Abstract
Background
Although pulmonary congestion can be quantified by lung ultrasonography (LUS) in heart failure (HF), little is known about LUS findings (B-lines) in different HF phenotypes. This prospective cohort study investigated the prevalence, clinical and echocardiographic correlates of B-lines in ambulatory HF patients with preserved (HFpEF) or reduced ejection fraction (HFrEF) compared with hypertensive patients. We related LUS findings to 12-month HF hospitalizations and all-cause mortality.
Methods & Results
We used LUS to examine hypertensive (n=111), HFpEF (n=46) and HFrEF (n=73) patients (median age 66, 56% male, 79% white, and median EF 55%) undergoing clinically indicated outpatient echocardiography. B-line number was quantified offline, across 8 chest zones, blinded to clinical and echocardiographic characteristics. The proportion of patients with ≥3 B-lines was lower in hypertensive patients (13.5%) compared to both HFrEF (45.2%, P<0.001) and HFpEF (34.8%, P=0.05). HF patients with ≥3 B-lines had a higher risk of the composite outcome (age- and sex-adjusted HR 2.62, 95% CI 1.15, 5.96; P=0.022).
Conclusions
When performed at the time of outpatient echocardiography, LUS findings of pulmonary congestion differ between patients with known HF and those with hypertension, and may be associated with adverse outcomes.
Keywords: Heart failure, lung ultrasound, B-lines, echocardiography
Introduction
Pulmonary congestion is the most common sign of heart failure (HF), the leading cause of hospitalization in the U.S. population over 65 years of age.1, 2 Patient-reported dyspnea is used both clinically and in trials as a surrogate marker of pulmonary edema because traditional methods for detecting and quantifying pulmonary congestion are insensitive.2, 3 However, both under- and over-treatment of congestion may occur if HF management is based on symptoms alone.4
Lung ultrasonography (LUS) has emerged as a non-invasive, semi-quantitative diagnostic tool for the assessment of extravascular lung water in HF.5, 6 B-lines on LUS are vertical, hyperechoic artifacts which arise from the pleural line and can be quantified with high interrater agreement.7 These sonographic findings have demonstrated higher sensitivity than the clinical examination or chest radiography in the identification of pulmonary edema in acute HF in patients presenting to Emergency Departments with undifferentiated dyspnea.7, 8
Although the role of LUS for the diagnosis of pulmonary congestion in acute HF is fairly well established, less is known about its potential utility in chronic HF and ambulatory patients at risk for HF.8–10 Hypertension represents one of the leading causes of HF, however the prevalence of LUS findings in patients with hypertension but without established HF is unknown. In addition, little is known about the prevalence of LUS findings in certain HF phenotypes, especially HFpEF, as compared with patients at risk for HF. We hypothesized that LUS findings would be prevalent in ambulatory patients with prior HF, regardless of ejection fraction, and differ from hypertensive patients who are at risk for HF. The primary objective of this study was to determine the prevalence, clinical and echocardiographic correlates of LUS findings in ambulatory patients with known HFpEF and HFrEF as compared to hypertensive patients without HF or coronary artery disease (CAD). In addition, we sought to relate LUS findings in patients with HF to 12-month adverse events.
Methods
Patient population
This was a prospective, single center, observational study of adults referred for clinically indicated outpatient transthoracic echocardiograms (TTEs) at an academic hospital between 2014 and 2015. Eligible patients were identified via the daily echocardiography laboratory schedule and review of electronic medical records (EMR). Of all patients with adequate quality data from standardized TTEs and lung ultrasound examinations performed, we identified 111 patients with hypertension (HTN) (defined as blood pressure >140/90 mmHg at time of echocardiography, history of hypertension, or taking blood pressure-lowering medications),11, 12 46 patients with Heart Failure with preserved Ejection Fraction (HFpEF) (defined as LVEF ≥50% and a prior clinical diagnosis of HF (based on EMR review by a single investigator (AAM)) and 73 patients with Heart Failure with reduced Ejection Fraction (defined as LVEF <50% and a prior clinical diagnosis of HF (based on EMR review by a single investigator (AAM)). For the purpose of sensitivity analyses hospital records of all HFpEF and HFrEF patients were reviewed by the same investigator (AAM) for the presence of HF according to Framingham criteria. Key exclusion criteria were: important lung conditions (e.g. pulmonary fibrosis, lung cancer, pneumonia), hemodialysis and same-day admission to the hospital. Additional exclusion criteria are detailed in the Supplements. This study complies with the Declaration of Helsinki, the locally appointed ethics committee approved the research protocol and informed consent was obtained from all study subjects.
Clinical and demographic data
Clinical and demographic data were collected from the subjects’ hospital records by a single investigator blinded to TTE and LUS data (AAM) (see Supplements for definitions). At the time of enrollment patients were asked whether they felt short of breath (a) while lying flat, and/or (b) climbing a flight of stairs over the past two weeks. Patients were then categorized as having no dyspnea or any dyspnea if they answered ‘yes’ to either (a) or (b).
Echocardiographic analysis
Echocardiograms were performed with standard ultrasound equipment by three major vendors (General Electric, Milwaukee, WI, USA; Phillips, Bothell, WA, USA; and Siemens, Erlangen, Germany) and 2–5 MHz phased array transducers. Digital B-mode TTE cine loops were analyzed offline using echocardiographic software (Syngo Dynamics, Siemens, Malvern, PA, USA) to assess cardiac structure and function. Each component of the echocardiographic image analysis was performed by one of three investigators (DRC, ESL, AAM) in accordance with current guidelines by the American Society of Echocardiography, blinded to LUS data (see Supplements).13, 14 Patients with prior mitral valve surgery or atrial fibrillation/flutter at the time of echocardiography were excluded from E/e’ analysis due to these factors’ interference with accurate E/e’ measurement, and those with prior atrial fibrillation/flutter were excluded from atrial size analyses due to their known association with increased atrial size.
