Abstract
OBJECTIVE
To identify perinatal risk factors that can distinguish arterial ischemic stroke from hypoxic–ischemic encephalopathy at birth.
METHODS
This is a cohort study of all neonates born at 35 weeks of gestation or greater admitted to our neonatal intensive care unit from January 1, 2010, to December 31, 2015, that compares neonates with stroke with those with hypoxic–ischemic encephalopathy undergoing whole-body hypothermia with abnormal brain magnetic resonance imaging.
RESULTS
During this 6-year period, there were 22 neonates with stroke and 47 with hypoxic–ischemic encephalopathy undergoing whole-body hypothermia with abnormal magnetic resonance imaging. Three neonates triaged to hypothermia initially thought to have hypoxic–ischemic encephalopathy were later diagnosed with stroke. All neonates with stroke had a negative thrombophilia workup. Neonates with stroke had a significantly higher incidence of seizures and increased initial platelet counts on univariate analysis. A multivariable model of variables with P<.1 on univariate analysis present within 6 hours of birth found significant increases in nonreassuring fetal heart rate tracings, sentinel events, low Apgar score at 5 minutes, and metabolic acidosis at birth with hypoxic–ischemic encephalopathy. Stroke was associated with a significantly increased initial platelet count.
CONCLUSION
Stroke is associated with increased initial platelet counts and is not associated with cesarean delivery for nonreassuring fetal heart rate tracings, sentinel events, or perinatal metabolic acidosis. Stroke is a form of neonatal brain injury not associated with perinatal risk factors that allow early identification.
Perinatal arterial ischemic stroke is important in the differential diagnosis of neonates presenting with seizures and encephalopathy in the newborn period. It is a leading known cause for cerebral palsy, accounting for 30% of children affected with hemiplegic cerebral palsy.1 With an estimated incidence of 17–93 per 100,000 live births, the incidence of stroke in the perinatal period rivals the incidence of stroke in adults (17–23/100,000).2
Although newborns with stroke are often systemically sick, their mothers are usually healthy.3 Up to 90% of newborns with stroke present acutely in the first 72 hours of life with neurologic symptoms, most commonly seizures.2 Hypoxic–ischemic encephalopathy occurs in 200–900 per 100,000 live births and may cause cerebral palsy.4 Neonates with hypoxic–ischemic encephalopathy typically present shortly after delivery with abnormalities in tone, posturing, and seizures.5 It can be difficult to differentiate stroke from hypoxic–ischemic encephalopathy in the neonate given overlapping presentations. Early assessment is required to initiate hypothermia within the first 6 hours of life for neonates with hypoxic–ischemic encephalopathy.4 There is no consensus regarding treatment of stroke aside from supportive therapy.6 Early differentiation between neonatal stroke and hypoxic–ischemic encephalopathy is paramount for triage and initiation of appropriate management. Our hypothesis is that stroke represents a distinct form of neonatal brain injury that can be differentiated from hypoxic–ischemic encephalopathy, and we sought to identify risk factors that may differentiate neonates with stroke and hypoxic–ischemic encephalopathy during the intra-partum period and within 6 hours of birth. If intra-partum events play an important role in the pathogenesis of stroke, risk factors will be shared with hypoxic–ischemic encephalopathy.
MATERIALS AND METHODS
This is a retrospective cohort study of all neonates born at 35 weeks of gestation or greater with arterial ischemic stroke and hypoxic–ischemic encephalopathy treated with hypothermia who had an abnormal brain magnetic resonance image (MRI) admitted to the neonatal intensive care unit at our institution from January 1, 2010, to December 31, 2015. This study was approved by our medical center institutional review board. We used the National Institute of Child Health and Human Development and the National Institute of Neurological Disorders and Stroke workshop definition of perinatal arterial ischemic stroke: “a group of heterogeneous conditions in which there is focal disruption of cerebral blood flow secondary to arterial or cerebral venous thrombosis or embolization, between 20 weeks fetal life through the 28th postnatal day, confirmed by neuroimaging or neuropathologic studies.”3 Neonates with stroke were compared with those with hypoxic–ischemic encephalopathy and abnormal brain MRI who were treated with whole-body hypothermia during the same period. Neonates receiving whole-body hypothermia who had a normal brain MRI were excluded to select for the most severe hypoxic–ischemic encephalopathy cases and compare MRI findings with stroke. Neonates were eligible for treatment with whole-body hypothermia if moderate to severe encephalopathy was present at birth. Diagnostic criteria to initiate whole-body hypothermia for neonates in our unit have been previously published.7 They were cooled to a rectal temperature of 33.5°C for 72 hours.5 Exclusion criteria for hypothermia treatment included greater than 6 hours of life, gestational age less than 35 weeks, severe growth restriction (birth weight less than 1,800 g), major congenital anomaly, severe persistent pulmonary hypertension with anticipated need for extracorporeal membrane oxygenation, coagulopathy with active bleeding, and suspected sepsis with severe hemodynamic compromise requiring large doses of vasopressors.
