Abstract
Background and objective
Obstructive sleep apnoea (OSA) is typically worse in the supine versus lateral sleeping position. One potential factor driving this observation is a fall in lung volume in the supine position which is expected by theory to increase a key OSA pathogenic factor: dynamic ventilatory control instability [i.e. loop gain]. We aimed to quantify dynamic loop gain in OSA patients in the lateral and supine positions, and to explore the relationship between change in dynamic loop gain and change in lung volume with position.
Methods
Data from 20 patients enrolled in previous studies on the effect of body position on OSA pathogenesis was retrospectively analysed. Dynamic loop gain was calculated from routinely collected polysomnographic signals using a previously validated mathematical model. Lung volumes were measured in the awake state with a nitrogen washout technique.
Results
Dynamic loop gain was significantly higher in the supine than in the lateral position (0.77±0.15 vs 0.68±0.14, P=0.012). Supine functional residual capacity (FRC) was significantly lower than lateral FRC (81.0±15.4 vs 87.3±18.4% of the seated FRC, P=0.021). The reduced FRC we observed on moving to the supine position was predicted by theory to increase loop gain by 10.2 (0.6, 17.1)%, a value similar to the observed increase of 8.4 (−1.5, 31.0)%.
Conclusion
Dynamic loop gain increased by a small but statistically significant amount when moving from the lateral to supine position and this may, in part, contribute to the worsening of OSA in the supine sleeping position.
Keywords: obstructive sleep apnoea, ventilatory control, lung volume, upper airway physiology, body position
INTRODUCTION
Obstructive sleep apnoea (OSA) is a common condition in which repetitive upper airway obstruction during sleep leads to frequent oxygen desaturation and recurrent arousals. In approximately half of all patients with OSA, the frequency of respiratory events doubles with a change from lateral to supine sleeping position (i.e. supine predominant OSA) 1. Furthermore, about a third of these supine predominant patients experience OSA exclusively in the supine position (i.e. supine-isolated OSA)2, 3.
Until recently, the mechanisms through which body position influences OSA severity were poorly understood. The available evidence suggests that both the degree5 and site6 of upper-airway collapse, are key determinants in the development of positional OSA. Specifically, Moon et al.6 demonstrated that patients are twice as likely to have positional versus non-positional OSA if they experience both retropalatal and retrolingual obstruction in the supine position. Furthermore, we recently demonstrated that lateral positioning improves anatomy/collapsibility under both passive and active conditions as well as increases lung volume5. However, it is clear that the pathogenesis of OSA involves both anatomical (i.e. small highly collapsible pharyngeal airway) and non-anatomical factors which include a hypersensitive ventilatory control system (i.e. high loop gain), a low arousal threshold and ineffective upper airway dilator muscles4. Importantly, in our previous study lateral positioning did not alter the arousal threshold, upper airway muscle responses, or steady-state loop gain (i.e. the ventilatory drive response to a sustained reduction in ventilation)5.
While an increase in lung volume may improve collapsibility, by directly stretching or dilating the pharyngeal airway through tracheal and mediastinal connections7–9, it also increases the stability of the ventilatory control system and reduces the propensity for central sleep apnea (lower dynamic loop gain, i.e. a reduction in the reflex ventilatory drive response to a transient hypopnea)10–13. Improved stability occurs via increased lung gas volume and improved capacity to buffer fluctuations in alveolar partial pressure of oxygen (PO2) and partial pressure of carbon dioxide (PCO2)11–13. Importantly, dynamic loop gain14–19, in addition to steady-state loop gain17, 20–23, has been implicated in the pathogenesis of OSA, and it is dynamic loop gain—not steady-state loop gain—that is expected to fall with increased lung volume12 on adoption of the lateral position..