Lung ultrasound imaging protocol and analysis
LUS examinations were performed by trained investigators (AAM, JP) at the time of TTE using the same ultrasound equipment and transducers. A standardized imaging protocol was employed using a phased array transducer at an imaging depth of 18 cm in supine position. Six-second clips were recorded for each of the 8 LUS zones (4 on each hemithorax) as recommended by an international guideline.5
All LUS analyses were performed offline by a single investigator (KHD) with experience in LUS analysis, blinded to clinical and TTE data. The maximum number of vertical B-lines in a single intercostal space was counted for each zone and the sum of all 8 zones was considered as the total number of B-lines as previously described.15, 16 Intra- and inter-rater agreement was assessed in a sample of 25 randomly selected patients as detailed in the Supplements.
Outcomes
HF patients were followed for a fixed time period of 12 months and time to first event was used for all outcome measures (composite of HF hospitalization or all-cause mortality). HF hospitalizations were confirmed through patient follow-up phone calls, contacting primary care physicians or cardiologists, and review of electronic medical records. All HF hospitalizations were adjudicated based on review of hospitalization records by a physician (EP), blinded to LUS findings, using pre-specified criteria employed in the adjudication of cardiovascular trials (see Supplements). No patients in this cohort underwent ventricular assist device placement or heart transplantation prior to an unplanned HF hospitalization. All-cause mortality was confirmed through the institution’s electronic medical records, the social security death index and obituaries.
Statistical analyses
Descriptive statistics for continuous variables are presented as medians (IQR) unless otherwise noted, and categorical variables as counts and percentages. We used global tests to compare baseline characteristics between the three groups (HTN, HFpEF, HFrEF) using Kruskal-Wallis and Pearson’s chi-squared or Fisher’s exact tests for continuous and categorical variables, respectively. For those characteristics where a significant difference was found via the global test, pairwise comparisons of each of the two heart failure groups against the reference HTN group were conducted using two-sample rank-sum tests for continuous variables and Pearson’s chi-square tests or Fisher’s exact test for categorical variables. Quantile (i.e. median) regression and logistic regression were used to determine the relationship between the study groups and LUS/echocardiographic measures after adjustment for age and sex as potential confounders with adjusted medians and standard errors shown. We again used global tests initially, followed by pairwise comparisons for those measures where a significant difference was found by the global test. The prognostic utility of B-lines in identifying subjects at risk for 1-year HF hospitalizations or death was assessed using receiver operating characteristics (ROC) curves. After determination of the B-line number in 8 zones with the highest sensitivity and specificity based on ROC analyses (Youden’s index), ≥3 B-lines was used as the cut-off value. Given the skewed distribution of B-lines, we reported proportions of B-lines by this cut-off (≥3 B-lines) and twice the cut-off (>6 B-lines).
In secondary analyses, we assessed differences across the two B-line groups (<3 B-lines vs. ≥3 B-lines) for echocardiographic characteristics with multivariable linear regression to determine the relationship between LUS and echocardiographic measures after adjustment for age and sex as potential confounders. Additional analyses of baseline characteristics for HF based on Framingham criteria and by B-line group are presented in the Supplements.
In exploratory analyses, Kaplan-Meier curves were estimated for HF patients for each B-line group to describe the temporal risk for the composite outcome. Unadjusted and adjusted Cox proportional hazard models were used to assess the relationship between B-lines (by group) in HF patients and risk for the composite outcome. Models were adjusted for potential confounding variables (age and sex). These covariates were chosen based on their clinical importance in relation to the outcome. The proportional hazards assumptions were assessed using Schoenfeld residuals.
Two-sided significance levels of 0.05 were used for all analyses and P values were not adjusted for multiplicity. Data were analyzed using Stata SE, version 14.2 (StataCorp, College Station, TX, USA 2015).
Results
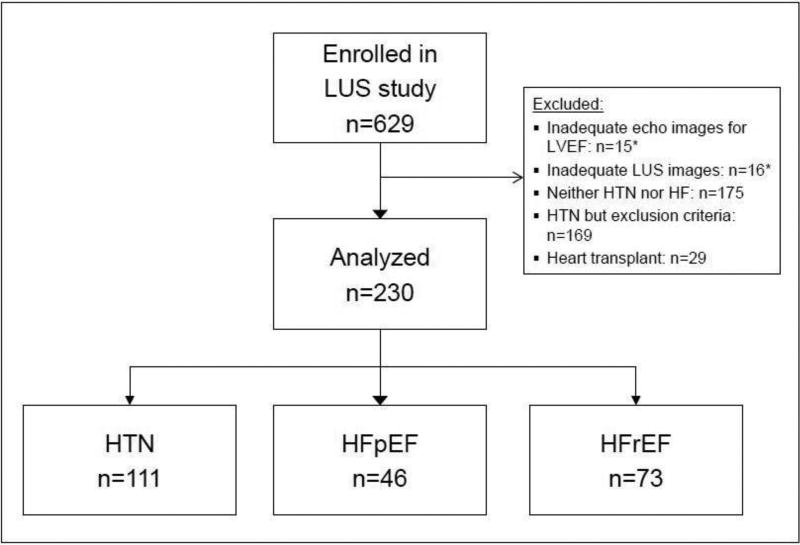
Of 629 patients enrolled, 603 (95.9%) had adequate images for both LVEF and 8-zone LUS analysis (Figure 1). A subset of this LUS cohort, 230 subjects, was included in this analysis: Patients with HTN without HF or CAD (n=111), patients with HFpEF (n=46) and HFrEF (n=73). The median age for all 230 subjects was 66 years (range 20–93), 56% were male, 79% white, and the median LVEF was 55% (IQR 46–62). Baseline characteristics for this cohort are summarized in Table 1.