Neonates with stroke and hypoxic–ischemic encephalopathy were identified through review of records from the neonatal intensive care unit and pediatric neurology clinic during this period, and clinical data were extracted from neonatal and maternal medical records. The diagnosis of chorioamnionitis was made if maternal fever and at least one other finding of fetal tachycardia, uterine tenderness, or purulent vaginal discharge were present; these mothers received intravenous antibiotics immediately on diagnosis. Nonreassuring fetal heart rate was diagnosed by the attending physician before performing an operative delivery (vacuum, forceps, or cesarean delivery). Fetal growth restriction was defined as an estimated fetal weight less than the 10th percentile for gestational age. Preeclampsia was defined as new-onset hypertension with protein-uria. Oligohydramnios was defined as an amniotic fluid index less than 5.0 cm with intact membranes during the admission in which delivery occurred. A sentinel event was an event occurring immediately before delivery that could lead to fetal hypoxia, including uterine rupture, placental abruption, fetal bradycardia, umbilical cord prolapse, shoulder dystocia, or maternal cardiac arrest.8 All neonates with stroke underwent a thrombophilia workup, which typically included protein C and S activity as a percent of normal for term neonates, activated protein C resistance ratio, antithrombin III levels, homocysteine levels, lipoprotein a levels, prothrombin 20210a mutation, dilute Russell viper venom time, and anticardiolipin antibody titers.
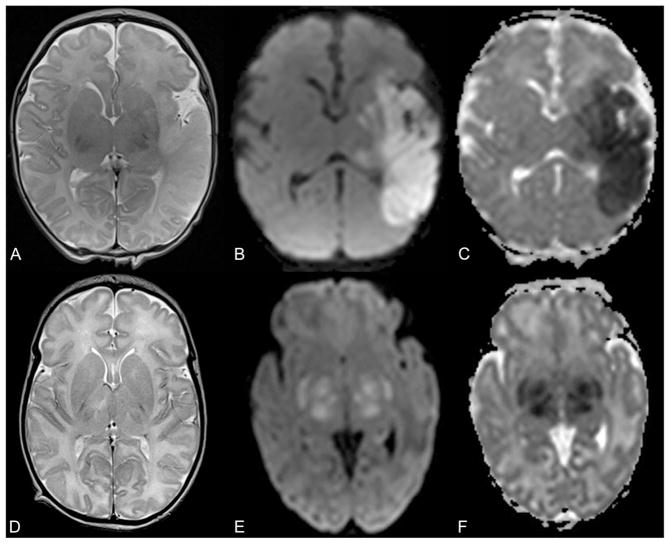
A pediatric neuroradiologist (A.P.) reviewed all brain MRI studies including conventional (T1- and T2-weighted) sequences as well as diffusion tensor imaging (trace of diffusion and apparent diffusion coefficient maps). Arterial ischemic stroke was diagnosed when an ischemic lesion (restricted diffusion including bright signal on trace of diffusion and low apparent diffusion coefficient values) was visualized in the vascular territory of the anterior, middle, or posterior cerebral arteries (Fig. 1A–C). Because we used diffusion changes as a criterion for the imaging diagnosis of stroke, and diffusion-weighted imaging changes typically normalize within 10–14 days after injury, this lets us time stroke to within 10–14 days previous to the MRI study.9 Magnetic resonance imaging abnormalities diagnostic of hypoxic–ischemic encephalopathy were focal or diffuse hyperintense T2 signal with or without changes in diffusion characteristics in cortical gray matter, subcortical white matter (Fig. 1D), basal ganglia (Fig. 1E–F), thalami (Fig. 1E–F), and hippocampi. Hypoxic–ischemic injury in a para-sagittal or watershed distribution was classified as gray and white matter abnormality in the territories of the anterior and middle or middle and posterior cerebral arteries, respectively. Hypoxic–ischemic injury in the perirolandic region was classified as gray and white matter abnormality in the territory of the middle cerebral artery. Periventricular white matter injury was classified as changes in signal intensity or diffusion of the white matter within the middle cerebral artery territory.
Fig. 1.
