Abstract
Trichodesmium plays a significant role in the oligotrophic oceans, fixing nitrogen in an area corresponding to half of the Earth’s surface, representing up to 50% of new production in some oligotrophic tropical and subtropical oceans. Whilst Trichodesmium blooms at the surface exhibit a strong dependence on diazotrophy, colonies at depth or at the surface after a mixing event could be utilising additional N-sources. We conducted experiments to establish how acclimation to varying N-sources affects the growth, elemental composition, light absorption coefficient, N2 fixation, PSII electron transport rate and the relationship between net and gross photosynthetic O2 exchange in T. erythraeum IMS101. To do this, cultures were acclimated to growth medium containing NH4+ and NO3- (replete concentrations) or N2 only (diazotrophic control). The light dependencies of O2 evolution and O2 uptake were measured using membrane inlet mass spectrometry (MIMS), while PSII electron transport rates were measured from fluorescence light curves (FLCs). We found that at a saturating light intensity, Trichodesmium growth was ~ 10% and 13% lower when grown on N2 than with NH4+ and NO3-, respectively. Oxygen uptake increased linearly with net photosynthesis across all light intensities ranging from darkness to 1100 μmol photons m-2 s-1. The maximum rates and initial slopes of light response curves for C-specific gross and net photosynthesis and the slope of the relationship between gross and net photosynthesis increased significantly under non-diazotrophic conditions. We attribute these observations to a reduced expenditure of reductant and ATP for nitrogenase activity under non-diazotrophic conditions which allows NADPH and ATP to be re-directed to CO2 fixation and/or biosynthesis. The energy and reductant conserved through utilising additional N-sources could enhance Trichodesmium’s productivity and growth and have major implications for its role in ocean C and N cycles.
Introduction
In marine ecosystems, phytoplankton primary production is often limited by the bioavailability of fixed N [1–3], where N-sources (e.g. NO3-, NO2-, NH4+, urea etc) are quickly depleted by fast growing phytoplankton [4]. A significant fraction (~ 25 Tg N yr-1) of N in the euphotic zone is lost via sedimentation to the deep ocean as particulate organic nitrogen (PON), making NO3- concentrations higher at greater depth [5–7]. Whilst areas of upwelling transport NO3- into the euphotic zone, there are vast regions of the oligotrophic open oceans that are dependent on the input of new N from N2-fixing cyanobacteria. Among the most important marine diazotrophs are Trichodesmium sp., which can form extensive surface blooms in the tropical and subtropical oceans [8–12].
Previous studies have highlighted Trichodesmium’s capacity to assimilate various forms of combined N-sources [13–17]. It is commonly assumed that Trichodesmium obtains most of its nitrogen quota from N2 fixation, however field-based measurements of N2 fixation show wide temporal and spatial variability [18]. The causes of this variability remain unclear, but environmental factors such as the availability of combined nitrogen may be a contributing factor.
Diazotrophy
Diazotrophic cyanobacteria are able to meet their daily nitrogen quota by fixing dinitrogen (N2).
| (1) |
While N2 fixation is an extremely energy demanding process, Trichodesmium incurs additional costs related to the protection of nitrogenase from the irreversible inhibition of photosynthetically evolved O2 [9, 19, 20]. The separation of O2 evolution and N2 fixation is regulated over a diurnal cycle of N2 fixation and photosynthesis [21], involving daily synthesis and degradation of nitrogenase [22, 23] and alternation of photosynthetic activity states [24]. Temporal separation occurs over short timescales, where peak rates of photosynthesis (~ 10 am) and N2 (~ 12 pm) fixation vary over a diel period. Spatial separation occurs via diazocytes, which are reversibly specialised cells for nitrogen fixation [25, 26]. Diazocytes contain the necessary proteins to perform photosynthetic CO2 fixation and N2 fixation. However, it has been suggested that when fixing N2, cells increase cyclic electron transport around PSI to enhance ATP synthesis [21, 24], thus allowing the cells to meet the energetic demands of N2 fixation (Eq 1).
Uptake of additional N-sources
Like other facultative diazotrophic cyanobacteria spp., Trichodesmium can exploit other forms of nitrogen including NH4+, NO3-, urea and amino acids [16, 27]. These N compounds are transported into the cell via permeases, metabolised to NH4+ and then incorporated into carbon skeletons through the glutamine synthetase (GS) and glutamine 2-oxoglutarate aminotransferase (GOGAT) pathways. This process is mediated by nitrate reductase (Eq 2) and nitrite reductase (Eq 3).
| (2) |
| (3) |
For cyanobacteria, nitrate reductase is located in the cytosol and uses NADPH to catalyse the transfer of two electrons. The NO2- formed by nitrate reductase is further reduced to NH4+ via the transfer of six electrons. Thus, the reduction of NO3- to NH4+ can be expressed as;
| (4) |
Amino acids are synthesised from ammonia (NH3) via the GS-GOGAT pathway. The initial GS pathway requires ATP and glutamate as a substrate;
| (5) |
where glutamine is subsequently transformed to 2-oxoglutarate and reduced using NADPH, forming two moles of glutamate.
| (6) |
Thus, for every mole of glutamate produced, one mole each of NH3, NADPH, ATP and 2-oxoglutarate are required. Additionally, ATP is required for the active transport of inorganic NH4+ or NO3- into the cell [28]. Different N-sources require different amounts of energy and reductant and as such can be ordered into a hierarchy of energy requirements; where diazotrophy requires the highest investment of electrons and ATP, followed by NO3-, NO2- and then NH3.
Utilising additional N-sources
Global warming is increasing sea surface temperatures (SSTs) which is enhancing water stratification and decreasing vertical mixing [29], potentially increasing the area of N-limited oceans. Whilst detrimental to many phytoplankton, a reduced flux of NO3- into the upper mixed layer will increase the competitive advantage of diazotrophs for other limiting nutrients (i.e. Fe or P). Trichodesmium colonies have been observed migrating to the nutricline [30, 31] to facilitate the luxury uptake of polyphosphates before returning to the surface. Whilst at these depths, cells are exposed to NO3- concentrations greater than those at the surface. As such, Trichodesmium colonies may be assimilating and storing (i.e. cyanophycin granules) more combined N than the blooms frequently measured on the surface [32]. This could have major implications for growth rates, primary productivity and biogeochemical cycles [33].
Our approach comprises a systematic experiment where T. erythraeum IMS101 was grown over long durations, at three N-source treatments, with controlled and well-defined growth conditions, ensuring fully acclimated, balanced growth had been achieved. Our aims were to assess the response of T. erythraeum IMS101 growth, light dependency of gross and net O2 photosynthesis, PSII electron transport rates and elemental composition to different N-sources; investigating the physiological cost of performing diazotrophy.
Materials and methods
T. erythraeum IMS101 was semi-continuously cultured to achieve fully acclimated balanced growth at three N-source treatments (N2, NH4+ and NO3-), at a targeted 380 μatm CO2 concentration, saturating light intensity (400 μmol photons m-2 s-1), 12:12 light:dark (L:D) cycle and optimal temperature (26 °C ± 0.2) (3 treatments in total) for ~ 2 months (~ 30 generations).
Experimental setup
Cultures were acclimated to the CO2 and light intensity for ~ 4 months (~ 60 generations) under diazotrophic conditions before the addition of NH4+ or NO3-. Cultures were gradually enriched over a 2/3-week period by increasing the dilution ratio of YBCII media containing NH4+ or NO3- (100 μM).
T. erythraeum IMS101 was grown using YBCII medium [34] at 1.5 L volumes in 2 L pyrex bottles that were acid-washed and autoclaved prior to culturing. Daily growth rates were quantified from changes in baseline fluorescence (Fo) measured between 09:00 to 10:30 on dark-adapted cultures (20 minutes) using a FRRfII FastAct Fluorometer System (Chelsea Technologies Group Ltd, UK). Cultures were regarded as fully acclimated and in balanced growth when both the slope of the linear regression of ln Fo versus time and the ratio of live cell to acetone extracted (method detailed below) baseline fluorescence (Fo) were constant following every dilution with fresh YBCII medium. Cultures were kept at the upper section of the exponential growth phase through periodic dilution with new growth media at 3–5 day intervals. Illumination was provided side-on by fluorescent tubes (Sylvania Luxline Plus FHQ49/T5/840). Cultures were constantly mixed using magnetic PTFE stirrer bars and aerated with a filtered (0.2μm pore) air mixture at a rate of ~ 200 mL s-1. The CO2 concentration was regulated (± 2 μatm) by mass flow controllers (Bronkhorst, Newmarket, UK). CO2-free air was supplied by an oil free compressor (Bambi Air, UK) via a soda lime gas-tight column which was mixed with a 10% CO2 in-air mixture from a gas cylinder (BOC Industrial Gases, UK). The CO2 concentration was continuously monitored and recorded by an infra-red gas analyser (Li-Cor Li-820, Nebraska USA), calibrated weekly by a standard gas (BOC Industrial Gases).
Throughout all culturing, the inorganic carbon chemistry (S1 File) and dissolved inorganic NH4+ and NO3- concentrations (S2 File) were determined prior to diluting with fresh media. Samples for elemental composition, photosynthesis-light response curves, fluorescence light curves (FLC), in vivo light absorption and acetylene reduction assays were collected at the same time of day, approximately 4 and 6 hours into the photo-phase of the L:D cycle.
Measuring O2 exchange by membrane inlet mass spectrometry (MIMS)
Light dependent rates of O2 production and consumption were measured with a membrane inlet mass spectrometer (MIMS), using an 18O2 technique modified from McKew et al. [35] (S3 File). MIMS measurements consisted of three biological replicates per treatment (S4 File). Chlorophyll a concentrations at the point of sampling ranged from 80 to 245 μg Chla L-1.
Changes in 16O2 and 18O2 and thus O2 consumption (Uo) and O2 evolution (Eo) were calculated using the following equations [36];
| (7) |
| (8) |
where Uo is the rate of O2 consumption calculated from the decrease of 18O2 over time (i.e. Δ 18O2/Δt), which takes into account the relative concentration of 18O2 compared to 16O2 (i.e. 1 + 16O2/18O2) and Eo is the rate of gross O2 evolution calculated from the increase in 16O2 over time (Δ16O2/Δt), where the decline of 18O2 (i.e. Δ18O2/Δt) and 18O2 is corrected for relative to the concentration of 16O2. Chlorophyll a- and C-specific rates were obtained by dividing U0 and E0 by the concentration of Chla and particulate organic carbon, respectively. Rates were multiplied by 1.073 to spectrally correct to the culturing LEDs (S1 Fig).
