Abstract
Preventing or delaying Alzheimer disease (AD) through lifestyle interventions will come from a better understanding of the mechanistic underpinnings of (1) why a significant proportion of elderly remain cognitively normal with AD pathologies (ADP), i.e., amyloid or tau; and (2) why some elderly individuals do not have significant ADP. In the last decades, concepts such as brain reserve, cognitive reserve, and more recently brain maintenance have been proposed along with more general notions such as (neuro)protection and compensation. It is currently unclear how to effectively apply these concepts in the new field of preclinical AD specifically separating the 2 distinct mechanisms of coping with pathology vs avoiding pathology. We propose a simplistic conceptual framework that builds on existing concepts using the nomenclature of resistance in the context of avoiding pathology, i.e., remaining cognitively normal without significant ADP, and resilience in the context of coping with pathology, i.e., remaining cognitively normal despite significant ADP. In the context of preclinical AD studies, we (1) define these concepts and provide recommendations (and common scenarios) for their use; (2) discuss how to employ this terminology in the context of investigating mechanisms and factors; (3) highlight the complementarity and clarity they provide to existing concepts; and (4) discuss different study designs and methodologies. The application of the proposed framework for framing hypotheses, study design, and interpretation of results and mechanisms can provide a consistent framework and nomenclature for researchers to reach consensus on identifying factors that may prevent ADP or delay the onset of cognitive impairment.
Alzheimer disease (AD) is associated with the deposition of β-amyloid (Aβ) into plaques and hyperphosphorylated tau as neurofibrillary tangles. These 2 AD pathology (ADP)–related changes are the drivers of neuronal dysfunction (hypometabolism and brain atrophy) and subsequent cognitive impairment seen with AD. The recent availability of imaging and CSF biomarkers to assess ADP and neuronal dysfunction has brought us closer to understanding the mechanistic underpinnings of AD. Biomarkers have been proposed for staging of individuals in preclinical stages of AD for research studies, i.e., in cognitively normal individuals with AD-related changes.1–3 These research developments allow for classification of individuals as positive or negative for significant levels of ADP, i.e., presence/absence or abnormal/normal levels of Aβ (A+/A−) or tau (T+/T−). These new frameworks for preclinical disease have accelerated the development of therapeutics4,5 and have also brought substantial research interest to the field of reserve, resilience, and protective factors.
Why are some individuals with AD pathologies cognitively normal?
Since the initial observations of the disconnect between the degree of pathology and cognition,6–9 there has been tremendous interest in understanding the mechanisms underlying resilience. Furthermore, studies have shown that lifestyle, genetic, and brain factors play an important role in delaying or slowing cognitive decline.10–18 In this context, the reserve hypothesis has been the most widely used approach to resilience, including its active10,11 and passive forms.6,19 Other notions such as brain maintenance20 may also be useful in capturing some aspects of resilience.
Why do some individuals have lower burden of AD pathologies?
There is emerging evidence that some lifestyle and behavioral factors may slow or halt the progression of ADP.21–30 This concept is distinct from the concept of pathology and cognition disconnect and not acknowledged in the concept of reserve. It fundamentally aims to explain how some individuals are able to slow ADP progression. For example, recent evidence supports the idea that certain individuals, referred to as exceptional agers, do not have significant ADP even at advanced ages due to better lifestyles.31
While there have been varied terminologies to investigate the above mentioned ideas, including cognitive10,19 and brain reserve,6,32 brain maintenance,20 or general notions such as (neuro)protection and compensation, the use of varied concepts and terminologies across publications has led to a lack of consensus across studies. It has importantly led to a lack of common ground for interpreting study results and for conveying hypotheses/ideas, which is more apparent in preclinical AD biomarker studies. With this in mind, our primary goal was to propose a simplistic conceptual framework for preclinical studies in AD that can aid with framing of hypotheses, understanding mechanisms, and interpreting results, especially in AD biomarker studies. We do not aim to propose new concepts but rather propose a framework that highlights the complementarity and clarity of existing concepts. The simplistic framework proposed here can aid with both conveying the results and moving the field toward a common goal using consistent nomenclature. The secondary aim was to discuss different study designs and methodologies that can be employed to investigate these ideas and illustrate their application in the literature.
Terminology
Resistance vs resilience to AD: Avoiding vs coping
A substantial proportion of people remain cognitively normal throughout their lifetime, some with ADP at autopsy or in vivo imaging (∼30%) and some without ADP as outlined in the Introduction.33–37 Here, we propose a simplistic framework of resistance and resilience that provides a conceptual distinction between these 2 aspects. We refer to resistance in the context of avoiding pathology, i.e., remaining cognitively normal with low ADP. We refer to resilience in the context of coping with pathology, i.e., remaining cognitively normal despite substantial ADP. This distinction of nomenclature will help advance our understanding of the genetic, behavioral, and brain mechanisms underlying the maintenance of normal cognition in the context of preclinical AD.
Definitions and common scenarios
Resilience denotes the ability to cope in the face of adversity. Resilience to AD thus may represent an individual's ability to sustain a better-than-expected cognitive performance in relation to the degree of ADP (see, for example, reference 16). The mechanisms underlying resilience may explain higher than expected cognitive performance. Note that we are considering resilience in the context of AD pathway and therefore require the elevation of Aβ or A+.
Resistance denotes “the act of resisting, opposing, or withstanding.” Resistance to AD will thus imply avoiding the appearance of ADP. In preclinical AD studies, resistance could be translated as individuals with absence or lower than expected levels of ADP. Therefore, the mechanisms underlying resistance may explain lower than expected ADP levels.