Axial T2-weighted magnetic resonance (MR) image (A), trace of diffusion map (B), and apparent diffusion coefficient (ADC) map (C) of a 3-day-old neonate with arterial ischemic stroke show hyperintense T2 signal of the cortical gray and subcortical white matter within the vascular territory of the left middle cerebral artery with absence of the normal cortical T2-hypointese signal and matching bright signal on trace of diffusion map and low ADC values (dark on C) representing restricted diffusion in the infarcted brain tissue. Axial T2-weighted MR image (D) of a 9-day-old neonate with hypoxic–ischemic encephalopathy and hypothermia therapy reveals global T2-hyperintense signal of the cerebral white matter. Axial trace of diffusion map (E) and ADC map (F) of a 6-day-old neonate with hypoxic–ischemic encephalopathy and hypothermia therapy show bright signal within the bilateral basal ganglia and thalami with matching low ADC values (dark on F) representing cytotoxic edema.
Univariate analysis was performed using Student t test to evaluate continuous variables and χ2 or Fisher exact test where appropriate for categorical variables. The Wilcoxon matched pair signed-rank test compared nonnormally distributed data such as gravidity, parity, and nucleated red blood cell counts. All statistical tests were performed at the .05 level of statistical significance. Logistic regression models were fit to estimate the relationship between variables present within 6 hours of birth and stroke. Most parsimonious multivariable logistic regression model was selected using the lowest value of Akaike Information Criterion. Receiver operator characteristic curves were generated and the sensitivity, specificity, and positive and negative predictive values were calculated. Stata 14 was used for statistical analysis.
RESULTS
During this 6-year period, 22 neonates were diagnosed with stroke and 47 of 164 (28.7%) neonates treated with whole-body hypothermia for suspected hypoxic–ischemic encephalopathy had an abnormal brain MRI. These 47 formed the comparison group. Of these, 6 of 22 (27.3%) of neonates with stroke and 13 of 47 (27.7%) of neonates with hypoxic–ischemic encephalopathy were delivered in our institution with the others being transported from hospitals across our state. All neonates with suspected hypoxic–ischemic encephalopathy born at outside institutions were admitted here to begin hypothermia treatment within 6 hours of birth, although these neonates commonly have the warmer turned off during transport so that the cooling process is begun before admission. During this period there were 18,344 deliveries within our institution for an incidence of stroke of 6 of 18,344 (32.7 per 100,000 live births, 95% confidence interval [CI] 12–71.2/100,000 live births) and an incidence of hypothermia-treated hypoxic–ischemic encephalopathy with abnormal MRI of 13 of 18,344 (71.0/100,000 live births, 95% CI 37.7–121.2/100,000 live births) for inborn neonates. In univariate analysis, mothers who delivered a neonate with stroke or hypoxic–ischemic encephalopathy did not differ by age, parity, or race. The hypoxic–ischemic encephalopathy group had a significantly higher incidence of nonreassuring fetal heart rate tracing, cesarean delivery, and sentinel events (Table 1). There were no vacuum or forceps deliveries in either group. The single sentinel event in the stroke group was a shoulder dystocia. The 14 sentinel events in the hypoxic–ischemic encephalopathy group included seven cases of abruption, three uterine ruptures, and one case each of maternal seizure, shoulder dystocia, tight true knot in the umbilical cord, and a large unexplained fetal–maternal hemorrhage. Abruption occurred in none of the neonates with stroke and in 14.9% of neonates with hypoxic–ischemic encephalopathy. There was no difference in intrapartum oxytocin use, oligohydramnios, fetal growth restriction, or intrapartum complications, including preeclampsia and chorioamnionitis. A majority of the neonates in both the stroke and hypoxic–ischemic encephalopathy groups was male (Table 2).
Table 1.
Univariate Analysis of Maternal Clinical Data Comparing Pregnancies in Which the Neonate Was Diagnosed With Perinatal Arterial Ischemic Stroke With Those Treated With Whole-Body Hypothermia for Hypoxic–Ischemic Encephalopathy With Abnormal Brain Magnetic Resonance Imaging
| Arterial Ischemic Stroke (n=22) | Hypoxic–Ischemic Encephalopathy (n=47) | P | |
|---|---|---|---|
| Maternal age (y) | 28.7±5.3 | 29.2±6.5 | .77 |
| Nulliparity | 11 (50) | 31 (66) | .21 |
| Race | .85 | ||
| White | 12 (54.5) | 22 (46.8) | |
| Black | 4 (18.2) | 13 (27.7) | |
| Hispanic | 2 (9.1) | 2 (4.5) | |
| Asian | 1 (4.5) | 3 (6.4) | |
| Other | 3 (13.6) | 6 (12.8) | |
| Cesarean delivery | 10 (45.5) | 37 (78.7) | .01* |
| Oxytocin | 9 (40.9) | 11 (23.4) | .14 |
| Preeclampsia | 1 (4.5) | 2 (4.5) | .96 |
| Meconium | 7 (31.8) | 18 (38.3) | .60 |
| Fetal growth restriction | 1 (4.5) | 2 (4.5) | .96 |
| Oligohydramnios | 1 (4.5) | 1 (2.1) | .54 |
| Clinical chorioamnionitis | 4 (18.2) | 4 (8.5) | .26 |
| Abruption | 0 | 7 (14.9) | .09 |
| Nonreassuring fetal heart rate | 4 (18.2) | 32 (68.1) | <.001* |
| Sentinel event | 1 (4.5) | 14 (29.8) | .03* |
Data are mean±standard deviation or n (%) unless otherwise specified.
