normal group were fed with the particular test supplements for 14 days before the induction of DM. The test supplements were administered to the respective rats in the posttreatment group after the induction of diabetic condition. The animals were freely allowed to access the diets and water ad libitum throughout the 12 weeks of the experiment. The animals were sacrificed under chloral hydrate general anesthesia (7% chloral hydrate 6 mL/kg of body weight) after overnight fasting (8-12 hours) at the end of the experimental period.7 All aspects of this study were conducted according to the standards of the Animal Ethics Committee of the Institution (Chiang Mai University Ethical Certification No. 23/2558).
Determination of Plasma Insulin Level
Blood samples were collected in microcentrifuge tubes coated with ethylene diamine tetraacetic acid. The plasma insulin concentration was determined using Sandwich ELISA (enzyme-linked immunosorbent assay) kit as per the manufacturer’s instructions (Rat/Mouse Insulin ELISA kit) (EMD Millipore, Cat No. EZRMI-13K).
Quantification of Plasma Glucose
The plasma glucose concentration was determined once a month by an enzymatic colorimetric method using the commercial kit (ERBA diagnostic Mannheim GmbH, Germany). The blood samples were collected in the microcentrifuge tubes containing 2.5 mg/mL of sodium fluoride (NaF). The experiment was performed as per the manufacturer’s instructions. Glucose concentration was calculated by comparing with the glucose standard curve.
Measurement of Hemoglobin A1c
The blood glycated hemoglobin (HbA1c) was determined at the laboratory service center of Faculty of Medicine, Chiang Mai University by turbidimetric immunoassay method using whole blood (Roche Diagnostics Ltd).
Profiling of Selected Cytokines
The selected circulating cytokines (interferon-γ [IFN-γ], interleukin-6 [IL-6], IL-17, transforming growth factor–β1 [TGF-β1], and IL-10) profile was measured using commercial rat IFN-γ (Cat No. DY585), IL-6 (Cat No. DY506), IL-17 (Cat No. DY421), TGF-β1 (Cat No. DY1679), and IL-10 (Cat. No. DY522) ELISA kit purchased from R&D Systems, Minneapolis, MN, USA. The serum samples were obtained from experimental rats and were subjected to ELISA as per the manufacturer’s instructions.
Profiling of Antioxidant Enzymes and Malondialdehyde
The antioxidant enzyme (total superoxide dismutase [SOD], Mn-SOD, Cu-Zn-SOD, glutathione peroxidase [GPx], catalase) levels in the blood samples of experimental rats was measured. Three forms of SOD were assayed. GPx was determined according to the previous description with some modification.21 Catalase activity was determined by measuring the rate of disappearance of H2O2.21 Malondialdehyde (MDA) level in serum was determined according to the previous description with some modification.21,22
Statistical Analysis
All in vitro experiments were executed in triplicates, and all data were expressed as mean ± standard deviation (SD). All in vivo experiments were expressed as mean ± standard error (SE). Dissimilarities between the mean values of multiple groups were evaluated by 1-way analysis of variance (ANOVA) using Duncan’s test. The variations were considered significantly different when P value was <.05.
Results
The Amino Acid Profile of FHJ and Dry H. erinaceus
A sum of 21 amino acids and GABA were observed in both dried mushroom and its fermented juice. Dried mushroom showed a high content of phenylalanine, leucine, lysine, glutamine, isoleucine, glutamic acid, and tyrosine than that of the FHJ. The amount of GABA was found to be higher in FHJ than that of the raw mushroom because of the biotransformation of glutamic acid to GABA by the starter culture, L. fermentum HP3 (Table 2).
Table 2.
Amino Acid Profile and GABA Content of Dry Hericium erinaceus and FHJ
| Amino Acid | Dry H erinaceus (mg/100 g) | FHJa (mg/100 mL) |
|---|---|---|
| GABA | 22.00 | 211.00 |
| Alanine | 160.90 | 17.70 |
| Arginine | <5 | <5 |
| Aspartic acid | 204.30 | 23.60 |
| Cystine | 48.44 | <5 |
| Glutamic acid | 329.50 | 15.40 |
| Glutamine | 350.00 | 37.00 |
| Glycine | 107.30 | 7.90 |
| Histidine | 254.10 | <5 |
| Hydroxylysine | <5 | <5 |
| Hydroxyproline | <5 | <5 |
| Isoleucine | 346.60 | 36.20 |
| Leucine | 771.00 | 76.80 |
| Lysine | 436.60 | 33.20 |
| Methionine | 10.08 | <5 |
| Phenylalanine | 824.10 | 96.50 |
| Proline | 131.90 | 10.40 |
| Serine | 38.99 | <5 |
| Threonine | 61.95 | <5 |
| Tryptophan | 32.09 | <5 |
| Tyrosine | 324.20 | 69.90 |
| Valine | 247.30 | 25.30 |
Abbreviations: FHJ, fermented Hericium erinaceus juice; GABA, γ-aminobutyric acid.
aFHJ was prepared using 10% (w/v) dried H erinaceus juice.