It is important to note that both terms are used in the context of AD including when we are discussing populations at risk. While this is implicit in the definition of resilience (the presence of ADP would be required), this should be more carefully considered in the definition of resistance to ADP. For example, being Aβ-negative alone does not imply resistance to ADP; however, being APOE4-positive with lower than expected Aβ levels implies resistance. Similarly, being older than 85 years with low/no Aβ or tau implies resistance. In other words, studies assessing resistance to AD should include either individuals at risk or methods that can indicate lower than expected ADP levels. Thus, the study of resistance would rely on known effects of risk factors on pathologic processes (e.g., APOE4 and age). While the present article focuses on preclinical AD, the concepts of resistance and resilience may be extended from aging to dementia, which will help characterize the underlying mechanisms.
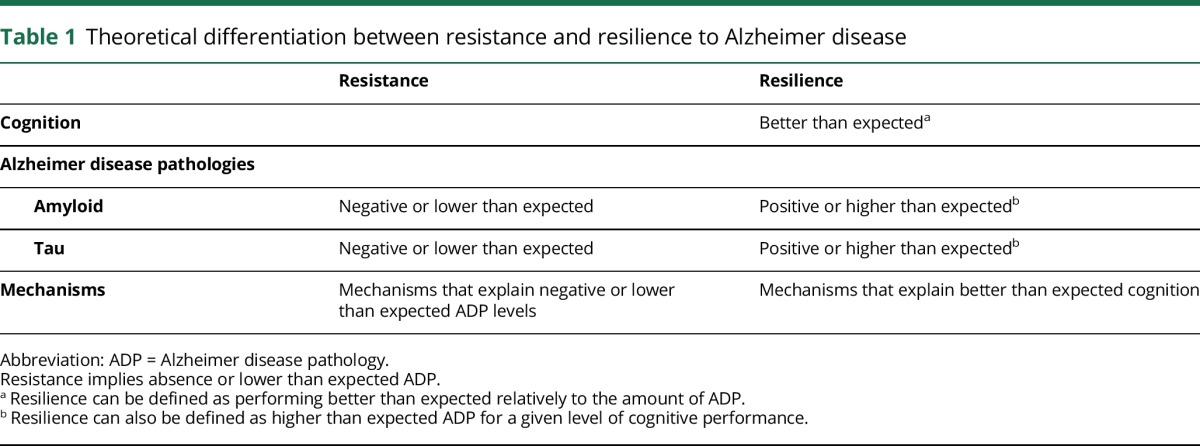
In table 1, we present the broad definitions of resistance and resilience. These definitions clarify and differentiate the 2 concepts, make them testable using AD biomarkers, and facilitate their use in both cross-sectional and longitudinal studies. Note that, especially in the case of resilience, the terminology used to describe the data and study should not be confounded with the method used to approach the concept. The choice of methodology would be made to optimally test the researchers’ proposed hypothesis. For example, a longitudinal approach rather than a cross-sectional approach might help distinguish an individual who is early in disease process from an individual who is truly coping with pathology (i.e., resilient). However, in most cases, the results may drive how the data would be presented and described. In such cases, the 2 frameworks would help convey if individuals were truly coping with pathology, i.e., resilience, or if there were differences in the ADP progression between individuals, i.e., resistance. Some approaches are discussed in the Study design and methodologies section.
Table 1.
Theoretical differentiation between resistance and resilience to Alzheimer disease
Given the existing terminology to explain mechanisms, one may argue against the need for new terminology. However, the varied terminology as discussed below and also classification of the existing terminologies into one of the 2 bins of resistance and resilience make the use of these terms appealing. These broader terms are particularly helpful while studying each specific process along the preclinical AD trajectories and relevant for AD prevention.
To disambiguate the terms of resistance and resilience, extensively used in other fields such as psychology, it will be useful to refer to resistance to AD and resilience to AD in preclinical AD studies. These terms can be further refined depending on primary outcomes of interest; for example, resistance or resilience to Aβ when studying Aβ markers or cognitive resilience when cognition is the primary outcome (tables 2 and 3). The term protective can be used when discussing factors and mechanisms contributing to resilience and resistance; for example, protective genes when discussing genes that confer protection against Aβ deposition. In the context of mechanisms, we propose the terms brain resistance and brain resilience to AD, which are discussed below. In table 2, we present common scenarios seen typically in studies to make a clear distinction between the uses of the 2 terminologies.
Table 2.
