Abstract
Background:
Small bowel involvement in Crohn’s disease (CD) is frequently proximal to the ileocecal valve and inaccessible by conventional ileocolonoscopy (IC). Small bowel capsule endoscopy (SBCE) is among the prime modalities for assessment of small bowel disease in these patients. Intestinal ultrasound (IUS) is an accurate bedside fast and low-cost diagnostic modality utilized in CD for both diagnosis and monitoring. The aim of this study was to examine the accuracy of IUS in patients with suspected CD after a negative IC, and to evaluate the correlation of IUS with SBCE, inflammatory biomarkers and other cross-sectional imaging techniques.
Methods:
Prospective single center study in which patients with suspected CD underwent IUS and SBCE examinations within 3 days. IUS results were blindly compared with SBCE that served as the gold standard. A post hoc comparison was performed of IUS and SBCE results and available cross-sectional imaging results (computed tomography or magnetic resonance enterography) as well as inflammatory biomarkers if measured. The study cohort was followed for 1 year. In case of discordance between the IUS and SBCE results, the diagnosis at 1 year was reported.
Results:
Fifty patients were included in the study. The diagnostic yield of both IUS and SBCE for the diagnosis of small bowel CD was 38%. The IUS findings significantly correlated to small bowel inflammation detected by SBCE (r = 0.532, p < 0.001), with fair sensitivity and specificity (72% and 84%). Cross-sectional imaging results significantly correlated to IUS as well (r = 0.46, p = 0.018). Follow up was available in 8 of the 10 cases of discordance between IUS and SBCE. In all of these cases, diagnosis of CD was not fully established at the end of the follow up.
Conclusions:
The diagnostic yield of CE and IUS for detection of CD in patients with negative ileocolonoscopy was similar. IUS can be a useful diagnostic tool in suspected CD when IC is negative.
Keywords: intestinal ultrasound, Crohn’s disease, small bowel capsule endoscopy
Introduction
Technological advances in the field of ultrasonography, as well as growing experience of ultrsonographers, have contributed to the placement of ultrasound as a clinically important, noninvasive imaging modality in Crohn’s disease (CD). High-resolution assessment of the mucosal, submucosal and muscular layers, as well as visualization of possible intestinal and extraintestinal pathologies (stenosis, abscesses or fistulas) can be attained clearly using intestinal US (IUS).1–4 IUS is one of the diagnostic modalities suggested by the European Crohn’s and Colitis Organization (ECCO) guidelines on the management of CD,5 and can be used for the initial evaluation of patients with clinically suspected CD. Economic models have proved the cost effectiveness of combining ileocolonoscopy (IC) and IUS for the diagnosis of CD.6 Thickening of the bowel wall is the most significant ultrasonographic feature of CD, found to be a valid marker of inflammation in CD.7 Other ultrasonographic signs including pseudo stratification of the bowel wall, mesenteric and adipose hypertrophy, enlargement of lymph nodes and enhancement of blood flow in the bowel wall.8
The diagnosis of CD relies upon the combination of clinical, endoscopic, cross-sectional imaging and histologic findings. IC is usually the first modality used when CD is suspected.9 However, when the disease is limited to more proximal small bowel sections, or when technical complications limit the use of IC, other imaging modalities are used. Small bowel capsule endoscopy (SBCE) enables evaluation of the whole of the small bowel in almost 100% of cases,10 and was found to have superior sensitivity and specificity in comparison to cross-sectional imaging modalities.11,12 SBCE can detect active mucosal inflammation in over 80% of patients with CD, even those in clinical remission.13 Previously unknown proximal small bowel inflammation can be detected in over 50% of patients with CD.14 Nevertheless, the use of SBCE is limited due to high cost and low but existing risk of capsule retention.15
The diagnostic yield of small intestine contrast ultrasonography (SICUS) for the diagnosis of active CD was demonstrated to be similar to that of SBCE in a meta-analysis including five studies.12 However, SICUS is a highly demanding and relatively time-consuming procedure, which is performed only in a few specialized centers. Bedside point of care IUS is more often preformed, as an auxiliary diagnostic tool used by a gastroenterologist. Data regarding the added diagnostic yield of IUS in cases of suspected CD are scarce. Therefore, the aim of our study was to compare the diagnostic accuracy of IUS with SBCE in patients with suspected CD after a negative IC, and to evaluate the correlation of these findings with inflammatory biomarker levels and other cross-sectional imaging methods.