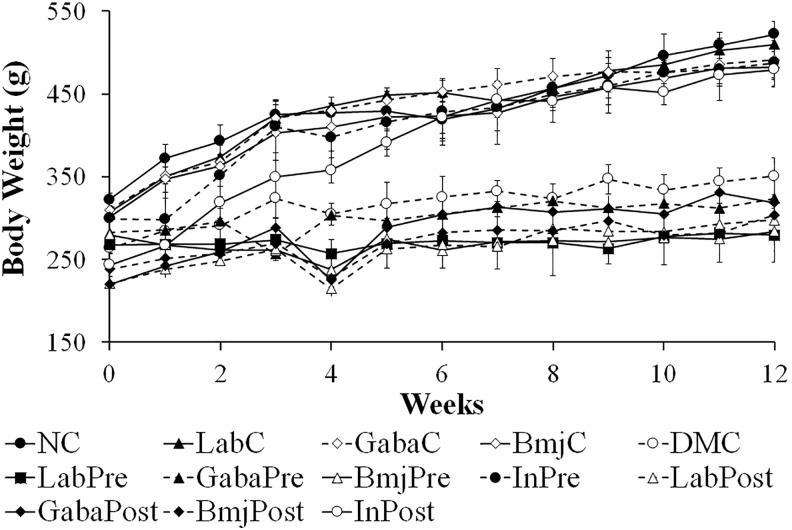
Impact of Treatment on Body Weight of the Animal
Body weight (BW) was recorded every week throughout the experimental period (Figure 1). After DM induction, the rapid decline of BW is one of the common symptoms of DM. The initial BW of rats was found to be anything between 260 and 300 g and then gradually increased to 480 to 530 g in normal rats (NC, LabC, GabaC, and BmjC) and insulin treatment groups (InPre, and InPost). In contrast, the BW of the diabetic rats (LabPre, GabaPre, BmjPre, LabPost, GabaPost, and BmjPost) were found to fluctuate throughout the experiment period. The BW of GabaPre, LabPost, GabaPost, BmjPost group rats showed a slight increase when compared to that of the LabPre and BmjPre group animals.
Figure 1.
Alteration in the body mass of the experimental animals.
Changes in Plasma Glucose Level
The effects of test supplements on the fasting plasma glucose levels in experimental rats are summarized in Table 3. The fasting plasma glucose levels in normal rats (LabC, BmjC, and GabaC) was not affected by the administration of test supplements including LAB, BMJ, and FHJ containing GABA and LAB, respectively. No significant differences in plasma glucose levels were observed between the DM groups at the baseline (0 months).
Table 3.
Plasma Glucose Level, Progress of Hyperglycemia, and Reduction of Plasma Glucose in Normal and DM Rats at the End of Experiment (3 Months).
| Groups | Treatment Code | Plasma Glucose (mg/dL) | Hyperglycemia Progress (%) (Within Group)a | Reduction of Plasma Glucose (%) (Compared With DMC)b |
|---|---|---|---|---|
| Normal rat | NC | 162.35 | — | — |
| LabC | 157.07 | — | — | |
| GabaC | 134.64 | — | — | |
| BmjC | 158.65 | — | — | |
| DM rat (control) | DMC | 547.41 | +60.13 | 00.00 |
| DM rat (pretreatment) | LabPre | 481.45 | +8.21 | 12.05 |
| GabaPre | 363.00 | −13.38 | 33.69 | |
| BmjPre | 373.85 | −10.39 | 31.70 | |
| InPre | 98.69 | −78.74 | 81.97 | |
| DM rat (posttreatment) | LabPost | 470.12 | +61.13 | 14.12 |
| GabaPost | 365.59 | +21.37 | 33.21 | |
| BmjPost | 435.64 | +41.51 | 20.42 | |
| InPost | 119.50 | −60.31 | 78.17 |
Abbreviations: LAB, lactic acid bacteria; FHJ, fermented Hericium erinaceus juice; BMJ, boiled mushroom juice; GABA, γ-aminobutyric acid; DM, diabetes mellitus; NC, normal control; LabC, LAB supplemented to naive rat; GabaC, FHJ supplemented to naive rat; BmjC, BMJ supplemented to naive rat; DMC, DM rat control; LabPre, LAB supplemented to naive rat and induced DM; GabaPre, FHJ supplemented to naive rat and induced DM; BmjPre, BMJ supplemented to naive rat and induced DM; InPre, insulin supplemented to naive rat and induced DM; LabPost, LAB supplemented to DM rat; GabaPost, FHJ supplemented to DM rat; BmjPost, BMJ supplemented to DM rat; InPost, insulin supplemented to DM rat.
aIndicates the increase (+) or decrease (−) in the percentage of progress of hyperglycemia in DM rats within the respective group.
bIndicates the reduction of plasma glucose level (%) in DM rats of each group when compared with that of the DMC group.
In the pretreatment study, the plasma glucose levels were significantly reduced in GabaPre and BmjPre group animals when compared with that of the DMC group (P < .05) at the end of the experiment (third month) (Table 3). The experimental animals in GabaPre group displayed the lowest level of plasma glucose (363.00 mg/dL) compared with BmjPre group (373.85 mg/dL, P > .05) and LabPre group (481.45 mg/dL, P < .05). While the plasma glucose level of the InPre group was found to be comparable to that of the normal rats. Plasma glucose levels in the pretreatment groups was not analogous to the normal control group. The progressive hyperglycemia (percentage of progress of hyperglycemia in DM rats at the end of the third month of the experiment compared with the respective group) in DM rats at the end of the experiment were found to be significantly (P < .05) lower (−13.38% and −10.39%) in GabaPre and BmjPre groups than that of the DMC group (+60.13%), respectively. Lowering of plasma glucose (percentage of difference between the plasma glucose level in the DM rats of the treatment group and DMC group at the end of the third month of the experiment) in DM rats of GabaPre and BmjPre groups were found to be higher than that of the LabPre group (33.69%, 31.70%, and 12.05%, respectively) (Table 3).