Common scenarios and recommendations to use the terms resistance and resilience
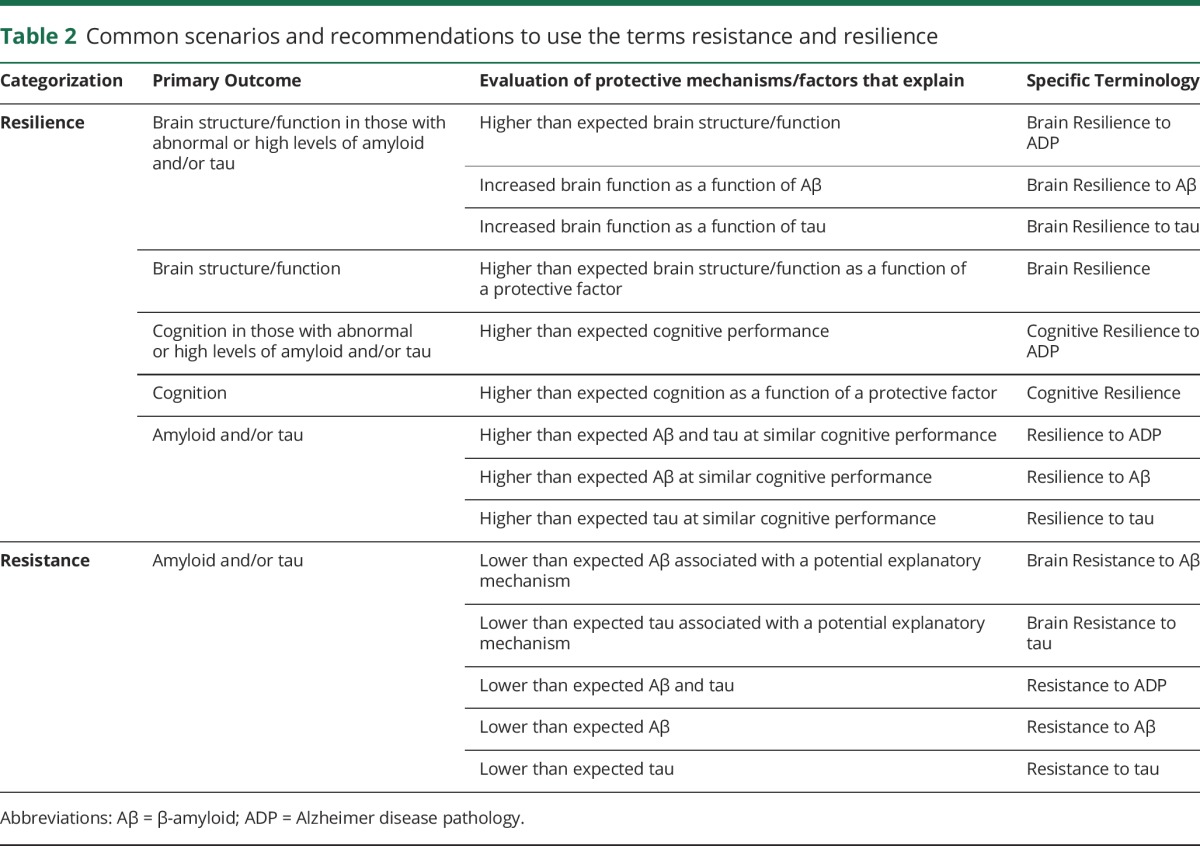
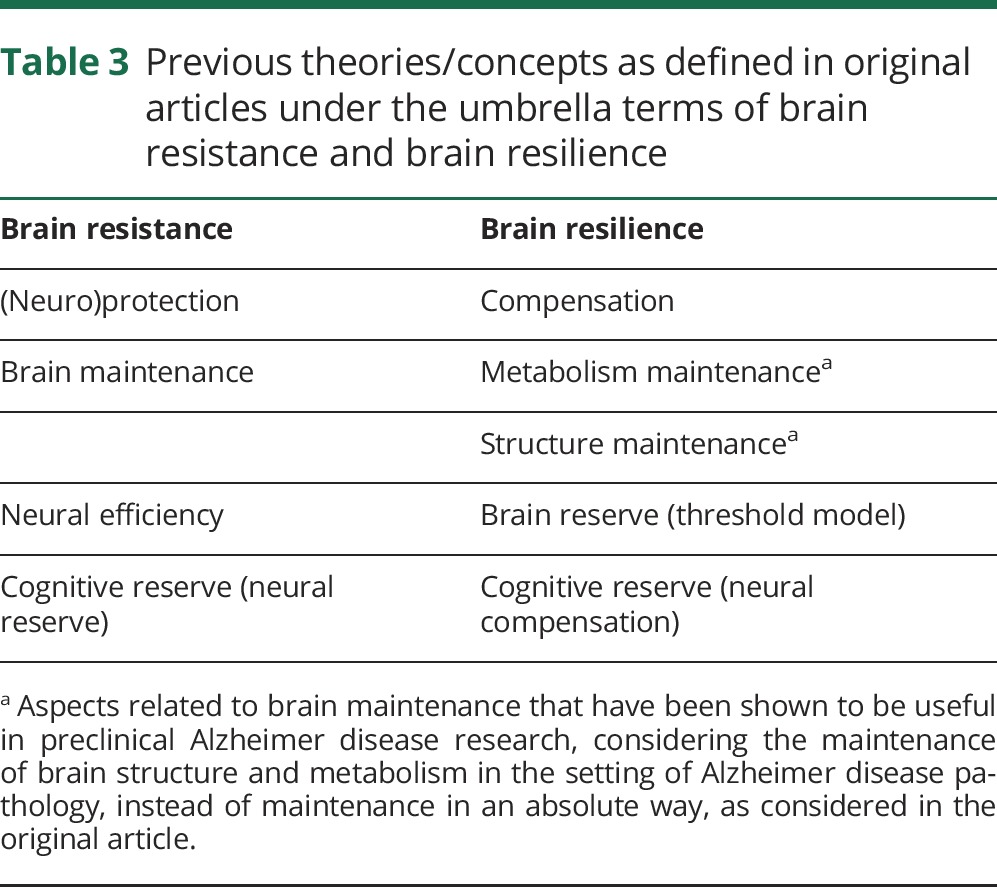
Table 3.
