Abstract
Background:
The emergence of novel antiprogrammed cell death protein-1 (PD-1) inhibitors in non-small cell lung cancers (NSCLC) has revolutionized the therapeutic landscape of this disease. Although overall survival (OS) has improved in the first- and second-line therapy settings for advanced NSCLC, the benefit is not universal. In a climate of global scrutiny for healthcare costs and potential for toxicities related to immunotherapy, appropriate patient selection is crucial. The aim of this study was to evaluate potential prognostic and predictive biomarkers interferon-gamma (IFN-γ), tumour necrosis factor-alpha (TNF-α) and a panel of interleukins (ILs) in the peripheral blood, and assess any correlation with response to anti-PD-1 inhibition, progression-free survival and OS in NSCLC patients.
Methods:
We prospectively studied 26 NSCLC patients that received immunotherapy (either pembrolizumab or nivolumab). IFN-γ, TNF-α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10 and IL-12 were analyzed by flow cytometry at the time of diagnosis and at 3 months after initiation of anti-PD-1 inhibition.
Results:
Increased cytokine values (IFN-γ, TNF-α, IL-1β, IL-2, IL-4, IL-6 and IL-8) at the time of diagnosis and at 3 months after initiation of treatment were significantly correlated with improved response to immunotherapy and prolonged OS. There was no correlation between cytokine levels and programmed cell death ligand-1 (PD-L1) expression.
Conclusions:
Increased IFN-γ, TNF-α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10 and IL-12 levels resulted in better response to NSCLC anti-PD-1 inhibition and longer survival, and this could potentially play an important role in selecting patients that would benefit from anti-PD-1 inhibitors.
Keywords: anti-PD-1 treatment, interferon-γ, interleukins, non-small lung cancer, predictive markers, tumour necrosis factor
Introduction
Immune checkpoint inhibitors have revolutionized the therapeutic landscape of advanced non-small cell lung cancer (NSCLC). Clinical trials have reported favourable responses using programmed cell death protein-1 (PD-1)/programmed cell death ligand-1 (PD-L1) blockade as monotherapy or in combination with chemotherapy.1–4 On the basis of these favourable results, the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) approved nivolumab and pembrolizumab as second-line therapies in advanced NSCLC.5,6 The FDA has also recently approved atezolizumab for the management of previously treated patients with advanced NSCLC.7
Despite prolonged overall survival (OS) in advanced NSCLC, the benefit of immunotherapy is not universal.8 Currently, PD-L1 is the only biomarker available in clinical practice to identify patients that would be eligible for anti-PD-1 inhibition.9
Although several studies have addressed the prognostic role of PD-L1 expression in NSCLC,10 the results remain controversial.11
In a climate of global scrutiny for healthcare costs and potential for toxicities related to immunotherapy, appropriate patient selection is crucial for maximizing clinical benefit and cost effectiveness from this novel treatment. Therefore, identification of prognostic biomarkers for patient selection in pragmatic clinical settings would be of great value in optimizing and personalizing immunotherapy; their use would facilitate identification of patients most likely to benefit from nivolumab and pembrolizumab in terms of clinical response, progression-free survival (PFS) and OS.
In recent years, the role of interleukins (ILs), interferons (IFNs) and tumour necrosis factor-alpha (TNF-α) has been extensively explored in immune regulation in tumour development, autoimmune diseases, allergy and asthma. IL involvement in lung cancer pathogenesis and progression has been thoroughly explored.12
The aim of our study was to investigate whether a defined cytokine panel (IFN-γ, TNF-α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12) can play a prognostic or predictive role in NSCLC patients treated with anti-PD-1 inhibition, with the view to assess any potential correlation between their serum levels and response to treatment response/disease progression.
Materials and methods
This was a prospective study approved by the ethics committee of the G. Papanikolaou Hospital in Thessaloniki, Greece (registration number 93/17) and was conducted in accordance with Good Clinical Practice (GCP) guidelines. All patients provided written informed consent before inclusion in the study.
Patients and samples
We prospectively enrolled patients that met the following criteria: age ⩾18 years, histologically confirmed diagnosis of advanced or metastatic NSCLC, PD-L1 expression detected by immunohistochemistry, Eastern Cooperative Oncology Group performance status 0–1 (inclusive), adequate organ function and capacity to make an informed decision. All patients were negative for epidermal growth factor receptor (EGFR) mutation or anaplastic lymphoma kinase (ALK) translocation. Patients with a previous history of interstitial lung disease, systemic immunosuppressive therapy or active autoimmune disease were excluded.
