Abstract
In many species of phytoplankton, simple photoreceptors monitor ambient lighting. Photoreceptors provide a number of selective advantages including the ability to assess the time of day for circadian rhythms, seasonal changes, and the detection of excessive light intensities and harmful UV light. Photoreceptors also serve as depth gauges in the water column for behaviors such as diurnal vertical migration. Photoreceptors can be organized together with screening pigment into visible eyespots. In a wide variety of motile phytoplankton, including Chlamydomonas, Volvox, Euglena, and Kryptoperidinium, eyespots are light-sensitive organelles residing within the cell. Eyespots are composed of photoreceptor proteins and typically red to orange carotenoid screening pigments. This association of photosensory pigment with screening pigment allows for detection of light directionality, needed for light-guided behaviors such as positive and negative phototaxis. In Chlamydomonas, the eyespot is located in the chloroplast and Chlamydomonas expresses a number of photosensory pigments including the microbial channelrhodopsins (ChR1 and ChR2). Dinoflagellates are unicellular protists that are ecologically important constituents of the phytoplankton. They display a great deal of diversity in morphology, nutritional modes and symbioses, and can be photosynthetic or heterotrophic, feeding on smaller phytoplankton. Dinoflagellates, such as Kryptoperidinium foliaceum, have eyespots that are used for light-mediated tasks including phototaxis. Dinoflagellates belonging to the family Warnowiaceae have a more elaborate eye. Their eye-organelle, called an ocelloid, is a large, elaborate structure consisting of a focusing lens, highly ordered retinal membranes, and a shield of dark pigment. This complex eye-organelle is similar to multicellular camera eyes, such as our own. Unraveling the molecular makeup, structure and function of dinoflagellate eyes, as well as light-guided behaviors in phytoplankton can inform us about the selective forces that drove evolution in the important steps from light detection to vision. We show here that the evolution from simple photoreception to vision seems to have independently followed identical paths and principles in phytoplankton and animals, significantly strengthening our understanding of this important biological process.
Introduction
Phytoplankton are microscopic single-celled organisms and include unicellular algae and cyanobacteria. They are found in aquatic and marine environments, and they contribute significantly to global productivity through photosynthesis (Taylor 1987; Tomas 1996). Phytoplankton range in size from less than a micron to several millimeters for some large colonial species, such as Volvox. Despite their small size, phytoplankton display a remarkable diversity and complexity in the types of photosensory pigments and light-detecting systems that they use. Their eyespots may have reflective properties, use shading of light to obtain directionality, and lenses to focus light (Foster and Smyth 1980; Kreimer 1999; Hegemann 2008; Hegemann and Dieckmann 2011). In some phytoplankton, the single cell houses optical systems rivaling vertebrate eyes in complexity. Further, phytoplankton express a large number of photoreceptor proteins that use bound chromophores such as retinal in retinylidene proteins (microbial rhodopsin), flavin in flavoproteins, pterins and flavins in cryptochromes, and bilin in biliproteins such as phytochrome (Hegemann 2008; Hegemann and Dieckmann 2011).
Simple photoreceptors provide input for simple, and yet, important behaviors and more complex structures serve more advanced behaviors. Major parts of the evolutionary progression seen in animals, from nondirectional photoreception to high-resolution vision (Nilsson 2013), is mirrored within the single cells of phytoplankton. In both animals and phytoplankton, selection on the photoreceptive systems acts primarily on the fitness of the behavior, and this in turn causes selection on the performance of the sensory systems. Based on animal photoreceptive systems, the sensory tasks were placed into four major classes calling for gradually more complex structures (Nilsson 2013), and this classification can be successfully applied to photoreception in phytoplankton. The simplest task (class I) is nondirectional photoreception, used for monitoring variations in the general ambient intensity. This is followed by directional photoreception (class II) used for phototaxis and for body/cell orientation. Further elaboration leads to low-resolution vision (class III) used for orientation and habitat selection, and finally to high-resolution vision (class IV) required for detection and identification of prey, predators, and conspecifics. In phytoplankton, classes I and II are well represented, and as we shall see, there are also examples of higher classes.
Motile phytoplankton have evolved specialized photoreceptors for monitoring ambient lighting (class I). These simple photoreceptors function to regulate their exposure to intense sunlight and harmful UV irradiation as well as for assessing the time of day for circadian rhythms, shadow detection, seasonal changes such as day length and depth in the water column (Dodge and Crawford 1969; Foster and Smyth 1980; Hegemann 2008; Hegemann and Dieckmann 2011). These functions require expression of a photosensory pigment as well as signal transmission to a motile organelle, but structurally, such systems can be morphologically inconspicuous.
Many motile algae and motile stages of nonmotile algae, such as gametes, contain photoreceptors organized into a more complex structure called an eyespot or a stigma. The eyespot is an organelle that contains osmiophillic globules or granules that are made up of reddish-orange colored carotenoid pigment. Eyespots are common in flagellated phytoplankton, and have been found in Chlorophyceace, Euglenophyceae, Dinoflagellata, Haptophyta, Cryptophyceae, Prasinophyceae, Xanthophyceae, Chrysophyceae, Phaeophyceae (Dodge 1969, 1984; Hegemann 2008; Hegemann and Dieckmann 2011). This association of photosensory pigment with carotenoid pigment in eyespots allows for phytoplankton to detect light directionality (class II), which enables them to carryout more complex light-guided behaviors such as positive and negative phototaxis (photophobic responses). The addition of screening pigment does not exclude the use also for class I tasks. Information about the orientation of the cell in relation to the light source, as well as the intensity and quality of the light, directs visually guided movement of the cell (Foster and Smyth 1980; Kreimer 1999; Hegemann 2008; Hegemann and Dieckmann 2011). In certain phytoplankton, a visible eyespot is lacking, although a chloroplast or other structures in the cell body perform a shading function for directionality.
Eyespots can be located in the chloroplast and are often found close to the flagella, facilitating signaling between the sensor and effector organelles (Fig. 1A–C). Because phytoplankton lack a brain or a nervous system, the photoreceptive system is directly connected to the output system to control swimming behavior. Following light activation of the photosensory pigment, the light signal is converted into an electrical signal and/or chemical signal and this information is transmitted directly to the flagella. As yet, very little is known about the mechanisms that transmit and process this information within the cell to generate appropriate behaviors (Hartz et al. 2008, 2011).
Fig. 1.