In the posttreatment study, the DM rats of GabaPost treatment group showed significantly (P < .05) lower fasting plasma glucose level (365.59 mg/dL) than that of the DM rats in DMC group (547.41 mg/dL) at the end of the third month of the experiment. Likewise, BMJ treatment in diabetic rats significantly (P < .05) decreased the plasma glucose level (435.64 mg/dL) when compared with that of the DM rats in DMC group. LabPost group (470.12 mg/dL) showed no significant impact on fasting plasma glucose compared with that of the DMC group rats. Insulin treatment successfully corrected the fasting plasma glucose levels in diabetic rats. As shown in Table 3, the progressive hyperglycemia of DM rats in the posttreatment groups (GabaPost and BmjPost) were found to be lower when compared with that of the DMC group (+21.37%, +41.51%, and +60.13%, respectively). Plasma glucose lowering the ability of DM rats in posttreatment groups was found to be in a similar pattern as shown by the DM rats in the pretreatment study. As expected, the DM rats of GabaPost treatment group showed the highest percentage of plasma glucose lowering ability (33.21%, P < .05) compared with that of the DMC group rats.
Plasma Insulin, and HbA1c
The plasma insulin concentration was measured at before and after the treatment. The plasma insulin levels of normal rats were observed to be not affected by the test supplements (Table 4). The low plasma insulin concentration was detected in DMC group rats than in the NC group rats. In the pretreatment study, the plasma insulin level in DM rats of GabaPre group slightly increased than that of the DMC, LabPre, and BmjPre groups but there was no significant difference among them. The insulin injection in diabetes rats fully restored the plasma insulin levels in both pretreatment and posttreatment groups. In the posttreatment study, the plasma insulin concentrations in DM rats of LabPost, GabaPost, and BmjPost groups did not alter when compared to that of the DMC group (Table 4). HbA1c was measured at the end of the experiment. HbA1c in normal rats of NC, LabC, GabaC, and BmjC groups was in the normal nondiabetic range (Table 4). The increased amount of Hb1Ac (9.95% ± 0.82%) was observed in DM rats of DMC group. HbA1c in DM rats of insulin group (InPre and InPost) was found to be reduced (6.55% ± 0.34% and 5.83% ± 0.29%, respectively) when compared with that of the DMC group. Importantly, HbA1c in the DM rats of both GabaPre group (6.80% ± 1.16%) and GabaPost group (7.80% ± 1.42%) were found to be not significant when compared with that of the insulin treatment groups (Table 4). DM rats of BmjPost group showed a slight insignificant reduction in HbA1c range when compared with that of the DMC group. LAB intervention in both pretreatment and posttreatment study failed to reduce the HbA1c value in DM rats of LabPre and LabPost group (Table 4).
Table 4.
Summary of a Physiological Parameter of DM Rat and Normal Rat at the End of the Experiment (3 Months).a
| Groups | Treatment code | Assessed Parameters in DM Rats During Study | |
|---|---|---|---|
| Plasma Insulin (ng/mL) | HbA1c (%) | ||
| Normal rat | NC | 5.05 ± 1.39 | 4.87 ± 0.22 |
| LabC | 5.03 ± 1.03 | 4.67 ± 0.19 | |
| GabaC | 3.34 ± 0.91 | 5.63 ± 0.26 | |
| BmjC | 5.46 ± 1.30 | 4.87 ± 0.27 | |
| DM rat (control) | DMC | 1.10 ± 0.18 | 9.95 ± 0.82 |
| DM rat (pretreatment) | LabPre | 0.61 ± 0.16 | 11.08 ± 0.35 |
| GabaPre | 1.40 ± 0.86 | 6.80 ± 1.16 | |
| BmjPre | 0.94 ± 0.06 | 10.78 ± 0.48 | |
| InPre | 10.00 ± 0.00 | 6.55 ± 0.34 | |
| DM rat (posttreatment) | LabPost | 1.22 ± 0.53 | 10.60 ± 0.53 |
| GabaPost | 1.23 ± 0.64 | 7.80 ± 1.42 | |
| BmjPost | 1.08 ± 0.44 | 8.75 ± 1.39 | |
| InPost | 10.00 ± 0.00 | 5.83 ± 0.29 | |
Abbreviations: LAB, lactic acid bacteria; FHJ, fermented Hericium erinaceus juice; BMJ, boiled mushroom juice; GABA, γ-aminobutyric acid; DM, diabetes mellitus; NC, normal control; LabC, LAB supplemented to naive rat; GabaC, FHJ supplemented to naive rat; BmjC, BMJ supplemented to naive rat; DMC, DM rat control; LabPre, LAB supplemented to naive rat and induced DM; GabaPre, FHJ supplemented to naive rat and induced DM; BmjPre, BMJ supplemented to naive rat and induced DM; InPre, insulin supplemented to naive rat and induced DM; LabPost, LAB supplemented to DM rat; GabaPost, FHJ supplemented to DM rat; BmjPost, BMJ supplemented to DM rat; InPost, insulin supplemented to DM rat.
aData are presented as mean ± standard error.