Enrolled patients received either pembrolizumab as monotherapy (200 mg for chemotherapy-naïve patients, or 2 mg/kg for patients previously treated with chemotherapy every 3 weeks), or nivolumab administered intravenously at a dose of 3 mg/kg every 2 weeks. Agent choice was based on PD-L1 status and patients’ previous treatment history (first- or second-line setting).
PD-L1 immunohistochemistry
Detection of PD-L1 expression was performed by 22C3 pharma DxTM Dako test, in formalin-fixed, paraffin-embedded (FFPE) patients tissue samples, using Autostainer Link 48 (Dako Denmark A/S). The Tumour Proportion Score (TPS) was calculated by evaluating the percentage of PD-L1-positive tumour cells relative to all viable tumour cells present in the specimen.
Tumour PD-L1 expression was confirmed when staining of the tumour cell membrane (at any intensity) was observed at prespecified expression levels of 1% or higher and 50% or higher in a section that included at least 100 tumour cells that could be evaluated.
Grading of toxicity
Toxic effects were graded with the use of the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. Scheduled computed tomography or magnetic resonance imaging was performed every 9 weeks. Treatment continued until confirmed disease progression by investigator-assessed immune-related response criteria, unacceptable toxicity, or withdrawal of consent. Although immune-related response criteria were evaluated,13 the primary radiographic assessment was assessed by Response Evaluation Criteria in Solid Tumours (RECIST), version 1.1.14
Cytokine testing in blood by flow cytometry
Peripheral blood samples were tested for immune response at the following time points: (a) before the initiation of anti-PD-1 inhibition treatment; (b) after 3 months of anti-PD-1 inhibition or when radiologically confirmed disease progression was reported.
The 3-month follow-up period was decided on the basis of the essential time required for the immune system to convert from innate to adaptive response.15
Blood samples were collected from all patients before starting anti-PD-1 inhibitors and at 3 months after initiation of anti-PD-1 inhibitors or at radiologically confirmed disease progression. Serum samples were collected and processed using the same standardized protocol. Briefly, blood samples were left to coagulate at room temperature for 30 min, centrifuged at 3000 g for 20 min (4°C), and the supernatants were collected, made to aliquots, and stored in −80°C until assayed. Time interval between processing and freezing was no more than 2 h for each sample. None of the samples were thawed more than twice before analysis.
The following cytokines were measured in blood samples: IFN-γ, TNF-α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10 and IL-12. Serum levels of IFN-γ, TNF-α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10 and IL-12 were determined using a panel kit (AimPlex Biosciences, Pomona, USA) tested by flow cytometry analysis, using a BD FACS Calibur system (BD Biosciences, San Jose, CA, USA), according to the manufacturer’s recommendations and instructions. Specifically, 45 µl of serum sample and 45 µl beads of the panel kit were mixed and then incubated for 1 h in room temperature. After incubation, 0.5 ml of wash buffer was added, and the samples were centrifuged for 5 min. Samples were incubated first with biotin-conjugated monoclonal antibody (30 min) and then subsequently incubated with streptavidin-conjugated monoclonal antibody (20 min). Finally, wash reading buffer was added in all samples.
Statistical analysis
The response duration was defined as the time from first documented evidence of response until progression, according to RECIST. PFS and OS were defined as the time from the first dose of pembrolizumab/nivolumab to progression, according to RECIST, or death (for PFS) or death alone (for OS). We used the Kaplan–Meier method to calculate median values for the duration of response, PFS and OS. We performed Cox regression analysis to examine correlation of biomarker values with PFS and OS, while correlation of these values with patients’ response was performed by one-way ANOVA (analysis of variance) statistical test (IBM Statistical Package for Social Sciences version 20, Armonk, New York, United States).
Results
Patients’ characteristics
Α total of 26 patients were enrolled in the study. Median age was 66.5 years (age range 55–74 years). At the time of data cut off, the median duration of follow up was 6 months, and 16 patients continued to receive anti-PD-1 inhibitors.
Pembrolizumab was offered to 8/26 (31%) patients; 3/8 (37.5%) patients as first-line treatment, and 5/8 (62.5%) patients as second-line treatment. The remaining 18/26 (69%) patients received nivolumab as a second-line treatment. PD-L1 expression was ⩾1% in 13/26 patients (50%). Among them, 10/13 or 10/26 (38.5%) patients had PD-L1 expression in ⩾50% of tumour cells and they were incorporated in the PD-L1 ⩾1 group. Most patients were men and were current or former smokers. The clinical characteristics of patients included in this study are shown in Table 1. A median of six doses of nivolumab and four doses of pembrolizumab were administered.