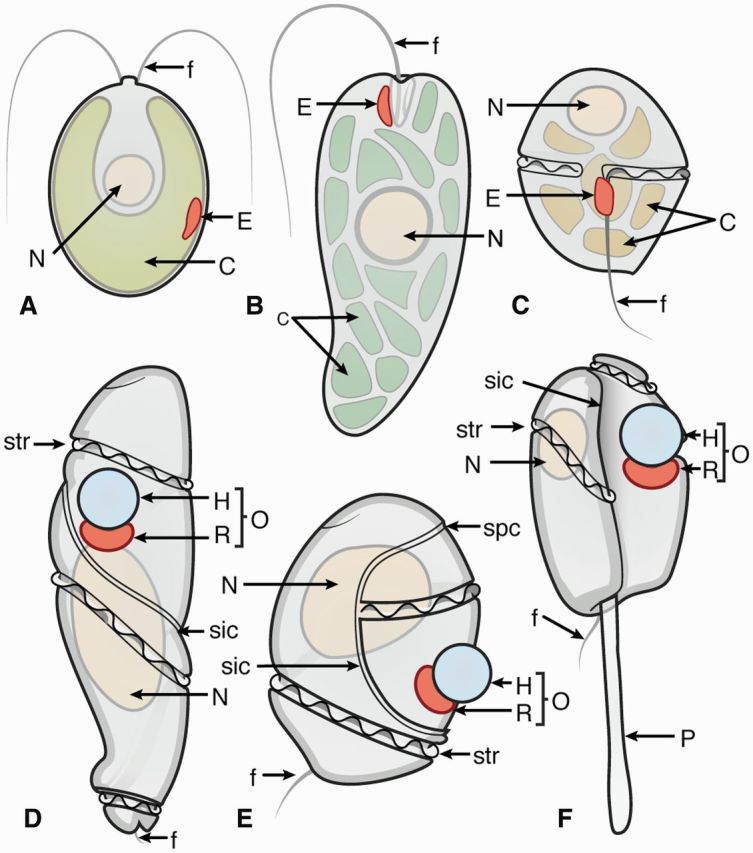
Schematic representations of eyespots and ocelloids in phytoplankton. (A)Chlamydomonas reinhardtii cell with pigmented eyespot (E) located within the chloroplast (C). Adapted from Hegemann and Dieckmann (2011). (B)Euglena gracilis cell with pigmented eyespot (E) in close proximity to the flagella (f). Adapted from Hegemann and Dieckmann (2011). (C)Kryptoperidinium foliaceum cell with pigmented eyespot (E) in close proximity to the flagella (f). Adapted from Dodge and Crawford (1969). (D)Warnowia cell with ocelloid (O). Adapted from Greuet (1987) and Kofoid and Swezy (1921). (E)Nematodinium cell with ocelloid (O). Adapted from Greuet (1987) and Kofoid and Swezy (1921). (F)Erythropsidinium cell with ocelloid (O) and piston (P). Adapted from Greuet (1987) and Kofoid and Swezy (1921). N, nucleus; E, eyespot; C, chloroplast; O, ocelloid; H, hyalosome; R, retinal body; P, piston; f, flagella; sic, intercingular sulcus; spc, precingular sulcus; str, cingular sulcus with transverse flagellum.
Photosensory pigments
Phytoplankton express a wide range of microbial rhodopsins in their photoreceptors (Hegemann 2008; Hegemann and Dieckmann 2011; Zhang et al. 2011). Rhodopsins function to absorb photons of light for cellular signaling and for energy conversion. Two distinct classes of rhodopsins exist; type I and type II rhodopsins. Bacteria, archaea, and protists express a type I (microbial) rhodopsins that are utilized for energy harvesting and sensory functions (Hoff et al. 1997; Giovannoni et al. 2005; Falb et al. 2008; Gonzalez et al. 2009; Gomez-Consarnau et al. 2010; Ernst et al. 2014), whereas metazoans express type II (animal) rhodopsins that are light-sensitive G-protein-coupled receptors (Palczewski et al. 2000; Palczewski 2006; Schmidt et al. 2011; Ernst et al. 2014). Type II rhodopsins are exclusively found in animals where it serves vision as well as nonvisual photoreception for tasks such as circadian clock regulation, pupil dilation, and as photoisomerases (Schmidt et al. 2011; Porter et al. 2012).
Type I and type II rhodopsins display similar topologies with seven transmembrane alpha-helices and with their C-terminus facing inside and their N-terminus facing outside the cell. The chromophore in both type I and type II rhodopsins is an aldehyde of vitamin A, retinal, bound to each protein via a Schiff base to a lysine residue in the seventh transmembrane domain (Ernst et al. 2014). However, despite these similarities in the microbial, type I rhodopsins, retinal is photoisomerized from all-trans to 13-cis and in the animal, type II rhodopsins, retinal is photoisomerized from the 11-cis to the all-trans configuration. Type II rhodopsins are also known to exist with three alternative versions of the vitamin-A chromophore: 3-dehydroretinal, 3-hydroxyretinal, and 4-hydroxyretinal, helping to extend the range of absorption peaks from 350 to 675 nm (Cronin et al. 2014). Type I and type II rhodopsins display very little amino acid identity, strongly suggesting that type I and type II rhodopsins evolved independently (Porter et al. 2012).
The type I rhodopsins known from phytoplankton include channelrhodopsins (ChR1 and ChR2), bacteriorhodopsin (BR, light-driven proton pumps), and sensory rhodopsins and they are derived from a common ancestor (Fig. 2) (Ernst et al. 2014). Phylogenetic trees of type I rhodopsins for several species, including dinoflagellates, reveal proton pumps as well as some rhodopsins more related to algal sensory rhodopsins (Slamovits et al. 2011; Hayakawa et al. 2015). The first microbial rhodopsin was discovered in archaeal species, Halobacterium salinarum and is a light-driven proton pump, BR (Oesterhelt and Stoeckenius 1971, 1973). This discovery was followed by the subsequent identification of halorhodopsin and sensory rhodopsin I and II (HR, SRI, and SRII). In H. salinarum, SRI and SRII function as phototaxis receptors in most conditions, but in conditions of high light, BR also mediates phototaxis (Hoff et al. 1997). Since the discovery of rhodopsins in H. salinarum, results from genome projects have led to the identification of a wide variety of photoreceptive proteins in archaea as well as marine, freshwater, and terrestrial bacteria and protists (unicellular eukaryotes), such as phytoplankton (Ruiz-González and Marín 2004; van der Horst and Hellingwerf 2004; Saranak and Foster 2005; Spudich 2006; Jekely 2009; Slamovits et al. 2011; Ernst et al. 2014). What began as a small group of microbial rhodopsins that function as proton pumps, chloride pumps, and as receptors for phototaxis has now expanded to include a large number of sensory photoreceptors such as cation channels, photosensors, and photoactivated enzymes (Ernst et al. 2014) (Fig. 2). For example, channelrhodopsins in Chlamydomonas are photosensitive cation channels. Further, enzymerhodopsins, with long C-term tails containing histidine kinase and cyclase domains, have been identified in Chlamydomonas and in the tiny marine green algae Ostreococcus tauri. The latter is one of the smallest eurkaryotes known, measuring only 0.8 µm in size. Additional photosensory pigments include photoactivated cyclases that contain blue-light receptors using flavin adenine dinucleotide (FAD, BLUF) and photoactivated adenylate cyclases as well as phototrophins, which are photoactivated serine/threonine kinases containing sensor domains for light, oxygen, and voltage (LOV). Neochromes containing LOV domains, a kinase and an N-terminal phytochrome domain have been identified in the filamentous green alga, Mougeotea. Phytochromes use a bilin chromophore. Finally, blue light activated transcription factors with LOV domains, aureochromes, have been identified in Vaucheria frigida (Xanthophyceae or yellow-green algae), Fucus distichus (Phaeophyceae or brown algae), and the marine diatom, Thalassiosira pseudonana (Dodge and Crawford 1969; Foster and Smyth 1980; Kreimer 1999; Hegemann 2008; Hegemann and Dieckmann 2011; Zhang et al. 2011; Ernst et al. 2014).
Fig. 2.