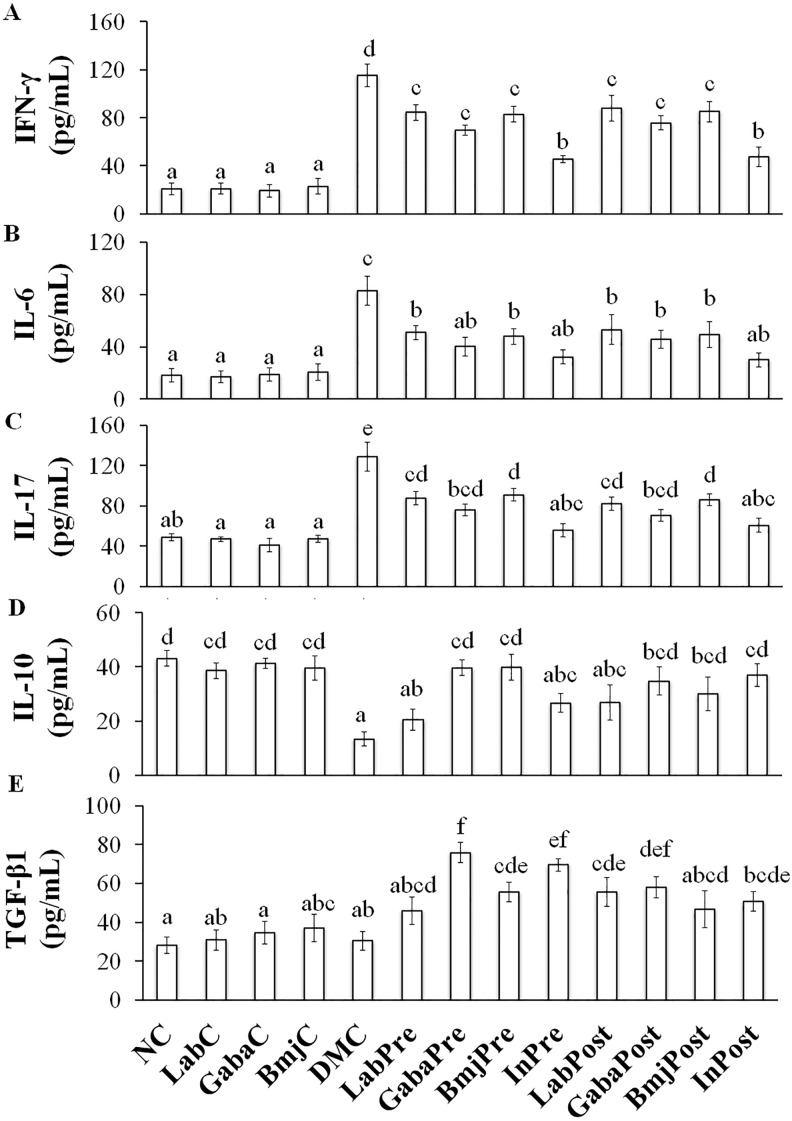
Regulation of Selected Cytokines
The expression level of inflammatory cytokines (IFN-γ, IL-6, and IL-17) in rats of normal group (LabC, GabaC, and BmjC) was observed to be not affected by the test supplements when compared to that of the NC group (Figure 2A-C). The DM control group rats showed significant increase in IFN-γ (115.19 ± 9.34 pg/mL), IL-6 (82.84 ± 11.30 pg/mL), and IL-17 (128.84 ± 14.20 pg/mL) levels. All the test supplements showed the significant suppressive effect on selected inflammatory cytokines in DM rats of both pretreatment and posttreatment group compared to that of the DMC group rats (Figure 2A-C). Selected anti-inflammatory cytokines (IL-10 and TGF-β1) levels in normal rat group (NC, LabC, GabaC, and BmjC) were found to be in a range of 38.56 ± 2.80 to 43.14 ± 2.91 pg/mL and 28.07 ± 4.20 to 37.05 ± 7.14 pg/mL, respectively. While, selected anti-inflammatory levels in DM rats of DMC group dropped to 13.35 ± 2.50 and 30.53 ± 4.85 pg/mL, respectively (Figure 2D-E). Notably, the intervention of FHJ (containing GABA and LAB) and insulin improved the production of IL-10 and TGF-β1 in DM rats of treatment group (GabaPre and GabaPro) than that of the other tested reagents.
Figure 2.
The regulation of selected cytokines in experimental animals. (A) interferon-γ (IFN-γ), (B) interleukin-6 (IL-6), (C) IL-17, (D) IL-10, (E) transforming growth factor–β1 (TGF-β1). a-f represents the significant variations among the experimental groups (P < .05).