P<.05.
Table 2.
Univariate Analysis of Neonatal Clinical Data Comparing Pregnancies in Which the Neonate Was Diagnosed With Perinatal Arterial Ischemic Stroke With Those Treated With Whole-Body Hypothermia for Hypoxic–Ischemic Encephalopathy With Abnormal Brain Magnetic Resonance Imaging
| Arterial Ischemic Stroke (n=22) | Hypoxic–Ischemic Encephalopathy (n=47) | P | |
|---|---|---|---|
| Gestational age (wk) | 39.7±0.8 | 38.9±1.6 | .03* |
| Birth weight (g) | 3,666±414 | 3,292±626 | .01* |
| Male sex | 16 (72.7) | 29 (61.7) | .37 |
| 1-min Apgar score less than 7 | 12 (54.5) | 46 (97.9) | <.001* |
| 5-min Apgar score less than 7 | 7 (31.8) | 41 (87.2) | <.001* |
| Umbilical artery pH | 7.15±0.19 (n=13) | 6.93±0.19 (n=40) | <.001* |
| Umbilical artery BD (mM) | 8.7±9.0 (n=11) | 14.9±8.5 (n=36) | .04* |
| Cord gas with pH less than 7.0 or BD greater than 12 mM | 2/13 (15.4) | 31/40 (77.5) | <.001* |
| Neonatal length of stay (d) | 10.7±7.5 | 24.6±17.7 | <.001* |
| Intraventricular hemorrhage | 1 (4.5) | 4 (8.5) | 1.0 |
| Periventricular white matter injury | 5 (22.7) | 7 (14.9) | .50 |
| Seizures | 18 (81.8) | 22 (46.8) | .008* |
| Positive blood culture | 0 | 2 (4.5) | 1.0 |
| Positive cerebrospinal fluid culture | 1 (4.5) | 0 | .32 |
| Initial neonatal WBC count (k/mm3) | 17.9±7.4 | 20.0±9.2 | .37 |
| Initial neonatal hematocrit (%) | 45.0±5.2 | 43.8±10.5 | .64 |
| Initial neonatal platelet count (k/mm3) | 215±76 | 153±62 | .001* |
| Initial neonatal serum glucose (mg/dL) | 75±24 | 104±84 | .14 |
| Initial nucleated RBCs (no./100 WBC) 50%; 5–95% | 110; 0–10,020 | 2,510; 220–34,688 | <.001* |
| Initial neonatal arterial pH | 7.30±0.18 | 7.10±0.18 | <.001* |
| Initial neonatal arterial BD (mM) | 6.3±9.4 | 17.1±8.1 | <.001* |
BD, base deficit; WBC, white blood cells; RBCs, red blood cells.
Data are mean±standard deviation or n (%) unless otherwise specified.
P<.05.
Neonates with stroke had a small increase in gestational age and birth weight that was statistically, but not clinically, significant (Table 2). Neonates with stroke had a significantly lower incidence of 1- and 5-minute Apgar scores less than 7. Umbilical arterial cord gases were obtained for 13 of 22 (59.1%) of the neonates with stroke and 40 of 47 (85.1%) with hypoxic–ischemic encephalopathy (Table 2). Neonates with stroke had a mean cord arterial pH and base deficit that was normal, but 2 of 13 (15.4%) had pH less than 7.0 or base deficit greater than 12 mM indicating metabolic acidosis. Neonates with hypoxic–ischemic encephalopathy had a significantly lower pH and higher base deficit in the umbilical arterial gas at birth, and 77.5% had pH less than 7.0 or base deficit greater than 12 mM. All neonates had an arterial gas shortly after birth, and again neonates with stroke had a normal pH and base deficit, whereas those with hypoxic–ischemic encephalopathy had a significant degree of metabolic acidosis. Three neonates (13.6% of neonates with stroke) who were initially thought to have hypoxic–ischemic encephalopathy underwent hypothermia and were later diagnosed with stroke; one of these neonates had severe metabolic acidosis at birth (cord pH 6.63, base deficit −33.8 mM), one had no cord gas but the initial arterial gas after birth showed pH 7.39 and base deficit 0.1 mM, and one had a normal cord gas with pH 7.29 and base deficit 1.1 mM. None of these neonates experienced known adverse effects as a result of hypothermia treatment. All neonates with stroke had a negative thrombophilia workup. Rates of intraventricular hemorrhage, periventricular white matter injury, positive blood cultures, positive cerebrospinal fluid cultures, initial neonatal white blood cell count, hematocrit, and glucose were all similar between both groups. Neonates with stroke were significantly more like to have seizures (Table 2). Of the 18 neonates with stroke who had a seizure, 12 (66.7%) had their initial seizure at greater than 12 hours of life. Of the 47 neonates with hypoxic–ischemic encephalopathy, 22 of 47 (46.8%) had seizures and 5 of 22 (22.7%) had their initial seizure at greater than 12 hours of life (P=.005).