Methods
Study population and procedure
In this prospective observational study, adults (⩾18 years) were assessed for suspected CD. A normal IC was needed in order to participate in the study. The decision to send the patients for SBCE was under the discretion of the treating gastroenterologist.
Patients were excluded if they were unable to understand or provide informed consent, or had any of the usual contraindications to SBCE.
After signing the informed consent, participants underwent SBCE, followed by IUS within 3 days of SBCE completion, by a single operator (DC), blind to the result of the SBCE or any other former laboratory or cross-sectional examination. Demographics and other medical data were collected from the patients’ medical file.
The study was approved by the Sheba medical center institutional ethics review board (SMC-2177-15).
Intestinal ultrasound
All examinations were performed using a Toshiba Xario ultrasound machine (Toshiba Inc., Japan) or a BK 3000 ultrasound machine (BK Ultrasound, Peabody, USA) with low frequency (2.5–6 MHz) curved array transducer enabling all abdominal quadrants to be examined for fill levels, potential pathological distension, motility and para intestinal structures such as abscesses. This was followed by examination using a high-resolution linear array transducer (6.0–12 MHz) for detailed examination of the bowel wall structure. All examinations were performed without any preceding preparation, using a consistent technique and protocol, beginning the examination with the proximal to distal colon, followed by complete examination of the small bowel. Assessment was performed for features of inflammation, especially bowel wall thickness (>4 mm for colon and >3 mm for small bowel) and type of complications (stenosis or penetrating complications including fistulas, or inflammatory masses).
Small bowel capsule endoscopy
PillCam SB3 video capsule endoscope (Medtronic, Minneapolis, MN, USA) was used in our study. Preparation included 24 h of clear liquids prior to the examination and an overnight 12 h fast. All images were reviewed using the RAPID 8 software (Medtronic) by an experienced board-certified gastroenterologist) UK, RE). Mucosal inflammation was quantified using the Lewis Score (LS).16 Normal exam was defined as LS less than 135, mild to moderate inflammation as LS of 135–790, and moderate to severe inflammation as LS at least 790.16 LS was calculated automatically (RAPID Reader, Medtronic).
Comparison of the results of bowel ultrasound to SBCE and other cross-sectional modalities
Abnormal SBCE was defined as LS greater than 135. Any major small bowel pathology on IUS (bowel wall thickening >3 mm and complications) was regarded as abnormal. We further compared pathologies limited to the third small bowel tertile on SBCE to pathologies located at the terminal ileum on IUS.
A post hoc comparison of IUS and SBCE results to available cross-sectional imaging results [computed tomography (CT) or magnetic resonance enterography] was performed. We included only exams that were performed within 3 months of the IUS and SBCE. We incorporated data from the final cross-sectional reports, establishing a positive diagnosis on well known signs of inflammation.17,18
The study cohort was followed for 1 year. In the case of discordance between the IUS and SBCE results, the diagnosis at 1 year was reported.
Inflammatory biomarkers
Fecal calprotectin (FCP) levels were measured using the Quantum blue calprotectin kit (Buhlmann Laboratories AG, Basel, Switzerland).
The reported value range is 30–300 µg/g. Levels over 100 µg/g were considered positive. C-reactive protein (CRP) levels over 5 mg/liter were considered elevated.
Statistical analysis
Descriptive statistics were presented as means ± standard deviations for continuous variables and percentages for categorical variables. We evaluated the correlation of abnormal IUS finding to abnormal SBCE (LS > 135) and to other cross-sectional imaging. Sensitivity, specificity, negative predictive value (NPV) and positive predictive value (PPV) as well as Spearman’s rank (r) correlation were calculated. Correlation r values less than 0.3 were considered as weak to low correlation, 0.3–0.49 as low to moderate, 0.5–0.69 as moderate, and 0.7 and over as strong correlation.19 A two-tailed p value up to 0.05 was considered statistically significant.