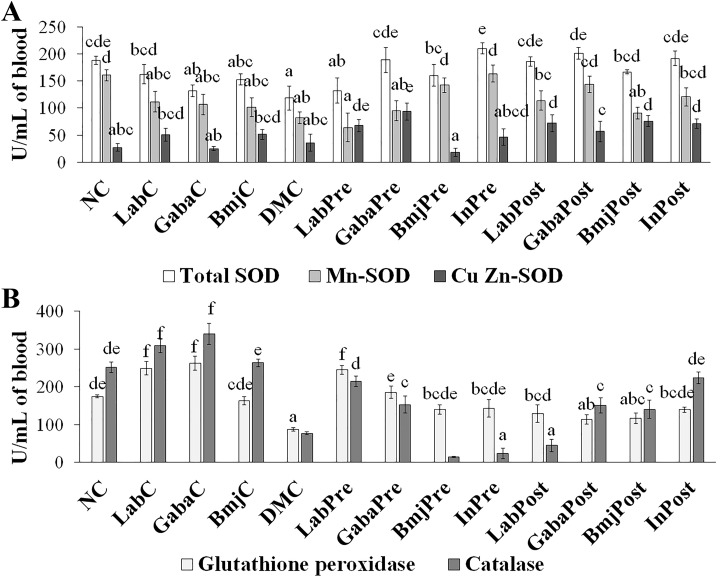
Regulation of Antioxidant Enzyme and MDA
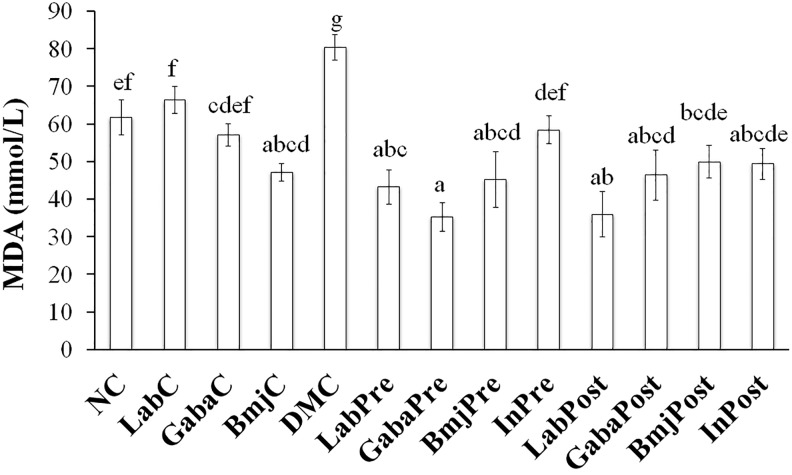
Three forms of SOD in rats of normal group were not affected by the test supplements when compared to that of the NC group rats (Figure 3A). While GPx and catalase were found to be not affected only in the normal rats of BmjC group when compared to that of the NC group rats (Figure 3B). LAB and FHJ significantly (P < .05) increased the GPx and catalase production in normal rats of LabC and GabaC group. The intervention of LAB significantly (P < .05) increased the Cu-Zn-SOD and GPx production in DM rats of both pretreatment and posttreatment group when compared with that of the DMC group. LAB significantly (P < .05) increased the catalase in DM rats of LabPre group and increased the total SOD in DM rats of LabPost group. Supplementation of FHJ increased the total SOD and catalase in DM rats of both GabaPre and GabaPost group than that of the DMC group, while Cu-Zn-SOD and GPx were found to be increased only in the DM rats of GabaPre group, and Mn-SOD was significantly increased only in the DM rats of GabaPost group. BMJ elevated the total SOD production in DM rats of both pretreatment and posttreatment group when compared with that of the DMC group, while Mn-SOD and GPx were significantly increased only in DM rats of pretreatment group, CuZn-SOD and catalase were increased only in the DM rats of the posttreatment group. Insulin increased the production of SOD and reduced the production of GPx in the DM rats of both InPre and InPost group. The intervention of test supplements showed a significant (P < .05) reduction of MDA levels in the DM rats of both pre- and posttreatment group when compared with that of the DMC group. LAB and FHJ supplementation also suppressed the production of MDA in DM rats of treatment group (LabPre, LabPost, GabaPre, and GabaPost) than that of the insulin group (InPre and InPost) rats (Figure 4).
Figure 3.
Effect of test supplements on the antioxidant enzyme levels of the experimental animals. (A) Changes in total superoxide dismutase (SOD), Mn-SOD, Cu-Zn-SOD levels. (B) Alterations in glutathione peroxidase, and catalase. a-f represents the significant variations among the experimental groups (P < .05).
Figure 4.
Effect of test supplements on the level of malondialdehyde (MDA) in experimental animals. a-g represents the significant variations among the experimental groups (P < .05).
Discussion
H. erinaceus has been reported to contain protein content (8.01 %) and free amino acids, mainly glutamic acid (1468.12 mg%).14 Researchers have reported the chemical composition of many medicinal mushrooms including H. erinaceus that consist of essential amino acids like threonine, methionine, leucine, valine, isoleucine, lysine, phenylalanine, histidine, and arginine.23 Some nonessential amino acids such as glutamic acid and aspartic acid were also found in H. erinaceus.23 In the present study, the amino acid composition of H. erinaceus was estimated before and after the fermentation. The results revealed that the fermentation by L. fermentum HP3 enhances the richness of GABA in H. erinaceus juice (Table 2) and the reason behind the nutritional nourishment was due to the conversion of glutamic acid to GABA by L. fermentum HP3.13
The impact of FHJ was assessed by administration of test supplements along with the normal standard diet to normal and DM-induced rats, and by measuring several parameters. BW index is one of the commonly used biomarkers for DM. Among the DM rats of treatment groups except for insulin treatment group, the BW of DM rats of GabaPre and GabaPost groups was slightly greater than that of the diabetic rats of other treatment groups, which indicated the potential efficiency of GABA on the reduction of metabolic disturbances (Figure 1).
Insulin is a physiological modulator of glucagon secretion. Both insulin and glucagon are primary endocrine factors responsible for regulating the glucose homeostasis in the body. The STZ injection destructs the pancreatic beta cells and leads to the deficiency of insulin secretion and the onset of DM.24,25 This leads to hyperglycemia due to the disturbance of autoregulation within the islets of Langerhans, which cause the hypersecretion of glucagon hormone.25 In the present study, a single dose of STZ efficiently induced DM in rats, which was confirmed by the progress of hyperglycemia (Table 3) and constant low plasma insulin concentration (Table 4) in DM rats of DMC group than that of the NC group rats throughout the experimental period. In both pre- and posttreatment groups, the intervention of FHJ (containing GABA and LAB) slightly increased the plasma insulin level in DM rats of treatment group when compared to that of the DM rats of DMC group (Table 4). The results suggested that the antihyperglycemic effect of GABA may not involve the activation of pancreatic insulin secretion.