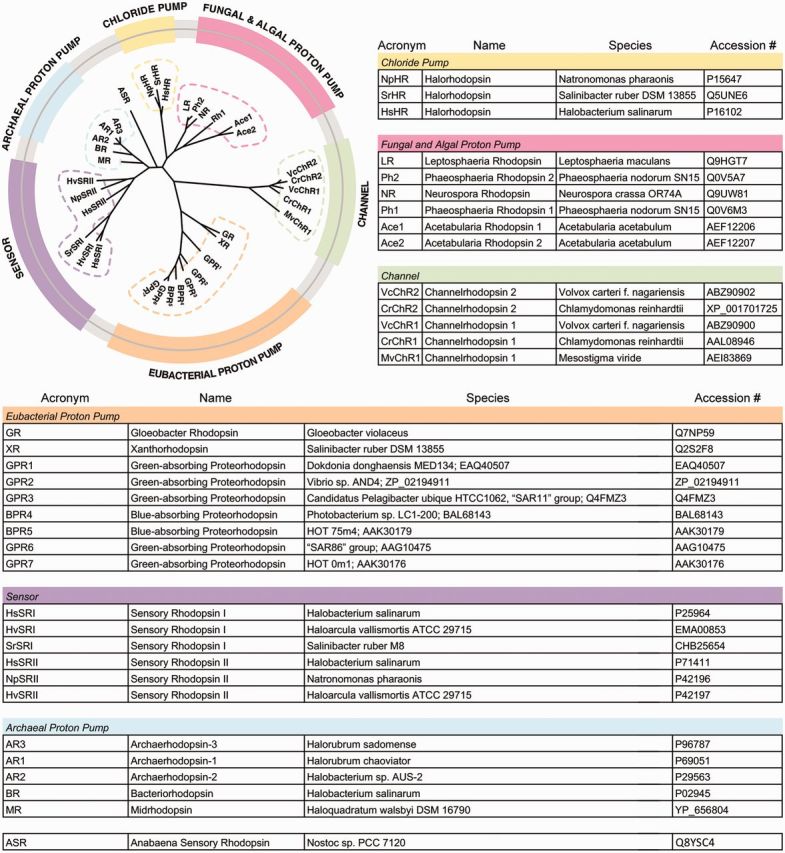
Phylogenetic tree of microbial rhodopsins (adapted from Ernst et al. 2014). Microbial rhodopsins shown are: chloride ion pumps; fungal and algal proton pumps; green, channelrhodopsins; Eubacteria proton pumps; purple, photosensors; archaeal proton pumps. Table with name of photosensory pigment, genus and species, and accession number (adapted from Ernst et al. 2014).
Proteorhodopsins were first found in marine bacterioplankton and are expressed in a large number of bacteria and archaea. In halophillic archaeal species, rhodopsins are thought to be used in the natural environment for photosensing as well for metabolic functions and marine bacteria in the open ocean are also thought to use proteorhodopsins energetically for ATP production (Hoff et al. 1997; Giovannoni et al. 2005; Falb et al. 2008; Gonzalez et al. 2009; Gomez-Consarnau et al. 2010; Slamovits et al. 2011; Ernst et al. 2014). Further, the heterotrophic, predatory dinoflagellate, Oxyrrhis marina, expresses proteorhodopsin, which is encoded by an abundantly expressed nuclear gene. Oxyrrhis marina is on an early branch of dinoflagellate evolution and it has been proposed that its proteorhodopsin may function as a light-stimulated proton pump for acidifying food vacuoles (Saldarriaga et al. 2003; Slamovits et al. 2011). In addition, O. marina displays positive phototaxis that is mediated by rhodopsin located in the outer cell membrane (Hartz et al. 2011).
Photoreception without screening pigment
Algal photoreception has been previously classified on the basis of functional characteristics, position in the cell, anatomy, and structure (Dodge 1969; Foster and Smyth 1980; Barsanti et al. 2012). For example, certain eyespots are adjacent to the flagella and others are located within the chloroplast (Fig. 1A–C). Here we follow the task classification developed for animal photoreceptive systems (Nilsson 2009, 2013) to reveal a striking similarity between single cell phytoplankton and metazoan animals. In phytoplankton, nondirectional photoreception (class I; Nilsson 2013) is represented in species where no carotenoid screening pigment is visible. Many phytoplankton sequences are now available and there is a growing number of examples of species that contain microbial rhodopsins and photosensory pigments but do not display a visible reddish-orange eyespot. Examples include the dinoflagellate, Prorocentrum donghaiense, the diatom, Pseudo-nitzschia granii and the hyptophyte, Phaeocystis globosa. In many species, the cellular location and the function of these newly identified photosensory pigments are not yet known. These photoreceptors may be able to monitor ambient light and may function for class I, nondirectional photoreception. These organisms may be able to regulate their exposure to high light intensities and harmful UV irradiation, assess the time of day for circadian rhythms, detect shade and shadows, and perceive seasonal changes such as day length as well as the depth the organism is in the water column (Dodge and Crawford 1969; Foster and Smyth 1980; Hegemann 2008; Hegemann and Dieckmann 2011). These photoreceptive functions offer a number of selective advantages. Phytoplankton near the ocean’s surface can be harmed by solar UV. Negative phototaxis in this intense environment causes migration to lower depths. If the phytoplankton are too deep, insufficient illumination causes a positive phototactic response, so the organisms move upward to an optimal position (Foster and Smyth 1980; Jekely et al. 2008).
The addition of screening pigment
Chloroplasts or other absorbing structures in the cell body may in some cases provide sufficient shading for phytoplankton to detect light directionality and carry out phototaxis. But in phytoplankton with a visible eyespot it is clear that photoreception must be directional, and thus belong to class II. These types of eyespots are evident in Chlamydomonas reinhardtii (Fig. 1A). Chlamydomonas is about 8–10 µm in diameter and is the most widely investigated member of the class of green algae, Chlorophyceae. Its eyespot is about 1 µm in size and its carotenoid-rich pigmented granules are hexagonally packed and organized into layers within the chloroplast. Some species have one or two layers and others have as many as eight layers of granules. The pigmented eyespot allows for directional light-sensitivity, and it is located in close proximity to the plasma membrane where ChR1 and ChR2 reside (Fig. 1A) (Hegemann 2008; Kreimer 2009; Hegemann and Dieckmann 2011). In addition to the ChR1 and ChR2 photosensory pigments, Chlamydomonas also expresses enzymerhodopsins, phototropins, and histidine kinase rhodopsins (UVA light receptor) (Hegemann 2008; Ernst et al. 2014). ChR1 and ChR2 play important roles in phototaxis, and Chlamydomonas swims in a smooth helical pattern with its flagella directed forward, with the eyespot scanning the environment (Hegemann and Dieckmann 2011).
Stacking of photoreceptor membrane
Some phytoplankton eyespots contain multilayered membrane structures with photoreceptive protein and carotenoid-rich granules organized into rows. In animals, stacked membranes are necessary for sufficient light sensitivity and exist in most directional photoreceptors and all photoreceptors used for spatial vision (Nilsson 2009, 2013). In the dinoflagellate, Kryptoperidinium foliaceum (formally called Glenodinium) stacked membranes are found in the eyespots (Figs. 1C and 5A, B). Dinoflagellates are unicellular protists that are ecologically important constituents of the phytoplankton. They display a great deal of diversity in morphology, nutritional modes and symbioses, and can be photosynthetic or heterotrophic, feeding on smaller phytoplankton (Taylor 1980, 1987; Colley and Trench 1983). The dinoflagellate Kryptoperidinium is photosynthetic and has eyespots that are used for light-mediated tasks such as phototaxis. The eyespot of Kryptoperidinium is about 3 µm wide and 6 µm long and is made up of two layers of granules, and extensive stacks of organized lamellar membrane structures (Fig. 5A and B) (Dodge 1969, 1984; Dodge and Crawford 1969). Kryptoperidinium displays phototaxis and this light-guided behavior is mediated by its eyespot with a peak spectral sensitivity about 500 nm (Moldrup and Garm 2012; Moldrup et al. 2013).