Table 1.
Patients’ characteristics.
| Pembrolizumab |
Nivolumab |
|
|---|---|---|
| n = 8 | n = 18 | |
| Age (years) | ||
| Median | 70 | 63 |
| Sex | ||
| Male | 8 (100%) | 17 (94%) |
| Female | 0 (0%) | 1 (6%) |
| Smoking status | ||
| Current or former smoker | 8 (100%) | 18 (100%) |
| Never smoked | — | — |
| Histology | ||
| Adenocarcinoma | 6 (75%) | 6 (33%) |
| Squamous | 2 (25%) | 12 (67%) |
| PD-L1 expression | ||
| 0% | 0 (0%) | 13 (72%) |
| ⩾1% | 8 (100%) | 5 (28%) |
| Response | ||
| Progressive disease | 2 (25%) | 7 (39%) |
| Stable disease | 0 (0%) | 9 (50%) |
| Partial response | 6 (75%) | 2 (11%) |
PD-L1, programmed cell death ligand-1.
The rate of confirmed objective response was 31% for all patients who received either nivolumab or pembrolizumab.
At the time of analysis, median PFS for all patients was 4.2 months (95% CI, 2.9–4.8) and OS was 17 months (95% CI, 12.5–23). Patients who expressed PD-L1 ⩾1% had better PFS compared with those with PD-L1 <1% [3.3 months (range 3.0–3.6) versus 5.6 months (range 5.1–6.0), respectively, p < 0.001]. Furthermore, patients with PD-L1 ⩾1% had a better response to treatment.
The most frequently reported treatment-related adverse events were low in severity and included fatigue (in 15% of patients) and hypothyroidism (in 8% of patients). Importantly, they did not lead to discontinuation of treatment or patients’ withdrawal from the study.
Cytokines results and clinical outcome to anti-PD-1 inhibition
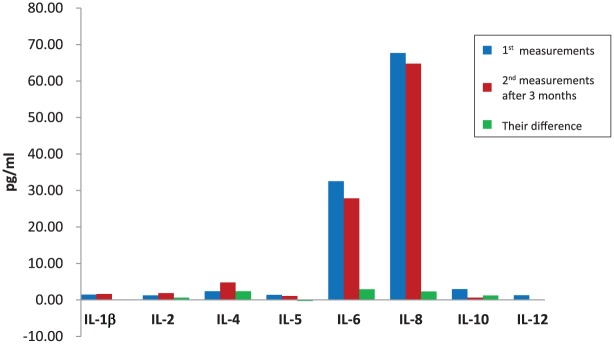
Levels of IFN-γ, TNF-α and ILs in the peripheral blood of patients were significantly associated with improved treatment response and survival at 3 months after initiation of anti-PD-1 inhibition (Table 2). Cytokine measurements were performed in all patients enrolled in the study, irrespective of their response to treatment. Fluctuations in the value of each biomarker (difference in the first and second measurement) were strongly associated with patients’ response and survival (Table 3). Increased cytokine values have been associated with improved response and prolonged survival. Levels of IFN-γ, TNF-α (Figure 1) and ILs (Figure 2) before anti-PD-1 inhibition, after 3 months, and their difference are shown in Figures 1 and 2 in a more understandable way.
Table 2.
First and second measurements in IFN-γ, TNF-α, cytokine levels, their mean values, standard deviation, mean lower and upper bound (95% confidence interval) and their statistical correlation with patient response.