The analysis was performed using IBM SPSS statistical software (version 20.0; Armonk, NY, USA).
Results
Fifty patients (21 men, 29 women, median age 20.8 years) were enrolled and underwent IUS and SBCE. All SBCE exams were complete, with the capsule reaching the cecum. All IUS exams were technically adequate.
Correlation of signs of inflammation on IUS to SBCE
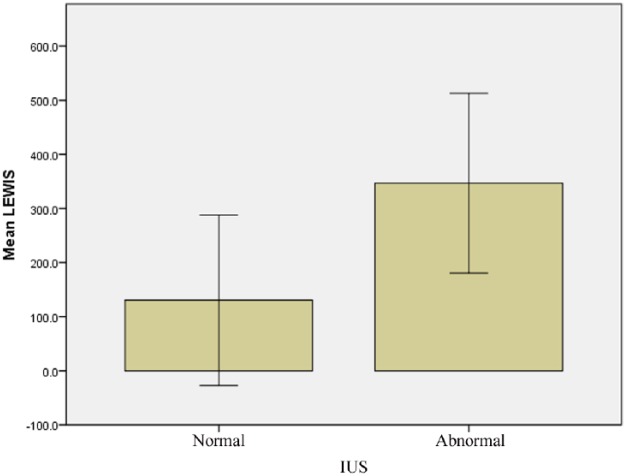
The diagnostic yield of SBCE for the diagnosis of small bowel CD was 38% (n = 19); similarly, signs of small bowel inflammation were detected in 38% of the exams by IUS. When considering SBCE as the referral gold standard exam, the correlation for detection of CD by IUS was moderate but significant (r = 0.532, p < 0.001) with fair sensitivity and specificity (72.2% and 84.4%) (Figure 1). The correlation for detection of CD in the distal ileum by IUS to that of SBCE in the distal part of the small bowel was similarly significant (r = 0.55, p < 0.001) (Table 1).
Figure 1.
Correlation of small bowel inflammation (bowel wall thickness >3 mm) detected by IUS with degree of small bowel inflammation (Lewis score). The correlation was significant.
IUS, intestinal ultrasound.
Table 1.
Correlation of signs of inflammation by IUS to SBCE in the entire small bowel and in the distal small bowel.
| Small bowel (%) | Distal small bowel (%) | |
|---|---|---|
| Sensitivity | 72.2 | 72.7 |
| Specificity | 84.4 | 86 |
| PPV | 72.2 | 76 |
| NPV | 84.5 | 83.3 |
| Correlation | 0.532* | 0.55* |
p < 0.001.
IUS, intestinal ultrasound; NPV, negative predictive value; PPV, positive predictive value; SBCE, small bowel capsule endoscopy.
In 3 of the 19 SBCE examinations (15%), the inflammatory findings were limited to the first and second part of the small bowel, with distal ileum sparing. IUS spotted two of the cases.
Correlation of IUS and SBCE to cross-sectional imaging
Cross-sectional imaging results were available in 25 participants (50%). The correlation of inflammation on cross-sectional imaging to that of IUS was significant (r = 0.46, p = 0.018) (Table 2).
Table 2.
Agreement between results of cross-sectional imaging to IUS.
| IUS cross-sectional agreement (%) | |
|---|---|
| Positive IUS and SBCE | 4 (66) |
| Negative IUS and SBCE | 10 (77) |
| Positive IUS negative SBCE | 1 (50) |
| Negative IUS positive SBCE | 4 (100) |
| Correlation IUS/cross-sectional imaging | r = 0.46 |
| p = 0.018 |
IUS, intestinal ultrasound; SBCE, small bowel capsule endoscopy.
In five cases, the IUS was normal, while the SBCE demonstrated pathologic small bowel findings consistent with CD. In four of five cases, other cross-sectional imaging results were also negative for CD.
One-year clinical follow up was available in four of five cases. In all the cases, the diagnosis of CD was not fully established at the end of the follow up.