By way of introduction, loop gain is a quotient used in engineering to quantify the inherent stability of a negative feedback loop system. In sleep apnoea physiology, loop gain describes the sensitivity of the negative feedback loop that controls ventilation. This feedback loop comprises two key components: The ‘plant’ determines the change in carbon dioxide (CO2) and oxygen (O2) in the arterial blood that occurs for any given fluctuation in ventilation. The ‘controller’ determines the increase in ventilatory drive that occurs with any given increase in PCO2. Loop gain can be measured/expressed as a steady-state value (steady-state loop gain) or as a function of time (dynamic loop gain). In both forms, loop gain is the ratio of ventilatory drive response to ventilatory disturbance, with the steady-state value representing the maximal long-term response to a persistent change in ventilation, while the dynamic loop gain reflects the response to a transient disturbance such as a brief hypopnea. The dynamic loop gain is affected by a number of physiological factors including lung volume according to the following equation13, 24, 25:
| Equation 1 |
Equation 1 highlights that dynamic loop gain is determined by four factors: (i) the chemosensitivity, reflecting the dynamic ventilatory response to hypercapnia, (ii) the alveolar PCO2 (reflecting the alveolar minus inspired gas gradient) (iii) a complex timing factor T, which includes the effect of the circulation time between lungs and chemoreceptors (delays in information transfer on the level of O2 and CO2 tensions) and the time constant of the lung and (iv) the lung gas volume (i.e. functional residual capacity, FRC). Chemosensitivity is also known as the ‘controller gain’, while the other three variables in parentheses collectively comprise what is termed ‘plant gain’, which defines how effectively and quickly a change in ventilation is converted into a change in alveolar and arterial PCO2. Equation 1 illustrates that the “dynamic” loop gain is inversely proportional to changes in lung volume. For example, if lung volume is doubled, “dynamic” loop gain will be halved.
Given that lung volume is a key factor that contributes to an individual’s dynamic loop gain, we examined whether: (1) the measured dynamic loop gain increases with a change from lateral to supine sleeping position and (2) the change in measured dynamic loop gain is consistent with the magnitude of the lung volume change, as predicted by theory11–13.
METHODS
Participants
We retrospectively analysed the dynamic loop gain of the 20 OSA patients who participated in two of our previous experiments examining the effect of body position on the physiological traits causing OSA. Briefly, patients were recruited through the Monash Lung and Sleep Department of Monash Health, a university teaching hospital in Melbourne, Australia. Patients were recruited on the basis of their diagnostic polysomnography with a requirement for the presence of OSA with an apnea/hypopnea index (AHI) of >5 events/hr. Additionally, patients were required to have spent at least 30 min in both the supine and lateral sleeping positions on their original diagnostic study. Ethics approval for this project was obtained through Monash Health Human Research Ethics Committee and patients provided informed consent prior to enrolment.
Experimental Design and Setup
Full details of the experimental design and set-up have been described previously, although none of the findings from the current analysis have been reported5, 26. Briefly, patients were selected on the basis of clinical polysomnographic parameters as described above. They then attended the Monash Lung and Sleep Department and had lung volume measurements made as described below. Following lung volume measurements, a proportion of the included patients underwent a physiological sleep study5.
Lung Volume Measurements
Two measurements of lung volume were made using a nitrogen gas washout method27 in each position: seated, supine and lateral (with position order randomized), with the average of the two measurements used for analysis. Patients breathed via a mouth-piece (nares occluded with a nose clip) that allowed for measurement of carbon dioxide (CO2) (NICO Cardiopulmonary Management System), oxygen (O2) (Ametek S-3A/I, Ametek Process Instruments, PA, USA) and ventilation. Fractional expired nitrogen was calculated from the fractional O2 and CO2 levels (FN2 = 1 − FO2 − FCO2). Once breathing acclimatized (~2–3 min), the inspired gas was switched from room air (79% nitrogen, N2) to 100% oxygen (0% nitrogen, N2), via the use of a non-rebreathing valve (Medium T shape 2-Way, Hans-Rudolph, MO, USA). When a steady-state expired FN2 was achieved, breathing was switched back to room air (‘wash-in’ phase) until a steady state was reached (constant FN2 within 1.5% between breaths, ~5–7 min). The time over which N2 increased was used to calculate FRC according to FRC = ΔVolN2/ΔFN2, where ΔVolN2 is the change in alveolar N2 volume from the start to end of the test, and ΔFN2 is the change in alveolar N2 concentration during this time (final FN2 − initial FN2). The wash-in phase, rather than the ‘washout’ phase, was used to avoid transient ventilation reductions that are possible with a rapid rise in PO2 at washout onset. With the bed positioned horizontally, patients were asked to lie in the lateral and supine positions. When lateral, the Frankfort plane was aligned with the craniocaudal axis of the body, perpendicular to the long axis of the bed. When lying supine, foam pads were used to position the head and neck in the Frankfort plane perpendicular to the bed. All lung volume measurements were made during wakefulness.