Photosynthesis-light (P-E) curves for gross (E0Chl(C)) and net photosynthesis (PnetChl(C) = E0Chl(C)-U0Chl(C)) were fitted to the equations from Platt and Jassby [37];
| (9) |
| (10) |
where E0mChl(C) and PnetmChl(C) are the maximum gross and net O2 evolution rates; αg Chl(C) and αnChl(C) are the initial light-limited slopes for gross and net photosynthesis; Rd is the dark respiration rate; and E is the light intensity (μmol photons m-2 s-1). Curve fitting was performed on each replicate separately to calculate mean (± S.E.) curve fit parameterisations (Sigmaplot 11.0).
The maximum quantum efficiency of gross (ɸmgross) and net (ɸmnet) O2 evolution was calculated as follows;
| (11) |
where the C-specific initial slope for gross (αgC) or net (αnC) O2 evolution was divided by the C-specific, effective light absorption coefficient (aeffC).
Measuring nitrogenase activity by acetylene reduction
Acetylene reduction rates were measured using gas chromatography (ATI Unicam 610 series). Gaseous samples were injected into the GC column head (60 °C), carried via N2 gas through a Porapak N column (100 °C) to a flame ionising detector (100 °C). Peak areas of acetylene and ethylene were quantified by an integrated chromatograph data acquisition unit (Shimadzu C-R8A Integrator) and were converted into concentrations via an acetylene and ethylene standard curve performed with standard gases (Scientific and Technical Gases Ltd., UK). Triplicate 6 mL samples of each biological replicate culture were placed into 12 mL exetainer, screw capped glass vials (Labco Ltd, UK). Exactly 1.2 mL of the headspace was removed and replaced with a 1.2 mL sample of acetylene (BOC Industrial Gases, UK) (headspace = 20% acetylene). The vials were gently inverted for 1 minute before 250 μL of headspace was injected into the GC column for an initial measurement of acetylene and ethylene concentrations (T0). Vials were incubated at 26 °C and 400 μmol photons m-2 s-1 in an aluminium temperature block and were gently inverted every 10 minutes to prevent trichomes from settling on the bottom or aggregating at the meniscus. After 1 hour, a second 250 μL gaseous headspace was injected into the GC column for the post-incubation measurement (T1). Temperature and pressure was measured during each set of measurements and accounted for in the calculations. The rate of ethylene production was calculated with the assumption that the concentrations of acetylene and ethylene within the media were always in equilibrium to those in the headspace;
| (12) |
where (ΔC2H2) is the ethylene production rate (μmol C2H4 h-1), C2H2(T0) and C2H2 (T1) are the ethylene concentrations in the headspace at the start (T0) and end (T1) of the incubation, V(I) is the volume of gaseous sample injected into the GC column (L-1) and t is the incubation time (min).
N2 fixation rates were calculated to a Chla (μmol N2 (mg Chla)-1 h-1) and total carbon (μmol N2 (mg C)-1 h-1) basis;
| (13) |
where ΔC2H2 (μmol h-1) is divided by the Chla or total carbon concentration (mg) and multiplied by 0.25 under the assumption that reduction of four moles of acetylene is equivalent to reduction of one mole of dinitrogen.
Fluorescence light curves (FLCs)
A 2 mL sample of each replicate culture was used to measure a fluorescence light curve (FLC) [38]. The FLCs were measured with a FRRfII FastAct Fluorometer System, using a white LED actinic light source (Chelsea Technologies Group Ltd, UK). Each FLC lasted 1 hour; comprising 12 light steps which ranged from 10 to 1600 μmol photon m-2 s-1, each lasting 5 minutes in duration. The FLCs provided measurements of the light absorption cross-section of PSII photochemistry (σPIIʹ), the average time constant for the re-opening of a closed PSII reaction centre (τfʹ) and the operating efficiency of PSII photochemistry (Fqʹ/Fmʹ);
| (14) |
where Fmʹ is the maximum fluorescence in the light-adapted state and Fʹ is the steady-state fluorescence at any point.
Photosystem II (PSII) electron transport rates were normalised to a Chla (mol e- (g Chla)-1 h-1) and total carbon (mol e- (g C)-1 h-1) basis;
| (15) |
where Fqʹ/Fmʹ is the operating efficiency of PSII photochemistry; E is the light intensity (mol photons m-2 s-1), aChl(C) is the Chla-specific (C-specific) effective light absorption (m2 g-1 Chla and m2 g-1 C, respectively), FAQPII is the fraction of absorbed photons directed to PSII, which was set to 0.5 [39], with the assumption that the quantum yield of electron transport of one trapped photon within a reaction centre is equal to 1 [40]; 3600 converts seconds to hours and SCF is a spectral correction factor of 1.194, which converts electron transport rates to the culturing LED spectrum (S1 Fig).
ETR curves were modelled using a P-E equation [37], performed on each individual replicate using a Marquardt–Levenberg least squares algorithm to generate the best fit (R2 > 0.993);
| (16) |
where ETRmʹ is the hypothetical Chla(C)-specific maximum electron transport rate that would be achieved if there was no photoinhibition (mol e- (g Chla(C))-1 h-1); αETR is the initial slope of the Chla(C)-specific ETR-light curve (mol e- (g Chla(C))-1 h-1 (μmol photons m-2 s-1)-1); βETR is the parameter that accounts for downregulation and/or photoinhibition at supra-optimal light intensities (mol e- (g Chla(C))-1 h-1 (μmol photons m-2 s-1)-1); and E is the light intensity (μmol photons m-2 s-1).
The realised maximum PSII electron transport rate in the presence of photoinhibition (ETRm), light intensity at which ETR is maximal (Eopt), the light-saturation parameter (Ek) and the light inhibition parameter (Ep) were calculated from the fitted parameters as follows:
| (17) |
| (18) |
| (19) |
| (20) |
The ratio of PSII electron transport to gross O2 evolution (E0) under light-limitation (Φeα) and light-saturation (Φem) were calculated as follow;
| (21) |
| (22) |
Cellular elemental composition and light absorption
Samples for determining particulate organic carbon (POC), nitrogen (PN) and phosphorus (PP) (S5 File), chlorophyll a (S6 File) and in vivo light absorption (S7 File) were collected with each MIMS measurement, with each sample being a biological replicate.
Modelling the in vivo light absorption from pigment absorption spectra
In vivo light absorption was reconstructed using the light absorption spectra of Chla and photoprotective carotenoids (PPC) taken from Woźniak et al. [41] and the light absorption spectra of phycourobilin (PUB1, PUB2, PUBx, PUB4, PUB5a, PUBb, PUB5d, PUB5g and PUB5j), phycoerythrin (PE1, PE2a, PE2b and PE3b), alloplastocyanin (APC) and plastocyanin (PC1 and PC2) taken from Küpper et al. [42] (S2 Fig).
The Chla-specific light absorption coefficient was modelled as the sum of the contribution of all pigments;
| (23) |
where aChlmod is the modelled in vivo light absorption at a specific wavelength (λ = 400–700 nm); βi is the contribution of each pigment to aChlmod and ai is the pigment-specific spectral absorption coefficient of pigment i, in m2 (g pigment i)-1.
The modelled in vivo light absorption spectra (aChlmod (λ)) was optimised to the measured spectra between 400 and 700 nm using a reduced sum of squares method (Sigmaplot 11.0). If a zero value was returned for a βi parameter, that pigment was removed from the model and the curve fit reapplied.
Results
Inorganic C-chemistry, growth rate and cell composition
Balanced growth of T. erythraeum IMS101 was 0.34 d-1 when grown on N2, increasing by 10% and 13% when grown in the presence of NH4+ and NO3-, respectively (Table 1). Particulate C:N, C:P and N:P ratios were all influenced by the presence of additional N-sources. When compared to the N2 treatment, C:N decreased by 36% and 43% for the NH4+ and NO3- treatments, respectively. Ratios of C:P and N:P were comparable between NH4+ and NO3- treatments, but were significantly lower (~ 60% and 35%, respectively) compared to the N2 treatment (Table 1). Ratios of Chla:C were 80% and 67% higher for the NH4+ and NO3- treatments than for the N2 treatment, while Chla:N was not significantly different between treatments (Table 1). Carbon and Chla-specific N2 fixation rates were highest for the N2 treatment, decreasing significantly by 84% and 80% (Chla-specific) and 73% and 68% (C-specific) for the NH4+ and NO3- treatments, respectively (Table 1).
Table 1. The median (± S.E.) balanced growth rates and mean elemental stoichiometry and N2 fixation rates for T. erythraeum IMS101 when acclimated to three N-source conditions (N2, NH4+ and NO3-), at a target CO2 concentration (380 μatm), saturating light intensity (400 μmol photons m-2 s-1) and optimal temperature (26 °C).
| Variables | Units | N2 | NH4+ | NO3- |
|---|---|---|---|---|
| Growth rate | d-1 | 0.340 (0.038)[A] | 0.375 (0.011)[B] | 0.384 (0.005)[B] |
| Elemental Stoichiometry | ||||
| C:N | mol:mol | 6.9 (0.7) | 4.4 (0.9) | 3.9 (0.7) |
| C:P | mol:mol | 122.6 (7.0)[B] | 47.9 (2.4)[A] | 36.9 (2.9)[A] |
| N:P | mol:mol | 18.1 (1.3)[B] | 11.8 (2.2) | 9.9 (1.0)[A] |
| Chla:C | mg:mol | 134 (8)[A] | 239 (4)[B] | 222 (2)[B] |
| Chla:N | mg:mol | 906 (43) | 1041 (209) | 855 (154) |
| N2 Fixation | ||||
| Chla-specific | μmol N (mg Chla)-1 h-1 | 14.75 (1.66)[B] | 2.35 (0.49)[A] | 2.84 (0.44)[A] |
| C-specific | μmol N (mg C)-1 h-1 | 0.16 (0.01)[B] | 0.04 (0.01)[A] | 0.05 (0.01)[A] |
Abbreviations; C:N, C:P and N:P ratios are mol:mol, Chla:C and Chla:N ratios are mg:mol (n = 3). Letters in parenthesis indicate significant differences between N-source treatments (One Way ANOVA, Tukey post hoc test; P < .05); where [B] is significantly greater than [A].
The inorganic carbon concentration, pH and alkalinity (AT) did not vary significantly amongst N-source treatments. Overall, CO2 drawdown ranged between 78 to 92 μatm from the target concentration (i.e. 380 μatm) for all N-source treatments (Table 2) and exhibited little variability over a diurnal cycle (S3 Fig). Inorganic N concentrations were > 1 μM for the N2 treatment and were ~ 8 μM for the NH4+ and NO3- treatments at the point of dilution (Table 2).