In five cases, the SBCE was normal, while bowel wall thickening was detected by the IUS. Cross-sectional imaging was available in only two of these cases. In one case, cross-sectional imaging results demonstrated bowel wall thickening (Table 2). One-year clinical follow up was available in four of the cases. In all of the cases, the diagnosis of CD was not fully established at the end of the follow up.
Correlation of inflammatory biomarkers
FCP and CRP levels were available in 27 patients. The correlation of FCP and CRP levels to the results of IUS, SBCE and cross-sectional imaging were nonsignificant (Table 3).
Table 3.
Correlation of inflammatory biomarkers with abnormal IUS, SBCE and cross-sectional imaging.
| FCP | CRP | |
|---|---|---|
| IUS | r = 0.04 | r = 0.12 |
| p = 0.86 | p = 0.6 | |
| SBCE | r = 0.1 | r = 0.18 |
| p = 0.3 | p = 0.7 | |
| Cross-sectional imaging | r = 0.1 | r = 0.09 |
| p = 0.6 | p = 0.5 |
IUS, intestinal ultrasound; SBCE, small bowel capsule endoscopy.
Discussion
In this prospective study, we have demonstrated that IUS can be considered as an additional diagnostic modality when the initial evaluation for CD is negative.
CD is a chronic, relapsing condition characterized by transmural inflammation of the intestine. For suspected CD, IC is the first-line procedure used to establish the diagnosis.5 However, adequate visualization of the terminal ileum can be achieved only in 85% of colonoscopies.20 The proximal small bowel seems to be affected in over 50% of patients with CD on capsule endoscopy examinations. In addition, up to 30% of newly diagnosed CD cases have disease limited to the small bowel beyond the reach of IC, necessitating the use of advanced endoscopic techniques to visualize and obtain histology.21 Therefore, a noninvasive or minimally invasive imaging method capable of assessing disease activity and severity in cases when IC is inadequate is warranted.
IUS is a readily available bedside diagnostic method that has a place both in making the initial diagnostic decision and in monitoring of IBD treatment. It does not necessitate preparation, does not require exposition to potentially harmful irradiation and is relatively inexpensive. Our group had previously demonstrated the feasibility of IUS for the detection of inflammation and complications in patients with IBD.8 In this study, we demonstrate a similar yield of IUS and SBCE for diagnosis of CD. The reasonable correlation with the SBCE findings highlights the place of IUS as a practical imaging modality that could be used as a first- or second-line diagnostic modality in CD.
The accuracy of IUS for the diagnosis and monitoring of CD has been evaluated in a meta-analysis, comparing IUS, CT, magnetic resonance imaging and scintigraphy. IUS was found to have high sensitivity and specificity, not significantly different from other the other cross-sectional imaging modalities.22 However, the use of IUS was limited in cases of small bowel disease proximal to the terminal ileum. In this study, we found that IUS can successfully demonstrate active inflammation in small bowel sections that are out of reach of IC, similarly to SBCE or other cross-sectional imaging modalities. Nevertheless, most of the prominent positive findings in our study were confined to the distal small bowel (though out of the reach of IC), where the ultrasonographic demonstration of small bowel disease is more likely. In this regard, we found that IUS successfully detected two of the three cases of inflammation confined to proximal small bowel. Although the numbers are small, limiting the possibility to draw a definite conclusion, IUS may also be a useful tool in more proximal small bowel disease. This fact may be explained by improved US technique (equipment and software) and operator’s experience.
In five cases, small bowel wall thickening was not detected by IUS, although mucosal signs of inflammation were found in the SBCE. Similarly, in most of these cases, bowel wall thickening was not detected by CT or magnetic resonance enterography. This observation could be explained by the fact that disease limited to the mucosa, especially in the proximal small bowel, might go undetected by cross-sectional imaging modalities.12 On the other hand, in five cases IUS detected small bowel thickening, while the small bowel was found to be normal on SBCE. Although endoscopy and SBCE are more sensitive for the detection of mucosal limited disease, IUS (like other cross-sectional imaging methods) has the advantage of visualization of all bowel layers.23 Therefore, our finding can be hypothetically explained by submucosal inflammation with minute mucosal involvement that went undetected by SBCE.