Polysomnography
Overnight polysomnography was performed at Monash Health, an academic sleep centre in Melbourne, Australia using a standard clinical montage. Body position was recorded with a body position sensor (part number 7000-0104-02, Compumedics, Abbotsford, Australia) placed over the sternum and attached to the thoracic inductance band with Velcro and tape and was verified with video monitoring, with manual correction as appropriate. The sensor uses a series of gravity referenced switches to output a signal indicating one of four possible positions – supine, left lateral, right lateral or prone. Sleep studies were staged and scored according to American Academy of Sleep Medicine criteria28. Dynamic loop gain analysis was performed on signals obtained from the scored polysomnographic data using our previously described and validated methods14.
Determining dynamic loop gain
Routine clinical polysomnographic signals were used to calculate dynamic loop gain. Data was exported from the Compumedics recording program as a European Data Format file and imported into Matlab (R2015a version 8.5.0197613 Mathworks Inc., Natick, MA) for manipulation. A software routine identified 7-minute periods of non-REM sleep containing one or more scored obstructive apnoeas/hypopnoeas. Nasal pressure was square-root transformed and taken as a surrogate of ventilatory flow and integrated and normalized by the mean to provide a ventilation signal for subsequent analysis. A categorical breath-by-breath time series of scored obstructed breaths and scored arousals was created. Using these data, a standard ventilatory control model was fit for each 7-minute epoch in order to determine the optimum set of system parameters (i.e. a gain, time constant and delay). Parameters were adjusted automatically such that the estimated ventilatory drive signal output from the model best matches the ventilation signal during unobstructed breaths (i.e. when ventilation reflects ventilatory drive). From these parameters, we can determine dynamic loop gain at any frequency of interest. Here we report the dynamic loop gain determined at 1 cycle/min (LG1) with all measurements for each 7-minute epoch then averaged to give a single value for each individual.
Statistical analysis
Statistical analysis was performed using SPSS (version 20, 2011, New York, USA) and Prism 6 (6.0e, La Jolla, USA). Continuous variables were expressed as means and standard deviations where normally distributed; and as median and interquartile range where not normally distributed. Comparisons were made using paired t-tests for normally distributed data, Wilcoxon signed rank or Mann-Whitney U tests for non-parametric data and Fisher’s exact test for categorical data. Bivariate correlations are made with Pearson’s r for normally distributed data and Spearman’s r (rs) for non-parametric data. A p-value of <0.05 was considered significant. In addition to the measured/observed changes in loop gain, the percentage predicted change in dynamic loop gain with position was also calculated from the observed percentage change in lung volume with position using Equation 1. The observed vs predicted changes in dynamic loop gain were then compared.
RESULTS
The demographics of the included patients are displayed in Table 1.
Table 1.
Baseline participant characteristics (n=20)
| Variable | |
|---|---|
| Age, years | 54.7 ± 12.9 |
| Sex, male, % | 55 |
| BMI, kg/m2, mean (SD) | 35.3 ± 6.7 |
| Neck Circumference (cm) | 43.1 ± 3.4 |
| Chest Circumference (cm) | 114.1 ± 9.3 |
| Waist Circumference (cm) | 115.9 ± 13.6 |
| Hip Circumference (cm) | 115.8 ± 14.9 |
| Total AHI, events/hr | 60.8 ± 23.7 |
| AHI Supine, events/hr | 79.0 ± 21.5 |
| AHI Non-Supine, events/hr | 45.0 ± 32.0 |
| % Supine Sleep | 44.2 ± 17.2 |
Values are means ± SD. Abbreviations: AHI: apnea-hypopnea index; BMI: body mass index.
Effects of body position on loop gain and lung volume
The supine lung volume was reduced by 9.5 (0.5, 14.8)% compared to the lateral lung volume. This reduction corresponded to an expected (or predicted) increase in dynamic loop gain of 10.2 (0.6, 17.1)% as calculated by using Equation 1 from the introductory section.