Previous theories/concepts as defined in original articles under the umbrella terms of brain resistance and brain resilience
Mechanisms and factors associated with the development vs the clinical expression of ADP
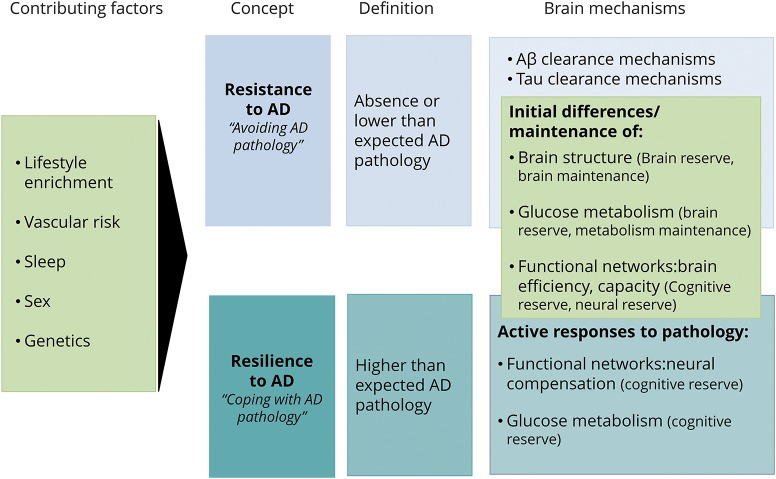
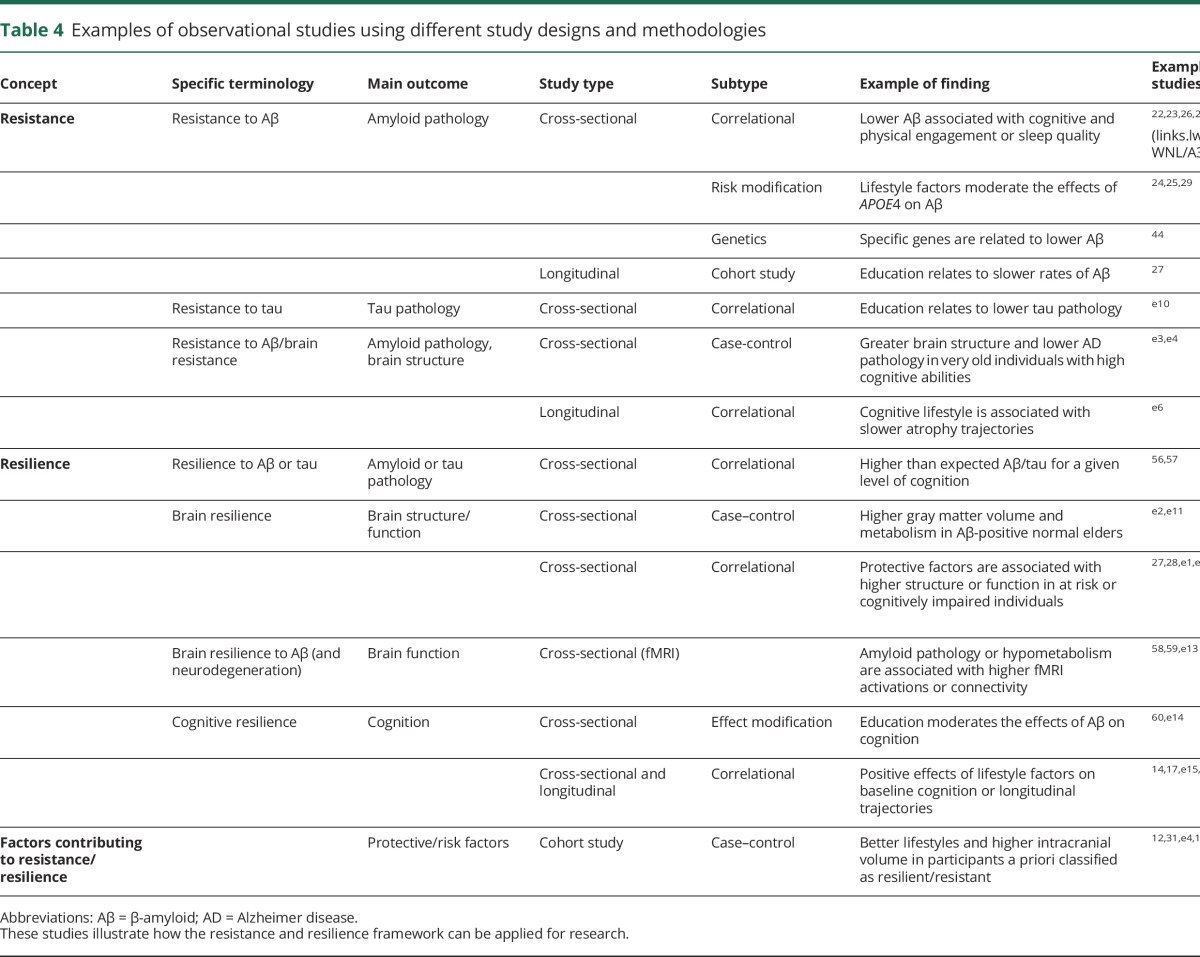
The figure summarizes the definitions of resilience and resistance and the contributing factors. It also illustrates previous theories within the framework of resistance and resilience, highlighting common and specific mechanisms. The present framework attempts to guide research into 2 sets of factors/mechanisms: (1) those associated with ADP processes and (2) those associated with the clinical expression of ADP. The first set of factors/mechanisms are associated with resistance to AD, should explain lower than expected ADP, and include amyloid and tau clearance mechanisms as well as structural and functional brain characteristics that may result in diminished ADP. The second set of factors/mechanisms underlie resilience to AD, should explain better than expected cognition in the face of ADP, and include structural and functional brain characteristics that may enable coping with ADP either through response mechanisms or through inherent brain efficiency/characteristics. Novel evidence supports this framework, suggesting that these 2 concepts may be underlain by distinct phenotypic traits. For example, resilience might be linked to the preservation of neuronal, synaptic elements and spine plasticity38,39 and resistance might be linked to enhanced Aβ clearance.40 Previous studies suggest that resistance and resilience may be promoted by genetic and lifestyle variables, including sex, APOE, vascular risk, current and lifelong cognitive and physical engagement, and sleep.17,37,38,41–46 For example, sleep acts possibly as a resistance mechanism (for a review, see reference 46) through amyloid clearance vs intellectual enrichment, which may act primarily as a resilience mechanism (see for example reference 47) with possible associations with lower ADP (for a review, see reference 48). Examples from the literature considering other factors and potential mechanisms are provided in table 4. There needs to be further research in clarifying the extent to which several important factors promote resistance vs resilience.