| Mean (pg/ml) | Standard deviation (pg/ml) | Standard error (pg/ml) | 95% confidence interval for mean (pg/ml) |
p value (correlation with response) |
||
|---|---|---|---|---|---|---|
| Lower bound | Upper bound | |||||
| IFN-γ 1st | 1.23 | 0.430 | 0.084 | 1.06 | 1.40 | 0.195 |
| IFN-γ 2nd | 12.38 | 23.257 | 4.561 | 2.99 | 21.78 | 0.000 |
| TNF-α 1st | 1.215 | 0.3663 | 0.0718 | 1.067 | 1.363 | 0.275 |
| TNF-α 2nd | 1.638 | 1.0632 | 0.2085 | 1.209 | 2.068 | 0.12 |
| IL-1β 1st | 1.462 | 0.6469 | 0.1269 | 1.200 | 1.723 | 0.265 |
| IL-1β 2nd | 1.615 | 1.3587 | 0.2665 | 1.067 | 2.164 | 0.087 |
| IL-2 1st | 1.231 | 0.4297 | 0.0843 | 1.057 | 1.404 | 0.193 |
| IL-2 2nd | 1.846 | 1.1897 | 0.2333 | 1.366 | 2.327 | 0.017 |
| IL-4 1st | 2.3846 | 2.48317 | 0.48699 | 1.3816 | 3.3876 | 0.029 |
| IL-4 2nd | 4.7692 | 9.04348 | 1.77357 | 1.1165 | 8.4220 | 0.04 |
| IL-5 1st | 1.3846 | 0.63730 | 0.12499 | 1.1272 | 1.6420 | 0.379 |
| IL-5 2nd | 1.0769 | 0.27175 | 0.05329 | 0.9672 | 1.1867 | 0.193 |
| IL-6 1st | 32.5385 | 33.03239 | 6.47818 | 19.1964 | 45.8805 | 0.084 |
| IL-6 2nd | 27.8462 | 33.46364 | 6.56276 | 14.3299 | 41.3624 | 0.401 |
| IL-8 1st | 2.9231 | 3.65429 | 0.71667 | 1.4471 | 4.3991 | 0.066 |
| IL-8 2nd | 67.6923 | 204.34183 | 40.07473 | −14.8431 | 150.2278 | 0.009 |
| IL-10 1st | 2.3077 | 2.05464 | 0.40295 | 1.4778 | 3.1376 | 0.058 |
| IL-10 2nd | 2.9615 | 1.85969 | 0.36472 | 2.2104 | 3.7127 | 0.495 |
| IL-12 1st | 1.2000 | 0.33466 | 0.06563 | 1.0648 | 1.3352 | 0.093 |
| IL-12 2nd | 1.2769 | 0.37556 | 0.07365 | 1.1252 | 1.4286 | 0.726 |
IFN, interferon; IL, interleukin; TNF, tumor necrosis factor.
Table 3.
Differences in first and second measurements in IFN-γ, TNF-α, cytokine levels, their mean values and their statistical correlation with patient response and overall survival.
| Mean values (pg/ml) |
p value (correlation with response) |
p value (correlation with survival) |
|
|---|---|---|---|
| DIFN-γ | 11.15 | 0.000 | 0.002 |
| DTNF-α | 0.423 | 0.006 | 0.009 |
| DIL-1β | 0.154 | 0.038 | 0.003 |
| DIL-2 | 0.62 | 0.011 | 0.002 |
| DIL-4 | 2.38 | 0.018 | 0.138 |
| DIL-5 | −0.31 | 0.090 | 0.018 |
| DIL-6 | −4.69 | 0.014 | 0.027 |
| DIL-8 | 64.7 | 0.008 | 0.015 |
| DIL-10 | 0.65 | 0.081 | 0.004 |
| DIL-12 | 0.7 | 0.482 | 0.001 |
DIFN-γ, difference in first and second measurements of IFN-γ; DTNF-α, difference in first and second measurements of TNF-α; DIL, difference in first and second measurements of interleukin; IFN-γ, interferon-gamma; IL, interleukin; TNF-α, tumor necrosis factor-alpha.
Figure 1.
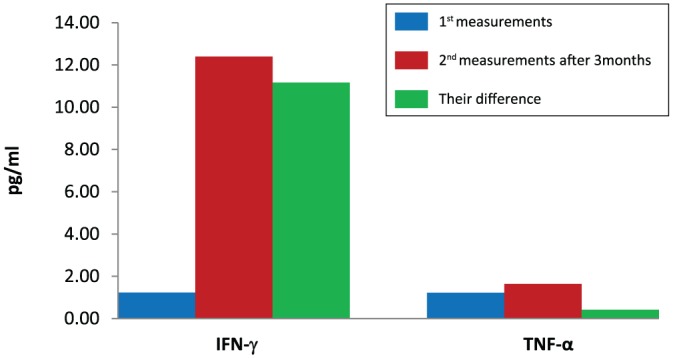
Levels of interferon-γ and tumour necrosis factor-α before immunotherapy, after 3 months, and their difference.
IFN-γ, interferon-gamma; TNF-α, tumour necrosis factor-alpha.
Figure 2.
Levels of interleukins before immunotherapy, after 3 months, and their difference.
There was no correlation between IFN-γ, TNF-α, cytokine levels and PFS.
