Abstract
Background
This is the first report to characterize the prevalence and genovar distribution of genital chlamydial infections among random heterosexual patients in the multi-ethnic Saratov Region, located in Southeast Russia.
Methods
Sixty-one clinical samples (cervical or urethral swabs) collected from a random cohort of 856 patients (7.1%) were C. trachomatis (CT) positive in commercial nucleic acid amplification tests (NAATs) and duplex TaqMan PCRs.
Results
Sequence analysis of the VDII region of the ompA gene revealed seven genovars of C. trachomatis in PCR-positive patients. The overall genovars were distributed as E (41.9%), G (21.6%), F (13.5%), K (9.5%), D (6.8%), J (4.1%), and H (2.7%). CT-positive samples were from males (n = 12, 19.7%), females (n = 42, 68.8%), and anonymous (n = 7, 11.5%) patients, with an age range of 19 to 45 years (average 26.4), including 12 different ethnic groups representative of this region. Most patients were infected with a single genovar (82%), while 18% were co-infected with either two or three genovars. The 1156 bp-fragment of the ompA gene was sequenced in 46 samples to determine single nucleotide polymorphisms (SNP) among isolates. SNP-based subtyping and phylogenetic reconstruction revealed the presence of 13 variants of the ompA gene, such as E (E1, E2, E6), G (G1, G2, G3, G5), F1, K, D (D1, Da2), J1, and H2. Differing genovar distribution was identified among urban (E>G>F) and rural (E>K) populations, and in Slavic (E>G>D) and non-Slavic (E>G>K) ethnic groups. Multilocus sequence typing (MLST) determined five sequences types (STs), such as ST4 (56%, 95% confidence interval, CI, 70.0 to 41.3), ST6 (10%, 95% CI 21.8 to 3.3), ST9 (22%, 95% CI 35.9 to 11.5), ST10 (2%, 95% CI 10.7 to 0.05) and ST38 (10%, 95% CI 21.8 to 3.3). Thus, the most common STs were ST4 and ST9.
Conclusion
C. trachomatis is a significant cause of morbidity among random heterosexual patients with genital chlamydial infections in the Saratov Region. Further studies should extend this investigation by describing trends in a larger population, both inside and outside of the Saratov Region to clarify some aspects for the actual application of C. trachomatis genotype analysis for disease control.
Introduction
Chlamydia trachomatis (CT) is one of the most commonly occurring sexually transmitted infections (STIs) in both young men and females with an annual estimate of 105.7 million new cases worldwide [1]. Because of its high epidemic potential, undiagnosed and untreated Chlamydial infection can result in a number of complications. The World Health Organization (WHO) estimates the cost-burden for treating chlamydia patients, especially among adolescents, is approximately $10 billion annually [2]. During the past two decades, several countries worldwide have focused studies on genotyping local CT cases to enhance the understanding of clonal diversification, genovar prevalence and evolution, level of transmission, and to address the role of co-infection with two or more CT variants. This knowledge could significantly improve the ability of national and trans-national surveillance programs to build global STI surveillance systems [1].
There is little information on CT genovar distribution in the Russian Federation besides the Moscow and St. Petersburg Regions [3,4,5]. National diagnostic laboratories have developed the capability to molecularly detect CT DNA in clinical specimens of chlamydial patients by highly sensitive nucleic acid amplification tests (NAATs) [6]. However, these detection systems do not provide genetic tools to discriminate CT genovars, even for the ompA gene polymorphism that has been generally accepted as a single-locus typing standard [7]. For further discrimination of C. trachomatis strains between and within genotypes, we additionally applied the multilocus sequence typing (MLST). This procedure is based on the analysis of polymorphism of housekeeping genes, and is widely used for genotyping many microorganisms, including the evaluation of Chlamydia diversity [8–11]. Although the determination of Chlamydia sequences types (STs) and genovars may not be essential for the clinical outcome and treatment schedule, it can certainly shed light on the global epidemiology of this pathogen. Nevertheless, a recent single nucleotide polymorphism (SNP) based study of Smelov et al. [5] revealed the association between the phenotypic diseases (lymphogranuloms venerium, urethritis and cervicitis, and ocular trachoma) and branches in phylogenetic tree. Another important question was whether, as others have previously reported [12–17] there are differences in the genovar’s distribution among different ethnicity groups that may reflect variations in the selection of certain genovars in different parts of the world. The goal of this study was to investigate the prevalence of C. trachomatis infection among patients in multi-ethnic European Region located in Southeast Russia. The relationships between CT genovar distributions, gender, age, nationality and place of residence of patients were examined. Our research revealed the most common CT sequences types (STs) in the Saratov Region.
Materials and methods
Setting
The Saratov Region population (~ 2.6 million people) has an approximately equal percentage of men (45.8%) and women (54.2%), with total of 22 ethnic groups (Slavic population of 87.6% of Russian descent, and non-Slavic ones, such as Kazakhs (3.1%), Tatars (2.2%), Ukrainians (1.7%), Jews (0.1%), Germans (0.3%) and other nationalities (5%). The urban population (~ 1.9 million) is located in a large Southeastern European part of Russia (S = 101200 km2), which borders Kazakhstan.
Clinical samples
Clinical samples (cervical or urethral swabs) from a random cohort of heterosexual patients (n = 856, women (n = 400) and men (n = 400) and anonymous (n = 56), from August, 2011 to January, 2012), who reported to one of seven different diagnostic laboratories of the Saratov Region for detection of chlamydia infection were screened for C. trachomatis to confirm current infection. Specimens were routinely collected and directly delivered for PCR testing using the Chlamydia Transport-Single Swab (COPAN, Italy). All the patients had symptoms of typical complaints for chlamydial infection, such as lower abdominal pain, pronounced vaginal discharge, frequent urination, post-coital bleeding, inter-menstrual bleeding, and others. Each patient provided written informed consent. This study was approved by the Human Bioethics Committee of the Saratov Scientific and Research Veterinary Institute No. IRB00008288 (http://ohrp.cit.nih.gov/search/IrbDtl.aspx).
C. trachomatis detection and typing
Total DNA was isolated from clinical specimens using the DNeasy Blood and Tissue Kit (QIAGEN GmbH, Hilden, Germany) according to the manufacturer’s instructions. DNA samples were routinely analyzed by conventional real-time PCR kits (Central Research Institute of Epidemiology, Moscow, Russia) that targets the presence of the C. trachomatis cryptic plasmid [6]. The samples were also analyzed by a duplex TaqMan PCR designed to simultaneously detect the cryptic plasmid and the 16S RNA gene of C. trachomatis (“C. trachomatis-RT-quantity", SYNTOL, Moscow, Russia). The results were verified by real-time PCR (Vector-Best, Novosibirsk, Russia) targeted to both cryptic plasmid gene and gyrA gene [6] coupled to DNA isolation with magnetic silica particles.