Table 2. The growth conditions (± S.E.) for T. erythraeum IMS101 when cultured under three N-source conditions (N2, NH4+ and NO3-), at a target CO2 concentration (380 μatm), saturating light intensity (400 μmol photons m-2 s-1) and optimal temperature (26 °C).
| Variables | Units | N2 | NH4+ | NO3- |
|---|---|---|---|---|
| pH | Total | 8.18 | 8.18 | 8.19 |
| H+ | nM | 6.6 (0.1) | 6.6 (0.1) | 6.4 (0.2) |
| AT | μM | 2483 (47) | 2427 (59) | 2482 (56) |
| TCO2 | μM | 2066 (41) | 2019 (51) | 2056 (44) |
| HCO3- | μM | 1762 (35) | 1723 (44) | 1746 (33) |
| CO32- | μM | 296 (7) | 288 (9) | 302 (12) |
| CO2 | μM | 8.3 (0.2) | 8.2 (0.3) | 8.0 (0.4) |
| pCO2 | μatm | 300 (8) | 296 (9) | 289 (7) |
| NH4+ | μM | 0.76 (0.13) | 8.33 (0.45) | 0.59 (0.07) |
| NO3- | μM | 0.07 (0.07) | 0.46 (0.07) | 8.24 (1.31) |
| n | 35 | 34 | 10 |
Individual pH values were converted to a H+ concentration, allowing a mean pH value to be calculated.
Light absorption
The effective light absorption coefficients were not significantly different between N-source treatments, nor were the modelled absorption coefficients significantly different to the measured coefficients; with modelled coefficients being only 1 to 3% higher across all N-source treatments (Table 3).
Table 3. The mean (± S.E.) measured and modelled effective light absorption coefficients and the relative contribution of each photosynthetic pigment to the total light absorption under the culturing LEDs within T. erythraeum IMS101, when acclimated to three N-sources (N2, NH4+ and NO3-), at a target CO2 concentration (380 μatm), saturating light intensity (400 μmol photons m-2 s-1) and optimal temperature (26 °C).
| Variables | Units | N2 | NH4+ | NO3- |
|---|---|---|---|---|
| aeffChl | m2 (g Chla)-1 | 9.9 (0.6) | 7.7 (0.9) | 8.1 (0.3) |
| aeffC | m2 (g C)-1 | 0.111 (0.013) | 0.154 (0.020) | 0.149 (0.006) |
| amodChl | m2 (g Chla)-1 | 10.0 (0.6) | 7.8 (0.9) | 8.3 (0.3) |
| amodC | m2 (g C)-1 | 0.112 (0.013) | 0.156 (0.019) | 0.154 (0.005) |
| Chla | % | 35.66 (0.41) | 36.74 (0.40) | 39.18 (2.14) |
| PPC | % | 30.64 (3.19) | 27.48 (3.05) | 27.44 (2.87) |
| PUB1 | % | 2.67 (1.36)[A] | 5.04 (4.67) | 10.11 (2.13)[B] |
| PUB2 | % | 1.34 (0.28)[B] | 1.63 (1.04) | 0.06 (0.06)[A] |
| PUBx | % | 0 | 0 | 0 |
| PUB4 | % | 0.02 (0.02) | 0 | 0 |
| PUB5a | % | 0.42 (0.21) | 0 | 0 |
| PUB5b | % | 0.24 (0.23) | 0 | 0 |
| PUB5d | % | 0.05 (0.03) | 0 | 0 |
| PUBg | % | 0.18 (0.18) | 0 | 0 |
| PUBj | % | 0.19 (0.19) | 0 | 0 |
| PE1 | % | 8.10 (3.73) | 7.51 (3.70) | 3.19 (2.01) |
| PE2a | % | 1.17 (0.75) | 0 | 0 |
| PE2b | % | 1.02 (0.72) | 0 | 0 |
| PE3b | % | 10.93 (2.28) | 13.14 (3.60) | 13.03 (0.92) |
| APC | % | 5.45 (1.30) | 5.17 (1.34) | 5.97 (1.17) |
| PC1 | % | 0.94 (0.57) | 0.19 (0.19) | 0 |
| PC2 | % | 2.22 (1.15) | 3.08 (1.61) | 1.02 (0.52) |
Light absorption coefficients were spectrally corrected to the culture LEDs and were normalised to a chlorophyll a (m2 g Chla-1) and carbon (m2 g C-1) basis. Abbreviations; aeffChl and aeffC are the measured Chla- and C-specific light absorption coefficients, while amodChl and amodC are the modelled Chla- and C-specific light absorption coefficients. amodChl and amodC were constructed from a range of pigment light absorption spectrums (λ = 400–700); comprising chlorophyll a (Chla), photoprotective carotenoids (PPC), phycourobilins (PUB1, PUB2, PUBx, PUB4, PUB5a, PUBb, PUB5d, PUB5g and PUB5j), phycoerythrin (PE1, PE2a, PE2b and PE3b), alloplastocyanin (APC) and plastocyanin (PC1 and PC2). Letters in parenthesis indicate significant differences between N-source treatments (One Way ANOVA, Tukey post hoc test; P < .05); where [B] is significantly greater than [A].
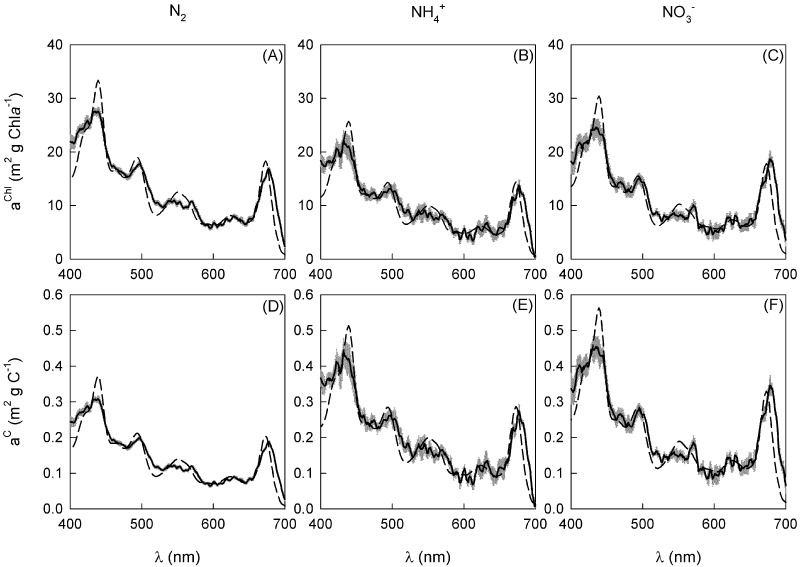
In vivo light absorption spectra (Fig 1) exhibited peaks at ~ 440 nm (Chla), ~ 490–500 nm (phycourobilin; PUB), ~ 540 and 568 nm (phycoerythrin; PE), ~ 620 nm (phycocyanin; PC), ~ 640 nm (allophycocyanin; APC) and ~ 675 nm (Chla) (Table 3). Chlorophyll a and photoprotective carotenoids (PPC) dominated light absorption, together accounting for ~ 65% of the total. PUB1 and PUB2 were the only pigments to exhibit significant differences, where relative to the N2 treatment, the contribution of PUB1 to the total light absorption increased by 7.4% whereas PUB2 decreased by 1.3% in the presence of NO3- (Table 3).
Fig 1. The mean (± S.E.) Chla (a-c) and C-specific (d-f) in vivo light absorption spectra for T. erythraeum IMS101 (n = 3).
Cultures were acclimated to three N-source treatments (N2, NH4+ and NO3-), at a target CO2 concentration (380 μatm), saturating light intensity (400 μmol photons m-2 s-1) and optimal temperature (26 °C). The solid black line is the measured light absorption spectra (grey area represents the S.E.) while the dashed line is the modelled light absorption spectra.
Light-dependence of O2 exchange
The C-specific maximum rate (E0mC) and initial slope (αgC) of light-dependent gross photosynthesis increased with additional N-sources (i.e. NH4+ and NO3-) and was highest for the NH4+ treatment relative to the N2 treatment (Table 4). There were also significant effects of additional N-sources on the Chla-specific maximum rate (E0mChl) and initial slope of light-dependent gross photosynthesis (αgChl) (S1 Table), however the effects were more pronounced when expressed as a C-specific rate, where E0mC increased by 143% from the N2 to the NH4+ treatment, while E0mChl increased by only 36%.
Table 4. The parameters (± S.E.) of the C-specific light-response curves for gross and net photosynthetic O2 evolution of T. erythraeum IMS101 (n = 3).
| Parameters | Units | N2 | NH4+ | NO3- |
|---|---|---|---|---|
| Gross O2 evolution | ||||
| E0mC | mmol O2 (g C)-1 h-1 | 6.05 (0.37)[A] | 14.71 (1.20)[C] | 10.98 (0.33)[B] |
| Ek | μmol photons m-2 s-1 | 238 (55) | 227 (44) | 255 (55) |
| αgC | μmol O2 (g C)-1 h-1 (μmol photons m-2 s-1)-1 | 27.9 (5.3)[A] | 67.7 (8.2)[B] | 49.8 (15.1) |
| ɸmgross | mol O2 (mol photons)-1 | 0.07 (0.01)[A] | 0.12 (0.01)[B] | 0.09 (0.03) |
| E0:Nfix | mol O2 (mol N2)-1 | 31 (4)[A] | 289 (32)[B] | 185 (57)[B] |
| Net Photosynthesis | ||||
| PnetmC | mmol O2 (g C)-1 h-1 | 3.75 (0.27)[A] | 11.48 (1.56)[B] | 9.59 (0.37)[B] |
| Ek | μmol photons m-2 s-1 | 250 (69) | 277 (8) | 220 (37) |
| αnC | μmol O2 (g C)-1 h-1 (μmol photons m-2 s-1)-1 | 16.8 (3.4)[A] | 41.5 (5.8)[B] | 46.1 (8.0)[B] |
| RdC | mmol O2 (g C)-1 h-1 | -1.63 (0.19) | -1.53 (0.28) | -1.16 (0.76) |
| ɸmnet | mol O2 (mol photons)-1 | 0.04 (0.01)[A] | 0.08 (0.02)[B] | 0.09 (0.01)[B] |
| Pnet:Nfix | mol O2 (mol N2)-1 | 18 (1)[A] | 207 (29)[B] | 163 (36)[B] |
| Gross (x) vs. Net (y) | ||||
| slope | Dimensionless | 0.60 (0.02)[A] | 0.82 (0.03)[B] | 0.83 (0.01)[B] |
Abbreviations; E0mC, the C-specific maximum gross O2 evolution rate; PnetmC, the C-specific maximum net O2 evolution rate; Ek, the light saturation parameter; αgC and αnC are the C-specific initial slopes the light response curve for net and gross photosynthesis; ɸmgross and ɸmnet are the maximum quantum efficiencies of gross and net O2 evolution; RdC, the C-specific dark respiration rate; slope, the gradient of the regression between PnetC and E0C; E0:Nfix and Pnet:Nfix, the ratio of gross and net photosynthesis to N2 fixation, where rates of E0 and Pnet were calculated at 400 μmol photons m-2 s-1, matching to light intensity of the N2 fixation incubations; slope, the gradient of the regression between PnetC and E0C. The r2 values of all curve fits were > 0.982. Letters in parenthesis indicate significant differences between CO2 treatments (One Way ANOVA, Tukey post hoc test; P < .05); where [B] is significantly greater than [A] and [C] is significantly greater than [B] and [A].