Figure. Relation between the concepts of resistance and resilience, brain mechanisms, and contributing factors.
Lifestyle factors, vascular risk, sleep, sex, and genetics are contributors to resistance and resilience. Mechanisms specific to resistance include those related to (Aβ) and tau clearance. Common mechanisms comprise initial differences in brain structure (brain reserve) and function (neural reserve–cognitive reserve) and brain maintenance processes, including the preservation of brain structure, glucose metabolism, and functional networks. Specific brain mechanisms of resilience include those that appear as an active response to pathology including neural compensation and compensatory glucose metabolism increases. Light blue indicates the concept, definition, and brain mechanisms associated with resistance to Alzheimer disease (AD). Dark blue indicates the concept, definition, and brain mechanisms associated with resilience to AD. Green indicates factors and mechanisms associated with both concepts. Aβ = β-amyloid.
Table 4.
Examples of observational studies using different study designs and methodologies
Brain resistance and brain resilience to explain brain mechanisms
The concepts of resilience and resistance can also be used to explain brain mechanisms. Brain resistance and brain resilience to AD refer to the brain processes underlying the ability to better resist or cope with pathology. From a theoretical perspective, existing concepts may fall in 1 of 2 categories (resistance or resilience). For instance, while the reserve concept stresses the way of coping with pathology (resilience), the brain maintenance concept focuses on the relative lack or postponement of brain changes as the key to preserving cognition in elderly (resistance) (adapted from Nyberg et al.20).
Existing concepts/theories that explain brain resistance
Brain resistance refers to the brain processes underlying the ability to better resist pathology. There have been a few existing concepts that focus on how some older adults have low or no pathology or age-related changes. The notion of neuroprotection refers to the maintenance of neuronal integrity against internal or external insults.49 In line with this idea, Nyberg et al.20 proposed the theory of brain maintenance, which considers the idea of preservation of brain structure (neuroprotection), preservation of task-related networks, along with the absence of significant pathologies as the best predictors of successful cognition. However, it has been shown that the use of maintenance to make reference to the preservation of some aspects of brain structure and function (instead of in an absolute way) might be useful in the context of resilience to AD (for example, metabolism maintenance).50 The concept of neural reserve19 also emphasizes strategies used when coping with task demands that can be identified in the absence of pathologic changes.
Existing concepts/theories that explain brain resilience
Brain resilience refers to the brain processes through which positive outcomes are achieved in the context of pathologic changes (adapted from Masten51) and it may include passive or active processes. For example, the theory of cognitive reserve is mechanistically explained by the ability to cope with pathology. The notion of compensation is used to refer to strategies used to compensate for cognitive decline and thus counteract the changes that occur during aging52,53 or pathology.19 The theory of cognitive reserve includes the notion of neural compensation to refer to an active response implying the use of new or alternate brain networks after pathology has affected those networks typically utilized.19 Passive processes, such as starting with a greater brain structure, may also play an important role in brain resilience. For example, the threshold models of brain reserve54 posit that there is a specific cutoff that sets the amount of brain damage that can be sustained before reaching a threshold for clinical symptoms, e.g., individuals with greater brain volumes may tolerate higher levels of Aβ deposition.
Potential common mechanisms
Common mechanisms include preexisting or better preserved/maintained brain characteristics that may be associated with diminished ADP or enhanced capacity to cope with ADP. For example, preexisting functional differences may result in greater lifelong neural efficiency, which may be associated with lower Aβ.55 On the other hand, these preexisting differences in neural efficiency may also help tolerate greater ADP. See figure 1 for further details.
Study design and methodologies
We provide examples of study designs that could use the present framework along with some specific examples and new approaches from the literature with specific focus on biomarker studies. (detailed information in table 4).
Study designs
Several approaches described here can be used to investigate protective factors/mechanisms that contribute to maintenance of normal cognition in the context of resistance vs resilience. However, both the recruitment mechanism and study design need to be carefully considered while interpreting the generalizability of the results. We present studies that include 3 sets of variables. (1) Protective factors or measures contributing to resilience and resistance: (1a) behavioral/lifestyle; (1b) genetic; (1c) cerebral (brain structure and function). While protective and conversely risk factors are numerous, here we focus on the 3 common categories of factors. We have included cerebral measurements as they can possibly reflect protective mechanisms. (2) Biomarkers or surrogates of ADP: CSF, imaging, or plasma biomarkers. These are key variables to define resistance and resilience to AD and may not be needed for studying cognitive resilience in individuals at risk. (3) Cognitive measurements are necessary to determine if the study participants are performing better than expected for studying resilience. Most study designs also use cognition to ensure that study participants are performing within a given range (in the context of resistance).
Cross-sectional study designs
Cross-sectional study designs can be used to assess the associations between hypothesized factors contributing to resistance or resilience (1), ADP (2), or cognition (3). A limitation of these studies is that they do not address causal relationships or change over time. A cross-sectional study design addressing the resistance hypothesis could test the relationship between early intellectual enrichment (1a) or APOE2 carriage (1b) and Aβ deposition (2). Findings in line with the resistance hypothesis may include associations between greater intellectual enrichment/APOE2 carriage and lower Aβ deposition.22,44 A cross-sectional study design showing participants with higher than expected ADP for a given level of cognition provides evidence consistent with the resilience hypothesis.56,57 Evidence of brain resilience, and notably of neural compensation, may come from studies evaluating changes in brain function as a function of ADP/neurodegeneration; for example, increased brain activations or brain connectivity at rest or during a cognitive task.58,59 Cross-sectional studies may also test the modification effect of factors contributing to resilience/resistance on the association of ADP with cognition. For example, the effects of Aβ deposition on cognitive performance might be minimized with higher education,60 and the effects of APOE4 on Aβ might be modified by intellectual enrichment variables or physical activity (for example, reference 29). Finally, cross-sectional studies that do not include AD biomarkers information may assess the relationship between lifestyle factors (1a) and brain structure or function (1c), thus investigating brain resilience. In the absence of AD biomarkers, the study framework and the interpretation of the results will determine the evidence of resistance or resilience (for example,e1 links.lww.com/WNL/A347).