Figure 1. Study flow chart.
LUS, Lung ultrasound; LVEF, Left ventricular ejection fraction; HTN, Hypertension; HFpEF, Heart failure with preserved EF; HFrEF, HF with reduced EF
*Of patients with inadequate ultrasound images, n=5 subjects had both inadequate echo and LUS images.
Table 1.
Patient characteristics by study group
| N | HTN (n=111) |
HFpEF (n=46) |
P value* |
HFrEF (n=73) |
P value† |
|
|---|---|---|---|---|---|---|
| Clinical characteristics | ||||||
| Age (years) | 230 | 62 (50, 71) | 72 (61, 81) | <0.001 | 70 (60, 76) | <0.001 |
| Men | 230 | 51 (46.0) | 25 (54.4) | 0.34 | 53 (72.6) | <0.001 |
| Race | 230 | 0.10 | 0.019 | |||
| White | 78 (70.3) | 39 (84.8) | 64 (87.7) | |||
| Black | 11 (9.9) | 4 (8.7) | 4 (5.5) | |||
| Other | 22 (19.8) | 3 (6.5) | 5 (6.9) | |||
| BMI (kg/m2) | 230 | 27 (24, 31) | 28 (26, 31) | NS | 27 (24, 30) | NS |
| Heart rate (beats/min) | 230 | 68 (58, 75) | 67 (60, 74) | NS | 70 (60, 80) | NS |
| Systolic BP (mmHg) | 218 | 134 (122, 147) | 132 (120, 146) | 0.37 | 122 (110, 132) | <0.001 |
| Diastolic BP (mmHg) | 218 | 76 (67, 82) | 71 (62, 77) | 0.037 | 72 (62, 79) | 0.036 |
| Any dyspnea | 217 | 47 (44.8) | 26 (57.8) | 0.14 | 46 (68.7) | 0.002 |
| Medical history | ||||||
| Hypertension | 230 | 111 (100) | 43 (93.5) | - | 61 (83.6) | - |
| Diabetes | 229 | 21 (19.1) | 13 (28.3) | 0.21 | 26 (35.6) | 0.012 |
| Myocardial infarction | 230 | - | 4 (9.1) | - | 23 (31.5) | - |
| Atrial fibrillation/flutter | 230 | - | 22 (47.8) | - | 37 (50.7) | - |
| CABG | 230 | - | 8 (17.4) | - | 19 (26.0) | - |
| PCI | 230 | - | 6 (13.0) | - | 15 (21.4) | - |
| Prior HF hospitalization | 230 | - | 20 (43.5) | - | 39 (53.4) | - |
| COPD | 230 | 4 (3.6) | 5 (10.9) | NS | 5 (6.9) | NS |
| Sleep apnea | 230 | 10 (9.0) | 11 (23.9) | 0.013 | 12 (16.4) | 0.13 |
| Active cancer | 189 | 35 (38.5) | 4 (10.0) | 0.001 | 0 | <0.001 |
| Laboratory data | ||||||
| Sodium (mmol/L) | 181 | 139 (137, 141) | 140 (138, 142) | NS | 139 (137, 142) | NS |
| Creatinine (mg/dl) | 182 | 0.9 (0.7, 1.1) | 1.0 (0.9, 1.3) | 0.006 | 1.2 (0.9, 1.4) | <0.001 |
| eGFR (ml/min/1.73m2) | 182 | 80 (60, 107) | 59 (42, 85) | 0.005 | 49 (40, 67) | <0.001 |
| Hemoglobin (g/dl) | 167 | 13.2 (11.5, 14.5) | 12.8 (11.3, 14.0) | NS | 12.9 (11.8, 13.9) | NS |
| Albumin (g/dL) | 145 | 4.3 (4.0, 4.4) | 4.1 (3.8, 4.4) | NS | 4.2 (3.9, 4.4) | NS |
| NT-proBNP ≥125 pg/ml (n, %) | 51 | - | 14 (82.4) | - | 32 (94.1) | - |
Categorical variables are presented as count (%) and continuous variables as median (IQR) unless otherwise noted.
P value comparing HTN and HFpEF
P value comparing HTN and HFrEF
HTN = Hypertension; HFpEF = Heart failure with preserved ejection fraction; HFrEF = HF with reduced ejection fraction; BMI = Body mass index; BP = Blood pressure; CABG = Coronary artery bypass graft; PCI = Percutaneous coronary intervention; COPD = Chronic obstructive pulmonary disease; eGFR = estimated glomerular filtration rate; NS = Global test not significant
Patients with HF were significantly older than those without HF (P<0.001 for both HFpEF and HFrEF). Patients with HFrEF were significantly more likely to be male, had lower systolic blood pressure and reported dyspnea more frequently, whereas HTN patients comprised a significantly higher proportion of patients with active cancer. HF patients had worse renal function than those in the HTN group.