The genotyping of C. trachomatis in the positive samples was performed by amplification and sequencing of the Variable Domain (VDII) of the ompA gene. Three forward and four reverse primers, previously designed by Quint et al. [18], were used in amplifications with all possible individual primer combinations (12 pairs) for each sample. These generated amplicons of 157 to 160 bp that were sequenced by using the same pair of primers. The consensus sequence of the reads from direct and reverse chains and NCBI Blast (http://blast.ncbi.nlm.nih.gov/Blast.cgi) were used to determine the CT genovar. Subsequently, samples with an identified genovar were used in an amplification reaction with the forward primer F1 (CGGTATTAGTATTTGCCGCTTTG) and B11 [19] that amplified a 1156 bp fragment of nearly full ompA gene. The amplicon was sequenced with the use of flanking and internal primers for the ompA gene. The subtyping of each genovar was based on the reference strains designated in the study of Lysén et al. [12]. The sequences of the ompA gene were aligned with the reference strains to identify single nucleotide polymorphisms and their impact on translated sequences.
Primer specificity and protocol validation for basic CT detection was implemented as described [20] using specific relevant DNA from referent Chlamydial strains (CT, Chlamydia pneumoniae, Chlamydia psittaci, Chlamydia abortus etc, kindly gifted by Dr Hasanova TA) in parallel with Cobas Amplicor (conventional PCR) and Cobas TagMan48 (real-time PCRs) (Roche Diagnostics, Branchburg, NJ, USA) approved by FDA [21].
All representative genovar sequences reported in this research were deposited in GenBank (accession numbers KU963174-KU963186). The evolutionary tree was inferred in MEGA 7 using the Neighbor-Joining method with 100 bootstrap replicate samples. ModelTest in MEGA7 was used to identify the most appropriate model of evolution (the Tamura 3-parameter method [22]). All positions containing gaps were eliminated. The tree was drawn using Figtree software (http://tree.bio.ed.ac.uk/software/figtree/).
MLST based on seven housekeeping genes (gatA, oppA, hfiX, gitA, enoA, hemN and fumC) were performed as described [4,8,9]. A consensus sequence was created from forward and reverse sequence reads, genes were concatenated, and queried against MLST sequences in the PubMLST database to find identical allelic profile known as STs (https://pubmlst.org). Multiple sequence alignments of the sequence output were created by ClustalW (http://www.ebi.ac.uk/Tools/msa/clustalw2/). Phylogenetic tree was constructed using the UMPGA hierarchical clustering method in MEGA 7 [22]. Strain clustering and SNP analyses were performed as described [9] to define the relationships between strains at the microevolutionary level.
Statistical analysis
The geographical, gender, age and ethnic origin data were analyzed using Graphpad Software. Categories of data were presented in the rows of each matrix (genovars D/E/F/G/H/J/K, and STs, ST4/ST6/ST9/ST10/ST38); marginal totals, reflecting the presence or absence of specific genovar and STs, were presented in the columns of the matrix (male/female, Rural/Urban, and Slavic/non-Slavic). Proportions of individuals, who have and do not have the specific genovar and STs inside each of the category, were compared. Results of ompA genovar and STs distribution was estimated using 95% confidence interval (95% CI). The significance of the differences in distribution of ompA genovar and STs between proportions of mono-infected and multiple-infected individuals was statistically compared by the Chi-square test for categorical data or the Fisher’s exact test when the number of samples was small. A p-value<0.05 was considered significant.
Results
Baseline subject characteristics enrolled
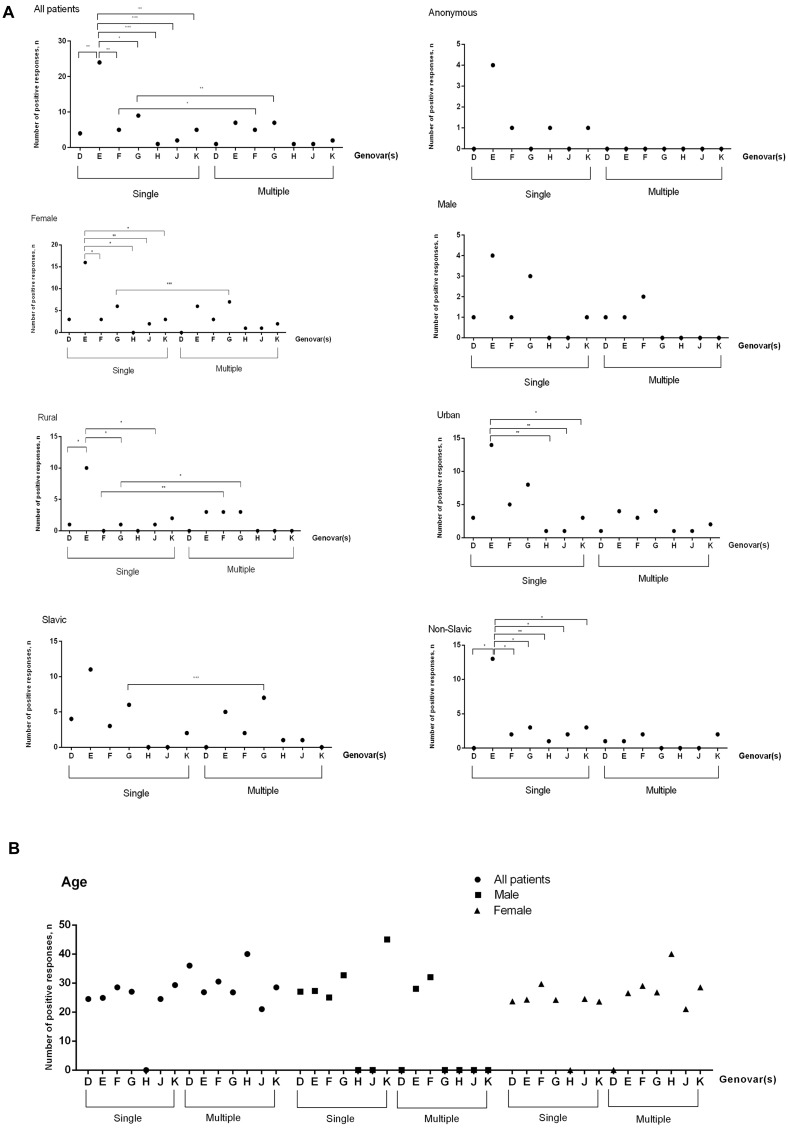
C. trachomatis DNA was detected in 61 of 856 samples tested (7.1%). The CT-positive patients’ ages ranged from 19 to 45 years (average 26.4), with a subset (11.5%) who gave no personal information (anonymous, n = 7 of 61) and 88.5% (54 of 61) who agreed to provide gender, age, place of residence and nationality data (S1 Table). The majority were women (42 of 54, 77.8%), while men contributed fewer samples (12 of 51, 22.2%). Urban citizens (42 of 61, 68.8%) were predominantly infected over the rural residents (19 of 61, 31.2%). At least 12 different ethnic groups were CT-infected (S1 Fig). In total, at least seven C. trachomatis genovars, D, E, F, G, H, J and K, were identified in the positive patients. The following serovar distribution was observed: E—41.9%, G—21.6%, F—13.5%, K—9.5%, D—6.8%, J—4.1%, and H—2.7% (74 DNA samples were genotyped). The majority of the patients (82%, 50 of 61) were infected with a single genovar, while 18% (11 of 61) demonstrated co-infection with either two or three CT genovars (Fig 1A). The distribution of CT genotypes in patients with monoinfection and among multiple-infected persons were similarly distributed, with E>G>F/K>D>J>H and E>G/F>K>D/H/J, respectively. However, in patients with mono-infection the presence of genovar E only was observed more frequently when compared to all other genovars detected, such as D, F and K (p<0.01), G (p<0.05) and genovars H and J (p<0.001). No statistically significant difference in CT genovar distribution was registered in multiple-infected patients (p>0.05). At least two genovars, F (p<0.05) and G (p<0.01) were found to be present more often among mono-infected and multiple-infected patients (Fig 1A, panel All patients).