Case-control study designs
Case-control study designs are convenient for identifying factors contributing to resistance or resilience by studying individuals who do not show an expected negative outcome in the setting of a given exposure, as compared to a control group. For example, while normal cognition (3) in the setting of Aβ deposition (2) implies resilience, absence of ADP/neurodegeneration (2) at very old age implies resistance. Usually, individuals are retrospectively assigned to a group (for example, reference 12). Several studies have mimicked such a study design by assigning groups cross-sectionally. Findings in line with the resilience hypothesis may include larger volumes in A+ as compared to A− normal elderlye2 (links.lww.com/WNL/A347). Results providing insights into the determinants of resistance include evidence of fewer risk factors and chronic conditions in very old adults without ADP31,e3,e4 or greater brain structure and lower ADP in very old individuals with unusually high cognitive performances, namely super agers.e3,e4 Limitations of these types of studies concern notably the sampling: due to their retrospective nature, they are especially prone to selection bias. The lack of representativeness of the sample also affects the generalizability of the findings. Moreover, temporal sequence between the outcome and exposure would be difficult to establish and thus they do not address causality.
Longitudinal study designs
Finally, longitudinal cohort studies might be used to evaluate the relationship between the hypothesized determinants of resistance and resilience and cognitive and biomarker changes longitudinally. This design would be particularly useful to investigate a sequence of events and provide relevant information about possible causation; for example, evaluation of a slower rate of cognitive decline with higher intellectual enrichmente5 (links.lww.com/WNL/A347). Findings from longitudinal studies in line with resistance hypothesis would show slower rates of atrophy or Aβ deposition with higher intellectual enrichment.27,e6 Evidence in line with cognitive resilience would come from studies showing effects of intellectual enrichment on cognitive trajectories but not on biomarker trajectories.e5 Possible selection bias can be seen with retrospective cohort studies in longitudinal study designs. In addition, longitudinal study designs are subject to bias due to differential loss to follow up (drop-out cases or loss to mortality).
Additional approaches specific to resilience
Several studies have reported factors that may contribute to resistance by studying individuals with exceptional cognitive capacities or lower than expected ADP, i.e., super agers or exceptional agers. One of the important challenges in the field of resilience, however, is to identify individuals who do not progress to AD, notably when long follow-up data are not available. Here we present recent approaches that have been developed to capture the notion of resilience including residual and risk approaches.
Residual approaches
Residual approaches study measurements reflect the discordance between ADP or neurodegeneration and cognition or between several AD processes (e.g., ADP and neurodegeneration). Such an approach reflects the discordance between predicted and observed measurements. These kinds of approaches were initially proposed within the framework of cognitive reservee7 (links.lww.com/WNL/A347) and have been used in several investigations.15,17,e8 A strength of this approach includes the quantification of resilience as a continuous variable allowing its use at different disease stages. It has recently shown great utility for clinical research, as it may predict cognitive decline.17 A shortcoming of this approach, however, is that it is reductionist. Resilience is defined by the error that is not explained in the model, and thus it depends on the large number of inputs included in the model (which may be incomplete or poor surrogates), which may lead to noisy residual measurements. To date, these approaches have been less informative about the underlying brain mechanisms, as often brain measurements (structure or function) are included as inputs in the model, which does not allow for studying brain measurements that contribute to resilience.
Risk approaches
Risk approaches rely on the assumption that known risk factors for AD (e.g., APOE4 or age) are related to negative outcomes (such as cognitive decline or ADP) (for example, references 12 and 31). These approaches allow for better characterization of brain mechanisms as individuals can be identified based solely on risk factors. The limitations are that the relationships between risk factors and outcomes are typically complex (especially with increasing age) and long follow-up times may be needed to reliably identify nondeclining individuals.
Discussion
Several concepts have been proposed to date aiming at explaining the disconnect between ADP levels and cognition. There is now emerging evidence that lifestyle and behavioral modifications can slow or halt ADP progression, which is theoretically distinct from the concept of pathology and cognition disconnect. The present article represents an effort to integrate previous concepts in a common framework and nomenclature that can aid researchers to investigate these 2 distinct aspects under the notions of resilience and resistance to AD. This framework will facilitate preclinical AD studies and aid in distinguishing between the behavioral/lifestyle, genetic, and brain determinants of resistance vs resilience. It complements previous approaches, where factors such as lifestyle variables are used as convenient proxies to study the concepts (e.g., cognitive reserve).
The application of the proposed framework for proposing hypotheses, study design, and interpretation of results in preclinical AD studies can provide a common research ground for understanding the mechanisms underlying the maintenance of normal cognition. This will ultimately help advance the field from observational research towards developing effective intervention and prevention strategies.
Acknowledgment
The authors thank Prof. W. Jagust, Dr. S. Landau, Dr. G. Chételat, Dr. A. Bejanin, Dr. D. Vidal-Piñeiro, and Prof. D. Bartrés-Faz for their scientific input.
Glossary
- Aβ
β-amyloid
- AD
Alzheimer disease
- ADP
Alzheimer disease pathology
Author contributions
E.M. Arenaza-Urquijo: study concept, interpretation of data, drafting the manuscript. P. Vemuri: study concept, interpretation of data, drafting the manuscript, revising the manuscript for content.