The correlation of IUS signs of small bowel inflammation to inflammatory markers was insignificant. Prior studies demonstrated poor correlation of CRP levels to inflammatory findings on SBCE and cross-sectional imaging.12 The low correlation of FCP with pathologic IUS and SBCE findings might be explained by the relatively low number of available FCP results.
Our study has limitations. First, this was a single-center study with one very well experienced ultrasonographist performing IUS. Second, only a minority of the study participants had a disease limited to the proximal small bowel. Therefore, it is possible that the accuracy of IUS could be worse is these cases, although IUS detected proximal disease in two of the three cases in the current study.
Another limitation relates to the relatively small amount of cross-sectional imaging results available. However, although only available in 50% of cases, significant correlation was found to IUS results, as demonstrated in previous studies
In summary, IUS can be a useful diagnostic tool in suspected CD when IC is negative. The availability and the relatively low cost of IUS places this imaging exam in the front line of the imaging modalities in CD.
Acknowledgments
Uri Kopylov and Rami Eliakim contributed equally to this work.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Uri Kopylov  https://orcid.org/0000-0002-7156-0588
https://orcid.org/0000-0002-7156-0588
Contributor Information
Dan Carter, Department of Gastroenterology, Chaim Sheba Medical Center, 2nd Sheba Rd, Tel Hashomer, Israel; Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
Lior H. Katz, Department of Gastroenterology, Chaim Sheba Medical Center, Ramat Gan, Israel Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel
Eytan Bardan, Department of Gastroenterology, Chaim Sheba Medical Center, Ramat Gan, Israel Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
Eti Salomon, Department of Gastroenterology, Chaim Sheba Medical Center, Ramat Gan, Israel.
Shulamit Goldstein, Department of Gastroenterology, Chaim Sheba Medical Center, Ramat Gan, Israel.
Shomron Ben Horin, Department of Gastroenterology, Chaim Sheba Medical Center, Ramat Gan, Israel Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
Uri Kopylov, Department of Gastroenterology, Chaim Sheba Medical Center, Ramat Gan, Israel Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
Rami Eliakim, Department of Gastroenterology, Chaim Sheba Medical Center, Ramat Gan, Israel Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
References
- 1. Fraquelli M, Colli A, Casazza G, et al. Role of US in detection of Crohn disease: meta-analysis. Radiology 2005; 236: 95–101. [DOI] [PubMed] [Google Scholar]
- 2. Parente F, Maconi G, Bollani S, et al. Bowel ultrasound in assessment of Crohn’s disease and detection of related small bowel strictures: a prospective comparative study versus x ray and intraoperative findings. Gut 2004; 50: 490–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rieder F, Zimmermann EM, Remzi FH, et al. Crohn’s disease complicated by strictures: a systematic review. Gut 2013; 62: 1072–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maconi G, Sampietro GM, Parente F, et al. Contrast radiology, computed tomography and ultrasonography in detecting internal fistulas and intra-abdominal abscesses in Crohn’s disease: a prospective comparative study. Am J Gastroenterol 2003; 98: 1545–1555. [DOI] [PubMed] [Google Scholar]
- 5. Gomollón F, Dignass A, Annese V, et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. J Crohns Colitis 2017; 11: 3–25. [DOI] [PubMed] [Google Scholar]
- 6. Maconi G, Bolzoni E, Giussani A, et al. Accuracy and cost of diagnostic strategies for patients with suspected Crohn’s disease. J Crohns Colitis 2014; 8: 1684–1692. [DOI] [PubMed] [Google Scholar]