For neonates with stroke, the initial brain MRI was performed between day 1 and day 23 (5th–95th percentile); day of life 3 was the 50th percentile for timing of the initial MRI. For neonates with hypoxic–ischemic encephalopathy, the initial MRIs were performed between day of life 8 and 21 (5th and 95th percentile); day of life 8 was the 50th percentile. All neonates with stroke had diffusion restriction on MRI showing that the injury occurred within the previous 10–14 days, because diffusion changes normalize after this period. Neonates with stroke were significantly more likely to have injury to the cortical gray matter within the vascular territory of the right and left middle cerebral artery (Table 3). Neonates with hypoxic–ischemic encephalopathy were significantly more likely to have changes in signal intensity or diffusion characteristics of the white matter within the vascular territories of the anterior and posterior cerebral arteries. There were no significant differences in injury or signal intensity of the gray matter within the vascular territories of the anterior or posterior cerebral artery, white matter within the vascular territory of the middle cerebral artery or basal ganglia, thalamus, or hippocampus.
Table 3.
Univariate Analysis of the Anatomic Region With Abnormal T2 Signal or Diffusion Characteristics on Brain Magnetic Resonance Imaging Results Comparing Neonates With Perinatal Arterial Ischemic Stroke With Those Treated With Whole-Body Hypothermia for Hypoxic–Ischemic Encephalopathy With Abnormal Brain Magnetic Resonance Imaging
| Arterial Ischemic Stroke (n=22) | Hypoxic–Ischemic Encephalopathy (n=47) | P | |
|---|---|---|---|
| Thalamus | 10 (45.5) | 23 (48.9) | .79 |
| Putamen | 8 (36.4) | 16 (34.0) | .85 |
| Caudate nucleus | 9 (40.9) | 12 (25.5) | .20 |
| Hippocampus | 3 (13.6) | 14 (29.8) | .15 |
| Anterior cerebral artery | |||
| Gray matter | 5 (22.7) | 15 (31.9) | .43 |
| White matter–right | 2 (9.1) | 22 (46.8) | .002* |
| White matter–left | 3 (13.6) | 22 (46.8) | .008* |
| Middle cerebral artery | |||
| Gray matter–right | 10 (45.5) | 8 (17.0) | .01* |
| Gray matter–left | 15 (68.2) | 9 (19.1) | <.001* |
| White matter–right | 12 (54.5) | 23 (48.9) | .66 |
| White matter–left | 16 (72.7) | 23 (48.9) | .06 |
| Posterior cerebral artery | |||
| Gray matter | 5 (22.7) | 17 (36.2) | .26 |
| White matter–right | 2 (9.1) | 21 (44.7) | .005* |
| White matter–left | 3 (13.6) | 22 (46.8) | .008* |
Data are n (%) unless otherwise specified.
P<.05.