Study funding
E.M.A.-U. was supported by La Fondation Thèrése et René Planiol and the EU's Horizon 2020 Research and Innovation Programme (Grant agreement 667696). P.V. was supported by NIH grants (R01 NS097495, R01 AG056366, and P50 AG16574/P1).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement J 2011;7:280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dubois B, Feldman HH, Jacova C, et al. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol 2014;13:614–629. [DOI] [PubMed] [Google Scholar]
- 3.Jack CR, Bennett DA, Blennow K, et al. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 2016;87:539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sperling RA, Rentz DM, Johnson KA, et al. The A4 study: stopping AD before symptoms begin? Sci Transl Med 2014;6:228fs13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bateman RJ, Benzinger TL, Berry S, et al. The DIAN-TU next generation Alzheimer's prevention trial: adaptive design and disease progression model. Alzheimers Dement 2017;13:8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katzman R, Terry R, DeTeresa R, et al. Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol 1988;23:138–144. [DOI] [PubMed] [Google Scholar]
- 7.Crystal H, Dickson D, Fuld P, et al. Clinico-pathologic studies in dementia: nondemented subjects with pathologically confirmed Alzheimer's disease. Neurology 1988;38:1682–1687. [DOI] [PubMed] [Google Scholar]
- 8.Morris JC, Storandt M, McKeel DW, et al. Cerebral amyloid deposition and diffuse plaques in “normal” aging: evidence for presymptomatic and very mild Alzheimer's disease. Neurology 1996;46:707–719. [DOI] [PubMed] [Google Scholar]
- 9.Neuropathology Group, Medical Research Council Cognitive Function and Aging Study (MRC CFAS). Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Lancet 2001;357:169–175. [DOI] [PubMed] [Google Scholar]
- 10.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc 2002;8:448–460. [PubMed] [Google Scholar]
- 11.Valenzuela MJ, Sachdev P. Brain reserve and dementia: a systematic review. Psychol Med 2006;36:441–454. [DOI] [PubMed] [Google Scholar]
- 12.Kaup AR, Nettiksimmons J, Harris TB, et al. Cognitive resilience to apolipoprotein E ε4: contributing factors in black and white older adults. JAMA Neurol 2015;72:340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amieva H, Mokri H, Le Goff M, et al. Compensatory mechanisms in higher-educated subjects with Alzheimer's disease: a study of 20 years of cognitive decline. Brain J Neurol 2014;137:1167–1175. [DOI] [PubMed] [Google Scholar]
- 14.Vemuri P, Lesnick TG, Przybelski SA, et al. Association of lifetime intellectual enrichment with cognitive decline in the older population. JAMA Neurol 2014;71:1017–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Negash S, Xie S, Davatzikos C, et al. Cognitive and functional resilience despite molecular evidence of Alzheimer's disease pathology. Alzheimers Dement 2013;9:e89–e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Negash S, Wilson RS, Leurgans SE, et al. Resilient brain aging: characterization of discordance between Alzheimer's disease pathology and cognition. Curr Alzheimer Res 2013;10:844–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hohman TJ, McLaren DG, Mormino EC, et al. Asymptomatic Alzheimer disease: defining resilience. Neurology 2016;87:2443–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson RS, Boyle PA, Yu L, Barnes LL, Schneider JA, Bennett DA. Life-span cognitive activity, neuropathologic burden, and cognitive aging. Neurology 2013;81:314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stern Y. Cognitive reserve. Neuropsychologia 2009;47:2015–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nyberg L, Lövdén M, Riklund K, Lindenberger U, Bäckman L. Memory aging and brain maintenance. Trends Cogn Sci 2012;16:292–305. [DOI] [PubMed] [Google Scholar]
- 21.Liang KY, Mintun MA, Fagan AM, et al. Exercise and Alzheimer's disease biomarkers in cognitively normal older adults. Ann Neurol 2010;68:311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landau SM, Marks SM, Mormino EC, et al. Association of lifetime cognitive engagement and low β-amyloid deposition. Arch Neurol. Epub 2012 Jan 23. [DOI] [PMC free article] [PubMed]
- 23.Wirth M, Haase CM, Villeneuve S, Vogel J, Jagust WJ. Neuroprotective pathways: lifestyle activity, brain pathology, and cognition in cognitively normal older adults. Neurobiol Aging 2014;35:1873–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Head D, Bugg JM, Goate AM, et al. Exercise engagement as a moderator of the effects of APOE genotype on amyloid deposition. Arch Neurol 2012;69:636–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown BM, Peiffer JJ, Taddei K, et al. Physical activity and amyloid-β plasma and brain levels: results from the Australian imaging, biomarkers and lifestyle study of ageing. Mol Psychiatry 2013;18:875–881. [DOI] [PubMed] [Google Scholar]
- 26.Okonkwo OC, Schultz SA, Oh JM, et al. Physical activity attenuates age-related biomarker alterations in preclinical AD. Neurology 2014;83:1753–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lo RY, Jagust WJ. Alzheimer's Disease Neuroimaging Initiative. Effect of cognitive reserve markers on Alzheimer pathologic progression. Alzheimer Dis Assoc Disord 2013;27:343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arenaza-Urquijo EM, Bejanin A, Gonneaud J, et al. Association between educational attainment and amyloid deposition across the spectrum from normal cognition to dementia: neuroimaging evidence for protection and compensation. Neurobiol Aging. Epub 2017 Jun 24. [DOI] [PubMed]