Fig. 5.
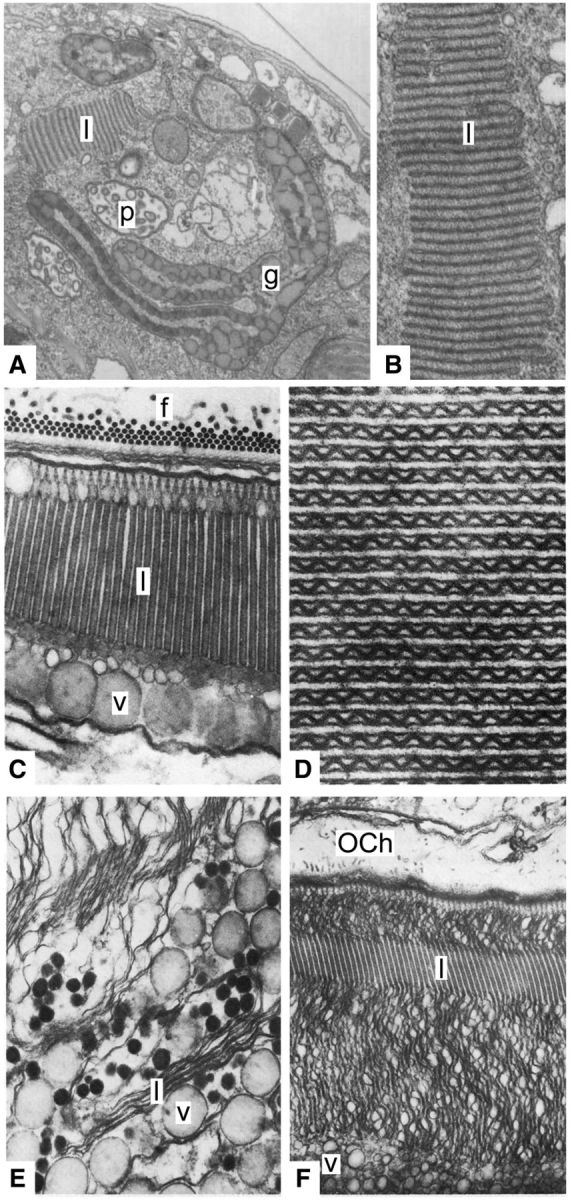
Ultrastructure of Kryptoperidinium foliaceum eyespot, Warnowia ocelloid and Erythropsidinium ocelloid. (A) Oblique longitudinal section through the Kryptoperidinium eyespot, showing the membranes of the lamellar body (l) pigment granules (g), and the pusule (p) (×21,000). (B) Higher magnification of the Kryptoperidinium eyespot membranes in the lamellar body (l) which consists of stacks of flattened vesicles (×62,500). (C)Warnowia ocelloid showing retinal body organization. Longitudinal section of Warnowia retinal body: f, fibrillar formations (made up of microtubules) on the floor of the ocelloid chamber; l, lamellae (paired thylakoids); v, vesicular layer (×30,000). (D)Warnowia ocelloid, transverse section of the paired thylakoids with the medium sinusoidal wall (×62,000). (E, F) Two stages of the reorganizing retinal body during cell division in Erythropsidinium. (E) Pairing of the thylakoids and pleating of the median boundary: v, vesicular layer; l, lamellae (×24,000). (F) Normalizing of the lamellar structure: l, lamellae; OCh, ocelloid chamber; v, vesicular layer. (A–B) reproduced from Dodge and Crawford (1969) with copyright permission from The Company of Biologists Ltd, (C–F) reproduced from Greuet (1987) with copyright permission from John Wiley & Sons Inc.
Another example of an eyespot with stacked membrane is found in the unicellular flagellate, Euglena. In Euglena, the carotenoid-rich granules are clustered around a reservoir and photoreceptor crystal that are in close proximity to the long flagellum (Fig. 1B) (Walne and Arnott 1967; Hegemann and Dieckmann 2011). In Euglena, light stimulation leads to activation of its photosensitive adenylate cyclase and an increase in cAMP (Hegemann 2008). Unlike Chlamydomonas, the eyespot in Euglena is not located in the chloroplast but is located very close to the flagella, promoting signaling for light-guided directional movement (Fig. 1A and B) (Walne and Arnott 1967; Hegemann 2008; Hegemann and Dieckmann 2011). Given the differences in the anatomy and photosensitive pigments expressed, it has been proposed that phototaxis in Euglena evolved independently from phototaxis in green algae (Hegemann and Dieckmann 2011). Another euglenoid, Peranema trichophorum, lacks chloroplasts and changes its shape (curling into a ball) to effectively capture and ingest prey. The cells glide and the body curves and straightens to turn, then, the cells curl into a ball with fast beating cilia when they consume prey. It has been proposed that rhodopsin mediates the curling behavior in P. trichophorum (Saranak and Foster 2005).
The stacked membranes will allow for more photosensory pigment (which is membrane bound) to be concentrated in the shadow of the eyespot, and thus significantly improve sensitivity despite the loss of light in the screening pigment (Nilsson 2013). The reason that some phytoplankton have stacked membrane in the eyespots, and others do not, could reflect use in different light intensities, different integration time (sensory speed), or different degrees of shading by the screening pigment.
Imaging optics
The most complex and elaborate type of optical system in phytoplankton is the ocelloid found in gymnodinoid dinoflagellates belonging to the Warnowiaceae. The Warnowiaceae family is made up of five genera: Erythropsidinium, Greuetodinium, Nematodinium, Proterythropsis, and Warnowia (Hoppenrath et al. 2009; Garate-Lizarraga 2012). In addition to ocelloids, warnowiids have additional organelles. For example, some warnowiids contain photosynthetic chloroplasts and some do not. Nematodinium and Proterythropsis possess nematocysts and Erythropsidinium and Greuetodinium have a unique piston capable of contractions (Figs. 1F and 3C). Greuetodinium has an even more complex ocelloid with multiple lenses, resembling a compound eye (Kofoid and Swezy 1921; Greuet 1987; Gomez 2008; Hoppenrath et al. 2009). Dinoflagellates are considered to be remarkable evolutionary experiments because of the tendency to transfer genes into their disproportionately huge genome (Hackett et al. 2004). Warnowiids are elusive creatures and, therefore, there is limited information about them. They are not often captured in plankton net tows, they are fragile and have not yet been successfully cultured. Therefore, studies about the warnowiid ocelloid and warnowiid behavior have been challenging.
Fig. 3.
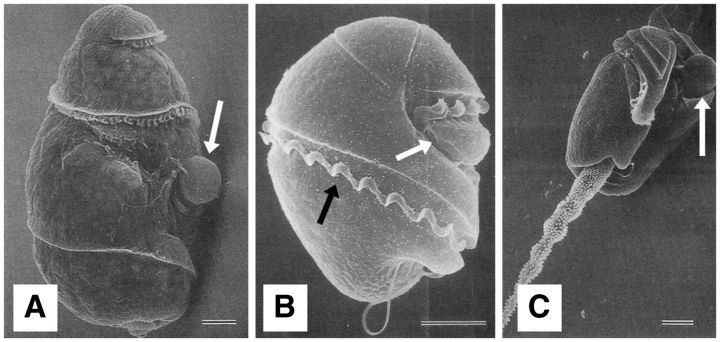
Scanning electron microscopic images of (A)Warnowia, (B)Nematodinium, and (C)Erythropsidinium. Black arrows indicate the transverse flagella, while white arrows indicate the ocelloid. Scale = 10 μm. Reproduced from Steidinger and Tangen (1996) with permission from Dr Haruyoshi Takayama.