Fig 1. Distribution of genovars in CT-positive patients.
(A) According to their gender, place of residence and ethnic origin. (B) According to their age in mono-infected and multiple-infected CT-positive samples. Analysis was done by Fisher’s exact test (two-tailed). Statistically significant differences are indicated by * (p<0.05) or by ** (p<0.01) or by *** (p<0.001). Slavic cohort included Russians, Byelorussians and Ukrainians, Non-Slavic cohort was presented by Caucasians, Jews, Kyrgyzs, Koreans, Moldavians, Germans, Mordovians, and anonymous.
Further in the group of infected patients (n = 61) we observed that genovars E and G were the most prevalent mono-infections for almost each category (Fig 1A). In the same category of mono-infected individuals, genovar E was observed more frequently in comparison with genovars F, H, K (p<0.05) and J (p<0.01) in female, genovars D, G and J (p<0.05) in rural, K (p<0.05), H & J (p<0.01) in urban, H (p<0.01) and the rest of genovars (p<0.05) in Non-Slavic population. There was no statistically significant difference (p>0.05) in distribution of genovars in such groups of mono-infected patients as male, anonymous, and Slavic individuals, as well as in all categories of multiple-infected patients. Among other genovars, genovar G was more often (p<0.05) observed in mono-infected than in multiple-infected patients, namely in female (p<0.001), rural (p<0.05) and Slavic (p<0.001) patients (Fig 1A). There was a statistically significant difference in distribution of genovar F in mono-infected and multiple-infected individuals in a single group of rural population (p<0.01). Overall, genovars D, F, J and K were identified occasionally in each group. Genovar H was rarely present and detected only in a single anonymous urban patient. The combination of genovars as D+F, E+F, E+G, E+K, F+G, F+K, E+H+G, and E+J+G was seen in the group of multiple-infected patients. The distribution of different genovars was similar in the group of male and female patients (Fig 1B).
ompA subtyping
Analysis of the 1156 bp-fragment of the ompA gene, comprising four variable (VD) and five constant (CD) domains, revealed 13 genetic variants of C. trachomatis in clinical samples of the PCR-positive patients (Table 1). Overall, seventeen substitutions in three variable (VDI, VDII and VDIV) and three constant (CDI, CDIII and CDIV) domains were identified across all genovars when compared to reference strains. Only five (29.4%) nonsynonymous substitutions were seen in the variable regions, such as: VDI—1, VDII—1 and VDIV—3. In contrast, 12 of 17 (70.6%) substitutions were located in constant domains, namely: CDI—9, CDIII—2, and CDIV—1. In fact, three of these 12 (25%) substitutions were silent (synonymous substitutions): 2—in CDI, and 1—in CDIV. The remaining nine (75%) substitutions would induce an amino acid change (non-synonymous substitution). Of the nine observed amino acid mutations, only one mutation significantly altered the amino acid’s general characteristics (Da2, K75E), which mutates a strongly positive amino acid (K) to a strongly negative amino acid (D).
Table 1. The ompA gene polymorphism found in 13 genetic variants of 61 clinical C. trachomatis specimens collected in Saratov Region, Russia, compared to reference sequencesa.
| Genotype (no. of cases/total of this subtyped genovar) | MOMP Region | Nucleotide change | Amino acid Change | Strain name (accession number of identical sequences in GenBank) |
|---|---|---|---|---|
| D1 (1/3) | Reference strain | D/B120 (X62918) | ||
| Da2 (2/3) | CDI | C132T | G44G | D/IC-Cal8 (X62920) |
| CDI | G154A | A52T | ||
| CDI | G184Ab T186G b | V62M b | ||
| CDI | C195T | Y65Y | ||
| CDI | A223G | K75E | ||
| CDI | T228A | T76T | ||
| CDI | T246C | F82F | ||
| CDI | G249C | Q83H | ||
| CDIII | A636T | S212S | ||
| E1 (18/22) | Reference strain | E/Bour (X52557) | ||
| E2 (3/22) | VDIV | G997A | A333T | E/IU-TC0755ut(ACI43896.1) |
| E6 (1/22) c | VDIV | G995A | S332N | DK-K31 (AM901187.1) |
| F1 (4/4) | Reference strain | F/IC-Cal3 (X52080) | ||
| G1 (1/6) | Reference strain | G/UW57 (AF063199) | ||
| G2 (1/6) | VDIV | T1003G | S335A | 9768 (CP001887.1) |
| G3 (3/6) | CDI VDII CDIII VDIV | T228A G487AG700C T1003A | T76T G163S E234Q S335T | I-149 (DQ116400.1) |
| G4 (0/6) | VDII | G487A | G163S | G/11222 (CP001888.1) |
| G5 (1/6) c | VDII VDIV | G487A T1003A | G163S S335T | This study |
| H1 (0/1) | Reference strain | H/UW4 (AF304857.1) | ||
| H2 (1/1) | VDI CDIV | A272G C850T | N91S L284L | ATCC VR-879 (JX564246.1) |
| J1 (3/3) | Reference strain | J/UW36 (DQ064292.1) | ||
| K (7/7) | Reference straind | K/UW31/Cx (AF063204) |
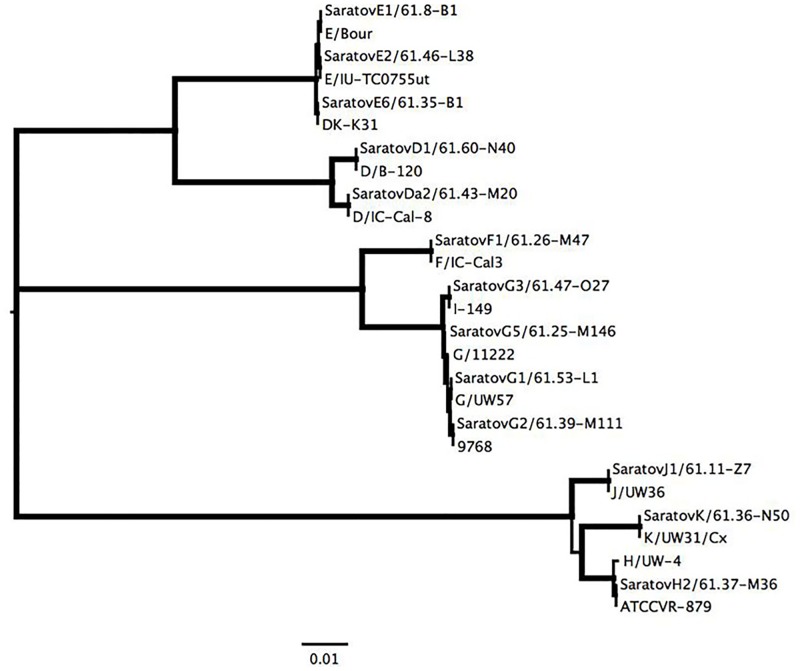
In the majority of the genovar E sequences, 18 of the 22 subtyped (81.8%) showed higher similarity to the reference strain E/Bour of subtype E1. However, we identified two additional subtypes of the E2 variant reported by Lysen et al. [12] with a single point mutation at position 997 (3 of 22 subtyped, 13.6%), and another variant with an SNP at position 995 (1 of 22, 4.6%), which was detected earlier in Europe [7,23]. This subtype was absent in the study by Lysén et al. [12] for genovar E, therefore, we designated it as E6 (Table 1). All E1, E2, and E6 sequences could be distiguished by a single SNP, and branched in a significant phylogenetic clade (Fig 2).