- 29.Wirth M, Villeneuve S, La Joie R, Marks SM, Jagust WJ. Gene-environment interactions: lifetime cognitive activity, APOE genotype, and beta-amyloid burden. J Neurosci 2014;34:8612–8617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schreiber S, Vogel J, Schwimmer HD, Marks SM, Schreiber F, Jagust W. Impact of lifestyle dimensions on brain pathology and cognition. Neurobiol Aging 2016;40:164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vemuri P, Knopman DS, Lesnick TG, et al. Evaluation of amyloid protective factors and Alzheimer disease neurodegeneration protective factors in elderly individuals. JAMA Neurol 2017;74:718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mortimer JA, Snowdon DA, Markesbery WR. Head circumference, education and risk of dementia: findings from the Nun Study. J Clin Exp Neuropsychol 2003;25:671–679. [DOI] [PubMed] [Google Scholar]
- 33.Jansen WJ, Ossenkoppele R, Knol DL, et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA 2015;313:1924–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer's disease. Ann Neurol 1999;45:358–368. [DOI] [PubMed] [Google Scholar]
- 35.Price JL, McKeel DW, Buckles VD, et al. Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging 2009;30:1026–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jack CR, Wiste HJ, Weigand SD, et al. Age-specific and sex-specific prevalence of cerebral β-amyloidosis, tauopathy, and neurodegeneration in cognitively unimpaired individuals aged 50–95 years: a cross-sectional study. Lancet Neurol 2017;16:435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Latimer CS, Keene CD, Flanagan ME, et al. Resistance to Alzheimer disease neuropathologic changes and apparent cognitive resilience in the Nun and Honolulu-Asia Aging Studies. J Neuropathol Exp Neurol 2017;76:458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perez-Nievas BG, Stein TD, Tai H-C, et al. Dissecting phenotypic traits linked to human resilience to Alzheimer's pathology. Brain J Neurol 2013;136:2510–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boros BD, Greathouse KM, Gentry EG, et al. Dendritic spines provide cognitive resilience against Alzheimer's disease. Ann Neurol 2017;82:602–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science 2013;342:373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sundermann EE, Maki PM, Rubin LH, et al. Female advantage in verbal memory: evidence of sex-specific cognitive reserve. Neurology 2016;87:1916–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valenzuela MJ, Matthews FE, Brayne C, et al. Multiple biological pathways link cognitive lifestyle to protection from dementia. Biol Psychiatry 2012;71:783–791. [DOI] [PubMed] [Google Scholar]
- 43.Corder EH, Saunders AM, Risch NJ, et al. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet 1994;7:180–184. [DOI] [PubMed] [Google Scholar]
- 44.Grothe MJ, Villeneuve S, Dyrba M, Bartrés-Faz D, Wirth M. Alzheimer's Disease Neuroimaging Initiative. Multimodal characterization of older APOE2 carriers reveals selective reduction of amyloid load. Neurology 2017;88:569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferrari C, Xu WL, Wang HX, et al. How can elderly apolipoprotein E ε4 carriers remain free from dementia? Neurobiol Aging 2013;34:13–21. [DOI] [PubMed] [Google Scholar]
- 46.Mander BA, Winer JR, Jagust WJ, Walker MP. Sleep: a novel mechanistic pathway, biomarker, and treatment target in the pathology of Alzheimer's disease? Trends Neurosci 2016;39:552–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vemuri P, Weigand SD, Przybelski SA, et al. Cognitive reserve and Alzheimer's disease biomarkers are independent determinants of cognition. Brain J Neurol 2011;134:1479–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arenaza-Urquijo EM, Wirth M, Chételat G. Cognitive reserve and lifestyle: moving towards preclinical Alzheimer's disease. Front Aging Neurosci 2015;7:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davis M, Mendelow AD, Perry RH, Chambers IR, James OF. Experimental stroke and neuroprotection in the aging rat brain. Stroke 1995;26:1072–1078. [DOI] [PubMed] [Google Scholar]
- 50.Förster S, Yousefi BH, Wester H-J, et al. Quantitative longitudinal interrelationships between brain metabolism and amyloid deposition during a 2-year follow-up in patients with early Alzheimer's disease. Eur J Nucl Med Mol Imaging 2012;39:1927–1936. [DOI] [PubMed] [Google Scholar]
- 51.Masten AS. Ordinary magic: resilience processes in development. Am Psychol 2001;56:227–238. [DOI] [PubMed] [Google Scholar]
- 52.Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging 2002;17:85–100. [DOI] [PubMed] [Google Scholar]
- 53.Grady CL, McIntosh AR, Beig S, Keightley ML, Burian H, Black SE. Evidence from functional neuroimaging of a compensatory prefrontal network in Alzheimer's disease. J Neurosci 2003;23:986–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Satz P. Brain reserve capacity on symptom onset after brain injury: A formulation and review of evidence for threshold theory. Neuropsychology 1993;7:273–295. [Google Scholar]
- 55.Jagust WJ, Mormino EC. Lifespan brain activity, β-amyloid, and Alzheimer's disease. Trends Cogn Sci 2011;15:520–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kemppainen NM, Aalto S, Karrasch M, et al. Cognitive reserve hypothesis: pittsburgh compound B and fluorodeoxyglucose positron emission tomography in relation to education in mild Alzheimer's disease. Ann Neurol 2008;63:112–118. [DOI] [PubMed] [Google Scholar]
- 57.Hoenig MC, Bischof GN, Hammes J, et al. Tau pathology and cognitive reserve in Alzheimer's disease. Neurobiol Aging 2017;57:1–7. [DOI] [PubMed] [Google Scholar]
- 58.Elman JA, Oh H, Madison CM, et al. Neural compensation in older people with brain amyloid-β deposition. Nat Neurosci 2014;17:1316–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Franzmeier N, Duering M, Weiner M, Dichgans M, Ewers M. Alzheimer's Disease Neuroimaging Initiative (ADNI). Left frontal cortex connectivity underlies cognitive reserve in prodromal Alzheimer disease. Neurology 2017;88:1054–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roe CM, Mintun MA, D'Angelo G, Xiong C, Grant EA, Morris JC. Alzheimer disease and cognitive reserve: variation of education effect with carbon 11-labeled Pittsburgh compound B uptake. Arch Neurol 2008;65:1467–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]