Moreover, statistical analysis revealed that PD-L1 expression correlated significantly with the difference in IFN-α levels, but not with the other cytokines (p = 0.03).
Cytokine levels initially increased before the first administration of anti-PD-1 inhibition and then decreased 3 months later.
Discussion
In this study, we evaluated a small series of patients with a confirmed diagnosis of NSCLC that received anti PD-1 inhibitors nivolumab or pembrolizumab; our sample included patients that had already received first-line chemotherapy as well as chemo-naïve patients.
Most clinical trials measure a single immune system parameter in a single tissue compartment (mostly IFN-γ-secreting T cells in the peripheral blood) and this may insufficiently capture the immunological signature correlating with the development of an appropriate antitumour response. More data suggest that multiple functions of antigen-specific T cells, rather than T-cell numbers per se, correlate with clinical outcome. This has been described in studies of protective immune response to microbial infections in both mice and humans.
Early studies supporting the existence of cancer immune editing revealed an important function for IFN-γ in suppressing tumour development in models of both tumour transplantation and primary tumour induction.16 In our study, IFN-γ levels have been associated with response to anti-PD-1 inhibitors, supporting its potential in suppressing tumour development.
Overall, IFNs are potent immunomodulators that shape patients’ immunity through direct actions on innate and adaptive lymphocytes, enhancement of natural killer cell cytotoxicity and augmentation of dendritic cell function, which are central to the initiation of adaptive immune responses that suppress tumour development.17
Soluble TNF-α, along with other proinflammatory factors, recruits and activates neutrophils, macrophages and lymphocytes at the sites of damage and infection.18
Certain ILs, including IL-4, IL-1β and IL-6 are involved in key mechanisms of tumourigenesis, reported to be a promising pathway towards cancer immunotherapy.19 The majority of ILs are secreted by CD4+ T-helper lymphocytes, as well as by monocytes, macrophages and endothelial cells. IL-12 can induce cell cycle arrest, apoptosis in human hepatocellular carcinoma cells, and effectively shift the tumour microenvironment from pro-oncogenic to antitumour through recruitment of immune cells and inhibiting stromal cell growth.20
Cytokine concentrations seem to be correlated with PFS. Tran and colleagues reported increased IL-8 concentrations were correlated with shorter PFS in patients with renal cell carcinoma treated with pazopanib.21 In our study, we found no correlation between cytokine levels and PFS, but this may be attributed to our small sample of patients. This is one of the limiting factors, as well as the fact that some of our patients have received first-line chemotherapy prior to anti-PD-1 inhibitors. The impact of conventional chemotherapy on cytokine levels on a background of second-line anti-PD-1 inhibition remains vague. Unfortunately, large phase III studies have been performed without taking this into account, as biomarkers have not been included in them. The recent negative trials on talactoferrin and anti-MUC-1 are examples of this lack of patient selection beforehand.22–24
Conclusion
Immune-checkpoint inhibition clearly has changed the therapeutic approach in NSCLC. Although intratumoural PD-L1 expression appears to identify patients eligible for anti-PD-1 treatment, it is unclear whether it will translate into a stand-alone and clinically meaningful biomarker predictive of clinical response. Cytokine serum levels could provide prognostic information and constitute predictive markers of immunotherapy benefit in patients with advanced NSCLC. Further studies of the predictive effects of these markers in bigger populations are warranted. Overall, the optimal predictive biomarker used in everyday clinical practice should be easily applicable in busy clinical settings, cost effective and provide an accurate prediction of a patient’s clinical response. New knowledge obtained from ongoing and future research will refine our approach for the meaningful clinical application of biomarker research.
Footnotes
Funding: The study was funded by the National Institute of Scholarship (ΙΚΥ) Fellowships of Excellence for Postgraduate Studies in Greece-Siemens Programme (grant number: 2016-017-0173-10666).
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Efimia Boutsikou, Pulmonary Department – Oncology Unit, ‘G. Papanikolaou’ Hospital, Aristotle University of Thessaloniki, Platonos 1G, Pefka, 57010, Thessaloniki, Greece.
Kalliopi Domvri, Pulmonary Department, ‘G. Papanikolaou’ Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece.
Georgia Hardavella, Department of Respiratory Medicine, King’s College Hospital, London, UK.
Dora Tsiouda, Pulmonary Department, ‘Theageneio’ Anticancer Hospital, Thessaloniki, Greece.
Konstantinos Zarogoulidis, Pulmonary Department, ‘G. Papanikolaou’ Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece.