The ocelloid is an elaborate eye-organelle that has been well characterized in Warnowia, Nematodinium, and Erythropsidinium (Figs. 1D–F and 3A–C). These large complex ocelloids range in size from about 10 to 15 µm. Ocelloids were reported in the Pouchetidae by Kofoid and Swezy in 1921 which were later renamed, Warnowiidae, by Lindemann (Kofoid and Swezy 1921; Lindemann 1928). The ultrastructure and function of ocelloids were extensively described by Greuet, Francis, and Mornin (Greuet 1965, 1968, 1969, 1970, 1978, 1987, 1977; Francis 1967; Mornin and Francis 1967). Recent studies focused on ocelloids have confirmed much of this original work and have provided renewed interest in and information about the molecular makeup of warnowiids (Gehring 2005, 2014; Gomez 2008; Leander 2008; Gomez et al. 2009; Hoppenrath et al. 2009; Garate-Lizarraga 2012; Gavelis et al. 2015; Hayakawa et al. 2015).
The ocelloid is composed of a hyalosome (lens), an ocellar chamber (vitreous body), and a melanosome containing a retinal body placed in a cup of dark pigment (Fig. 3A and B). The transparent hyalosome acts as a focusing lens (Francis 1967; Mornin and Francis 1967; Greuet 1978, 1987) (Fig. 4A and B). It is a complex layered structure containing a peripheral corneal region and a central crystalline body (Fig. 4A and B). The corneal layer is made up of a microtubular layer covering a layer of mitochondria. The crystalline body is formed by the superimposition of flat endoplasmic vesicles containing hyaline, which is the refractive agent secreted by the corneal layer. The hyalosome sits on a basal plate, and striated fibers are located along the basal plate and their contraction makes the hyalosome turn, orienting the ocelloid (Francis 1967; Mornin and Francis 1967; Greuet 1987). This suggests that the ocelloid is capable of changing its gaze, which is a feature otherwise only found in high-resolution animal eyes (Nilsson 2013).
Fig. 4.
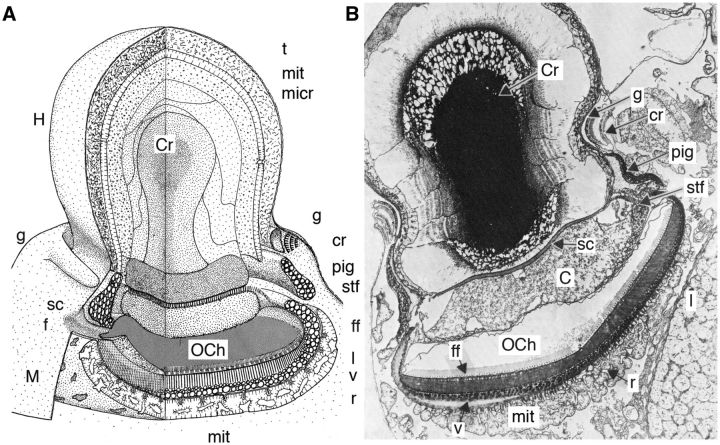
Anatomy of the Erythropsidinium ocelloid. (A) Schematic representation of the Erythropsidinium ocelloid and (B) longitudinal section of Erythropsidinium ocelloid (×3900). t, microtubular layer; mit, mitochondrion; micr, microcrystalline layer; g, periocelloid gallery; cr, constricting ring; pig, pigmentary ring; stf, striated fibre; ff, fibrillar formations on the floor of the ocelloid chamber (microtubules make up the fiber layer); l, highly ordered lamellar membranes of the retinal body (paired thylakoid membranes); v, vesicular layer of the retinal body (vesicles containing melanoid and carotenoid pigment); r, reticulum; mit, mitochondria; M, melanosome; f, ocelloid channel; sc, scalariforme plate; H, hyalosome; sc, scalariforme plate; Cr, crystalline body; OCh, ocelloid chamber; C, core. (A) Reproduced from Greuet (1968) with adapted labeling from Greuet (1987) with copyright permission from Elsevier and John Wiley & Sons Inc. (B) Reproduced from Greuet (1987) with copyright permission from John Wiley & Sons Inc.
The hyalosome is separated from the retinal body by the ocelloid chamber which is in close association with the flagella, suggesting direct transmission of signaling from the ocelloid to the flagella for visually guided movement (Fig. 4A and B). The ocelloid channel is an invagination which opens outside into the sulcus groove that houses the transverse flagellum. The ocelloid chamber communicates with the external sea water via the ocelloid channel opening. The ocelloid chamber is lined by the amphiesma, which is composed of a series of flattened vesicles (amphiesmal vesicles). It is in close association with the double membrane limiting the retinal body beneath. The floor of the ocelloid chamber consists of a paracrystalline fibrillar layer made up of microtubules (Fig. 4A and B) (Francis 1967; Mornin and Francis 1967; Greuet 1987).
Beneath the hyalosome, the ocelloid contains a melanosome composed of a precisely structured retinal body with highly ordered lamellar membranes, and dark pigmented cup lining the back of the retinal body. Around the edge there is also a ring of pigment shielding light from the side. The pigment ring is made up of vesicles containing dark melanoid and carotenoid pigment. The retinal body contains highly ordered lamellar membranes (Fig. 5C). Between the retinal body and the base of the ocelloid chamber is a layer of microtubules that make up the fiber layer. At the proximal surface of the fiber layer there is a conical cap to each membrane lamella in contact with the plastid membrane (Fig. 5C). At the base of the ocelloid, beneath the retinal body and the pigment layer, there is a layer with mitochondria and endoplasmic reticulum membranes (Fig. 4A and B) (Greuet 1987).
The plastid origin of the retinal body was shown during cell division by Greuet (1987). He showed that cytokinesis is preceded by the division of the ocelloid, and that the division of the melanosome follows the same progression as the division of algal chloroplasts. The thylakoids dedifferentiate and then reorganize in two steps. First, there is the pairing of the thylakoids and then the normalizing of the lamellar structure (Fig. 5E and F). More specifically, he showed that each lamella is formed by the coalescence of two thylakoids and is made up of three membranes (Fig. 5D–F). The central membrane is a thicker membrane with a sinuous period of 70 nm and he showed that all of the sinusoids of all the coupled thylakoids were in phase (Fig. 5D) (Greuet 1987). If we assume that the retinal body is the photoreceptive site, it is interesting to note that it is formed by modification of the thylakoid membranes, which normally house chlorophyll. In animal photoreceptor cells, the photoreceptive membrane is extended either by microvilli in rhabdomeric receptors or by cilia in ciliary receptors. The stack of thylakoid membranes observed in dinoflagellate ocelloids offers a third analogous structure for maximizing photosensory pigment density and thus bringing sensitivity to levels necessary for vision (Greuet 1987; Lamb et al. 2007; Colley 2010; Fain et al. 2010; Lamb 2013; Nilsson 2013).