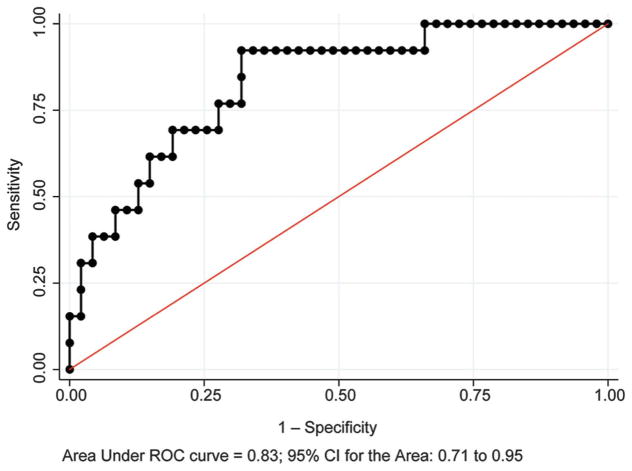
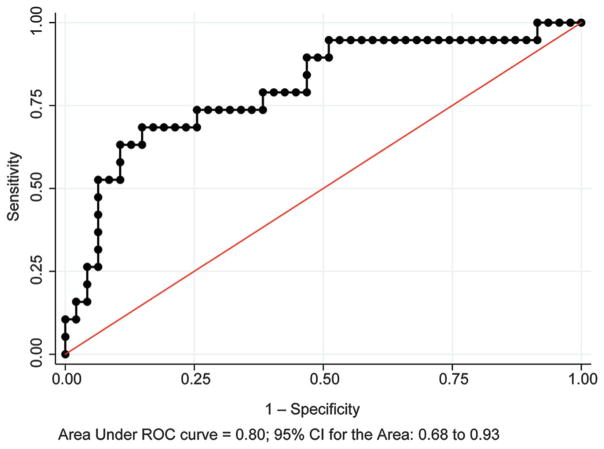
Neonates with stroke had significantly higher initial platelet counts and lower nucleated red blood cell counts. Logistic regression for the initial platelet count to distinguish stroke from hypoxic–ischemic encephalopathy, corrected for birth weight, showed area under receiver operator characteristic curve 0.80 (95% confidence interval [CI] 0.69–0.89) (Fig. 2A), sensitivity 52.6%, specificity 93.6%, positive predictive value 76.9%, and negative predictive value 83.0%. Logistic regression for the initial nucleated red blood cell count, corrected for birth weight, showed area under the receiver operator characteristic curve 0.83 (95% CI 0.71–0.95) (Fig. 3), sensitivity 38.5%, specificity 91.5%, positive predictive value 55.6%, and negative predictive value 84.3%. A multivariable model of variables with P<.1 on univariate analysis present within 6 hours of birth found significant increases in nonreassuring fetal heart rate tracings, sentinel events, Apgar score less than 7 at 5 minutes, and metabolic acidosis in cord gas at birth and the initial neonatal arterial gas in neonates with hypoxic–ischemic encephalopathy (Table 4). Stroke was associated with a significant increase in initial platelet count.
Fig. 2.
Receiver operating characteristic (ROC) curve for initial neonatal platelet count in distinguishing perinatal arterial ischemic stroke from hypoxic—ischemic encephalopathy treated with whole-body hypothermia with abnormal brain magnetic resonance imaging. CI, confidence interval.
Fig. 3.
Receiver operating characteristic (ROC) curve for initial neonatal nucleated red blood cell count in distinguishing perinatal arterial ischemic stroke from hypoxic–ischemic encephalopathy treated with whole-body hypothermia with abnormal brain magnetic resonance imaging. CI, confidence interval.
Table 4.
Odds Ratios Estimating the Strength of Association Between Variables Present Within 6 Hours of Birth and Perinatal Arterial Ischemic Stroke
| Variable | Unadjusted Estimates | Adjusted Estimates | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| OR | 95% CI | P | OR | 95% CI | P | |
| Nonreassuring fetal heart rate tracing | 0.10 | 0.03–0.37 | <.001 | 0.18 | 0.04–0.71 | .015 |
| Sentinel event | 0.11 | 0.01–0.93 | .043 | |||
| 5-min Apgar score less than 7 | 0.07 | 0.02–0.24 | <.001 | 0.12 | 0.03–0.51 | .004 |
| Cord pH (per 0.1 increment) | 2.19 | 1.13–4.25 | .021 | |||
| Initial neonatal pH (per 0.1 increment) | 1.90 | 1.20–3.00 | .006 | |||
| Initial platelet count (per 100 increment) | 3.80 | 1.42–10.19 | .008 | 2.50 | 0.72–8.68 | .149 |
| Initial nucleated red blood cell count (per 1,000 increment) | 0.63 | 0.22–1.85 | .402 | |||
| Birth weight (per 500 increment) | 1.86 | 1.13–3.07 | .015 | |||
OR, odds ratio; CI, confidence interval.
Adjusted estimates are from the most parsimonious model based on Akaike Information Criterion.
DISCUSSION
Our study found that 82% of neonates with stroke diagnosed in the neonatal period presented with seizures, similar to data from the International Pediatric Stroke Study of 248 neonates from 30 centers in which 72% of neonates with stroke presented with seizures.3 Most of the seizures associated with stroke occur within the first 3 days of life.10 The initial occurrence of the seizure after 12 hours of life, especially one with focal features, has been reported to distinguish perinatal stroke from generalized hypoxic–ischemic encephalopathy,11 and our study confirmed this finding with 66.7% of seizures occurring at greater than 12 hours of life in neonates with stroke as compared with only 22.7% in hypoxic–ischemic encephalopathy.