Fig 2. Phylogenetic tree demonstrating the relationship of Saratov ompA sequences to references strains from Table 1.
Thick branches, subtending all large clades, represent 100% bootstrap support. The scale at the bottom represents one substitution per site.
Of the 9 specimens with mono-infection with a second predominant genovar G determined by sequencing the short VDII region, we were able to amplify and sequence the large amplicon of the ompA gene from 6 samples (Table 1). Lysen et al. [12] described the subtypes G1-G4, and we identified three of these in our samples: G1 (1 of 6, 16.7%), G2 (1 of 6, 16.7%) and G3 (3 of 6, 50%). Moreover, we identified a novel subtype G5, which contained a combination of two previously described SNPS that were not present in the current version of GenBank. One SNP was located at position 487 of the VDII region and was observed in subtypes G3 and G4, and another one was positioned at 1003 of VDIV and was detected in G2 and G3 subtypes. Thus, the G5 subtype represents a combination of single SNPs of G2 and G4 variants that could be seen in the corresponding cluster of the genovar G variants on the dendrogram (Fig 2). The subtypes of the other genovars revealed the presence of well-known variants, which are also represented in the reference strains of Lysen et al. [12], such as D1, F1, J1 and K (no subtypes known), as well as more rare variants, such as Da2 and H2 (Table 1, Fig 2).
MLST analysis
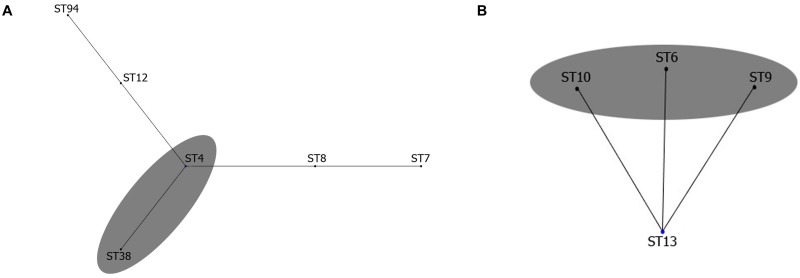
Five STs were determined by MLST (Table 2) among 13 genetic variants based on the ompA genotyping of C. trachomatis (Table 1). The majority of them (>91%), excluding ST38, were assigned to genetic lineages of two groups, such as Group I (ST6, ST9 and ST10, Fig 3A) and Group III (ST4, Fig 3B). Therefore, our STs could be allocated to two out of three non-overlapping clonal complexes identified early by Pannekoek et al. [9] from global collections (1959–2009 years) of urogenital, ocular and rectal strains of C. trachomatis [24]. The ST38 found by us here was not described in these studies [9,24]; however, this type was found later in Moscow region [4]. The ST38 belongs to Group III as CT strains of ST38 demonstrated identical alleles with six out of the seven loci found in ST4 and differed from latter ST by a single allele hflX (Table 2). In fact, both ST38 and ST4 have been found to be the members of a single clonal complex [4] of the relevant Group III in which the ST4 was a putative founder for ST38 [9]. Thus, the Saratov CT strains of ST38 together with those of ST4 were assigned to genetic lineage of Group III (Fig 3B). This rare ST38, which was discovered first in Moscow in 2005 [4], is related to CT isolates found exclusively in Europe (Sweden) in 2002 and in the UK in 2009 [24]. No ST13, the putative founder for Group I [4,9] was revealed in our study (Fig 3A). Almost all STs detected here (ST4, ST6, ST9 and ST38), except ST10, were present among the STs from the samples of the clinical cases in the Moscow region [4].
Table 2. The ompA and seven housekeeping genes MLST sequence types of Saratov and reference strains of C. trachomatis obtained from the PubMLST database (https://pubmlst.org).
| ST (MLST) | No. of CT strains | Allele | ompA genovar(s) | Reference strain(s) information | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abs. | % | gatA | oppA | hfiX | gitA | enoA | hemN | fumC | Name (accession number of identical sequences in PubMLST database | ompA genovar | Reference | ||
| 4 | 28 | 56 | 3 | 1 | 1 | 2 | 4 | 2 | 3 | E | E/Sweden2 (269) | E | 9,24 |
| 3 | 1 | 1 | 2 | 4 | 2 | 3 | SaratovE1/61.8-B1 | E | This study | ||||
| 3 | 1 | 1 | 2 | 4 | 2 | 3 | D | D/IC-Cal-8 (7) | D | 9 | |||
| 3 | 1 | 1 | 2 | 4 | 2 | 3 | SaratovDa2/61.43-M20 | D | This study | ||||
| 6 | 5 | 10 | 3 | 3 | 2 | 5 | 3 | 1 | 3 | K | K/UW-31 (14) | K | 9,24 |
| 3 | 3 | 2 | 5 | 3 | 1 | 3 | SaratovK/61.36-N50 | K | This study | ||||
| 9 | 11 | 22 | 3 | 3 | 2 | 4 | 3 | 2 | 3 | G | G/11074 (242) | G | 5,24 |
| 3 | 3 | 2 | 4 | 3 | 2 | 3 | SaratovG1/61.53-L1 | G | This study | ||||
| 3 | 3 | 2 | 4 | 3 | 2 | 3 | J | G/IOL-238 (16) | J | 9 | |||
| 3 | 3 | 2 | 4 | 3 | 2 | 3 | SaratovJ1/61.30-A32 | J | This study | ||||
| 10 | 1 | 2 | 3 | 3 | 2 | 1 | 3 | 2 | 3 | H | H/UW-4 (18) | H | 5,9,24 |
| 3 | 3 | 2 | 1 | 3 | 2 | 3 | SaratovH2/61.37-M36 | H | This study | ||||
| 38 | 5 | 10 | 3 | 1 | 2 | 2 | 4 | 2 | 3 | F | F/SW4 (239) | F | 5,24 |
| 3 | 1 | 2 | 2 | 4 | 2 | 3 | SaratovF/61.26-M47 | F | This study | ||||
| 94 | 0 | 0 | 3 | 4 | 1 | 32 | 4 | 2 | 3 | E | E/Bour (235) | E | 5,24 |
Fig 3. Clonal grouping of sequence types (STs) of C. trachomatis strains found in our study (marked with gray ovals).