A recent study examined the Erythropsidinium ocelloid and showed that it responded to light by changes in the morphology of the retinal body (Hayakawa et al. 2015). Hayakawa and coauthors isolated mRNA from Erythropsidinium and prepared a cDNA library and identified 800 ESTs. They identified an 88 amino acid sequence in Erythropsidinium that corresponds to the C-terminus of a BR proton pump (Hayakawa et al. 2015). The sequence is similar to that of a microbial rhodopsin proton pump identified in several algal species including a recent submission of sequence to NCBI from the dinoflagellate, P. donghaiense as well as sequences from two diatom species belonging to the genus, Pseudo-nitzschia, and the hyptophyte, P. globosa (Hegemann 2008; Hegemann and Dieckmann 2011; Ernst et al. 2014; Hayakawa et al. 2015). Hayakawa and coauthors examined the expression pattern for the microbial rhodopsin mRNA and showed mRNA labeling in the ocelloid. Further, they showed a weak spot of DAPI staining at a position they identify as the retinal body of the ocelloid (Hayakawa et al. 2015). They conclude that the DNA and the mRNA for the Erythropsidinium microbial rhodopsin gene are located in the retinal body of the ocelloid, and further suggest that the Erythropsidinium rhodopsin was acquired by horizontal gene transfer (Hayakawa et al. 2015). It has also been speculated that the ocelloid is an endosymbiont (Gavelis et al. 2015; Hayakawa et al. 2015). Microbial rhodopsins in other protists, such as Chlamydomonas are encoded by nuclear genes (Hegemann 2008; Hegemann and Dieckmann 2011). Therefore, further studies of Erythropsidinium BR are needed before substantiated conclusions can be reached. Precise localization of the BR gene within the cell is essential, as is demonstration that high concentrations of the BR protein is present in the retinal body. At this point, it is not possible to confidently state that a microbial rhodopsin is the principal photosensory pigment in the retinal body of the Erythropsidinium ocelloid.
Vision in warnowiids
Ocelloids are similar in morphology to the multicellular camera eyes in metazoans with a lens (hyalosome) and retinal structures (retinal body). Early studies showed that the ocelloid is capable of focusing images and, therefore, may enable organisms to carry out more complex tasks compared to those with eyespots. In 1967, David Francis assessed the refractive index and shape of the lens, and concluded: “Our experiments have shown that the Nematodinium lens is physically capable of focusing light on the inner part of the pigment cup, and therefore able to form images there of objects outside.” He continued: “It is suggested that functions involving image formation may be realized, even in the absence of a true retina and nervous system” (Francis 1967; Mornin and Francis 1967). The size of 10–15 μm makes the ocelloid an unusually large organelle, and just large enough to function as a camera type eye with focusing capabilities (Francis 1967; Mornin and Francis 1967; Dodge 1969; Greuet 1987; Nilsson 2013). Even though a more detailed optical analysis of the hyaloid lens is highly desirable, it seems inevitable from the structure that the ocelloid is an eye providing spatial vision. Assessment of the visually guided behaviors of warnowiids combined with structural and optical analyses is necessary for assessing important performance values such as spatial resolution (acuity), contrast sensitivity and temporal resolution (Land and Nilsson 2012; Cronin et al. 2014). The function of the hyalosome must be to allow spatial vision and to increase the sensitivity to high spatial frequencies. It cannot be a question of sensitivity without spatial resolution, because the hyaloid is roughly the same diameter as the retinoid, and thus would not increase sensitivity to extended light sources. A likely, but still sensational, possibility is that warnowiids use their ocelloid for visually guided predation on other phytoplankton. Some warnowiids carry out photosynthesis, and chloroplasts may or may not be evident. Warnowiids are also heterotrophic, and feed on other, smaller, plankton. Visually guided steering toward dark objects would allow them to attack other cells, for example, those with dark chloroplasts. However, at this point there is not sufficient information to say if warnowiids have low or high-resolution vision, (class III or IV; Nilsson 2009, 2013) that is, if they use their ocelloid for habitat selection or for seeing and interacting with other organisms.
Parallel evolution in phytoplankton and animals
In animals, high-resolution vision used for detecting and identifying prey, predators, and conspecifics has evolved in vertebrates, cephalopods, and arthropods, whereas low resolution vision for navigation and habitat selection is present in most animal phyla (Land and Nilsson 2012). High-resolution versions of both compound eyes and camera-type eyes in animals are likely to have originated shortly before the early Cambrian, some 530 million years ago, but the first low-resolution forerunners may have appeared as much as 100 million years earlier (Nilsson 2009). For dinoflagellate ocelloids it is not possible to make a comparable timing of their evolution. Ancestral dinoflagellates are thought to be represented in cysts called acritarchs. These fossilized cysts first appeared in the Precambrian Period (∼1.8 bya). Further, there is a possible record of a Precambrian dinoflagellate, Zosterosphaera tripunctata (Taylor 1980, 1987). However, tracking the evolutionary history of dinoflagellates is challenging. The fossil record is limited to those dinoflagellates with resistant cyst walls, and many dinoflagellates do not appear to produce such robust cysts. The earliest accepted record of a dinoflagellate is in the Silurian Period (∼440 mya) and is that of Arpylorus. Dinoflagellate fossils are abundant in the Triassic Period (∼250 mya) (Taylor 1980, 1987). However, when the first warnowiid ocelloid appeared in evolutionary history is not known. The optics and structures of an eye may only take a few hundred thousand generation to evolve (Nilsson and Pelger 1994), so eye evolution can occur quickly in evolutionary terms.
A comparison of photoreception and vision between animals and phytoplankton reveals striking similarities at all stages, despite one group being multicellular and the other unicellular. The structurally similar photosensory pigments, rhodopsins, have most likely evolved independently, and even though the photochemistry differs between animals and protists, both use vitamin-A derivatives. The next step toward more complex functions is the addition of screening pigment to obtain directionality for phototaxis. This step has also been taken independently in animals and phytoplankton. Further elaboration typically involves membrane stacking for increased sensitivity, and this has likewise appeared independently in animals and phytoplankton. The final steps introducing spatial vision and focusing optics also appears to have been taken independently in animals and phytoplankton. Remarkably, the evolutionary sequence of photoreceptive tasks from nondirectional photoreception to directional photoreception and then on to low resolution vision and high resolution vision (classes 1–4; Nilsson 2009, 2013) thus seems to have been followed in both animals and phytoplankton, with independent acquisition first of a photosensory pigment, then screening pigment, followed by membrane stacking and finally structures for spatial vision and focusing optics. Apparently this is the way eyes evolve in both multicellular and single cell organisms.
Acknowledgments
The authors thank their collaborators, Drs Jose Carvajal, Jules Jaffe, Mike Latz, John McGowen, and Greg Rouse, as well as the Hazardous Algal Blooms (HABs) monitoring, Coastal Marine Plankton Group: Ms Melissa Carter, Mary Hilbern, Kristi Seech, and Mr Matt Hubbell. Drs Peter Hegemann and Carol Dieckmann provided valuable discussions. The authors thank Dr Haruyoshi Takayama for the generous use of his images and the anonymous reviewer for insightful comments. The authors are grateful to Chad Smith and Alaina Heinen for producing the figures, editing and processing the manuscript.