The light saturation parameter (Ek = E0mC/αgC) of gross O2 evolution did not vary significantly between N-source treatments (Table 4) and was due to covariation of αgC and E0mC. The maximum quantum efficiency of gross O2 evolution (ɸmgross = αgC/aeffC) increased significantly by 76% from the N2 to NH4+ treatment (Table 4) and was due to the relatively constant aeffC and the significant increase in αgC.
Carbon-specific dark respiration rates (RdC) varied by ~ 24% and were slightly higher for the N2 and NH4+ treatments than the NO3- treatment (Table 4). Light-saturated net O2 evolution rates (PnetmC) approximately trebled and more than doubled from the N2 treatment to the NH4+ and NO3- treatments respectively (Table 4); with the initial slope (αnC) showing a similar pattern to PnetmC. This increase in αnC for the NH4+ and NO3- treatments resulted in the maximum quantum efficiency of net O2 evolution (ɸmnet = αnC/aeffC) increasing significantly by 86% and 100% respectively, relative to the N2 treatment (Table 4). The light saturation parameter (Ek = PnetmC/αgC) for net O2 evolution did not vary significantly between N-source treatments (Table 4).
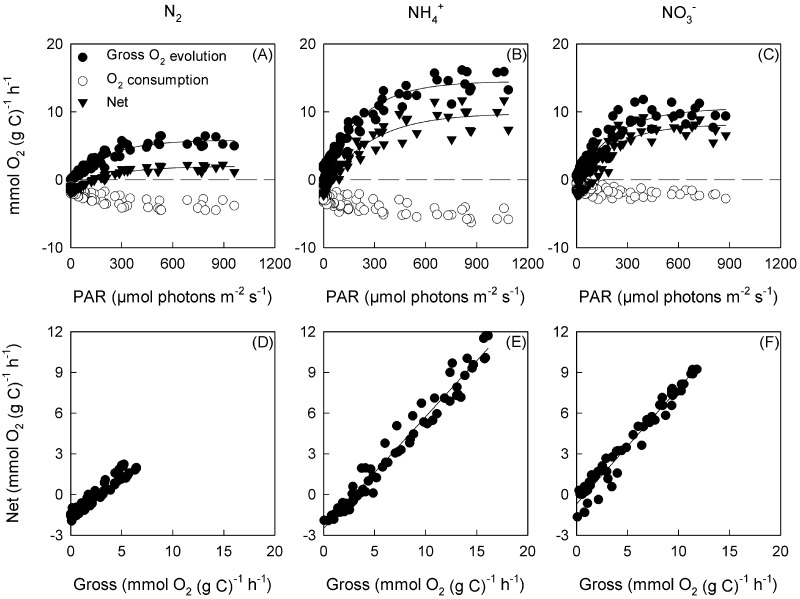
The relationship between net and gross O2 evolution was linear (Fig 2D–2F), with the slope increasing by approximately 40% when cultured in the presence of an additional N-source (Table 4). This linear relationship suggests that light-dependent O2 consumption (U0C) was a constant proportion of gross O2 evolution (E0C) and was independent of light intensity for all N-source treatments. Subtracting the slope from unity gave the ratio of light-driven U0C to E0C, which was significantly lower for the N2 treatment.
Fig 2. The C-specific light response curves for gross O2 evolution, O2 consumption, net photosynthesis (n = 3) (a-c) and the relationship between gross and net O2 evolution (d-f) for T. erythraeum IMS101.
Cultures were acclimated to three N-sources (N2, NH4+ and NO3-), at a target CO2 concentration (380 μatm), saturating light intensity (400 μmol photons m-2 s-1) and optimal temperature (26 °C). Chla-specific light response curves are shown in S4 Fig, while the light response curves for individual replicates are shown in S6–S8 Fig.
The ratio of gross photosynthesis (E0) to N2 fixation increased 9-fold and 6-fold for the NH4+ and NO3- treatments relative to the N2 treatment. In addition, the ratio of net photosynthesis (Pnet) to N2 fixation was 12-fold and 7-fold higher for the NH4+ and NO3- treatments relative to the N2 treatment (Table 4).
Light-dependence of PSII electron transport
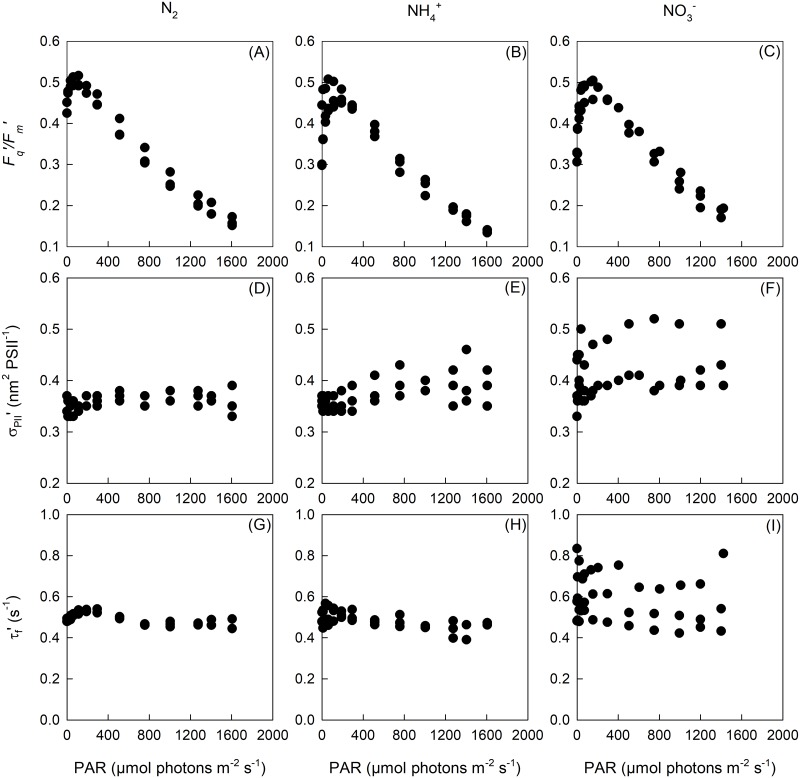
The operating efficiency of PSII photochemistry (Fq'/Fmʹ) increased at low light intensities, reaching a maximum at ~ 110 to 130 μmol photons m-2 s-1, before decreasing significantly with increasing light intensity (Fig 3). The light saturation parameter (Ek) and the light at which ETR was maximal (Eopt) were significantly higher for the N2 treatment than the NH4+ treatment. Conversely, the light inhibition parameter (Ep), absorption cross-section of PSII photochemistry (σPII) and the time constant for the re-opening of a closed PSII reaction centre (τf) in the dark-adapted state were not significantly different between N-source treatments. Furthermore, both σPIIʹ and τfʹ exhibited no light-dependency, remaining relatively constant across the entire range of actinic light intensities (Fig 3, Table 5).
Fig 3. The operating efficiency of PSII photochemistry (Fqʹ/Fmʹ) (a-c), light absorption cross-section of PSII photochemistry (σPIIʹ) (d-f) and average time constant for the re-opening of a closed PSII reaction centres (τfʹ) (g-i) across a range of actinic light intensities for T. erythraeum IMS101 (n = 3).
Cultures were acclimated to three N-sources (N2, NH4+ and NO3-), at a target CO2 concentration (380 μatm), saturating light intensity (400 μmol photons m-2 s-1) and optimal temperature (26 °C).
Table 5. The parameters (± S.E.) of the fluorescence light-response curves (FLCs) of T. erythraeum IMS101 (n = 3).
| Parameters | Units | N2 | NH4+ | NO3- |
|---|---|---|---|---|
| ETRmC | mmol e- (g C)-1 h-1 | 62.5 (16.7) | 70.3 (14.4) | 91.0 (4.9) |
| Ek | μmol photons m-2 s-1 | 465 (8)[B] | 421 (3)[A] | 447 (20) |
| αETRC | mmol e- (g C)-1 h-1 (μmol photons m-2 s-1)-1 | 0.133 (0.033) | 0.167 (0.033) | 0.200 (0.003) |
| βETRC | mmol e- (g C)-1 h-1 (μmol photons m-2 s-1)-1 | 5081 (55)[A] | 5577 (55)[C] | 5332 (14)[B] |
| Eopt | μmol photons m-2 s-1 | 1263 (22)[B] | 1144 (3)[A] | 1216 (54) |
| Ep | μmol photons m-2 s-1 | 0.99 (0.11) | 0.67 (0.08) | 0.83 (0.09) |
| Fv/Fm | Dimensionless | 0.44 (0.01) | 0.35 (0.05) | 0.32 (0.01) |
| σPII | nm2 PSII-1 | 0.353 (0.009) | 0.367 (0.003) | 0.380 (0.032) |
| τf | s-1 | 489 (5) | 494 (15) | 631 (105) |
| Φem | mol e- (mol O2)-1 | 10.5 (1.1)[B] | 5.7 (1.1)[A] | 7.9 (0.5) |
| Φeα | mol e- (mol O2)-1 | 5.2 (0.8) | 2.8 (0.2) | 4.5 (1.0) |
Abbreviations; ETRmC, the C-specific maximum electron transport rate; αETRC, the C-specific initial slope of the electron transport rate light response curve; βETRC, the C-specific light saturated slope of the electron transport rate light response curve; Ek, the light saturation parameter; Eopt, the light at which ETR is maximal; Ep, the light inhibition parameter; Fv/Fm, the maximum photochemical efficiency of PSII in the dark-adapted state; σPII, the absorption cross-section of PSII photochemistry in the dark-adapted state; τf, the average time constant for the re-opening of a closed PSII reaction centre in the dark-adapted state; Φem, the light saturated ratio of PSII electron transport to gross O2 evolution; Φeα, the light limited ratio of PSII electron transport to gross O2 evolution. The r2 values of all curve fits were > 0.977. Letters in parenthesis indicate significant differences between N-source treatments (One Way ANOVA, Tukey post hoc test; P < .05); where [B] is significantly greater than [A] and [C] is significantly greater than [B] and [A].