Supplementation of GABA (daily intraperitoneal injection: 20 µmol/mouse) protected and showed the regenerative effects on an islet beta cells and reverses diabetes in T1DM.6 GABA therapy prevents the progress of T1DM by suppression of insulitis (lymphocytic infiltration) and inflammatory cytokine production.6 In the present study, the results of pre- and post-treatment studies suggested that the supplementation of GABA potentially decreased the fasting plasma glucose level, thereby it impedes the progress of hyperglycemia (Table 3) and decreased the HbA1c concentration without enhancing the pancreatic insulin secretion (Table 4). Therefore, proposed mechanisms of antihyperglycemic effect of FHJ (containing GABA) in DM rats may involve the improvement of insulin sensitivity and / or reduction of glucagon secretion from pancreatic cells.
Tian et al9 reported the effect of GABA on obesity-related insulin resistance and type 2 DM and revealed that the ad libitum oral treatment with GABA (2 mg GABA per milliliter of water) improved the glucose tolerance and insulin sensitivity by significantly reducing the concentration of fasting blood glucose and inhibiting inflammation in high-fat diet–fed mice. They also reported the reduction of frequency of macrophage infiltrate, adipocyte size and epididymal fat mass in the adipose tissues of high-fat diet–fed mice as a result of oral treatment with GABA.9 Likewise, von Blankenfeld et al26 reported that the activation of GABAA receptors leads to depolarization of plasma membrane and then activate the voltage-gated Ca2+ channels that lead to increased cytosolic calcium, enhancing autocrine process and finally resulting in pancreatic insulin release.26 The exogenous GABA was absorbed through the intestinal epithelial membrane via the H+/GABA symport.27 Moreover, the intestinal absorption of GABA by co-transport with a proton is operated under the acidic condition. Therefore, supplementation of FHJ containing GABA and LAB may facilitate the adequate absorption of GABA via cotransport mechanism by the acid environment created by LAB through lactic acid production.
HbA1c, the key indicator of glycemic status over the long term, reflects average plasma glucose over the period of 8 to 12 weeks.28 HbA1c could be used as an objective measure of glycemic control. The supplementation of FHJ reduces the HbA1c level, which indicates the positive effect of FHJ in DM rats of GabaPre and GabaPost group (Table 4).
Supplementation of FHJ that contains GABA significantly reduces the circulating inflammatory cytokine (IL-1β, tumor necrosis factor–α [TNF-α], IFN-γ, and IL-12) levels and increases the anti-inflammatory cytokine, IL-10 level in STZ-induced DM rat. Soltani et al6 revealed that GABA suppressed the IFN-γ production of CD4+/CD8+ T cells and suppressed IL-12 production by macrophages in in vitro assays. Besides, GABA induces the increased production of TGF-β1 in the unstimulated splenic T cells and the activated splenic T cells by anti-CD3 and anti-CD28 antibodies.6 Tian et al29 investigated the effect of GABA pellet (600 µg of GABA released daily) implanted in the T1DM mouse model. Progression of the disease and the development of proinflammatory T cell responses were inhibited with the GABA treatment. Tian et al9 reported that oral treatment with GABA is effective in treating the obesity-related T2DM. The regulatory T cells (Tregs) are a potent inhibitor of macrophage activation and function. GABA promotes the proliferation and maturation of Tregs.9 GABAA receptors are expressed by the macrophages, T cells, and adipocytes, and these receptors may play a role in the therapy of DM.9,29 The possible mode of GABA action is through the activation of GABAA receptors on the macrophages and adipocytes to inhibit the macrophage activation and migration. Thereby it reduces the chronic inflammation in adipose tissues.
The aqueous extract of H. erinaceus (AEHE) significantly reduced the serum glucose level, increased the serum insulin level, and also exhibited antioxidant properties in the experimental DM rat model.30 Wang et al11 evaluated the health-promoting effect of H. erinaceus extract (fruiting bodies extract) on the blood glucose levels in an experimental diabetic rat model. They found that feeding of methanol extract of H erinaceus (HEM) to STZ-induced DM rats significantly diminishes the elevation of blood glucose levels.11 L. rhamnosus GR-1 and L. fermentum RC-14 are bile tolerant probiotics that can survive the gastrointestinal transit.31 The chronic inflammation induced by gut-derived endotoxin is one of the key incidents in the onset and development of T2DM, and Zhang et al32 found that L. casei reduced the endotoxin lipopolysaccharide release induced by STZ injection, and decreased the pro-inflammatory cytokines (IFN-γ and TNF-α).32 The probiotic bacterium L. casei Zhang also suppresses the hyperglycemia and prevents the T2DM onset via a microbiota-based bile acid-chloride exchange mechanism.32 T2DM rat fed with fermented Thai indigenous plant juice showed a slight reduction in the fasting plasma glucose level.33 The results of the current study suggested that L. fermentum HP3–mediated fermented H. erinaceus juice efficiently suppressed the pro-inflammatory markers and induced the anti-inflammatory markers (Figure 2), which indicates the protective role of FHJ against STZ-induced DM in rats.