The division into Group I (A) and Group III (B) was based on the EBURST analysis with other STs of these clonal complexes obtained from PubMLST/Chlamydiales database.
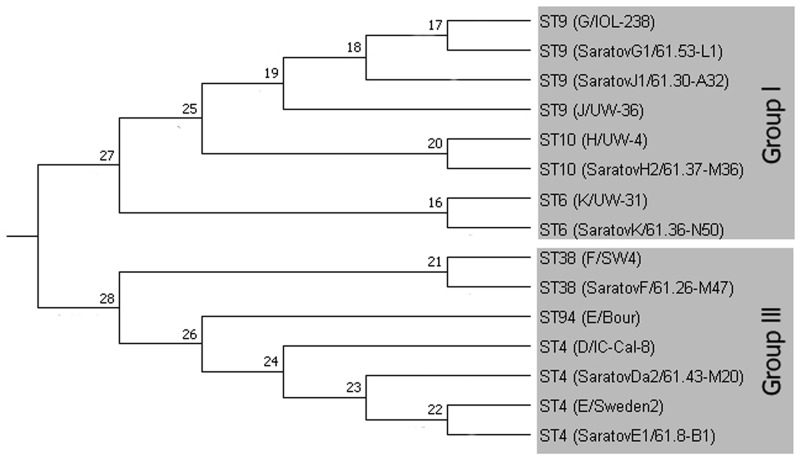
Interestingly, the Saratov CT samples of genovar E were grouped in ST4 only. They differed from E/Bour by two alleles (oppA and gitA) that resulted in their separation from the ST94 of E/Bour to a different cluster (Fig 4). There were no Saratov strains belonging to the ST of E/Bour, although the ompA types of all our strains of genovar E (subtype E1) were identical to this reference strain (Table 1, Fig 2).
Fig 4. Phylogenetic analyses of concatenated sequences of 7 housekeeping gene fragments of representative C. trachomatis strains.
Concatenated sequences were aligned and analyzed in MEGA 7. Phylogenetic tree was constructed using the UMPGA hierarchical clustering method model. Relevant STs & genotypes including in the clustering analyses are presented in Tables 1 & 2, respectively.
The group of mono-infected patients (n = 50) was also examined for STs distribution according to the gender, geographical and ethnic origin (S2 Fig), as well age. Overall, two STs, such as ST4 and ST9 were the most prevalent mono-infections among the patients tested. Both STs were found more often in male, female, urban and Slavic population in comparison with other STs. ST4 was observed more frequently over ST6, ST10 and ST38 (p<0.01) in female, but not to ST9 (p>0.05). ST4 was occurred more often than ST10 (p<0.01), as well as both ST6 and ST38 (p<0.05) in urban population. In Slavic individuals, ST4 significantly differed from ST38 (p<0.05), ST6 and ST10 (p<0.01).
ST4 was also found more often in other groups, such as anonymous, rural and Non-Slavic patients. In male and anonymous patients, ST4 demonstrated no significant difference in distribution than other STs (p>0.05). ST4 was more often observed in rural compared to other STs (p<0.05), including ST10 and ST38 (p<0.01). Further, ST4 was the most prevalent in Non-Slavic population in comparison to ST6 and ST38 (p<0.05), ST10 (p<0.01), but not to ST9 (p>0.05). The appearence of ST9 was statistically unsignificant in all groups (p>0.05). The ST4 group (patients average age = 25.3) consisted of the Saratov CT strains of the most frequently occurring genovars E and D (Table 2) that were clearly distinguished in ompA typing (Table 1, Fig 2). Nevertheless, the ST4 relevant loci of the representative Saratov CT strains were identical to the reference strains of ST4 of both genovars (Table 2). Similarly ST9 (patients average age = 26.6) was also formed by two separate genovars, namely G and J. The alleles of these strains corresponded to the reference strains of ST9 (Table 2). ST6 (patients average age = 29), ST10 (age was unknown, anonymous) and ST38 (average age = 28.5) were represented by only single genovars, such as K, H and F, respectively (Table 2). Each of them was related to the reference CT strain of the assigned ST.
There was no significant difference in distribution of Group I (average age = 27.2) and Group III (average age = 25.8) in the patients tested (p>0.05).
Discussion
Overall, the clinical isolates of CT, an obligate intracellular pathogen, have been divided into three biovars comprising 15 genovars, namely trachoma (genovars A-C), urogenital Chlamydial infection (genovars D-K), and lymphogranuloma venereum (L1-L3). These biovars differ in disease manifestation and severity [25]. Initially, this classification was based on the antigenic variations within the major outer membrane protein (MOMP) detectable by the MOMP-based micro immunofluorescence test [26]. Currently, the molecular inter- and intra-species differentiation of CT isolates relies on the variability of the four VDs of the ompA gene encoding the relevant epitopes of the major outer membrane protein (MOMP) [27]. Although new techniques for the discrimination of C. trachomatis isolates have been successfully developed, the ompA-based genotyping is still the most widely used method for obtaining information on CT genetic variations. There were numerous efforts to find correlates between mutations in the ompA gene and relevant changes in phenotype that influence the host immune response, adaptation of the pathogen to diverse host niches, impact on disease severity, and difference in the host susceptibility to CT [25]. Despite the fact that no direct correlation between ompA variability and disease severity has been established [11,28] intensive testing of CT isolates during the last decades demonstrated a clear difference in the ompA-based genovar distribution worldwide among different ethnicity groups, including Europe, Americas, Africa and Russia [5,12–17]. This observation may indirectly point towards the existence of selectivity of certain CT genovars with respect to different regions and communities. Thus, there were no cases of trachoma registered in Russia since late 1940s which resulted in the lack of detection of CT isolates of the A-C serovars [29–30]. The cases of lymphogranuloma venereum (serovars L1-L3) are also extremely rare, thus a strong prevalence of genital CT genovars (D-K) in this country [5] can be seen. Nevertheless, little is known about the distribution of CT genovars within Russian Regions.