In conjunction, we observed an increase in directly measured dynamic loop gain of 8.4 (−1.5, 31.0)% in the supine versus lateral position. Figure 1 demonstrates the model output for an individual patient in the supine and lateral position (7 minute epoch in each position). The individual data points for dynamic loop gain in the supine and non-supine body positions are displayed in Figure 2 with group data represented in Table 2.
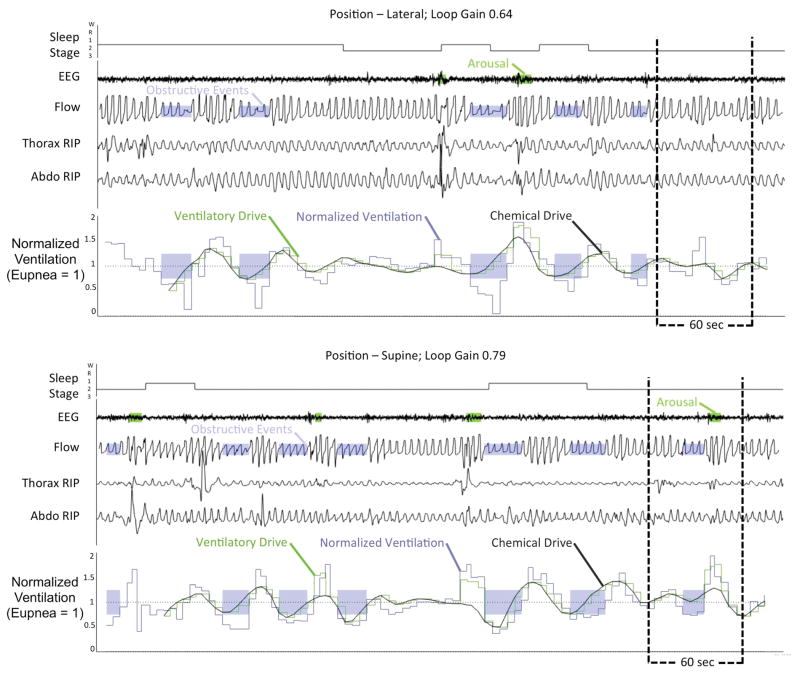
Figure 1.
Example of data output from standard ventilatory control model for an individual patient in the lateral and supine position. Shaded purple regions denote periods of obstruction while shaded green regions on the EEG trace represent scored respiratory arousals. The estimated chemical drive (solid smooth black line) is precisely superimposed on estimated ventilatory drive (green staircased line) is closely overlaid upon the observed ventilation (blue staircase line) in the absence of obstruction. Chemical drive should equal ventilatory drive except where there is an arousal. EEG – electroencephalogram, RIP – respiratory inductance plethysmography.
Figure 2.
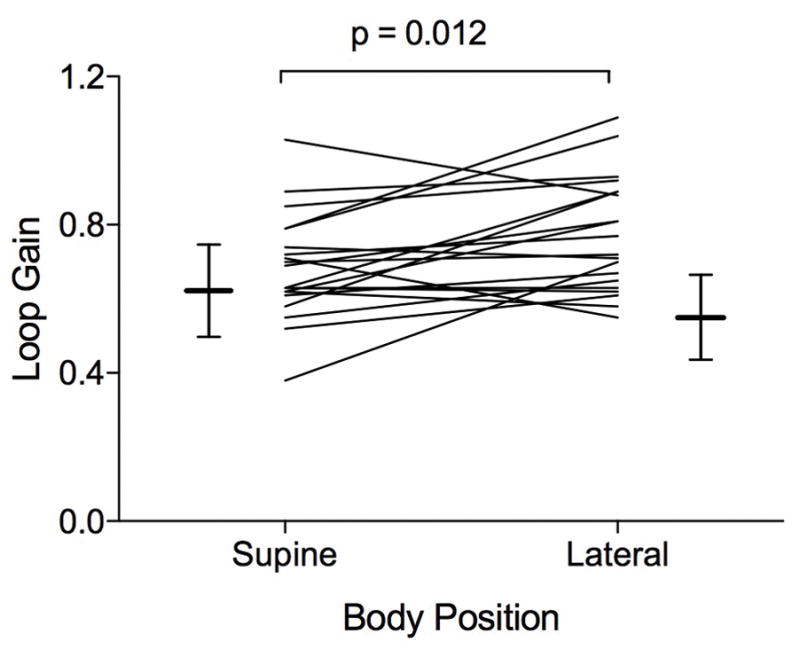
Loop gain values in the lateral and supine body positions.