Echocardiographic analyses: HTN vs. HFpEF, HTN vs. HFrEF
Among the 111 HTN patients, echocardiographic analysis showed normal sized ventricles and atria, as well as normal left and right ventricular function. Septal thickness was borderline increased, but LV mass index was within the normal range. Echocardiographic and LUS data by study group are presented in Table 2. Subjects with HFpEF demonstrated larger LV size, LV mass index, LA diameter, worse LVEF, LA emptying fraction and E/e’ than HTN group patients. There was no significant difference in LA volume index, right sided cardiac structure and function in the HTN group vs. those with HFpEF.
Table 2.
Lung ultrasound and echocardiographic measures by study group
| N | HTN (n=111) |
HFpEF (n=46) |
P Value* |
HFrEF (n=73) |
P value† |
|
|---|---|---|---|---|---|---|
| Lung ultrasound measures | ||||||
| ≥ 3 B-lines (n, %) | 230 | 15 (13.5) | 16 (34.8) | 0.05 | 33 (45.2) | <0.001 |
| B-line range | 230 | 0–6 | 0–16 | - | 0–17 | - |
| Echocardiographic measures | ||||||
| LV and LA structure | ||||||
| LV EDD (cm) | 226 | 4.5 (0.9) | 4.8 (0.2) | 0.015 | 5.3 (0.1) | <0.001 |
| LV ESD (cm) | 225 | 2.9 (0.1) | 3.2 (0.1) | 0.006 | 4.5 (0.1) | <0.001 |
| LV EDVi (ml/m2) | 229 | 47 (2) | 48 (3) | 0.45 | 65 (3) | <0.001 |
| LV ESVi (ml/m2) | 229 | 18 (1) | 21 (2) | 0.001 | 39 (2) | <0.001 |
| Septal wall thickness (cm) | 225 | 1.1 (0.02) | 1.2 (0.04) | NS | 1.1 (0.03) | NS |
| LV mass index (g/m2) | 225 | 81 (3) | 99 (5) | 0.002 | 120 (4) | <0.001 |
| LA diameter (cm)‡ | 167 | 3.7 (0.1) | 4.2 (0.1) | <0.001 | 4.2 (0.1) | <0.001 |
| LA volume index (ml/m2)‡ | 169 | 21 (1) | 22 (2) | NS | 24 (2) | NS |
| LV and LA function | ||||||
| LVEF (%) | 230 | 62 (0.01) | 55 (0.02) | <0.001 | 38 (0.01) | - |
| E/e' | 205 | 10 (0.5) | 11 (0.8) | 0.006 | 16 (0.7) | <0.001 |
| LA emptying fraction (%)‡ | 169 | 57 (1) | 47 (3) | <0.001 | 44 (2) | <0.001 |
| RV and RA structure | ||||||
| RV EDA (cm2) | 198 | 18.1 (0.8) | 17.9 (1.2) | NS | 19.0 (1.0) | NS |
| RA area (cm2)‡ | 165 | 16.5 (0.4) | 17.6 (0.9) | NS | 17.3 (0.8) | NS |
| RV function and pressure | ||||||
| RV FAC (%) | 196 | 42 (1) | 42 (2) | NS | 39 (2) | NS |
| TAPSE (cm) | 195 | 2.3 (0.1) | 2.2 (0.1) | 0.41 | 1.8 (0.1) | <0.001 |
| TR velocity (m/s) | 185 | 2.3 (0.1) | 2.4 (0.1) | NS | 2.4 (0.1) | NS |
| IVC diameter (cm) | 211 | 1.6 (0.1) | 1.7 (0.1) | NS | 1.9 (0.1) | NS |
Continuous variables are presented as estimated median (standard error) unless otherwise noted.
P value comparing HTN and HFpEF adjusting for age and sex
P value comparing HTN and HFrEF adjusting for age and sex
Patients without prior atrial fibrillation/flutter
HTN = Hypertension; HFpEF = Heart failure with preserved ejection fraction; HFrEF = HF with reduced EF; LVEF = Left ventricular EF; LV EDD = LV end diastolic diameter; LV ESD = LV end systolic diameter; LA = Left atrial; E/e’ = mitral inflow to mitral relaxation velocity ratio; RV EDA = Right ventricular end-diastolic area; RA = Right atrial; FAC = Fractional area change; TAPSE = Tricuspid annular plane systolic excursion; TR = Tricuspid regurgitant; IVC = Inferior vena cava; NS = Global test not significant
Subjects with HFrEF had larger LV, LA and RA size and worse LV systolic, diastolic and RV function than HTN patients. In addition, 10.8% of patients in the HTN group demonstrated elevated estimated LV filling pressures (average E/e’>14) as compared with 32.6% of HFpEF and 43.8% of HFrEF patients.14 E/e’ could not be analyzed in 25 (10.9%) study subjects.