Yeap et al34 reported that the fermented mung bean extracts significantly reduces the blood sugar levels and increased the plasma antioxidant levels in alloxan-induced hyperglycemic mice. Antihyperglycemic effect of fermented mung bean extracts might be due to the presence of high content of GABA and amino acid in the extract.34 GABA could alleviate the oxidative damage by increasing the total antioxidation capacity and reduced the MDA levels in the intestinal mucosa of heat stress-induced Wenchang chicken.35 Besides, Hu et al36 found that GABA significantly increases the activities of GPx, SOD, and decreased the MDA level in serum of pigs. The results of the present study also found that fermented H. erinaceus juice containing GABA increased the production of 3 forms of SOD, GPx, and catalase level in blood and reduced the serum MDA in DM rat.36
Conclusion
The antidiabetic property of L. fermentum HP3–mediated fermented H. erinaceus juice was evaluated in the present study. The supplementation of FHJ (containing GABA and LAB) positively regulated the plasma insulin, plasma glucose level, HbA1c, representative cytokines, and antioxidant system in experimental DM rats compared with that of the other supplements. The study revealed that the fermented H. erinaceus juice might be used as a food-based therapeutic supplement for the treatment of DM along with medication. Further in-depth research is required to reveal the molecular mechanism behind the glucose-lowering ability of FHJ (containing GABA and LAB).
Acknowledgments
We wish to acknowledge the Faculty of Pharmacy, Chiang Mai University, Thailand for the necessary provision.
Authors’ Note: The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the article.
Author Contributions: Sasimar Woraharn and Narissara Lailerd L were involved in the wet lab experiments and collection of data. Bhagavathi Sundaram Sivamaruthi S and Periyanaina Kesika were involved in the experimental design, data analysis, figures and table preparation, critical review of the data, and manuscript preparation. Sartjin Peerajan was responsible for the laboratory management and gathering data and processing. Chaiyasut Chaiyavat conceived and designed the experiments and finalize the manuscript.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: We gratefully acknowledge the Chiang Mai University grant (CMU-grant) for the support.
Ethical Approval: All aspects of this study were conducted according to the standards of the Animal Ethics Committee of the Institution (Chiang Mai University Ethical certification no. 23/2558).
References
- 1. Baskin DG, Figlewicz Lattemann D, Seeley RJ, Woods SC, Porte D, Jr, Schwartz MW. Insulin and leptin: dual adiposity signals to the brain for the regulation of food intake and body weight. Brain Res. 1999;848:114–123. [DOI] [PubMed] [Google Scholar]
- 2. Xu E, Kumar M, Zhang Y, et al. Intra-islet insulin suppresses glucagon release via GABA-GABAA receptor system. Cell Metab. 2006;3:47–58. [DOI] [PubMed] [Google Scholar]
- 3. Banks WA. Blood-brain barrier and energy balance. Obesity. 2006;14(suppl 5):234S–237S. [DOI] [PubMed] [Google Scholar]
- 4. van Loon LJC, Kruijshoop M, Menheere PP, Wagenmakers AJ, Saris WH, Keizer HA. Amino acid ingestion strongly enhances insulin secretion in patients with long-term type 2 diabetes. Diabetes Care. 2003;26:625–630. [DOI] [PubMed] [Google Scholar]
- 5. Newsholme P, Bender K, Kiely A, Brennan L. Amino acid metabolism, insulin secretion and diabetes. Biochem Soc Trans. 2007;35(pt 5):1180–1186. [DOI] [PubMed] [Google Scholar]
- 6. Soltani N, Qiu H, Aleksic M, et al. GABA exerts protective and regenerative effects on islet beta cells and reverses diabetes. Proc Natl Acad Sci USA. 2011;108:11692–11697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Adeghate E, Ponery AS. GABA in the endocrine pancreas: cellular localization and function in normal and diabetic rats. Tissue Cell. 2002;34:1–6. [DOI] [PubMed] [Google Scholar]
- 8. Dong H, Kumar M, Zhang Y, et al. Gamma-aminobutyric acid up- and downregulates insulin secretion from beta cells in concert with changes in glucose concentration. Diabetologia. 2006;49:697–705. [DOI] [PubMed] [Google Scholar]
- 9. Tian J, Dang HN, Yong J, et al. Oral treatment with γ-aminobutyric acid improves glucose tolerance and insulin sensitivity by inhibiting inflammation in high fat diet-fed mice. PLoS One. 2011;6:e25338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Friedman M. Chemistry, nutrition, and health-promoting properties of Hericium erinaceus (lion’s mane) mushroom fruiting bodies and mycelia and their bioactive compounds. J Agric Food Chem. 2015;63:7108–7123. [DOI] [PubMed] [Google Scholar]
- 11. Wang JC, Hu SH, Wang JT, Chen KS, Chia YN. Hypoglycemic effect of extract of Hericium erinaceus . J Sci Food Agric. 2005;85:641–646. [Google Scholar]
- 12. Woraharn S, Lailerd N, Sivamaruthi BS, et al. Development of fermented Hericium erinaceus juice with high content of l-glutamine and l-glutamic acid. Int J Food Sci Technol. 2015;50:2104–2112. [Google Scholar]
- 13. Woraharn S, Lailerd N, Sivamaruthi BS, et al. Evaluation of factors that influence the l-glutamic and γ-aminobutyric acid production during Hericium erinaceus fermentation by lactic acid bacteria. Cyta-J Food. 2016;14:47–54. [Google Scholar]