Table 2.
Lung volume and loop gain measure in the supine and lateral position
| Parameter | Lateral | Supine | P-Value |
|---|---|---|---|
| FRC (% of seated volume) | 87.3 ± 18.4 | 81.0 ± 15.4 | 0.021 |
| Loop gain, LG1 | 0.68 ± 0.14 | 0.77 ± 0.15 | 0.012 |
| Time constant, s | 107.2 ± 42.0 | 109.8 ± 29.3 | 0.855 |
| Chemoreflex delay, s | 10.8 [9.45 to 12.24] | 10.35 [9.03 to 11.0] | 0.079 |
| Tn, s | 37.93 [34.54 to 41.65] | 38.08 [31.61 to 42.74] | 0.370 |
Values are means ± SD. or medians [interquartile range]. Paired samples t-test for parametric paired data, Wilcoxon signed rank for non-parametric paired data. Abbreviations: LG: loop gain, ventilatory response to 1 cycle/min disturbance; Tn: natural cycling period. Arousal threshold is the estimated ventilatory drive preceding scored EEG arousal from sleep38.
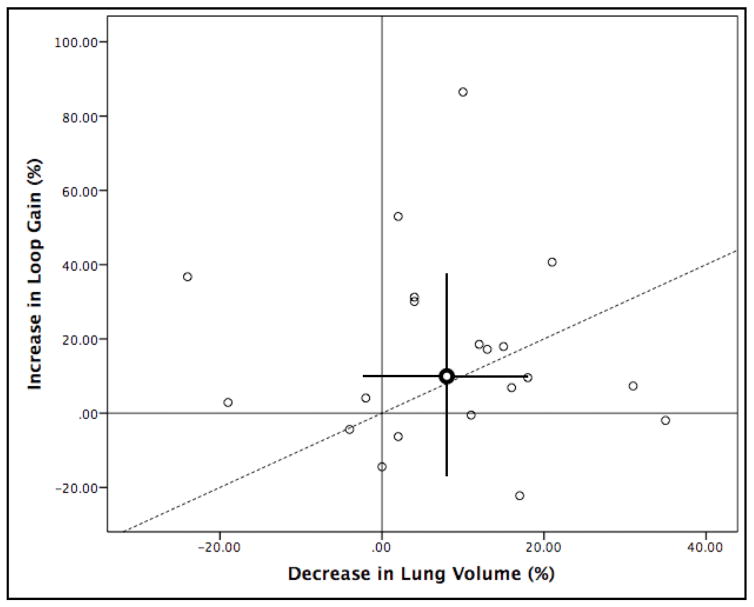
As a group, the magnitude of observed increase in loop gain was not statistically different to that which was expected/predicted based on the reduction in lung volume (p=0.5), see Figure 3. However, there was no correlation between the measured change in dynamic loop gain and the expected change in dynamic loop gain based on lung volume (Spearman’s r=−0.370, p=0.173). Additionally, there was no significant correlation between the lateral FRC and lateral dynamic loop gain (r=0.270, p=0.250), the supine FRC and the supine dynamic loop gain (r=−0.063, p=0.904) or the change in FRC and the change in dynamic loop gain (rs =0.041, p=0.862). There was no statistically significant correlation between FRC and chemoreflex delay in either the supine (r=0.172, p=0.469) or lateral (r=0.411, p=0.072) position. The change in FRC was also not statistically significantly correlated with change in circulation time/chemoreflex delay (rs=0.214, p=0.364).
Figure 3.
Relationship between percent increase in loop gain versus percent decrease in lung volume with change from lateral to supine sleeping position. Bold data point is the mean change in loop gain and lung volume with error bars representing the standard deviation vertically for loop gain and horizontally for lung volume. Dashed line is the line of identity.
DISCUSSION
The supine sleeping position is a well-documented exacerbating factor for OSA. Previous studies have demonstrated that body position affects anatomical contributors to airway collapse without altering non-anatomical factors such as steady-state loop gain29. However, the effect of body position on dynamic loop gain has not been previously investigated. The major finding of the current study is that dynamic loop gain increases by a significant yet small amount (~10%) as patients move from the lateral to supine position, which is similar to the change in lung volume between positions (~10%). Such an increase in an individual’s dynamic loop gain is likely to play a relatively minor role in the worsening of OSA in the supine versus lateral position that is observed in approximately 60% of all OSA patients.