Lung ultrasound analyses: HTN vs. HFpEF, HTN vs. HFrEF
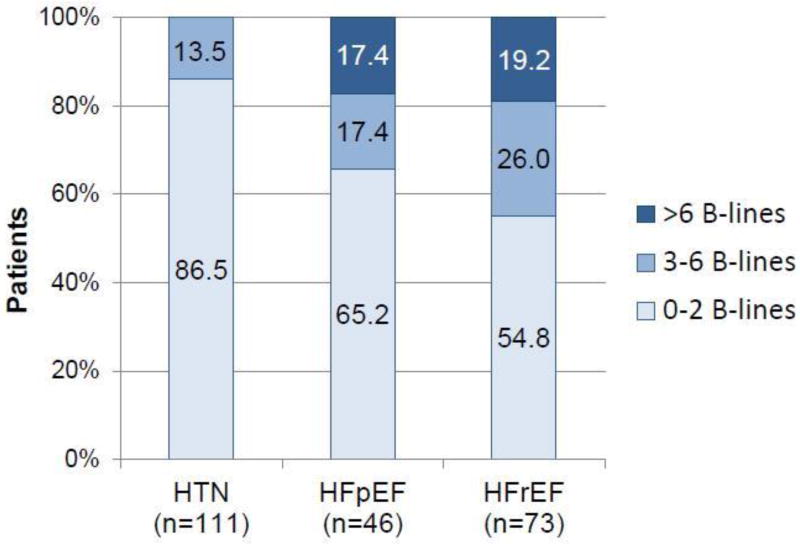
Among all 230 patients, the sum of B-lines in 8 zones ranged from 0 to 17 (median 1, IQR 0–3), with ranges from 0–6 in the HTN, 0–16 in the HFpEF and 0–17 in the HFrEF group. Compared with 13.5% of HTN patients, 34.8% of HFpEF and 45.2% of HFrEF patients had ≥3 B-lines across 8 lung zones (adjusted P=0.05 and P<0.001 respectively) (Table 2, Figure 2). Sensitivity analyses for the prevalence of B-lines for patients with HFpEF and HFrEF according to Framingham criteria were performed and are presented in the Supplements (Table S1). Using Framingham criteria, the HFrEF group remained unchanged and 39 patients were classified as having HFpEF. By this definition, the proportion of patients with ≥3 B-lines compared to HTN patients was 38.5% in HFpEF (adjusted P=0.037).
Figure 2. Number of B-lines by study group (n=230).
HTN, Hypertension; HFpEF, Heart failure with preserved ejection fraction; HFrEF, HF with reduced EF.
B-line cut-off value and analyses by B-line group
Based on ROC analyses, the optimal cut-off value of ≥3 B-lines had a sensitivity of 60.7% and specificity of 64.8% for identifying patients at risk for the composite of HF hospitalization or all-cause mortality within 12 months.
Patient demographic data by LUS group (<3 B-lines vs. ≥3 B-lines) are summarized in the supplemental Table S2. In the overall cohort, there were no significant differences with respect to race, BMI, vital signs or albumin level across B-line groups. Subjects in the groups with higher B-line numbers were older, more likely to report dyspnea and had a larger LV mass index (Table S3). 32.7% of patients with normal E/e’ who were in sinus rhythm during the echocardiogram demonstrated ≥3 B-lines on LUS and 28.6% of patients with elevated LV filling pressures, as estimated by E/e’ >14, had <3 B-lines on LUS. E/e’ could not be assessed in 16.8% of HF patients.
Outcomes
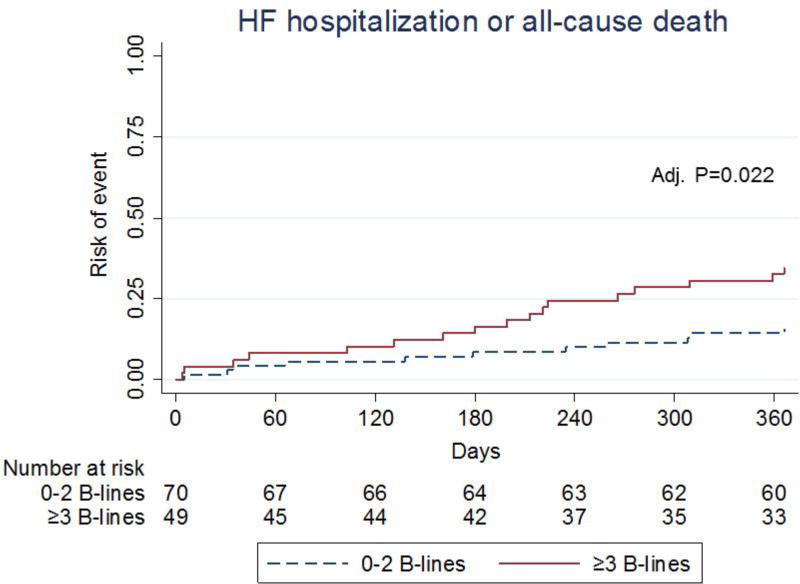
In the subset of patients with HF, there were 28 primary outcome events during the 12-month follow up period. The unadjusted and adjusted risk of the composite outcome of HF hospitalization and all-cause mortality is shown in Table 3. HF patients with ≥3 B-lines had a higher risk (age and sex adjusted HR 2.62 (95% CI: 1.15, 5.96; P=0.022) of the primary outcome when compared with those with <3 B-lines (Figure 3). No significant violations of the proportional hazards assumptions were detected (all P>0.05). There was no significant interaction between BMI and B-line number (P= 0.828) or ejection fraction and B-line number (P=0.282). In the subset of patients with available NT-proBNP (n=51) the relationship between B-lines and the primary outcome was not modified by NT-proBNP level (P=0.44 for interaction).
Table 3.