- 14. Choi MA, Park NY, Woo SM, Jeong YJ, Shin SR. Characteristics of Hericium erinaceus and its extracts. Korean J Food Preserv. 2003;10:560–564. [Google Scholar]
- 15. Honda K, Moto M, Uchida N, He F, Hashizume N. Anti-diabetic effects of lactic acid bacteria in normal and type 2 diabetic mice. J Clin Biochem Nutr. 2012;51:96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tzeng TF, Hong TY, Tzeng YC, Liou SS, Liu IM. Consumption of polyphenol-rich Zingiber zerumbet rhizome extracts protects against the breakdown of the blood-retinal barrier and retinal inflammation induced by diabetes. Nutrients. 2015;7:7821–7841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Widyawati T, Yusoff NA, Asmawi MZ, Ahmad M. Antihyperglycemic effect of methanol extract of Syzygium polyanthum (Wight.) leaf in streptozotocin-induced diabetic rats. Nutrients. 2015;7:7764–7780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Association of Analytical Chemists. Official Methods of Analysis. 17th ed Gaithersburg, MD: Association of Analytical Chemists; 2000. [Google Scholar]
- 19. Hagiwara H, Seki T, Ariga T. The effect of pre-germinated brown rice intake on blood glucose and PAI-1 levels in streptozotocin-induced diabetic rats. Biosci Biotechnol Biochem. 2004;68:444–447. [DOI] [PubMed] [Google Scholar]
- 20. Królczyk G, Laskiewicz J, Sobocki J, Matyja A, Kolasińska-Kloch W, Thor PJ. The effects of baclofen on the feeding behaviour and body weight of vagally stimulated rats. J Physiol Pharmacol. 2005;56:121–131. [PubMed] [Google Scholar]
- 21. Kaya S, Sütçü R, Cetin ES, Aridogan BC, Delibaş N, Demirci M. Lipid peroxidation level and antioxidant enzyme activities in the blood of patients with acute and chronic fascioliasis. Int J Infect Dis. 2007;11:251–255. [DOI] [PubMed] [Google Scholar]
- 22. Ho WY, Liang WS, Yeap SK, Beh BK, Yousr AHN, Alitheen NB. In vitro and in vivo antioxidant activity of Vernonia amygdalina water extract. Afr J Biotechnol. 2012;11:4090–4094. [Google Scholar]
- 23. Cohen N, Cohen J, Asatiani MD, et al. Chemical composition and nutritional and medicinal value of fruit bodies and submerged cultured mycelia of culinary-medicinal higher basidiomycetes mushrooms. Int J Med Mushrooms. 2014;16:273–291. [DOI] [PubMed] [Google Scholar]
- 24. Akbarzadeh A, Norouzian D, Mehrabi MR, et al. Induction of diabetes by streptozotocin in rats. Indian J Clin Biochem. 2007;22:60–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ozougwu JC, Obimba KC, Belonwu CD, Unakalamba CB. The pathogenesis and pathophysiology of type 1 and type 2 diabetes mellitus. J Physiol Pathophysiol. 2013;4:46–57. [Google Scholar]
- 26. von Blankenfeld G, Turner J, Ahnert-Hilger G, et al. Expression of functional GABAA receptors in neuroendocrine gastropancreatic cells. Pflugers Arch. 1995;430:381–388. [DOI] [PubMed] [Google Scholar]
- 27. Thwaites DT, Basterfield L, McCleave PM, Carter SM, Simmons NL. Br J Pharmacol. 2000;129:457–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. World Health Organization. Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus. Abbreviated report of a WHO Consultation. http://www.who.int/diabetes/publications/diagnosis_diabetes2011/en/. Accessed March 2, 2018. [PubMed]
- 29. Tian J, Lu Y, Zhang H, Chau CH, Dang HN, Kaufman DL. Gamma-aminobutyric acid inhibits T cell autoimmunity and the development of inflammatory responses in a mouse type 1 diabetes model. J Immunol. 2004;173:5298–5304. [DOI] [PubMed] [Google Scholar]
- 30. Liang B, Guo Z, Xie F, Zhao A. Antihyperglycemic and antihyperlipidemic activities of aqueous extract of Hericium erinaceus in experimental diabetic rats. BMC Complement Altern Med. 2013;13:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gardiner GE, Heinemann C, Baroja ML, et al. Oral administration of the probiotic combination Lactobacillus rhamnosus GR-1 and L. fermentum RC-14 for human intestinal applications. Int Dairy J. 2002;12:191–196. [Google Scholar]
- 32. Zhang Y, Guo X, Guo J, et al. Lactobacillus casei reduces susceptibility to type 2 diabetes via microbiota-mediated body chloride ion influx. Sci Rep. 2014;4:5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chaiyasut C, Kusirisin W, Lailerd N, Lerttrakarnnon P, Suttajit M, Srichairatanakool S. Effects of phenolic compounds of fermented Thai indigenous plants on oxidative stress in streptozotocin-induced diabetic rats. Evid Based Complement Alternat Med. 2011;2011:749307 doi:10.1155/2011/749307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yeap SK, Mohd Ali N, Mohd Yusof H, et al. Antihyperglycemic effects of fermented and nonfermented mung bean extracts on alloxan-induced-diabetic mice. J Biomed Biotechnol. 2012;2012:285430 doi:10.1155/2012/285430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen Z, Tang J, Sun YQ, Xie J. Protective effect of γ-aminobutyric acid on antioxidation function in intestinal mucosa of Wenchang chicken induced by heat stress. J Anim Plant Sci. 2013;23:1634–1641. [Google Scholar]
- 36. Hu J, Zou X, Cao D, Dong J, Sun K. Effect of γ-aminobutyric acid on serum biochemical parameter of growing and finishing hogs in summer. Feed Ind. 2008;29:9–11. [Google Scholar]