The major finding of this study is that the respiratory control system is slightly less stable when patients move from the lateral to the supine sleeping position. This important finding suggests a minor contribution of ventilatory control instability to the observed worsening in OSA severity when patients lie in the supine position. The magnitude of dynamic loop gain change is small (an increase of 8.4 [−1.5, 31.0]% as patients move from lateral to supine) and when patients sleep in the lateral position their lateral dynamic loop gain is 0.68 ± 0.14, which is still considered a high value15. As such, the contribution of ventilatory control instability to the change in OSA severity with body position is likely to be much less important than the previously reported anatomical contributions5.
Importantly, we have previously demonstrated that the steady state loop gain is unchanged with body position. The contrary findings regarding dynamic loop gain change and steady state loop gain change highlight an important difference between these two parameters. Firstly, steady state loop gain, as the name suggests, describes the long term or steady-state increase in ventilatory drive that occurs with a persistent reduction in ventilation. Because it is relevant over a longer time scale (i.e. whether steady-state ventilatory drive lies below the arousal threshold) it is not affected by the lung gas stores which may dampen oscillations in PCO2 for tens of seconds, but not over several minutes. However, the lung gas stores are an important determinant of PCO2 changes at a time scale relevant to dynamic loop gain (ventilatory stability).
Although the group mean magnitude of change in dynamic loop gain was small, Figure 2 demonstrates that for some individual patients, dynamic loop gain worsened by up to 30% when moving from lateral to supine. Although our study did not include patients with central sleep apnea, it may be that such large increases in dynamic loop gain underpin the worsening in central sleep apnea seen when patients move from the lateral to the supine position10. In a study by Szollosi and colleagues10, the almost 50% worsening in central sleep apnoea severity between lateral and supine positions was not thought to be due to anatomical factors, as the ratio of mixed to central apneas remained unchanged by sleeping position. The authors proposed a difference in pulmonary oxygen stores with body position as a putative mechanism and this would be borne out by a subsequent change in loop gain. Further to this, Sands and colleagues demonstrated an improvement in dynamic loop gain with an increase in lung volume associated with CPAP usage13. More research is required to elucidate the exact mechanism(s) behind the dependence of central sleep apnoea severity with sleeping position, but improvement change in dynamic loop gain is a plausible explanation.
The finding that the average change in dynamic loop gain with position (~10%) was similar in magnitude to that expected from the average change in lung volume (~10%) is consistent with our hypothesis that positional dynamic loop gain changes are likely the result of positional changes on plant gain (given that lung volume is one of the three factors that comprise the plant gain term – see Equation 1). Furthermore, the change in lung volume with body position has been observed by others.30, 31 However, we found the changes in awake lung volume did not significantly correlate with changes in dynamic loop gain, which was unexpected. One possible explanation for this finding is that the lung volume measurements we made were in the awake state prior to the sleep study. Previous studies have demonstrated a small but significant change in lung volume at both sleep onset and at the onset of apnoea32. In a study by Stadler and colleagues, sleep onset corresponded with a 61mL reduction and apnea onset with a further reduction of 90mL. It may be that awake lung volume differences do not accurately represent the lung volume differences during sleep, particularly with added inter- and intra-subject variability when differences in respiratory muscle tone and inspiratory flow limitation are taken into consideration. Accurately measuring lung volume during sleep, particularly during periods of respiratory disturbance in OSA patients, remains an ongoing challenge for the field.