In this study, the prevalence and CT genovar and STs distribution was carefully investigated in a cohort of patients in the Saratov Region, which is one of the 46 large regions of Russian Federation. This region is close to East (Belarus, Ukraine, Moldova) and South (Caucasus Region) European regions on the southwestern border and Kazakhstan on the southeastern border. Overall, this crossroad location between Europe and Asia dictates a multi-ethnic population and high migration area that may explain the relatively high prevalence of chlamydial infection (72.1 per 100,000 people) [31]. This specific geographic setting provided an opportunity to identify the pattern of representative genovars and compare it with the worldwide distribution of most prevalent strains, such as D, E, F and G together with three other genovars, H, J and K, typical for chlamydial genital infection [5,7,12–20,23,32–34]. In contrast to previous surveys from other countries, which revealed that the heterosexual population was infected with the dominating genovars D, E, G, or E alone, or E followed by F [7,15,33], the Saratov Region had a strikingly low representation of the genovar D with the prevalent domination of genovar E followed by G and F (Fig 1A). Similar CT genovar distribution has been recently observed in Greece in male patients with urethritis [14]. Likewise, it was shown recently that in Slovenia and Kharkiv Region (Ukraine) that genovars E, G and F were the most prevalent, although in a different proportion [13,20].
In addition, there was a significant difference in genovar’s distribution among the assessing population and CT patients of European and American women who were mostly infected with either genovars D or Ia. In our study, women demonstrated almost the same genital genovars, i.e. D, E, F, G, J and K, except Ia as shown for American, European and Russian St. Petersburg female patients [5].
Our data also show a slightly different genovar distribution from that in the Moscow Region [3,4], located in the northwest of the Saratov Region. In Moscow, genovar E was also the most dominant and accounted for about 40% of CT cases followed by G variants; however, genovar K was the third prevalent CT variant in the current research (Fig 1A). Moreover, we found more varieties in the genovar E subtypes, which were represented in the Saratov Region by E1, E2 and E6. The latter subtype contains a characteristic G995A SNP (Table 1), which was first detected in chlamydia patients in Sweden [23]. Nevertheless, both Saratov and Moscow Regions shared G1, G2, and G3 genovars, as well as a rare replacement T1003A existing in the G5 variant (Table 1) [4]. Also both Regions showed the presence of two identical variants of genovar D, D1 and Da2, as well as H2 and F1 subtypes (Table 1) [4]. In contrast to SNP at position 1063 reported by Ikryannikova et al. [4] for Moscow Region variants of the serovar K, all our seven CT samples of this genovar were identical to the reference strain K/UW31/Cx [12]. Moreover, our study revealed the presence of the J1 subtype, which was not found in two surveys in the Moscow Region [3,4]. Thus, a comparison of genovar distribution in two geographically close areas, Moscow and Saratov Regions, revealed a marked difference in genotypic prevalence. On the other hand, the observed prevalence of genovars E and F corresponds well with with the previous speculations on possible biological advantages for these genovars [7]. Similar assumptions were made for the second most prevalent genovar G (Fig 1A), which is thought to have an increased ability to overcome the host immune defense and possesses an increased transmission [12,13,17,20,23,33–35]. Unfortunately, we could not compare our data with CT genovar distribution from the bordering regions of Kazakhstan due to the lack of such studies for this country.
We applied MLST technique to estimate the diversity of 46 chlamydia samples from Saratov Region, and found five STs. A comparative analysis of their allele profiles employing seven housekeeping genes (Table 2) together with constructed phylogeny tree from the relevant concatenated sequences (Fig 4) demonstrated the presence of two types of strains belonging to Groups identified early [4,9]: (i) Group I was consisted of ST6, ST9 and ST10 (Fig 3A); (ii) Group III was represented by ST4 and ST38 (Fig 3B). ST4 was found to be a putative founder for ST38 clonal complex [4], while ST6, ST9 and ST10 were the founders for other subgroups that were not present in our set of the samples [4,9]. Also we did not uncover the presence of ST13, which was assigned as putative founder for ST6, ST9 and ST10 [4,9]. Nevertheless, this ST13 together with both ST6 and ST9 were recently found in Moscow Region that is relatively close to us geographically [4].
Surprisingly, in assessing population there were significantly less STs (only five STs in the current survey, Table 2) in comparison with those revealed among 58 CT positive patients from St. Petersburg, another Russian Region, located in the northern part of the country and bordering Europe [5]. In consisting with the recently reported classification based on both SNPs in the ompA gene and seven housekeeping genes [5], all CT strains associated with non-invasive urogenital disease were grouped within the Haplotype 2. No strains belonging either to the Haplotype 1, associated with invasive urogenital disease caused by LGV, or the Haplotype 3 connected with trachoma caused by typical A-C serovars were found.
Surprisingly, there were no CT strains associated directly with E/Bour (ST94), although all Saratov CT strains were related to STs of strains that are widely distributed around the globe [13]. Initially, ompA typing of CT strains from Saratov Region showed the prevalence of variants identical to E1 subtype of CT with E/Bour as the reference representative [12]. However, our strains of the genovar E were MLST identified as ST4 and were related to the ST4 of another reference strain of this genovar, such as isolate SWEDEN2 (Table 2). The latter seems to be a putative founder for ST94 (Fig 3B). Notably, E/Bour and other C. trachomatis genovar E1 strains could not be differentiated either by ompA typing or MLST analysis based on only five housekeeping genes typing [11]. In contrast the seven genes MLST used by us was able to discriminate such cases. This finding could be very useful for molecular epidemiology of CT to know better the population genetic structure. Furthermore, understanding the diversity of CT strains circulating in different regions of the world should be helpful in evaluation of association between CT genotype and the disease. Nevertheless, with the massive sequencing capabilities available, it is becoming increasingly important to connect the knowledge of the global diversification of CT with clinical data.
We also demonstrated three strong trends for the contingent of CT-positive patients in our Region: (i) descending age, (ii) prevalence of females compared with males and (iii) a high frequency (18%) of mixed or co-infection (Fig 1A) that slightly surpassed a 15% range reported in other parts of the world [18,19,32,36,37]. A previous survey in 2005–2007 in both Russia and Saratov Region demonstrated a reversal of statistics with older men as the predominantly infected CT-positive patients [38], which is also similar to data found in other international reports [13,15]. Prevalence of female patients under 30 years old with a clear tendency to further decrease was observed in the current study, which corresponded to the global trend [16,17,33]. The detection of mixed infection is important for understanding C. trachomatis evolution because of the competitive potential each genovar has in pathogenesis, as well as to increase the quality of Chlamydia diagnostics, partner notification and transmission.