A study of 60 mother–child pairs with perinatal stroke found one or more thrombophilic abnormalities in 50% of children and 55% of mothers.12 Mothers and neonates frequently did not share the same prothrombotic factor or factors, calling into question the clinical significance of these abnormalities. A study evaluating 79 neonates with stroke found thrombotic abnormalities in only 14% of the neonates, and none of the mothers had a stroke in relation to delivery.13 None of the mothers in our study had any intrapartum thromboembolic complications, and all neonates had a negative thrombophilia workup.
Clinical chorioamnionitis has been identified in 5–27% of cases of stroke with an odds ratio of 3.5 and has been linked with the development of cerebral palsy.14,15 Perinatal infections have been theorized to promote thrombotic episodes in the maternal or fetal circulation.3 In our study only 18% of neonates with stroke had clinical chorioamnionitis, 4.5% had cerebrospinal fluid infection, and none had a positive blood culture showing that most cases of stroke are not associated with perinatal infection.
Most neonates with perinatal stroke do not have severe perinatal asphyxia.14 Sentinel events occurred in only 4.5% of neonates with stroke as compared with 29.8% of neonates with hypoxic–ischemic encephalopathy in our study, which is similar to the findings of sentinel events in 3.8% of stroke and 22% of hypoxic–ischemic encephalopathy in another recent study.13 The International Pediatric Stroke Study found birth asphyxia in 8%.3 Another study of 60 neonates with stroke found hypoxic–ischemic encephalopathy occurred concomitantly in 8.3% and that the majority of stroke occurred after uncomplicated vaginal delivery and elective cesarean delivery, suggesting that the thromboembolic event may have preceded birth by hours, days, or weeks.12 Stroke can co-occur with hypoxic–ischemic encephalopathy and both may possible share some link with intrapartum hypoxia–ischemia as possibly occurred with the three neonates with stroke who received hypothermia in our study16; however, only 15.4% of neonates with arterial ischemic stroke had metabolic acidosis (pH less than 7.0 and base deficit greater than 12 mM) in umbilical arterial blood at birth as compared with 77.5% of neonates with hypoxic–ischemic encephalopathy.
No differences were found for the white matter of the middle cerebral artery territory, which is the most common location of perinatal stroke, because of the high incidence of injury in both groups. The success of therapeutic hypothermia in decreasing brain tissue injury and hence the small number of neonates with hypoxic–ischemic encephalopathy and injury of the perirolandic regions or watershed areas in our cohort is the most likely explanation for the significantly higher percentage of changes in signal intensity or diffusion characteristics of the gray matter within the middle cerebral artery territory in newborns with stroke.
Many neonates did not have placental pathology sent, limiting our ability to evaluate this key piece of information. The MRIs of neonates with stroke were performed on average 5 days earlier than those with hypoxic–ischemic encephalopathy. Although pseudo-normalization of diffusion data has been shown after 10 days in neonates with hypoxic–ischemic encephalopathy and hypothermia therapy,17 the evaluation of conventional MRI sequences should prevent this difference from affecting the numbers of neonates diagnosed into either group.
In conclusion, overlapping regions of injury may lead to overlap in pediatric neurologic symptoms, but these entities are distinct. Although stroke is associated with an increase in initial platelet count and lacks an association with cesarean deliveries for nonreassuring fetal heart rate tracings, sentinel events, low Apgar scores, and metabolic acidosis in the cord gas at delivery, and the initial neonatal arterial gas as does hypoxic–ischemic encephalopathy, these clinical features may help differentiate stroke from hypoxic–ischemic encephalopathy in the neurologically abnormal newborn, but they cannot be used to identify neonates with stroke in the general population. This shows that stroke is a form of perinatal brain injury not associated with the typical intrapartum risk factors associated with hypoxic–ischemic encephalopathy. As new neuroprotective therapies emerge, early identification of stroke will be critical to ensure targeted therapies and minimal risk.
Acknowledgments
Dr. Graham is supported by the Cerebral Palsy International Research Foundation.
Footnotes
Presented at the 36th Annual Pregnancy Meeting for the Society for Maternal-Fetal Medicine, February 1–6, 2016, Atlanta, Georgia.
Financial Disclosure
The authors did not report any potential conflicts of interest.