Funding
This work was supported by National Institutes of Health [R01 EY008768 to N.J.C.], the Retina Research Foundation and the RRF/M.D. Matthews Research Professorship (to N.J.C.), National Institutes of Health [P30 EY016665 (Core grant, Department of Ophthalmology and Visual Sciences, to N.J.C.)] and the Research to Prevent Blindness (RPB) foundation (Department of Ophthalmology and Visual Sciences, to N.J.C.), The Swedish Research Council (to D.E.N.), and the Knut and Alice Wallenberg foundation (to D.E.N.).
References
- Barsanti L, Evangelista V, Passarelli V, Frassanito AM, Gualtieri P. 2012. Fundamental questions and concepts about photoreception and the case of Euglena gracilis. Integr Biol 4:22–36. [DOI] [PubMed] [Google Scholar]
- Colley NJ. 2010. Retinal degeneration through the eye of the fly In: Dartt DA, Besharse JC, Dana R, editors. Encyclopedia of the eye. Vol. 4 Oxford: Elsevier/Academic Press; p. 54–61. [Google Scholar]
- Colley NJ, Trench RK. 1983. Selectivity in phagocytosis and persistance of symbiotic algae in the scyphistoma stage of the jellyfish Cassiopeia xamachana. Proc Roy Soc B 219:61–82. [DOI] [PubMed] [Google Scholar]
- Cronin TW, Johnsen S, Marshall NJ, Warrant EJ. 2014. Visual ecology. Oxford: Princeton University Press. [Google Scholar]
- Dodge JD. 1969. A review of the fine structure of algal eyespots. Br Phycol J 4:199–210. [Google Scholar]
- Dodge JD. 1984. The functional and phylogenetic significance of dinoflagellate eyespots. Biosystems 16:259–67. [DOI] [PubMed] [Google Scholar]
- Dodge JD, Crawford RM. 1969. Observations on the fine structure of the eyespot and associated organelles in the dinoflagellate glenodinium foliaceum. J Cell Sci 5:479–93. [DOI] [PubMed] [Google Scholar]
- Ernst OP, Lodowski DT, Elstner M, Hegemann P, Brown LS, Kandori H. 2014. Microbial and animal rhodopsins: structures, functions, and molecular mechanisms. Chem Rev 114:126–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain GL, Hardie R, Laughlin SB. 2010. Phototransduction and the evolution of photoreceptors. Curr Biol 20:R114–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falb M, Muller K, Konigsmaier L, Oberwinkler T, Horn P, von Gronau S, Gonzalez O, Pfeiffer F, Bornberg-Bauer E, Oesterhelt D. 2008. Metabolism of halophilic archaea. Extremophiles 12:177–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster KW, Smyth RD. 1980. Light antennas in phototactic algae. Microbiol Rev 44:572–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis D. 1967. On the eyespot of the dinoflagellate, Nematodinium. J Exp Biol 47:495–501. [DOI] [PubMed] [Google Scholar]
- Garate-Lizarraga I. 2012. New record of three species of the family Warnowiaceae (Dinophyceae) in the Gulf of California. Revista De Biologia Marina Y Oceanografia 47:581–6. [Google Scholar]
- Gavelis GS, Hayakawa S, White RA, 3rd, Gojobori T, Suttle CA, Keeling PJ, Leander BS. 2015. Eye-like ocelloids are built from different endosymbiotically acquired components. Nature 523:204–7. [DOI] [PubMed] [Google Scholar]
- Gehring WJ. 2005. New perspectives on eye development and the evolution of eyes and photoreceptors. J Hered 96:171–84. [DOI] [PubMed] [Google Scholar]
- Gehring WJ. 2014. The evolution of vision. Wiley Interdiscip Rev Dev Biol 3:1–40. [DOI] [PubMed] [Google Scholar]
- Giovannoni SJ, Bibbs L, Cho JC, Stapels MD, Desiderio R, Vergin KL, Rappe MS, Laney S, Wilhelm LJ, Tripp HJ, et al. 2005. Proteorhodopsin in the ubiquitous marine bacterium SAR11. Nature 438:82–5. [DOI] [PubMed] [Google Scholar]
- Gomez F. 2008. Erythropsidinium (Gymnodiniales, Dinophyceae) in the Pacific Ocean, a unique dinoflagellate with an ocelloid and a piston. Eur J Protistol 44:291–8. [DOI] [PubMed] [Google Scholar]
- Gomez F, Lopez-Garcia P, Moreira D. 2009. Molecular phylogeny of the ocelloid-bearing dinoflagellates erythropsidinium and warnowia (warnowiaceae, dinophyceae). J Eukaryot Microbiol 56:440–5. [DOI] [PubMed] [Google Scholar]
- Gomez-Consarnau L, Akram N, Lindell K, Pedersen A, Neutze R, Milton DL, Gonzalez JM, Pinhassi J. 2010. Proteorhodopsin phototrophy promotes survival of marine bacteria during starvation. PLoS Biol 8:e1000358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez O, Gronau S, Pfeiffer F, Mendoza E, Zimmer R, Oesterhelt D. 2009. Systems analysis of bioenergetics and growth of the extreme halophile Halobacterium salinarum. PLoS Comput Biol 5:e1000332.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greuet C. 1965. Structure fine de l'ocelle d'Erythropsis pavillardi Hertwig, Peridinien Warnowiidae Lindemaan. Cr. hebd. Seances de l'Academie des Sciences Paris 261:1904–7. [Google Scholar]
- Greuet C. 1968. Organisation ultrastructurale de l'ocelle de deux Peridiniens Warnowiidae, Erythropsis pavillardi Kofoid et Swezy et Warnowia pulchra Schiller. Protistologica 4:209–30. [Google Scholar]
- Greuet C. 1969. Etude morphologique et ultrastructurale du trophonte d'Erythropsis pavillardi Kofoid et Swezy. Protistologica 5:481–503. [Google Scholar]
- Greuet C. 1970. Ultrastructure de l'ocelle du Dinoflagellé Nematodium comparée à celles d'autres représentants de la famille des Warnowiidae. Proceedings of 7th International Congress on EM, Grenoble. p. 385–6. [Google Scholar]
- Greuet C. 1977. Structural and ultrastructural evolution of ocelloid of Erythropsidinium-Pavillardi-Kofoid-and-Swezy (Dinoflagellate Warnowiidae, Lindemann) during division and palintomic divisions. Protistologica 13:127–43. [Google Scholar]
- Greuet C. 1978. Organisation ultrastructurale d'ocelloide de Nematodinium. Aspect phylogenetique de l'evolution du photorecepteur des peridiniens Warnowiidae Lindemann. Cytobiologie 17:114–36. [PubMed] [Google Scholar]
- Greuet C. 1987. Complex organelles In: Taylor FJR, editor. The biology of dinoflagellates. Vol. xxii.Oxford, Boston (MA: ): Blackwell Scientific; p. 119–42. [Google Scholar]
- Hackett JD, Anderson DM, Erdner DL, Bhattacharya D. 2004. Dinoflagellates: a remarkable evolutionary experiment. Am J Bot 91:1523–34. [DOI] [PubMed] [Google Scholar]
- Hartz AJ, Sherr BF, Sherr EB. 2008. Using inhibitors to investigate the involvement of cell signaling in predation by marine phagotrophic protists. J Eukaryot Microbiol 55:18–21. [DOI] [PubMed] [Google Scholar]
- Hartz AJ, Sherr BF, Sherr EB. 2011. Photoresponse in the heterotrophic marine dinoflagellate Oxyrrhis marina. J Eukaryot Microbiol 58:171–7. [DOI] [PubMed] [Google Scholar]