Primary outcome by B-line group in patients with HF (n=119)
| Group 1: 0–2 B-lines (n=70) |
Group 2: ≥3 B-lines (n=49) |
Unadjusted HR (95% CI) P value |
Adjusted* HR (95% CI) P value |
|
|---|---|---|---|---|
| Primary outcome | ||||
| 12-month HF hospitalization (%) | 8 (11.4) | 13 (26.5) | 2.57 (1.06, 6.20) 0.036 | 3.03 (1.16, 7.87) 0.023 |
| Death (%) | 5 (7.1) | 5 (10.2) | 1.60 (0.46, 5.53) 0.46 | 1.04 (0.27, 3.98) 0.95 |
| Composite outcome (first event) (%) | 11 (15.7) | 17 (34.7) | 2.46 (1.15, 5.26) 0.020 | 2.62 (1.15, 5.96) 0.022 |
Adjusted model: Adjusted for age and sex
Loss to follow up: Group 1: n=2 (2.9%), Group 2: n=2 (4.1%)
Figure 3. Cumulative incidence of HF hospitalization or death by B-line group in patients with heart failure (HFpEF or HFrEF) (n=119).
Discussion
This prospective study of adult patients with HTN or HF referred for outpatient echocardiography and concomitantly performed 8-zone lung ultrasonography has three principal findings. First, the number of B-lines was higher in patients with either HFpEF or HFrEF than in hypertensive patients who are at risk for HF. Second, patients with HFpEF or HFrEF were more likely to demonstrate worse measures of cardiac structure and function, known to be of prognostic importance, than those with hypertension. Third, in an exploratory analysis a higher number of sonographic B-lines identified those HF patients with a nearly three-fold risk for HF hospitalizations or death over 12 months, independent of age and sex.
Spectrum of pulmonary congestion in HFpEF and HFrEF
Dichotomous categories for the presence or absence of pulmonary edema have been suggested to classify patients with acute HF, among other classifications.17 However, traditional methods to identify and quantify pulmonary edema are known to be insensitive.3, 8 This issue becomes even more challenging in the management of chronic HF patients in the outpatient setting. Prior data on LUS findings in chronic HF patients are sparse and were either limited to patients with HFrEF or did not stratify analyses by ejection fraction.15, 18, 19 However, two recent studies using LUS with pocket ultrasound devices for the detection of pulmonary congestion suggest that chronic HF patients may in fact exhibit a spectrum of pulmonary congestion.15, 18 Importantly, higher degrees of pulmonary congestion identified by LUS may be undetectable by auscultation in up to 80% of patients but are associated with an up to four-fold risk of 6 month HF hospitalizations or death.15 In this current study, we found that ambulatory patients with HFpEF undergoing LUS with standard echocardiographic equipment at the time of echocardiography may demonstrate a similar spectrum of pulmonary congestion as those with HFrEF. This is an important finding given the challenges in establishing the diagnosis of HFpEF and identifying those at higher risk for decompensation.
Obese patients, who are prone to develop HFpEF, may exhibit low natriuretic peptide levels even in the presence of HF. We found that BMI did not vary significantly across B-line groups, suggesting that with standard echocardiographic equipment B-lines can be detected despite obesity. Our findings are similar to those of a prior study in 97 ambulatory patients with HFrEF in Brazil, in which BMI did not differ significantly in patients with higher vs. lower B-line number (although these were analyzed in 28 zones in contrast to 8 in our study).19 Both 8- and 28-zone methods have been used in the assessment of patients with HF and are recommended by an international guideline, while a lower number of zones can be performed more rapidly and may provide similar information.5, 9
Interestingly, patients with HTN who are at risk for HF demonstrated a low level of B-lines (≤6 Blines in all cases). Nearly half of HTN patients reported dyspnea and while their symptoms could be due to a number of reasons, it is conceivable that a subset of these patients may have subclinical HF or pulmonary disease. Alternative etiologies for B-lines, such as interstitial lung disease, should also be a consideration in the interpretation of LUS findings. Larger studies are needed to investigate the utility of LUS in ambulatory patients at risk for HF and the association between LUS and adverse outcomes.
LUS and echocardiographic measures
E/e’ is a commonly used echocardiographic marker of LV filling pressures and is recommended as a core measure by recent guidelines.14 A correlation between E/e’ and LUS findings of pulmonary congestion in ambulatory patients with HF has been previously demonstrated.19 However, prior reports in patients with unexplained dyspnea or advanced HF suggest that E/e’ may provide misleading information about left-sided filling pressures and should be interpreted with caution for the diagnosis of decompensated HF.20, 21 Moreover, not all patients with sonographic findings indicating pulmonary congestion report dyspnea.22 LUS, thus, may provide an additional piece of information when evaluating HF patients in the outpatient setting and, in conjunction with echocardiography, could paint a more comprehensive picture by adding quantitative information regarding the degree of pulmonary congestion in these patients. LUS findings may prove especially useful in patients with known or suspected HFpEF, including those with obesity.
LUS findings and outcomes in patients with HF
Prior data on the prognostic utility of LUS findings in ambulatory patients with chronic HF are sparse.9, 23 Findings from two studies in mixed cohorts of outpatients with HFrEF and HFpEF suggested that ≥3 B-lines on 5- or 8-zone LUS with a pocket ultrasound device may identify patients at a nearly four-fold risk for 6 month HF hospitalization or death.15, 18 Our findings expand on these earlier investigations and suggest that the presence of subclinical congestion in patients with chronic HF, even when assessed on 8-zone LUS during routine outpatient echocardiography, may provide important prognostic information. Our data further suggest that HF patients with 0–2 B-lines may represent a low risk population. The incremental value of LUS in patients with HFpEF and HFrEF over known risk markers should be investigated in larger, well-designed outcome trials and cut-off values for B-lines should be validated.