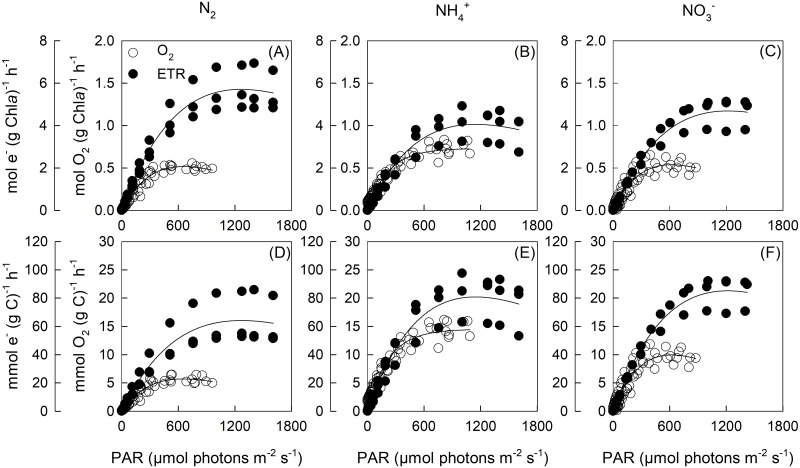
The light intensity at which ETR was maximal (Eopt) was significantly lower (by ~ 120 μmol photons m-2 s-1) for the NH4+ treatment relative to the N2 treatment (Fig 4). The Chla and C-specific maximum electron transport rate and initial slope (αETR) of the ETR-light curves were not significantly different between N-source treatments (Table 5, S2 Table). In contrast, the light-saturated photoinhibition slopes (βETR) were significantly different, with β increasing by 5% and 10% for the NO3- and NH4+ treatments, relative to the N2 treatment (Table 5).
Fig 4. Concurrent Chla (a-c) and C-specific (d-f) gross O2 evolution rates and PSII electron transport rates (ETR) for T. erythraeum IMS101 (n = 3).
Cultures were acclimated to three N-sources (N2, NH4+ and NO3-), at a target CO2 concentration (380 μatm), saturating light intensity (400 μmol photons m-2 s-1) and optimal temperature (26 °C).
The ratio of PSII electron transport to gross O2 evolution under light-limitation (Φeα) was ~ 4 and did not vary significantly between N-source treatments. Light saturated ratios (Φem) increased relative to Φeα for all N-source treatments, with the N2 treatment being 46% and 35% higher than the NH4+ and NO3- treatments, respectively (Table 5).
Discussion
Effect of acclimation to variation of N-sources on growth rates and elemental stoichiometry
Growth rates achieved under diazotrophic conditions were similar to most previous studies [23, 43–45], as was the increase in growth rate observed under non-diazotrophic conditions [23, 43], which we attribute to the lowered demand of NADPH and ATP for nitrogenase activity, where NADPH and ATP could be re-directed to CO2 fixation and/or biosynthesis. Our data shows that at saturating light intensity, the energetic cost of diazotrophy constrains Trichodesmium growth by ~ 13%. However, in a natural system, potential changes to inorganic carbon chemistry (influencing the activity of the carbon concentrating mechanism (CCM)), temperature (influencing enzyme activity), or other key nutrients (i.e. Fe, P), all of which were controlled in our experiments, will almost certainly influence this estimate.
The decrease in C:N, C:P and N:P under non-diazotrophic conditions is consistent with previous findings [43]. The high C:N under diazotrophic conditions may be due to accumulation of stored glycogen, whereas the decrease in C:N under non-diazotrophic conditions is likely due to high cellular N concentrations, likely due to the luxury uptake of NH4+ and NO3-, where surplus N is stored within cyanophycin granules [26]. Given the concurrent decrease in C:N, C:P and N:P under non-diazotrophic conditions, it is likely that utilising NH4+ or NO3- as a N-source enables Trichodesmium cells of low carbon biomass to maintain a Chla concentration comparable to diazotrophic conditions. This is supported by previous observation made by Eichner et al. [43] and is also reflected by the higher Chla:C yet comparable Chla:N ratios for NH4+ or NO3- treatments.
Growing evidence points towards nitrogenase being expressed in subsets of cells within filaments, called diazocytes [21, 25]. To date, no translocation transport mechanisms for N compounds have been observed in Trichodesmium, leading to suggestions that diazocytes release N into the external medium for use by neighbouring cells [25, 46]. This is partially supported by observations of Trichodesmium exhibiting a high capacity for NH4+ uptake during active N2 fixation [17, 18]. While such mechanisms may exist, we did not observe significant concentrations of dissolved inorganic NO3- or NH4+ in the medium of our control treatment.
Effect of acclimation to different N-sources on gross photosynthesis
We show an effect of N-source on C-specific light saturated gross O2 evolution rates. The more than two-fold increase in the maximum O2 evolution rate and initial slope when T. erythraeum IMS101 was grown on NH4+ or NO3- than when growing diazotrophically was largely due to differences in the ratio of Chla:C as chlorophyll a-specific photosynthetic parameters varied by only 36% between N2 and NH4+ treatments.
The increase of C-specific gross O2 evolution rates when Trichodesmium is supplied with NH4+ or NO3- may be due to an increase in the maximum rate of CO2 fixation and/or to an increase in PSII concentration. Previous studies report high PSI:PSII ratios under diazotrophic conditions (ranging between 1.3 to 4) [47–51], which would allow cyclic photophosphorylation in diazocytes to provide most of the ATP required for N2 fixation, with glycolysis and the Kreb’s cycle providing the required reducing equivalent. It may be that under non-diazotrophic conditions and with lower nitrogenase activity, Trichodesmium enhances linear electron transport to increase NADPH production; a pathway that generates more evolved O2.
Effect of acclimation to different N-sources on N2 fixation
Nitrogenase activity declined significantly by 81–84% when Trichodesmium was cultured in the presence of an additional N-source. Despite being cultured under N-replete concentrations, Trichodesmium cells in the NH4+ and NO3- treatments exhibited a baseline rate of N2 fixation. Similarly, Milligan et al. [52] reported a ~ 85% decrease when Trichodesmium was cultured in 100 μM of NO3- for 2 weeks and Holl and Montoya [44] reported a 66% decrease when cultured in 20 μM of NO3-, accrediting 8% of total N assimilation to diazotrophy despite the presence of additional N-sources. Maintaining the capability to perform N2 fixation under non-diazotrophic conditions, albeit at a reduced rate, could reflect Trichodesmium’s natural environment and act a potential safeguard mechanism to variable light and nutrient regimes.
Noting that 16 moles of ATP are consumed per mole of N2 fixed (Eq 1) and that 2.56 moles of ATP can be produced per mole of O2 evolved by photophosphorylation linked LPET [53], we calculated that T. erythraeum IMS101 may use 20% of the ATP that could be generated from gross O2 evolution to support the observed N2 fixation rate during diazotrophic growth:
| (24) |
This proportion decreases to 2% and 4% for the NO3- and NH4+ treatments, respectively, where the ratio of E0:Nfix increases to 289 for the NO3- treatment and 185 in the NH4+ treatment, versus 31 in the N2 treatment (Table 4).
Studies on natural populations of Trichodesmium spp. have shown that the addition of NO3- (100 μM) in the morning can cause a gradual decrease of N2 fixation over the photic period [22]. Further studies have also shown that addition of glutamine (10 μM) immediately decreases N2 fixation rates, indicating a direct effect on enzyme activity as opposed to enzyme synthesis [54]. These observations have been accredited to accumulation of N-containing metabolites acting as potential inhibitors to the specific activity rather than abundance of nitrogenase [22, 54].
It is well known that intracellular nitrogen pools have a role in regulating nitrogenase activity in diazotrophs [55, 56]. Dinitrogenase reductase catalyses the reduction of N2 to NH4+, which is assimilated into glutamine (gln) and then into glutamate (glu) via the glutamine synthetase (GS, EC 6.3.1.2)/glutamate synthase (GOGAT) pathway [54]. The intracellular pools of NH4+, glu and gln have been identified as important feedback regulators of N uptake and metabolism, with GS activity in Trichodesmium being sensitive to both intra- and extracellular N concentrations [55]. It could be hypothesised that the activity of nitrogenase is influenced by internally recycled N (e.g. NH4+ and gln), while the synthesis of nitrogenase is influenced by newly assimilated N (e.g. NO3-).
Effect of acclimation to different N-sources on light-stimulated O2 consumption and the relationship between net and gross O2 evolution
Net photosynthesis was significantly lower for the N2 treatment than for the NH4+ and NO3- treatments. Despite slight variations in E0C, the difference in net photosynthesis was principally driven by O2 consumption. Approximately 68%, 32% and 29% (N2, NH4+ and NO3-, respectively) of E0C was consumed by O2 consuming processes, which is comparable to previous observations [43, 52].
Several processes demand ATP in excess of the ATP:NADPH produced through linear photophosphorylation; two most notably being N2 fixation and the operation of the CCM [57]. In this study, the carbon chemistry of all cultures was closely regulated to ensure that variation in O2 consumption and net photosynthesis was due to the N-source treatments only. Linearity between gross O2 evolution (E0) and O2 consumption was observed across all N-source treatments, suggesting that light-dependent O2 consumption is linked to balancing ATP to NADPH production, as opposed to serving as a mechanism to dissipate excitation energy.
Diazotrophic cells consume more O2 per evolved O2 across the entire range of actinic light intensities than the NH4+ and NO3- treatments. This suggests a higher rate of water-water cycling due to either Mehler activity or operation of plastoquinone terminal oxidase when N2 is being fixed. To maintain a sufficient supply of ATP relative to NADPH, Trichodesmium may utilise pseudocyclic photophosphorylation linked to the Mehler reaction to augment the ATP generated by linear electron transfer from water to NADP+ in addition to ATP produced by cyclic electron flow around PSI.
Measurements of O2 evolution, ETR and N2 fixation were all made at one time of day (4 to 6 hours into the photo-phase of a 12:12 L:D cycle) and as such cannot be extrapolated to a diel response given the reports of temporal separation of photosynthesis and N2 fixation in Trichodesmium [21].