Conclusions
Thus, data obtained in this study represent a first report on monitoring the basic trends in CT genovar and STs prevalence in the Saratov Region of Russia in order to improve local and national STI services. Our future research will extend this investigation by describing trends in a larger population, both inside and outside of the Saratov Region to clarify some aspects for the actual application of C. trachomatis genotype analysis for disease control and prevention. Ideally, genotyping analysis should be connected with clinical data in both symptomatic and asymptomatic patients, as well as in different groups including adolescens, pregnants, infants, sexual minorities, etc. Future perspective is also connected with unraveling the genetic variability of both host and pathogen in parallel.
Supporting information
(DOC)
(DOC)
The anonymous patients were included as well. The analysis was performed by using 95% confidence interval (95% CI). Statistically significant differences are indicated by (p<0.05). Slavic cohort included Russians, Byelorussians and Ukrainians, Non-Slavic cohort was presented by Caucasians, Jews, Kyrgyzs, Koreans, Moldavians, Germans, Mordovians, and anonymous.
(TIF)
Acknowledgments
We are very grateful to Prof. Karl A. Western (NIAID/NIH) for his constant encouragement during the progress of this study. We thank Dr. Anna M. Lyapina for her help with statistical analysis. We also thank Dr. Ivan V. Druzhkin and Edward A. Fedotov for clinical specimens kindly gifted.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Russian Science Foundation, Project No. 17-16-01099 (for Prof. V.A. Feodorova) and the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, Baltimore, MD, 21205, USA (for Prof. T.C. Quinn). BioInfoExperts LLC provided financial support in the form of a salary for S.L. Lamers. The specific role of this author is articulated in the ‘author contributions’ section. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization. Strategies and laboratory methods for strengthening surveillance of sexually transmitted infection. 1. Sexually transmitted diseases—prevention and control. 2. Sexually transmitted diseases—epidemiology. 3. HIV infections. 4. Clinical laboratory techniques. 5. Sentinel surveillance. I.UNAIDS/WHO Working Group on Global HIV/AIDS/STI Surveillance. WHO, 2012 ISBN: 978 92 4 150447 8. [Google Scholar]
- 2.Chiaradonna C. The Chlamydia cascade: enhanced STD prevention strategies for adolescents. J Pediatr Adolesc Gynecol. 2008; 21(5):233–1. http://dx.doi.org/10.1016/j.jpag.2007.10.005 [DOI] [PubMed] [Google Scholar]
- 3.Sharkeev VI, Torshin IY, Shkarupeta MM, Akopian TA, Lazarev VN, Govorun VM. Identification of serovar-specific single-nucleotide polymorphisms of C. trachomatis omp1 gene. Bull Exp Biol Med. 2005; 140(2):222–7. [DOI] [PubMed] [Google Scholar]
- 4.Ikryannikova LN, Shkarupeta MM, Shitikov EA, Il’ina EN, Govorun VM. Comparative evaluation of new typing schemes for urogenital Chlamydia trachomatis isolates. FEMS Immunol Med Microbiol. 2010; 59(2):188–196. https://doi.org/10.1111/j.1574-695X.2010.00678.x [DOI] [PubMed] [Google Scholar]
- 5.Smelov V, Vrbanac A, van Ess EF, Noz MP, Wan R, Eklund C, et al. Chlamydia trachomatis strain types have diversified regionally and globally with evidence for recombination across geographic divides. Front Microbiol. 2017; 13(8):2195 doi: 10.3389/fmicb.2017.02195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shipitsyna E, Hadad R, Ryzhkova O, Savicheva A, Domeika M, Unemo M. First reported case of the Swedish new variant of Chlamydia trachomatis (nvCT) in Eastern Europe (Russia), and evaluation of Russian nucleic acid amplification tests regarding their ability to detect nvCT. Acta Derm Venereol. 2012; 92(3):330–1. https://doi.org/10.2340/00015555-1269. [DOI] [PubMed] [Google Scholar]
- 7.Nunes A, Borrego MJ, Nunes B, Florindo C, Gomes JP. Evolutionary dynamics of ompA, the gene encoding the Chlamydia trachomatis key antigen. J Bacteriol. 2009; 191(23): 7182–92. https://doi.org/10.1128/JB.00895-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klint M, Fuxelius HH, Goldkuhl RR, Skarin H, Rutemark C, Andersson SG, et al. High-resolution genotyping of Chlamydia trachomatis strains by multilocus sequence analysis. J Clin Microbiol. 2007; 45(5):1410–4. doi: 10.1128/JCM.02301-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pannekoek Y, Morelli G, Kusecek B, Morré SA, Ossewaarde JM, Langerak AA, et al. Multi locus sequence typing of Chlamydiales: clonal groupings within the obligate intracellular bacteria Chlamydia trachomatis. BMC Microbiol. 2008; 8:42 doi: 10.1186/1471-2180-8-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dahlberg J, Hadad R, Elfving K, Larsson I, Isaksson J, Magnuson A, et al. Ten years transmission of the new variant of Chlamydia trachomatis in Sweden: prevalence of infections and associated complications. Sex Transm Infect. 2017; doi: 10.1136/sextrans-2016-052992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gravningen K, Christerson L, Furberg AS, Simonsen GS, Ödman K, Ståhlsten A, et al. Multilocus sequence typing of genital Chlamydia trachomatis in Norway reveals multiple new sequence types and a large genetic diversity. PLoS One. 2012; 7(3):e34452 doi: 10.1371/journal.pone.0034452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lysén M, Osterlund A, Rubin CJ, Persson T, Persson I, Herrmann B. Characterization of ompA genotypes by sequence analysis of DNA from all detected cases of Chlamydia trachomatis infections during 1 year of contact tracing in a Swedish County. J Clin Microbiol. 2004; 42(4):1641–7. https://doi.org/10.1128/JCM.42.4.1641-1647.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bilozorova OА. Prevalent genotypes of Chlamydia trachomatis in patients with urogenital chlamydiosis in Kharkiv region. The Journal of V.N.Karazin Kharkiv National University. Series: Biology. 2010; 11:55–9. [Google Scholar]