References
- 1.Raju TN, Nelson KB, Ferriero D, Lynch JK NICHD-NINDS Perinatal Stroke Workshop Participants. Ischemic perinatal stroke: summary of a workshop sponsored by the National Institute of Child Health and Human Development and the National Institute of Neurological Disorders and Stroke. Pediatrics. 2007;120:609–16. doi: 10.1542/peds.2007-0336. [DOI] [PubMed] [Google Scholar]
- 2.Schneider AT, Kissela B, Woo D, Kleindorfer D, Alwell K, Miller R, et al. Ischemic stroke subtypes: a population-based study of incidence rates among blacks and whites. Stroke. 2004;35:1552–6. doi: 10.1161/01.STR.0000129335.28301.f5. [DOI] [PubMed] [Google Scholar]
- 3.Kirton A, Armstrong-wells J, Chang T, Deveber G, Rivkin MJ, Hernandez M, et al. Symptomatic neonatal arterial ischemic stroke: the International Pediatric Stroke Study. Pediatrics. 2011;128:e1402–10. doi: 10.1542/peds.2011-1148. [DOI] [PubMed] [Google Scholar]
- 4.Lee AC, Kozuki N, Blencowe H, Vos T, Bahalim A, Darmstadt GL, et al. Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatr Res. 2013;74(suppl 1):50–72. doi: 10.1038/pr.2013.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol. 1976;33:696–705. doi: 10.1001/archneur.1976.00500100030012. [DOI] [PubMed] [Google Scholar]
- 6.Rutherford M, Ramenghi LA, Edwards AD, Brocklehurst P, Halliday H, Levene M, et al. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic-ischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol. 2010;9:39–45. doi: 10.1016/S1474-4422(09)70295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wayock CP, Meserole RL, Saria S, Jennings JM, Huisman TA, Northington FJ, et al. Perinatal risk factors for severe injury in neonates treated with whole-body hypothermia for encephalopathy. Am J Obstet Gynecol. 2014;211:41e1–8. doi: 10.1016/j.ajog.2014.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American College of Obstetricians and Gynecologists, American Academy of Pediatrics. Neonatal encephalopathy and neurologic outcome. 2. Washington (DC): American College of Obstetricians and Gynecologists; 2014. [Google Scholar]
- 9.Allen LM, Hasso AN, Handwerker J, Farid H. Sequence-specific MR imaging findings that are useful in dating ischemic stroke. Radiographics. 2012;32:1285–97. doi: 10.1148/rg.325115760. [DOI] [PubMed] [Google Scholar]
- 10.Lee J, Croen LA, Lindan C, Nash KB, Yoshida CK, Ferriero DM, et al. Predictors of outcome in perinatal arterial stroke: a population-based study. Ann Neurol. 2005;58:303–8. doi: 10.1002/ana.20557. [DOI] [PubMed] [Google Scholar]
- 11.Rafay MF, Cortez MA, de Veber GA, Tan-Dy C, Al-Futaisi A, Yoon W, et al. Predictive value of clinical and EEG features in the diagnosis of stroke and hypoxic ischemic encephalopathy in neonates with seizures. Stroke. 2009;40:2401–7. doi: 10.1161/STROKEAHA.109.547281. [DOI] [PubMed] [Google Scholar]
- 12.Curry CJ, Bhullar S, Holmes J, Delozier CD, Roeder ER, Hutchison HT. Risk factors for perinatal arterial stroke: a study of 60 mother-child pairs. Pediatr Neurol. 2007;37:99–107. doi: 10.1016/j.pediatrneurol.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Martinez-Biarge M, Cheong JL, Diez-Sebastian J, Mercuri E, Dubowitz LM, Cowan FM. Risk Factors for Neonatal Arterial Ischemic Stroke: The Importance of the Intrapartum Period. J Pediatr. 2016;173:62–68e1. doi: 10.1016/j.jpeds.2016.02.064. [DOI] [PubMed] [Google Scholar]
- 14.Cheong JL, Cowan FM. Neonatal arterial ischaemic stroke: obstetric issues. Semin Fetal Neonatal Med. 2009;14:267–71. doi: 10.1016/j.siny.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 15.Wu YW, Colford JM., Jr Chorioamnionitis as a risk factor for cerebral palsy. JAMA. 2000;284:1417–24. doi: 10.1001/jama.284.11.1417. [DOI] [PubMed] [Google Scholar]
- 16.Ramaswamy V, Miller SP, Barkovich AJ, Partridge JC, Ferriero DM. Perinatal stroke in term infants with neonatal encephalopathy. Neurology. 2004;62:2088–91. doi: 10.1212/01.wnl.0000129909.77753.c4. [DOI] [PubMed] [Google Scholar]
- 17.Bednarek N, Mathur A, Inder T, Wilkinson J, Neil J. Impact of therapeutic hypothermia on MRI diffusion changes in neonatal encephalopathy. Neurology. 2012;78:1420–7. doi: 10.1212/WNL.0b013e318253d589. [DOI] [PMC free article] [PubMed] [Google Scholar]