- 7. Wilkens R, Hagemann-Madsen RH, Peters DA, et al. Validity of contrast enhanced ultrasonography and dynamic contrast enhanced MR enterography in the assessment of transmural activity and fibrosis in Crohn’s disease. J Crohns Colitis. Epub ahead of print 3 August 2017. DOI: 10.1093/ecco-jcc/jjx111. [DOI] [PubMed] [Google Scholar]
- 8. Carter D, Eliakim R. The feasibility of bedside bowel ultrasound performed by a gastroenterologist for detection and follow-up of inflammatory bowel disease. Isr Med Assoc J 2017; 19: 139–142. [PubMed] [Google Scholar]
- 9. Carter D, Eliakim R. Current role of endoscopy in inflammatory bowel disease diagnosis and management. Curr Opin Gastroenterol 2014; 30: 370–377. [DOI] [PubMed] [Google Scholar]
- 10. Bourreille A, Ignjatovic A, Aabakken L, et al. Role of small-bowel endoscopy in the management of patients with inflammatory bowel disease: an international OMED-ECCO consensus. Endoscopy 2009; 41: 618–637. [DOI] [PubMed] [Google Scholar]
- 11. Dionisio PM, Gurudu SR, Leighton JA, et al. Capsule endoscopy has a significantly higher diagnostic yield in patients with suspected and established small-bowel Crohn’s disease: a meta-analysis. Am J Gastroenterol 2010; 105: 1240–1248. [DOI] [PubMed] [Google Scholar]
- 12. Kopylov U, Yung DE, Engel T, et al. Diagnostic yield of capsule endoscopy versus magnetic resonance enterography and small bowel contrast ultrasound in the evaluation of small bowel Crohn’s disease: Systematic review and meta-analysis. Dig Liver Dis 2017; 49: 854–863. [DOI] [PubMed] [Google Scholar]
- 13. Kopylov U, Yablecovitch D, Lahat A, et al. Detection of small bowel mucosal healing and deep remission in patients with known small bowel Crohn’s disease using biomarkers, capsule endoscopy, and imaging. Am J Gastroenterol 2015; 110: 1316–1323. [DOI] [PubMed] [Google Scholar]
- 14. Greener T, Klang E, Yablecovitch D, et al. The impact of magnetic resonance enterography and capsule endoscopy on the re-classification of disease in patients with known Crohn’s disease: a prospective Israeli IBD Research Nucleus (IIRN) Study. J Crohns Colitis 2016; 10: 525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kopylov U, Nemeth A, Koulaouzidis A, et al. Small bowel capsule endoscopy in the management of established Crohn’s disease: clinical impact, safety, and correlation with inflammatory biomarkers. Inflamm Bowel Dis 2015; 21: 93–100. [DOI] [PubMed] [Google Scholar]
- 16. Gralnek IM, Defranchis R, Seidman E, et al. Development of a capsule endoscopy scoring index for small bowel mucosal inflammatory change. Aliment Pharmacol Ther 2008; 27: 146–154. [DOI] [PubMed] [Google Scholar]
- 17. Morris MS, Chu DI. Imaging for inflammatory bowel disease. Surg Clin North Am 2015; 95: 1143–1158. [DOI] [PubMed] [Google Scholar]
- 18. Baker ME, Hara AK, Platt JF, et al. CT enterography for Crohn’s disease: optimal technique and imaging issues. Abdom Imaging 2015; 40: 938–952. [DOI] [PubMed] [Google Scholar]
- 19. Fleiss J. Statistical methods for rates and proportions. 2nd ed. New York: John Wiley & Sons, 1981. [Google Scholar]
- 20. Coremans G, Rutgeerts P, Geboes K, et al. The value of ileoscopy with biopsy in the diagnosis of intestinal Crohn’s disease. Gastrointest Endosc 1984; 30: 167–172. [DOI] [PubMed] [Google Scholar]
- 21. Annunziata ML, Caviglia R, Papparella LG, et al. Upper gastrointestinal involvement of Crohn’s disease: a prospective study on the role of upper endoscopy in the diagnostic work-up. Dig Dis Sci 2012; 57: 1618–1623. [DOI] [PubMed] [Google Scholar]
- 22. Horsthuis K, Bipat S, Bennink RJ, et al. Inflammatory bowel disease diagnosed with US, MR, scintigraphy, and CT: meta-analysis of prospective studies. Radiology 2008; 247: 64–79. [DOI] [PubMed] [Google Scholar]
- 23. Kaushal P, Somwaru AS, Charabaty A, et al. MR enterography of inflammatory bowel disease with endoscopic correlation. Radiographics 2017; 37: 116–131. [DOI] [PubMed] [Google Scholar]