Limitations
This was a small, although well-characterized, sample enrolled at a single center which may affect generalizability. Laboratory results were not uniformly available in all patients, and were collected up to 6 months prior to or 7 days after the ultrasound examination, including NT-proBNP. Due to limited natriuretic peptide data and a high prevalence of atrial fibrillation/flutter more recent HFpEF definitions could not be applied. However, our HFpEF group had a similar rate of prior HF hospitalizations than our HFrEF group. In addition, results were similar when Framingham criteria were used (Table S1). Data regarding home medications were not collected in this study. The HTN group had a higher rate of active cancer than the two HF groups. However, patients with cancer involving the chest were excluded based on careful review of the EMR and recent imaging studies (frequently computed tomography for staging purposes). Due to the design of this current analysis we could not estimate positive and negative predictive values of B-line number for the exclusion of HF in ambulatory patients. Future investigations should address this question. In addition, the data driven cut-off values determined by this current study should be validated in different datasets in the future.
Conclusions
When performed at the time of outpatient echocardiography, LUS findings of pulmonary congestion differ between patients with known HF and those with hypertension who are at risk for HF. LUS estimation of pulmonary congestion may provide complementary information to traditional echocardiographic measures of cardiac structure and function in patients with known or suspected HF.
Supplementary Material
Highlights.
Lung ultrasound B-lines differ between hypertensive patients and those with HF.
B-lines are associated with echocardiographic markers of cardiovascular risk.
In ambulatory HF patients B-lines may be associated with adverse events.
Acknowledgments
Funding sources
This work was supported by grants from the National Heart, Lung and Blood Institute (grant number R00-HL-107642) (SC), the Ellison Foundation (SC) and the William F. Milton Fund (EP). The writing of this manuscript was supported by a grant from the National Heart, Lung and Blood Institute (grant number K23HL123533) (EP). The sponsors had no input or contribution in the development of the research and manuscript.
Abbreviations
- HF
Heart failure
- LUS
Lung ultrasound
- HFpEF
HF with preserved ejection fraction
- HFrEF
HF with reduced ejection fraction
- CAD
Coronary artery disease
- TTE
Transthoracic echocardiogram
- EMR
Electronic medical records
- HTN
Hypertension
- LVEF
Left ventricular ejection fraction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Pina IL, Trogdon JG. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–19. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gheorghiade M, Follath F, Ponikowski P, Barsuk JH, Blair JE, Cleland JG, Dickstein K, Drazner MH, Fonarow GC, Jaarsma T, Jondeau G, Sendon JL, Mebazaa A, Metra M, Nieminen M, Pang PS, Seferovic P, Stevenson LW, van Veldhuisen DJ, Zannad F, Anker SD, Rhodes A, McMurray JJ, Filippatos G. Assessing and grading congestion in acute heart failure: a scientific statement from the acute heart failure committee of the heart failure association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine. Eur J Heart Fail. 2010;12:423–33. doi: 10.1093/eurjhf/hfq045. [DOI] [PubMed] [Google Scholar]
- 3.Platz E, Jhund PS, Campbell RT, McMurray JJ. Assessment and prevalence of pulmonary oedema in contemporary acute heart failure trials: a systematic review. Eur J Heart Fail. 2015;17:906–16. doi: 10.1002/ejhf.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butler J, Gheorghiade M, Metra M. Moving away from symptoms-based heart failure treatment: misperceptions and real risks for patients with heart failure. Eur J Heart Fail. 2016;18:350–2. doi: 10.1002/ejhf.507. [DOI] [PubMed] [Google Scholar]
- 5.Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, Melniker L, Gargani L, Noble VE, Via G, Dean A, Tsung JW, Soldati G, Copetti R, Bouhemad B, Reissig A, Agricola E, Rouby JJ, Arbelot C, Liteplo A, Sargsyan A, Silva F, Hoppmann R, Breitkreutz R, Seibel A, Neri L, Storti E, Petrovic T (ICC-LUS) ILCoLUI-LfICCoLU. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38:577–91. doi: 10.1007/s00134-012-2513-4. [DOI] [PubMed] [Google Scholar]
- 6.Picano E, Pellikka PA. Ultrasound of extravascular lung water: a new standard for pulmonary congestion. Eur Heart J. 2016;37:2097–104. doi: 10.1093/eurheartj/ehw164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pivetta E, Goffi A, Lupia E, Tizzani M, Porrino G, Ferreri E, Volpicelli G, Balzaretti P, Banderali A, Iacobucci A, Locatelli S, Casoli G, Stone MB, Maule MM, Baldi I, Merletti F, Cibinel GA. Lung Ultrasound-Implemented Diagnosis of Acute Decompensated Heart Failure in the ED: A SIMEU Multicenter Study. Chest. 2015;148:202–10. doi: 10.1378/chest.14-2608. [DOI] [PubMed] [Google Scholar]