Effect of acclimation to different N-sources on electron transport rates and photophysiology
Like Eichner et al. [43], we observed a negligible effect of N-source on many photo-physiological parameters, including Fqʹ/Fmʹ, σPII and τf. Trichodesmium exhibited a light response typical for most cyanobacteria, where the dark-adapted photochemical yield is significantly affected by respiratory electron flow [58]. This results from a proportion of PSII reaction centres remaining in a closed state despite being in the dark and is imposed by a reduction in the plastoquinone (PQ) pool, which prevents the oxidation of QA-. Moving from darkness to a low light intensity increases the electron flux through PSI, alleviates the bottleneck of electron transport through the Cyt b6f complex, thereby increasing Fqʹ/Fmʹ and decreasing the re-oxidation time of QA-. Addition factors such as higher downregulation under dark-adapted conditions may also contribute to the increase in Fqʹ/Fmʹ under low light intensities.
Ratio of electron transport to gross O2 evolution
Electrons are transferred from PSII (where O2 is evolved) to an intermediate plastoquinone pool and eventually to ferredoxin to produce NADPH [59]. A minimum of four moles of electrons are transported through PSII for each mole of O2 evolved at PSII. Most higher plants exhibit a linear correlation between gross O2 evolution and electron transport rate [60]. In microalgae, this relationship is often ambiguous, especially at high light intensities where the relationship can become non-linear [61, 62].
Here we show that at low light intensities, the ratio of PSII electron transport to gross O2 evolution (Φeα) is close to a 4:1 ratio for all N-sources treatments. However, when light intensities exceed 150 μmol photons m-2 s-1, Φe declines as ETR saturates at a higher light intensity (~ 900 μmol photons m-2 s-1) than E0 (~ 400 μmol photons m-2 s-1). Similar responses have been reported for diatoms [63], microalgae [64] and the Baltic cyanobacteria, Nostoc [65]. Few studies have measured O2 production rates in Trichodesmium [47, 66] and to our knowledge none have reported concurrent PSII electron transport rates.
Interestingly, we calculated a higher Φe for the N2 cultures than for the NH4+ and NO3- cultures, irrespective of using the light-limited or -saturated rates. This may be due to overestimating the proportion of light absorbed by PSII in the non-diazotrophic growth conditions (i.e. NH4+ and NO3-) relative to the diazotrophic condition. Here we assumed that 50% of absorbed light was directed to PSII reaction centres and 50% to PSI reaction centres (i.e. FAQPII of 0.5). It’s likely that FAQPII was overestimated for diazotrophic treatment (i.e. N2) which may have had a higher ratio of PSI:PSII to support significant rates of cyclic photophosphorylation. In addition, non-diazotrophic cells may undergo more pronounced state transitions with phycobilin proteins being redistributed between PSII and PSI. Finally, a Φe > 4 could be accredited to cyclic electron flow around PSII, which may act a mechanism to dissipate excess excitation energy under high light [67].
Implications for future oligotrophic oceans
In N-limited regions of the oligotrophic open ocean, diazotrophy provides a competitive advantage by allowing cells to access N2 as an N-source against faster growing phytoplankton that rely on fixed N. Current ocean models predict a poleward shift in the 20 °C isotherm which could extend Trichodesmium’s niche into higher latitudes. On a global scale, this niche expansion is driven by increased SSTs; however, on regional scales persistence in an area may be dictated by Trichodesmium’s response to fluctuating nutrient regimes.
At the surface in oligotrophic waters, Trichodesmium is unlikely to encounter NO2-, NO3- or NH4+ concentrations in excess of 0.1 μM [68], except during mixing events. While Trichodesmium is commonly observed in the upper meters of the water column [69], observations have been recorded down to 200 m depth [70]. Thus, Trichodesmium colonies and free trichomes are able to migrate to the nutricline [30, 31]. Such vertical migration has been suggested to allow luxury uptake of phosphates before colonies return to the surface. In addition to encountering phosphates, Trichodesmium will also encounter high concentrations of NO3- in the nutricline. As such, NO3- uptake is likely at these greater depths or at the surface after a mixing event.
Mulholland et al. [17] reported significant NO3- uptake rates with the addition of 1 μM NO3- to the growth media. Furthermore, Karl et al. [30] showed that concentrations of dissolved NH4+ reached 1.5 μM L-1 and dissolved organic N (DON) reaching 13 μM L-1 during a natural bloom of Trichodesmium spp. in the North Pacific gyre. These concentrations are far greater than typical oceanic N pools and could therefore be high enough to inhibit N2 fixation rates [44]. It’s therefore possible that Trichodesmium colonies at depth may be utilising more combined N-sources than the blooms frequently measured on the surface. The energy and reductant conserved through utilising additional N-sources could significantly enhance Trichodesmium’s productivity and growth which could have major implications for biogeochemical cycles.
Our results indicate the need to seek more information on the potential for natural populations of Trichodesmium to uptake fixed N-sources (e.g. NO3-, NH4+, labile dissolved organic nitrogen (DON)) at concentrations that migrating colonies or trichomes experience in the nutricline or that are encountered transiently after deep mixing events. The potential significance of Trichodesmium assimilating fixed N is indicated by a modelling study by McGillicuddy [33] which concluded that to obtain realistic simulations of biomass and export production Trichodesmium populations in the North Atlantic must utilise fixed N. Specifically, this study indicated that 15–20% of the N quota of Trichodesmium could be due to uptake of NO3- and NH4+. Furthermore, although uptake of NO3-, NH4+ or DON will decrease N2 fixation rates in the short-term, as these N-sources are depleted over longer time periods, the increase in Trichodesmium biomass may lead to increased N2 fixation and greater competition for other nutrients including Fe and P.
Supporting information
(A) The fluorescence excitation was measured on a 2 mL concentrated sample treated with 20 μM DCMU (final concentration) [71]. Trichodesmium cells were acclimated to 150 μmol photons m-2 s-1 on a 14:10 light:dark cycle, 26 °C and ambient CO2. The sample was measured using a FluorWin fluorometer scanning between 400 to 715 nm at a 1 nm resolution, with the monochromator on the detector set to 730 nm emission [72]. Spectral correction factors were calculated using the FastPro8. (B) An example of an in vivo light absorption spectra of T. erythraeum IMS101 when spectrally corrected to the Culture, MIMS or FRRf LED spectra.
(TIF)
(A) The light absorption spectra of chlorophyll a (Chla), photoprotectant carotenoid (PPC), phycoerythrin (PE), plastocyanin (PC) and alloplastocyanin (APC) pigments. (B) The light absorption spectra of phycourobilin (PUB) pigments. Each pigment spectra was normalised to the maximum peak (λ = 400–700 nm).
(TIF)
The pH and TCO2 was measured directly, while the pCO2 concentrations were calculated via CO2SYS using the same constants as described in Boatman et al. [45] and S1 File.
(TIF)
Cultures were acclimated to three N-sources (N2, NH4+ and NO3-), at a target CO2 concentration (380 μatm), saturating light intensity (400 μmol photons m-2 s-1) and optimal temperature (26 °C).
(TIF)
Cultures were acclimated to three N-sources (N2, NH4+ and NO3-), at a target CO2 concentration (380 μatm), saturating light intensity (400 μmol photons m-2 s-1) and optimal temperature (26 °C). Pigments include chlorophyll a (Chla), photoprotectant carotenoid (PPC), phycourobilins (PUB1, PUB2, PUBx, PUB4, PUB5a, PUBb, PUB5d, PUB5g and PUB5j), phycoerythrin (PE1, PE2a, PE2b and PE3b), alloplastocyanin (APC) and plastocyanin (PC1 and PC2).
(TIF)
Cultures were acclimated to N2-only, at a target CO2 concentration (380 μatm), saturating light intensity (400 μmol photons m-2 s-1) and optimal temperature (26 °C).
(TIF)
Cultures were acclimated to a replete NH4+ concentration, at a target CO2 concentration (380 μatm), saturating light intensity (400 μmol photons m-2 s-1) and optimal temperature (26 °C).
(TIF)
Cultures were acclimated to a replete NO3- concentration, at a target CO2 concentration (380 μatm), saturating light intensity (400 μmol photons m-2 s-1) and optimal temperature (26 °C).
(TIF)
Abbreviations; E0mChl, the Chla -specific maximum gross O2 evolution rate; PmChl, the Chla -specific maximum net O2 evolution rate; αgChl and αnChl are the Chla -specific initial slopes the light response curve for net and gross photosynthesis; RdChl, the Chla-specific dark respiration rate. The r2 values of all curve fits were > 0.982. Letters in parenthesis indicate significant differences between CO2 treatments (One Way ANOVA, Tukey post hoc test; P < .05); where [B] is significantly greater than [A] and [C] is significantly greater than [B] and [A].
(PDF)
Abbreviations; ETRmChl, the Chla-specific maximum electron transport rate; αETRChl, the Chla-specific initial slope of the electron transport rate light response curve; βETRChl, the Chla-specific light saturated slope of the electron transport rate light response curve. The r2 values of all curve fits were > 0.977. Letters in parenthesis indicate significant differences between N-source treatments (One Way ANOVA, Tukey post hoc test; P < .05); where [B] is significantly greater than [A] and [C] is significantly greater than [B] and [A].
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
Tobias Boatman was supported by a UK Natural Environment Research Council PhD studentship (NE/J500379/1 DTB).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Tobias Boatman was supported by a UK Natural Environment Research Council PhD studentship (NE/J500379/1 DTB). Funding obtained by RJG and TL.