Theodoros Kontakiotis, Pulmonary Department, ‘G. Papanikolaou’ Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece.
References
- 1. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015; 373: 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015; 373: 1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016; 387: 1540–1550. [DOI] [PubMed] [Google Scholar]
- 4. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017; 389: 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kazandjian D, Suzman DL, Blumenthal G, et al. FDA approval summary: nivolumab for the treatment of metastatic non-small cell lung cancer with progression on or after platinum-based chemotherapy. Oncologist 2016; 21: 634–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pai-Scherf L, Blumenthal GM, Li H, et al. FDA approval summary: pembrolizumab for treatment of metastatic non-small cell lung cancer: first-line therapy and beyond. Oncologist 2017; 22: 1392–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weinstock C, Khozin S, Suzman D, et al. U.S. food and drug administration approval summary: atezolizumab for metastatic non-small cell lung cancer. Clin Cancer Res 2017; 23: 4534–4539. [DOI] [PubMed] [Google Scholar]
- 8. Garde-Noguera J, Martorell PM, de Julián M, et al. Predictive and prognostic clinical and pathological factors of nivolumab efficacy in non-small-cell lung cancer patients. Clin Transl Oncol 2018. DOI: 10.1007/s12094-017-1829-5. [DOI] [PubMed] [Google Scholar]
- 9. Aguiar PN, Jr, De Mello RA, Hall P, et al. PD-L1 expression as a predictive biomarker in advanced non-small-cell lung cancer: updated survival data. Immunotherapy 2017; 9: 499–506. [DOI] [PubMed] [Google Scholar]
- 10. Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016; 17: 1497–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang M, Li G, Wang Y, et al. PD-L1 expression in lung cancer and its correlation with driver mutations: a meta-analysis. Sci Rep 2017; 7: 10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Strieter RM, Polverini PJ, Arenberg DA, et al. Role of C-X-C chemokines as regulators of angiogenesis in lung cancer. J Leukoc Biol 1995; 57: 752–762. [DOI] [PubMed] [Google Scholar]
- 13. Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 2009; 15: 7412–7420. [DOI] [PubMed] [Google Scholar]
- 14. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 15. Ziauddin J, Schneider DS. Where does innate immunity stop and adaptive immunity begin? Cell Host Microbe 2012; 12: 394–395. [DOI] [PubMed] [Google Scholar]
- 16. Shankaran V, Ikeda H, Bruce AT, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 2001; 410: 1107–1111. [DOI] [PubMed] [Google Scholar]
- 17. O’Neill DW, Adams S, Bhardwaj N. Manipulating dendritic cell biology for the active immunotherapy of cancer. Blood 2004; 104: 2235–2246. [DOI] [PubMed] [Google Scholar]
- 18. Sethi G, Sung B, Aggarwal BB. TNF: a master switch for inflammation to cancer. Front Biosci 2008; 13: 5094–5107. [DOI] [PubMed] [Google Scholar]
- 19. Sahoo A, Im SH. Interleukin and interleukin receptor diversity: role of alternative splicing. Int Rev Immunol 2010; 29: 77–109. [DOI] [PubMed] [Google Scholar]
- 20. Weiss JM, Subleski JJ, Wigginton JM, et al. Immunotherapy of cancer by IL-12-based cytokine combinations. Expert Opin Biol Ther 2007; 7: 1705–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tran HT, Liu Y, Zurita AJ, et al. Prognostic or predictive plasma cytokines and angiogenic factors for patients treated with pazopanib for metastatic renal-cell cancer: a retrospective analysis of phase 2 and phase 3 trials. Lancet Oncol 2012; 13: 827–837. [DOI] [PubMed] [Google Scholar]
- 22. Digumarti R, Wang Y, Raman G, et al. A randomized, double-blind, placebo-controlled, phase II study of oral talactoferrin in combination with carboplatin and paclitaxel in previously untreated locally advanced or metastatic non-small cell lung cancer. J Thorac Oncol 2011; 6: 1098–1103. [DOI] [PubMed] [Google Scholar]
- 23. Kelly RJ, Giaccone G. The role of talactoferrin alpha in the treatment of non-small cell lung cancer. Expert Opin Biol Ther 2010; 10: 1379–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ramlau R, Quoix E, Rolski J, et al. A phase II study of Tg4010 (Mva-Muc1-Il2) in association with chemotherapy in patients with stage III/IV non-small cell lung cancer. J Thorac Oncol 2008; 3: 735–744. [DOI] [PubMed] [Google Scholar]