- 8.Martindale JL, Wakai A, Collins SP, Levy PD, Diercks D, Hiestand BC, Fermann GJ, deSouza I, Sinert R. Diagnosing Acute Heart Failure in the Emergency Department: A Systematic Review and Meta-analysis. Acad Emerg Med. 2016;23:223–42. doi: 10.1111/acem.12878. [DOI] [PubMed] [Google Scholar]
- 9.Platz E, Merz AA, Jhund PS, Vazir A, Campbell R, McMurray JJ. Dynamic changes and prognostic value of pulmonary congestion by lung ultrasound in acute and chronic heart failure: a systematic review. Eur J Heart Fail. 2017 doi: 10.1002/ejhf.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Price S, Platz E, Cullen L, Tavazzi G, Christ M, Cowie MR, Maisel AS, Masip J, Miro O, McMurray JJ, Peacock WF, Martin-Sanchez FJ, Di Somma S, Bueno H, Zeymer U, Mueller C Acute Heart Failure Study Group of the European Society of Cardiology Acute Cardiovascular Care A. Expert consensus document: Echocardiography and lung ultrasonography for the assessment and management of acute heart failure. Nat Rev Cardiol. 2017;14:427–440. doi: 10.1038/nrcardio.2017.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F, Redon J, Dominiczak A, Narkiewicz K, Nilsson PM, Burnier M, Viigimaa M, Ambrosioni E, Caufield M, Coca A, Olsen MH, Schmieder RE, Tsioufis C, van de Borne P, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Clement DL, Coca A, Gillebert TC, Tendera M, Rosei EA, Ambrosioni E, Anker SD, Bauersachs J, Hitij JB, Caulfield M, De Buyzere M, De Geest S, Derumeaux GA, Erdine S, Farsang C, Funck-Brentano C, Gerc V, Germano G, Gielen S, Haller H, Hoes AW, Jordan J, Kahan T, Komajda M, Lovic D, Mahrholdt H, Olsen MH, Ostergren J, Parati G, Perk J, Polonia J, Popescu BA, Reiner Z, Ryden L, Sirenko Y, Stanton A, Struijker-Boudier H, Tsioufis C, van de Borne P, Vlachopoulos C, Volpe M, Wood DA. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2013;34:2159–219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 12.Goncalves A, Hung CL, Claggett B, Nochioka K, Cheng S, Kitzman DW, Shah AM, Solomon SD. Left Atrial Structure and Function Across the Spectrum of Cardiovascular Risk in the Elderly: The Atherosclerosis Risk in Communities Study. Circ Cardiovasc Imaging. 2016;9:e004010. doi: 10.1161/CIRCIMAGING.115.004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the american society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. 2015;28:1–39. e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Platz E, Lewis EF, Uno H, Peck J, Pivetta E, Merz AA, Hempel D, Wilson C, Frasure SE, Jhund PS, Cheng S, Solomon SD. Detection and prognostic value of pulmonary congestion by lung ultrasound in ambulatory heart failure patients. Eur Heart J. 2016;37:1244–51. doi: 10.1093/eurheartj/ehv745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Platz E, Hempel D, Pivetta E, Rivero J, Solomon SD. Echocardiographic and lung ultrasound characteristics in ambulatory patients with dyspnea or prior heart failure. Echocardiography. 2014;31:133–9. doi: 10.1111/echo.12346. [DOI] [PubMed] [Google Scholar]
- 17.Logeart D, Isnard R, Resche-Rigon M, Seronde MF, de Groote P, Jondeau G, Galinier M, Mulak G, Donal E, Delahaye F, Juilliere Y, Damy T, Jourdain P, Bauer F, Eicher JC, Neuder Y, Trochu JN Cardiology HFotFSo. Current aspects of the spectrum of acute heart failure syndromes in a real-life setting: the OFICA study. Eur J Heart Fail. 2013;15:465–76. doi: 10.1093/eurjhf/hfs189. [DOI] [PubMed] [Google Scholar]
- 18.Gustafsson M, Alehagen U, Johansson P. Imaging Congestion With a Pocket Ultrasound Device: Prognostic Implications in Patients With Chronic Heart Failure. J Card Fail. 2015;21:548–54. doi: 10.1016/j.cardfail.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Miglioranza MH, Gargani L, Sant'anna RT, Rover MM, Martins VM, Mantovani A, Weber C, Moraes MA, Feldman CJ, Kalil RA, Sicari R, Picano E, Leiria TL. Lung ultrasound for the evaluation of pulmonary congestion in outpatients: a comparison with clinical assessment, natriuretic peptides, and echocardiography. JACC Cardiovasc Imaging. 2013;6:1141–51. doi: 10.1016/j.jcmg.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Solomon SD, Stevenson LW. Recalibrating the barometer: is it time to take a critical look at noninvasive approaches to measuring filling pressures? Circulation. 2009;119:13–5. doi: 10.1161/CIRCULATIONAHA.108.823591. [DOI] [PubMed] [Google Scholar]
- 21.Santos M, Rivero J, McCullough SD, West E, Opotowsky AR, Waxman AB, Systrom DM, Shah AM. E/e' Ratio in Patients With Unexplained Dyspnea: Lack of Accuracy in Estimating Left Ventricular Filling Pressure. Circ Heart Fail. 2015;8:749–56. doi: 10.1161/CIRCHEARTFAILURE.115.002161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zoccali C, Torino C, Tripepi R, Tripepi G, D'Arrigo G, Postorino M, Gargani L, Sicari R, Picano E, Mallamaci F. Pulmonary congestion predicts cardiac events and mortality in ESRD. J Am Soc Nephrol. 2013;24:639–46. doi: 10.1681/ASN.2012100990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miglioranza MH, Picano E, Badano LP, Sant'Anna R, Rover M, Zaffaroni F, Sicari R, Kalil RK, Leiria TL, Gargani L. Pulmonary congestion evaluated by lung ultrasound predicts decompensation in heart failure outpatients. Int J Cardiol. 2017;240:271–278. doi: 10.1016/j.ijcard.2017.02.150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.