References
- 1.Moore CM, Mills MM, Langlois R, Milne A, Achterberg EP, La Roche J, et al. Relative influence of nitrogen and phosphorus availability on phytoplankton physiology and productivity in the oligotrophic sub-tropical North Atlantic Ocean. Limnology and Oceanography. 2008;53(1):291–305. [Google Scholar]
- 2.Moore JK, Doney SC, Lindsay K, Mahowald N, Michaels AF. Nitrogen fixation amplifies the ocean biogeochemical response to decadal timescale variations in mineral dust deposition. Tellus B. 2006;58(5):560–72. [Google Scholar]
- 3.Vitousek PM, Howarth RW. Nitrogen limitation on land and in the sea: how can it occur? Biogeochemistry. 1991;13(2):87–115. [Google Scholar]
- 4.Dugdale R, Goering J. Uptake of new and regenerated forms of nitrogen in primary productivity. Limnology and Oceanography. 1967:196–206. [Google Scholar]
- 5.Gruber N, Sarmiento JL. Global patterns of marine nitrogen fixation and denitrification. Global Biogeochemical Cycles. 1997;11(2):235–66. [Google Scholar]
- 6.Coles VJ, Hood RR, Pascual M, Capone DG. Modeling the impact of Trichodesmium and nitrogen fixation in the Atlantic Ocean. Journal of Geophysical Research. 2004;109:C06007. [Google Scholar]
- 7.Hood RR, Coles VJ, Capone DG. Modeling the distribution of Trichodesmium and nitrogen fixation in the Atlantic Ocean. Journal of Geophysical Research. 2004;109(6):L06301. [Google Scholar]
- 8.Campbell L, Carpenter E, Montoya J, Kustka A, Capone D. Picoplankton community structure within and outside a Trichodesmium bloom in the southwestern Pacific Ocean. Vie et milieu. 2005;55(3–4):185–95. [Google Scholar]
- 9.Capone DG, Zehr JP, Paerl HW, Bergman B, Carpenter EJ. Trichodesmium, a globally significant marine cyanobacterium. Science. 1997;276(5316):1221–9. doi: 10.1126/science.276.5316.1221 [Google Scholar]
- 10.Carpenter EJ, Capone DG. Nitrogen fixation in Trichodesmium blooms. Marine Pelagic Cyanobacteria: Trichodesmium and other Diazotrophs. 1992;362:211–7. [Google Scholar]
- 11.Zehr JP, Bench SR, Carter BJ, Hewson I, Niazi F, Shi T, et al. Globally distributed uncultivated oceanic N2-fixing cyanobacteria lack oxygenic photosystem II. Science. 2008;322(5904):1110–2. doi: 10.1126/science.1165340 [DOI] [PubMed] [Google Scholar]
- 12.Zehr JP, Waterbury JB, Turner PJ, Montoya JP, Omoregie E, Steward GF, et al. Unicellular cyanobacteria fix N2 in the subtropical North Pacific Ocean. Nature. 2001;412(6847):635–7. doi: 10.1038/35088063 [DOI] [PubMed] [Google Scholar]
- 13.Goering JJ, Dugdale RC, Menzel DW. Estimates of in situ rates of nitrogen uptake by Trichodesmium sp. in the tropical Atlantic Ocean. Limnology and Oceanography. 1966:614–20. [Google Scholar]
- 14.Carpenter EJ, McCarthy JJ. Nitrogen fixation and uptake of combined nitrogenous nutrients by Oscillatoria (Trichodesmium) thiebautii in the western Sargasso Sea. Limnology and Oceanography. 1975;20(3):389–401. [Google Scholar]
- 15.Glibert P, Banahan S. Uptake of combined nitrogen sources by Trichodesmium and pelagic microplankton in the Caribbean Sea: comparative uptake capacity and nutritional status. EOS. 1988;69(1089):3996–4000. [Google Scholar]
- 16.Mulholland MR, Ohki K, Capone DG. Nitrogen utilization and metabolism relative to patterns of N2 fixation in cultures of Trichodesmium INIBB1067. Journal of Phycology. 1999;35(5):977–88. doi: 10.1046/j.1529-8817.1999.3550977.x [Google Scholar]
- 17.Mulholland MR, Ohki K, Capone DG. Nutrient controls on nitrogen uptake and metabolism by natural populations and cultures of Trichodesmium (Cyanobacteria). Journal of Phycology. 2001;37(6):1001–9. [Google Scholar]
- 18.Mulholland MR, Capone DG. Nitrogen fixation, uptake and metabolism in natural and cultured populations of Trichodesmium spp. Marine Ecology Progress Series. 1999;188:33–49. [Google Scholar]
- 19.Zehr JP, Jenkins BD, Short SM, Steward GF. Nitrogenase gene diversity and microbial community structure: a cross system comparison. Environmental Microbiology. 2003;5(7):539–54. [DOI] [PubMed] [Google Scholar]
- 20.Großkopf T, LaRoche J. Direct and indirect costs of dinitrogen fixation in Crocosphaera watsonii WH8501 and possible implications for the nitrogen cycle. Frontiers in Microbiology. 2012;3(236):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berman-Frank I, Lundgren P, Chen YB, Küpper H, Kolber Z, Bergman B, et al. Segregation of nitrogen fixation and oxygenic photosynthesis in the marine cyanobacterium Trichodesmium. Science. 2001;294(5546):1534–7. doi: 10.1126/science.1064082 [DOI] [PubMed] [Google Scholar]
- 22.Capone DG, O’Neil JM, Zehr J, Carpenter EJ. Basis for diel variation in nitrogenase activity in the marine planktonic cyanobacterium Trichodesmium thiebautii. Applied and Environmental Microbiology. 1990;56(11):3532–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sandh G, Ran L, Xu L, Sundqvist G, Bulone V, Bergman B. Comparative proteomic profiles of the marine cyanobacterium Trichodesmium erythraeum IMS101 under different nitrogen regimes. Proteomics. 2011;11(3):406–19. doi: 10.1002/pmic.201000382 [DOI] [PubMed] [Google Scholar]
- 24.Küpper H, Ferimazova N, Setlík I, Berman-Frank I. Traffic lights in Trichodesmium. regulation of photosynthesis for nitrogen fixation studied by chlorophyll fluorescence kinetic microscopy. Plant Physiology. 2004;135(4):2120–33. doi: 10.1104/pp.104.045963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bergman B, Sandh G, Lin S, Larsson J, Carpenter EJ. Trichodesmium–a widespread marine cyanobacterium with unusual nitrogen fixation properties. FEMS Microbiology Reviews. 2012;37(3):286–302. doi: 10.1111/j.1574-6976.2012.00352.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Finzi-Hart JA, Pett-Ridge J, Weber PK, Popa R, Fallon SJ, Gunderson T, et al. Fixation and fate of C and N in the cyanobacterium Trichodesmium using nanometer-scale secondary ion mass spectrometry. Proceedings of the National Academy of Sciences. 2009;106(15):6345–50. doi: 10.1073/pnas.0810547106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohki K, Zehr JP, Falkowski PG, Fujita Y. Regulation of nitrogen-fixation by different nitrogen sources in the marine non-heterocystous cyanobacterium Trichodesmium sp. NIBB1067. Archives of Microbiology. 1991;156(5):335–7. [Google Scholar]
- 28.Helbling EW, VillafañE V, Holm-Hansen O. Effects of ultraviolet radiation on Antarctic marine phytoplankton photosynthesis with particular attention to the influence of mixing: Wiley Online Library; 1994. [Google Scholar]
- 29.Doney SC. Oceanography: Plankton in a warmer world. Nature. 2006;444(7120):695–6. doi: 10.1038/444695a [DOI] [PubMed] [Google Scholar]
- 30.Karl D, Michaels A, Bergman B, Capone D, Carpenter E, Letelier R, et al. Dinitrogen fixation in the world’s oceans. Biogeochemistry. 2002;57(1):47–98. [Google Scholar]
- 31.Villareal TA, Carpenter EJ. Buoyancy regulation and the potential for vertical migration in the oceanic cyanobacterium Trichodesmium. Microbial Ecology. 2003;45(1):1–10. doi: 10.1007/s00248-002-1012-5 [DOI] [PubMed] [Google Scholar]
- 32.Davis CS, McGillicuddy DJ. Transatlantic abundance of the N2-Fixing colonial cyanobacterium Trichodesmium. Science. 2006;312(5779):1517–20. doi: 10.1126/science.1123570 [DOI] [PubMed] [Google Scholar]
- 33.McGillicuddy DJ. Do Trichodesmium spp. populations in the North Atlantic export most of the nitrogen they fix? Global Biogeochemical Cycles. 2014;28(2):103–14. [Google Scholar]
- 34.Chen YB, Zehr JP, Mellon M. Growth and nitrogen fixation of the diazotrophic filamentous nonheterocystous cyanobacterium Trichodesmium Sp. IMS 101 in defined media: evidence for a circadian rhythm. Journal of Phycology. 1996;32(6):916–23. [Google Scholar]
- 35.McKew BA, Davey P, Finch SJ, Hopkins J, Lefebvre SC, Metodiev MV, et al. The trade-off between the light-harvesting and photoprotective functions of fucoxanthin-chlorophyll proteins dominates light acclimation in Emiliania huxleyi (clone CCMP 1516). New Phytologist. 2013. [DOI] [PubMed] [Google Scholar]
- 36.Radmer RJ, Kok B. Photoreduction of O2 primes and replaces CO2 assimilation. Plant Physiology. 1976;58(3):336–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Platt T, Jassby AD. The relationship between photosynthesis and light for natural assemblages of coastal marine phytoplankton. Journal of Phycology. 1976;12(4):421–30. doi: 10.1111/j.1529-8817.1976.tb02866.x [Google Scholar]
- 38.Kolber ZS, Van Dover C, Niederman R, Falkowski P. Bacterial photosynthesis in surface waters of the open ocean. Nature. 2000;407(6801):177–9. doi: 10.1038/35025044 [DOI] [PubMed] [Google Scholar]
- 39.Johnsen G, Sakshaug E. Biooptical characteristics of PSII and PSI in 33 species (13 pigment groups) of marine phytoplankton, and the relevance for pulse-amplitude-modulated and fast-repetition-rate fluorometry. Journal of Phycology. 2007;43(6):1236–51. [Google Scholar]
- 40.Kromkamp JC, Forster RM. The use of variable fluorescence measurements in aquatic ecosystems: differences between multiple and single turnover measuring protocols and suggested terminology. European Journal of Phycology. 2003;38(2):103–12. [Google Scholar]
- 41.Woźniak B, Dera J, Ficek D, Majchrowski R, Kaczmarek S, Ostrowska M, et al. Modelling the influence of acclimation on the absorption properties of marine phytoplankton. Oceanologia. 1999;(41 (2)):187–210. [Google Scholar]
- 42.Küpper H, Andresen E, Wiegert S, Šimek M, Leitenmaier B, Šetlík I. Reversible coupling of individual phycobiliprotein isoforms during state transitions in the cyanobacterium Trichodesmium analysed by single-cell fluorescence kinetic measurements. Biochimica et Biophysica Acta (BBA)-Bioenergetics. 2009;1787(3):155–67. [DOI] [PubMed] [Google Scholar]
- 43.Eichner M, Kranz SA, Rost B. Combined effects of different CO2 levels and N sources on the diazotrophic cyanobacterium Trichodesmium. Physiologia Plantarum. 2014;152(2):316–30. doi: 10.1111/ppl.12172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holl CM, Montoya JP. Interations between nitrate uptake and nitrogen fixation in continuous cultures of the marine Diazotroph Trichodesmium (Cyanobacteria). Journal of Phycology. 2005;41(6):1178–83. doi: 10.1111/j.1529-8817.2005.00146.x [Google Scholar]