- 14.Papadogeorgakis H, Pittaras TE, Papaparaskevas J, Pitiriga V, Katsambas A, Tsakris A. Chlamydia trachomatis serovar distribution and Neisseria gonorrhoeae coinfection in male patients with urethritis in Greece. J Clin Microbiol. 2010; 48(6): 2231–4. https://doi.org/10.1128/JCM.00586-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gharsallah H, Frikha-Gargouri O, Sellami H, Besbes F, Znazen A, Hammami A. Chlamydia trachomatis genovar distribution in clinical urogenital specimens from Tunisian patients: high prevalence of C. trachomatis genovar E and mixed infections. BMC Infect Dis. 2012; November 30;12:333 https://doi.org/10.1186/1471-2334-12-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kapil R, Press CG, Hwang ML, Brown L, Geisler WM. Investigating the epidemiology of repeat Chlamydia trachomatis detection after treatment by using C. trachomatis ompA genotyping. J Clin Microbiol. 2015; 53(2): 546–9. https://doi.org/10.1128/JCM.02483-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giffard PM, Brenner NC, Tabrizi SN, Garland SM, Holt DC, Andersson P, et al. Chlamydia trachomatis genotypes in a cross-sectional study of urogenital samples from remote Northern and Central Australia. BMJ Open. 2016; 6(1): e009624 https://doi.org/10.1136/bmjopen-2015-009624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quint KD, van Doorn LJ, Kleter B, de Koning MN, van den Munckhof HA, Morre SA, et al. A highly sensitive, multiplex broad-spectrum PCR-DNA-enzyme immunoassay and reverse hybridization assay for rapid detection and identification of Chlamydia trachomatis serovars. J Mol Diagn. 2007; 9(5):631–8. https://doi.org/10.2353/jmoldx.2007.070011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dean D, Oudens E, Bolan G, Padian N, Schachter J. Major outer membrane protein variants of Chlamydia trachomatis are associated with severe upper genital tract infections and histopathology in San Francisco. J Infect Dis. 1995; 172(4): 1013–22. [DOI] [PubMed] [Google Scholar]
- 20.Kese D, Potocnik M, Maticic M, Kogoj R. Genotyping of Chlamydia trachomatis directly from urogenital and conjunctiva samples using an ompA gene pyrosequencing-based assay. FEMS Immunol Med Microbiol. 2011; 63(2): 210–6. https://doi.org/10.1111/j.1574-695X.2011.00843.x [DOI] [PubMed] [Google Scholar]
- 21.Emmadi R, Boonyaratanakornkit JB, Selvarangan R, Shyamala V, Zimmer BL, Williams L, et al. Molecular methods and platforms for infectious diseases testing a review of FDA-approved and cleared assays. J Mol Diagn. 2011; 13(6): 583–604. https://doi.org/10.1016/j.jmoldx.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016; https://doi.org/10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jurstrand M, Falk L, Fredlund H, Lindberg M, Olcen P, Andersson S, et al. Characterization of Chlamydia trachomatis omp1 genotypes among sexually transmitted disease patients in Sweden. J Clin Microbiol. 2001; 39(11): 3915–9. https://doi.org/10.1128/JCM.39.11.3915-3919.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris SR, Clarke IN, Seth-Smith HM, Solomon AW, Cutcliffe LT, Marsh P, et al. Whole-genome analysis of diverse Chlamydia trachomatis strains identifies phylogenetic relationships masked by current clinical typing. Nat Genet. 2012; 44:413–9, S1 doi: 10.1038/ng.2214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdelsamed H, Peters J, Byrne G. Genetic variation in Chlamydia trachomatis and their hosts: impact on disease severity and tissue tropism. Future Microbiol. 2013; 8(9):1129–1146. doi: 10.2217/fmb.13.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang SP, Grayston JT. Immunologic relationship between genital TRIC, lymphogranuloma venereum, and related organisms in a new microtiter indirect immunofluorescence test. Am J Ophthalmol. 1970; 70(3):367–374. [DOI] [PubMed] [Google Scholar]
- 27.Byrne GI. Chlamydia trachomatis strains and virulence: rethinking links to infection prevalence and disease severity. J Infect Dis. 2010; 201(Suppl. 2):S126–S133. doi: 10.1086/652398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ward H, Alexander S, Carder C, Dean G, French P, Ivens D, et al. The prevalence of lymphogranuloma venereum infection in men who have sex with men: results of a multicentre case finding study. Sex Transm Infect. 2009; 85(3):173–5. doi: 10.1136/sti.2008.035311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ibragimov NG. Participation of the medical community of Bashkiria in the prevention of trachoma. Vestn Oftalmol. 1978; (4):77–8. [PubMed] [Google Scholar]
- 30.Efremenko AA. Russian prerevolutionary dissertations on trachoma. Vestn Oftalmol. 1956; 69(5):94–5. [PubMed] [Google Scholar]
- 31.Tikhomirov AL, Sarsaniya SI. 2007. Urogenital chlamydiosis. Practical brochure for obstetricians and gynecologists. pp. 55.
- 32.Morré SA, Rozendaal L, van Valkengoed IG, Boeke AJ, van Voorst Vader PC, Schirm J, et al. Urogenital Chlamydia trachomatis serovars in men and women with a symptomatic or asymptomatic infection: an association with clinical manifestations. J Clin Microbiol. 2000; 38(6): 2292–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bax CJ, Quint KD, Peters RP, Ouburg S, Oostvogel PM, Mutsaers JA, et al. Analyses of multiple-site and concurrent Chlamydia trachomatis serovar infections, and serovar tissue tropism for urogenital versus rectal specimens in male and female patients. Sex Transm Infect. 2011; 87(6): 503–7. https://doi.org/10.1136/sti.2010.048173. [DOI] [PubMed] [Google Scholar]
- 34.Herrmann B, Isaksson J, Ryberg M, Tångrot J, Saleh I, Versteeg B, et al. Global multilocus sequence type analysis of Chlamydia trachomatis strains from 16 countries. J Clin Microbiol. 2015; 53(7): 2172–9. https://doi.org/10.1128/JCM.00249-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nunes A, Nogueira PJ, Borrego MJ, Gomes JP. Adaptive evolution of the Chlamydia trachomatis dominant antigen reveals distinct evolutionary scenarios for B- and T-cell epitopes: worldwide survey. PLoS One. 2010; 5(10). https://doi.org/10.1371/journal.pone.0013171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brunham RC, Kimani J, Bwayo J, Maitha G, Maclean I, Yang C, et al. The epidemiology of Chlamydia trachomatis within a sexually transmitted diseases core group. J Infect Dis. 1996; 173(4):950–6. [DOI] [PubMed] [Google Scholar]
- 37.Stevens MP, Twin J, Fairley CK, Donovan B, Tan SE, Yu J, et al. Development and evaluation of an ompA quantitative real-time PCR assay for Chlamydia trachomatis serovar determination. J Clin Microbiol. 2010; 48(6):2060–5. https://doi.org/10.1128/JCM.02308-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fedorova VA, Alikberov ShA, Eliseev II, Bannikova VA, Grashkin VA. Comparative study of the efficiency of methods for serodiagnosis of urogenital chlamydiasis. Klin Lab Diagn. 2010; 4:45–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
The anonymous patients were included as well. The analysis was performed by using 95% confidence interval (95% CI). Statistically significant differences are indicated by (p<0.05). Slavic cohort included Russians, Byelorussians and Ukrainians, Non-Slavic cohort was presented by Caucasians, Jews, Kyrgyzs, Koreans, Moldavians, Germans, Mordovians, and anonymous.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.