- Hayakawa S, Takaku Y, Hwang JS, Horiguchi T, Suga H, Gehring W, Ikeo K, Gojobori T. 2015. Function and evolutionary origin of unicellular camera-type eye structure. PLoS One 10:e0118415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegemann P. 2008. Algal sensory photoreceptors. Annu Rev Plant Biol 59:167–89. [DOI] [PubMed] [Google Scholar]
- Hegemann P., Dieckmann C. 2011. Algal eyes In: eLS 1-7. John Wiley & Sons, Ltd, Ltd, Chichester. [Google Scholar]
- Hoff WD, Jung KH, Spudich JL. 1997. Molecular mechanism of photosignaling by archaeal sensory rhodopsins. Annu Rev Biophys Biomol Struct 26:223–58. [DOI] [PubMed] [Google Scholar]
- Hoppenrath M, Bachvaroff TR, Handy SM, Delwiche CF, Leander BS. 2009. Molecular phylogeny of ocelloid-bearing dinoflagellates (Warnowiaceae) as inferred from SSU and LSU rDNA sequences. BMC Evol Biol 9:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jekely G. 2009. Evolution of phototaxis. Philos Trans R Soc B 364:2795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jekely G, Colombelli J, Hausen H, Guy K, Stelzer E, Nedelec F, Arendt D. 2008. Mechanism of phototaxis in marine zooplankton. Nature 456:395–9. [DOI] [PubMed] [Google Scholar]
- Kofoid CA, Swezy O. 1921. The free-living, unarmoured Dinoflagellates. Mem Univ Calif 5:1–562. [Google Scholar]
- Kreimer G. 1999. Reflective properties of different eyespot types in dinoflagellates. Protist 150:311–23. [DOI] [PubMed] [Google Scholar]
- Kreimer G. 2009. The green algal eyespot apparatus: a primordial visual system and more? Curr Genet 55:19–43. [DOI] [PubMed] [Google Scholar]
- Lamb TD. 2013. Evolution of phototransduction, vertebrate photoreceptors and retina. Prog Retin Eye Res 36:52–119. [DOI] [PubMed] [Google Scholar]
- Lamb TD, Collin SP, Pugh EN., Jr. 2007. Evolution of the vertebrate eye: opsins, photoreceptors, retina and eye cup. Nat Rev Neurosci 8:960–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land MF, Nilsson DE. 2012. Animal Eyes, Second Edition. (Oxford University Press, New York; 2012).
- Leander BS. 2008. Different modes of convergent evolution reflect phylogenetic distances: a reply to Arendt and Reznick. Trends Ecol Evol 23:481–2. Author reply 483–4. [DOI] [PubMed] [Google Scholar]
- Lindemann E. 1928. Abteilung Peridineae (Dinoflagellatae), in Die natürlichen Pflanzenfamilien nebst ihren Gattungen und wichtigeren Arten insbesondere den Nutzpflanze. (eds. A. Engler & K.A. Prantl) 3–104 (Engelmann, Leipzig; 1928).
- Moldrup M, Garm A. 2012. Spectral sensitivity of phototaxis in the dinoflagellate Kryptoperidinium foliaceum and their reaction to physical encounters. J Exp Biol 215:2342–6. [DOI] [PubMed] [Google Scholar]
- Moldrup M, Moestrup O, Hansen PJ. 2013. Loss of phototaxis and degeneration of an eyespot in long-term algal cultures: evidence from ultrastructure and behaviour in the dinoflagellate Kryptoperidinium foliaceum. J Eukaryot Microbiol 60:327–34. [DOI] [PubMed] [Google Scholar]
- Mornin L, Francis D. 1967. The fine structure of Nematodinium armatum, a naked dinoflagellate. J Microsc 6:759–72. [Google Scholar]
- Nilsson DE. 2009. The evolution of eyes and visually guided behaviour. Philos Trans R Soc B 364:2833–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson DE. 2013. Eye evolution and its functional basis. Vis Neurosci 30:5–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson DE, Pelger S. 1994. A pessimistic estimate of the time required for an eye to evolve. Proc Biol Sci 256:53–8. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D, Stoeckenius W. 1971. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat New Biol 233:149–52. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D, Stoeckenius W. 1973. Functions of a new photoreceptor membrane. Proc Natl Acad Sci USA 70:2853–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski K. 2006. G protein-coupled receptor rhodopsin. Annu Rev Biochem 75:743–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, et al. 2000. Crystal structure of rhodopsin: a G protein-coupled receptor. Science 289:739–45. [DOI] [PubMed] [Google Scholar]
- Porter ML, Blasic JR, Bok MJ, Cameron EG, Pringle T, Cronin TW,, Robinson PR. 2012. Shedding new light on opsin evolution. Proc Biol Sci 279:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-González MX, Marín I. 2004. New Insights into the evolutionary history of type 1 rhodopsins. J Mol Evol 58:348–58. [DOI] [PubMed] [Google Scholar]
- Saldarriaga JF, McEwan ML, Fast NM, Taylor FJ, Keeling PJ. 2003. Multiple protein phylogenies show that Oxyrrhis marina and Perkinsus marinus are early branches of the dinoflagellate lineage. Int J Syst Evol Microbiol 53:355–65. [DOI] [PubMed] [Google Scholar]
- Saranak J, Foster KW. 2005. Photoreceptor for curling behavior in Peranema trichophorum and evolution of eukaryotic rhodopsins. Eukaryot Cell 4:1605–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TM, Chen SK, Hattar S. 2011. Intrinsically photosensitive retinal ganglion cells: many subtypes, diverse functions. Trends Neurosci 34:572–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamovits CH, Okamoto N, Burri L, James ER, Keeling PJ. 2011. A bacterial proteorhodopsin proton pump in marine eukaryotes. Nat Commun 2:183.. [DOI] [PubMed] [Google Scholar]
- Spudich JL. 2006. The multitalented microbial sensory rhodopsins. Trends Microbiol 14:480–7. [DOI] [PubMed] [Google Scholar]
- Steidinger KA., Tangen K. 1996. Dinoflagellates In: Tomas CR, editor. Identifying marine diatoms and dinoflagellates. San Diego (CA: ): Academic Press; p. 387–584. [Google Scholar]
- Taylor FJ. 1980. On dinoflagellate evolution. Biosystems 13:65–108. [DOI] [PubMed] [Google Scholar]
- Taylor FJR. 1987. The biology of dinoflagellates. Vol. xii Oxford, Boston (MA: ): Blackwell Scientific Publications; p. 785. [Google Scholar]
- Tomas CR. 1996. Identifying marine diatoms and dinoflagellates. Vol. xiii San Diego (CA: ): Academic Press; p. 598. [Google Scholar]
- van der Horst MA, Hellingwerf KJ. 2004. Photoreceptor proteins, “star actors of modern times”: a review of the functional dynamics in the structure of representative members of six different photoreceptor families. Accounts Chem Res 37:13–20. [DOI] [PubMed] [Google Scholar]
- Walne PL, Arnott HJ. 1967. The comparative ultrastructure and possible function of eyespots: Euglena granulata and Chlamydomonas eugametos. Planta 77:325–53. [DOI] [PubMed] [Google Scholar]
- Zhang F, Vierock J, Yizhar O, Fenno LE, Tsunoda S, Kianianmomeni A, Prigge M, Berndt A, Cushman J, Polle J, et al. 2011. The microbial opsin family of optogenetic tools. Cell 147:1446–57. [DOI] [PMC free article] [PubMed] [Google Scholar]