- 45.Boatman TG, Lawson T, Geider RJ. A Key Marine Diazotroph in a Changing Ocean: The Interacting Effects of Temperature, CO2 and Light on the Growth of Trichodesmium erythraeum IMS101. PLoS ONE. 2017;12(1):e0168796 doi: 10.1371/journal.pone.0168796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mulholland MR, Capone DG. The nitrogen physiology of the marine N2-fixing cyanobacteria Trichodesmium spp. Trends in Plant Science. 2000;5(4):148–53. doi: 10.1016/s1360-1385(00)01576-4 [DOI] [PubMed] [Google Scholar]
- 47.Levitan O, Rosenberg G, Setlik I, Setlikova E, Grigel J, Klepetar J, et al. Elevated CO2 enhances nitrogen fixation and growth in the marine cyanobacterium Trichodesmium. Global Change Biology. 2007;13(2):531–8. doi: 10.1111/j.1365-2486.2006.01314.x [Google Scholar]
- 48.Levitan O, Sudhaus S, LaRoche J, Berman-Frank I. The influence of pCO2 and temperature on gene expression of carbon and nitrogen pathways in Trichodesmium IMS101. PLoS ONE. 2010;5(12):e15104 doi: 10.1371/journal.pone.0015104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown CM, MacKinnon JD, Cockshutt AM, Villareal TA, Campbell DA. Flux capacities and acclimation costs in Trichodesmium from the Gulf of Mexico. Marine Biology. 2008;154(3):413–22. [Google Scholar]
- 50.Berman-Frank I, Cullen JT, Shaked Y, Sherrell RMF, P.G. Iron availability, cellular iron quotas, and nitrogen fixation in Trichodesmium. Limnology and Oceanography. 2001;46(6):1249–60. [Google Scholar]
- 51.Berman-Frank I, Quigg A, Finkel ZV, Irwin AJ, Haramaty L. Nitrogen-fixation strategies and Fe requirements in cyanobacteria. Limnology and Oceanography. 2007;52(5):2260–9. [Google Scholar]
- 52.Milligan AJ, Berman-Frank I, Gerchman Y, Dismukes GC, Falkowski PG. Light-dependent oxygen consumption in nitrogen-fixing cyanobacteria plays a key role in nitrogenase protection. Journal of Phycology. 2007;43(5):845–52. [Google Scholar]
- 53.Baker NR, Harbinson J, Kramer DM. Determining the limitations and regulation of photosynthetic energy transduction in leaves. Plant, Cell and Environment. 2007;30(9):1107–25. doi: 10.1111/j.1365-3040.2007.01680.x [DOI] [PubMed] [Google Scholar]
- 54.Mulholland MR, Capone DG. Stoichiometry of nitrogen and carbon utilization in cultured populations of Trichodesmium IMS101: implications for growth. Limnology and Oceanography. 2001;46(2):436–43. [Google Scholar]
- 55.Guerrero M, Lara C. Assimilation of inorganic nitrogen. The Cyanobacteria. 1987:163–86. [Google Scholar]
- 56.Luque I, Flores E, Herrero A. Molecular mechanism for the operation of nitrogen control in cyanobacteria. The EMBO journal. 1994;13(12):2862–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raven JA, Johnston AM. Mechanisms of inorganic-carbon acquisition in marine phytoplankton and their implications for the use of other resources. Limnology and Oceanography. 1991;36(8):1701–14. [Google Scholar]
- 58.Campbell D, Hurry V, Clarke AK, Gustafsson P, Oquist G. Chlorophyll fluorescence analysis of cyanobacterial photosynthesis and acclimation. Microbiology and Molecular Biology Reviews. 1998;62(3):667–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Edwards G, Walker DA. C3, C4: mechanisms, and cellular and environmental regulation, of photosynthesis: Blackwell Scientific Publications; 1983. [Google Scholar]
- 60.Fryer MJ, Andrews JR, Oxborough K, Blowers DA, Baker NR. Relationship between CO2 assimilation, photosynthetic electron transport, and active O2 metabolism in leaves of maize in the field during periods of low temperature. Plant Physiology. 1998;116(2):571–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carr H, Björk M. A methodological comparison of photosynthetic oxygen evolution and estimated electron transport rate in tropical Ulva (Chlorophyceae) species under different light and inorganic carbon conditions. Journal of Phycology. 2003;39(6):1125–31. [Google Scholar]
- 62.Suggett DJ, MacIntyre HL, Kana TM, Geider RJ. Comparing electron transport with gas exchange: parameterising exchange rates between alternative photosynthetic currencies for eukaryotic phytoplankton. Aquatic Microbial Ecology. 2009;56:147–62. [Google Scholar]
- 63.Geel C, Versluis W, Snel JF. Estimation of oxygen evolution by marine phytoplankton from measurement of the efficiency of Photosystem II electron flow. Photosynthesis Research. 1997;51(1):61–70. [Google Scholar]
- 64.Flameling IA, Kromkamp J. Light dependence of quantum yields for PSII charge separation and oxygen evolution in eucaryotic algae. Limnology and Oceanography. 1998;43(2):284–97. [Google Scholar]
- 65.Sundberg B, Campbell D, Palmqvist K. Predicting CO2 gain and photosynthetic light acclimation from fluorescence yield and quenching in cyano-lichens. Planta. 1997;201(2):138–45. [Google Scholar]
- 66.Kranz SA, Levitan O, Richter KU, Prášil O, Berman-Frank I, Rost B. Combined effects of CO2 and light on the N2-fixing cyanobacterium Trichodesmium IMS101: physiological responses. Plant Physiology. 2010;154(1):334–45. doi: 10.1104/pp.110.159145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Falkowski PG, Wyman K, Ley AC, Mauzerall DC. Relationship of steady-state photosynthesis to fluorescence in eucaryotic algae. Biochimica et Biophysica Acta (BBA)-Bioenergetics. 1986;849(2):183–92. [Google Scholar]
- 68.Morel A. Available, usable, and stored radiant energy in relation to marine photosynthesis. Deep Sea Research. 1978;25(8):673–88. [Google Scholar]
- 69.Breitbarth E, Wohlers J, Klas J, LaRoche J, Peeken I. Nitrogen fixation and growth rates of Trichodesmium IMS-101 as a function of light intensity. Marine Ecology Progress Series. 2008;359:25–36. [Google Scholar]
- 70.Letelier RM, Karl DM. Role of Trichodesmium spp. in the productivity of the subtropical North Pacific Ocean. Marine Ecology Progress Series. 1996;133:263–73. [Google Scholar]
- 71.Silsbe GM, Oxborough K, Suggett DJ, Forster RM, Ihnken S, Komárek O, et al. Toward autonomous measurements of photosynthetic electron transport rates: An evaluation of active fluorescence‐based measurements of photochemistry. Limnology and Oceanography: Methods. 2015;13(3):138–55. [Google Scholar]
- 72.Suggett DJ, MacIntyre HL, Geider RJ. Evaluation of biophysical and optical determinations of light absorption by photosystem II in phytoplankton. Limnology and Oceanography: Methods. 2004;2:316–32. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) The fluorescence excitation was measured on a 2 mL concentrated sample treated with 20 μM DCMU (final concentration) [71]. Trichodesmium cells were acclimated to 150 μmol photons m-2 s-1 on a 14:10 light:dark cycle, 26 °C and ambient CO2. The sample was measured using a FluorWin fluorometer scanning between 400 to 715 nm at a 1 nm resolution, with the monochromator on the detector set to 730 nm emission [72]. Spectral correction factors were calculated using the FastPro8. (B) An example of an in vivo light absorption spectra of T. erythraeum IMS101 when spectrally corrected to the Culture, MIMS or FRRf LED spectra.
(TIF)
(A) The light absorption spectra of chlorophyll a (Chla), photoprotectant carotenoid (PPC), phycoerythrin (PE), plastocyanin (PC) and alloplastocyanin (APC) pigments. (B) The light absorption spectra of phycourobilin (PUB) pigments. Each pigment spectra was normalised to the maximum peak (λ = 400–700 nm).
(TIF)
The pH and TCO2 was measured directly, while the pCO2 concentrations were calculated via CO2SYS using the same constants as described in Boatman et al. [45] and S1 File.
(TIF)
Cultures were acclimated to three N-sources (N2, NH4+ and NO3-), at a target CO2 concentration (380 μatm), saturating light intensity (400 μmol photons m-2 s-1) and optimal temperature (26 °C).
(TIF)
Cultures were acclimated to three N-sources (N2, NH4+ and NO3-), at a target CO2 concentration (380 μatm), saturating light intensity (400 μmol photons m-2 s-1) and optimal temperature (26 °C). Pigments include chlorophyll a (Chla), photoprotectant carotenoid (PPC), phycourobilins (PUB1, PUB2, PUBx, PUB4, PUB5a, PUBb, PUB5d, PUB5g and PUB5j), phycoerythrin (PE1, PE2a, PE2b and PE3b), alloplastocyanin (APC) and plastocyanin (PC1 and PC2).
(TIF)
Cultures were acclimated to N2-only, at a target CO2 concentration (380 μatm), saturating light intensity (400 μmol photons m-2 s-1) and optimal temperature (26 °C).
(TIF)
Cultures were acclimated to a replete NH4+ concentration, at a target CO2 concentration (380 μatm), saturating light intensity (400 μmol photons m-2 s-1) and optimal temperature (26 °C).
(TIF)
Cultures were acclimated to a replete NO3- concentration, at a target CO2 concentration (380 μatm), saturating light intensity (400 μmol photons m-2 s-1) and optimal temperature (26 °C).
(TIF)
Abbreviations; E0mChl, the Chla -specific maximum gross O2 evolution rate; PmChl, the Chla -specific maximum net O2 evolution rate; αgChl and αnChl are the Chla -specific initial slopes the light response curve for net and gross photosynthesis; RdChl, the Chla-specific dark respiration rate. The r2 values of all curve fits were > 0.982. Letters in parenthesis indicate significant differences between CO2 treatments (One Way ANOVA, Tukey post hoc test; P < .05); where [B] is significantly greater than [A] and [C] is significantly greater than [B] and [A].
(PDF)
Abbreviations; ETRmChl, the Chla-specific maximum electron transport rate; αETRChl, the Chla-specific initial slope of the electron transport rate light response curve; βETRChl, the Chla-specific light saturated slope of the electron transport rate light response curve. The r2 values of all curve fits were > 0.977. Letters in parenthesis indicate significant differences between N-source treatments (One Way ANOVA, Tukey post hoc test; P < .05); where [B] is significantly greater than [A] and [C] is significantly greater than [B] and [A].
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.