We also considered how changes in body position could affect the other parameters that determine ventilatory stability (Equation 1). Firstly, there has been no study performed to date that explores the effect of body position on the ventilatory sensitivity to hypoxia and hypercapnia. Secondly, chemoreflex or circulatory delay (Equation 1), i.e. the time for blood to travel from the lungs to the chemoreceptors could potentially be affected by body position. However, we found no significant difference in chemoreflex delay in our study 10.35 [9.03 to 11.0] seconds vs. 10.8 [9.45 to 12.24] seconds. Although no studies have explored the effect of body position on circulatory delay time in OSA patients specifically, there are conflicting findings from studies examining the effects of lateral vs. supine positioning on haemodynamic parameters in normal subjects and patients undergoing cardiac testing. Lange and colleagues found no difference in cardiac output between supine and lateral positions suggesting that circulatory delay should remain unchanged by sleeping position33. Alternatively, Jones and colleagues found a lower blood pressure, heart rate and rate pressure product in the lateral vs. supine position which suggested that circulatory delay might be increased with lateral positioning34. Our study suggests that an increase in the circulatory delay is unlikely to occur in OSA patients. Thirdly, the alveolar CO2 tension (Equation 1) is unlikely to be affected, unless mean ventilation or metabolic rate is altered. Finally, it is also possible that ventilation-perfusion matching may be adversely affected, particularly in patients with obesity and restrictive lung disease in the lateral versus supine position, acting not only to lower effective lung volumes but also raising dynamic loop gain via hypoxic augmentation of chemosensitivity35, 36. Since the size of the positional change in dynamic loop gain matches, on average, the change in lung volume, such effects in OSA are unlikely.
As we have acknowledged, the major limitation of our study is the use of awake lung volume measurements, whereas it is known that both sleep onset and apnea onset reduce lung volume significantly. Another limitation of the study is inherent to the measurement of body position. Our patients had body position recorded using a position sensor that was corrected with video monitoring. The position sensor, however, does not take into account head position during sleep which is known to influence OSA severity37. In addition, the body position in which dynamic loop gain was measured was not randomized and patients were allowed to freely choose their sleeping position during the sleep study. However, only patients who recorded at least 30 min supine and 30 min non-supine were included in the study. As such, the sampling of dynamic loop gain may have been biased by individuals who preferred to sleep predominantly in a particular body position. However, Table 1 demonstrates that on average patients spent 44.2 ± 17.2% of their sleep study in the supine position. Given that our patients spent an almost equal amount of time in both sleeping positions, there is unlikely to be any sampling error our results. Furthermore, the time spent supine was not related to the change in loop gain observed (r=0.06 p=0.8).
In conclusion, we have demonstrated that moving from the lateral to supine sleeping position results in a small but significant reduction in ventilatory control system stability as measured by dynamic loop gain. The change in dynamic loop gain was of a similar magnitude to the reduction in awake lung volume that results from a change in body position. We conclude that the changes observed in dynamic loop gain with body position make only a minor contribution to the increase in frequency of respiratory events when OSA patients adopt the supine sleeping posture.
Summary at a glance.
We explored whether increased ventilatory control instability (i.e. loop gain) is responsible for the observed increase in respiratory event frequency in the supine sleeping position in obstructive sleep apnoea patients. Loop gain is significantly increased in the supine compared to lateral sleeping position, although the magnitude of change is small.
Acknowledgments
Dr. Landry is supported by NeuroSleep a National Health and Medical Research Council of Australia (NHMRC) Centre of Research Excellence. Dr. Sands is supported by the American Heart Association (15SDG25890059), an NHMRC Early Career Fellowship and R.G. Menzies award (1053201), an American Thoracic Society Foundation Unrestricted Grant, and the National Institute of Health (R01HL128658, 2R01HL102321, P01HL10050580). Dr. Edwards was supported by the National Health and Medical Research Council (NHMRC) of Australia’s CJ Martin Overseas Biomedical Fellowship (1035115) and is now supported by a Heart Foundation of Australia Future Leader Fellowship (101167). Dr. Terrill is supported by the NHMRC (1064163).
Footnotes
Disclosure statement
GH and SJ have received equipment to support research from Resmed, Philips Respironics and Air Liquide Healthcare.
References
- 1.Joosten SA, O’Driscoll DM, Berger PJ, Hamilton GS. Supine position related obstructive sleep apnea in adults: Pathogenesis and treatment. Sleep medicine reviews. 2013;18:7–17. doi: 10.1016/j.smrv.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Mador MJ, Kufel TJ, Magalang UJ, Rajesh SK, Watwe V, Grant BJB. Prevalence of positional sleep apnea in patients undergoing polysomnography. Chest. 2005;128:2130–7. doi: 10.1378/chest.128.4.2130. [DOI] [PubMed] [Google Scholar]
- 3.Joosten SA, Hamza K, Sands S, Turton A, Berger P, Hamilton G. Phenotypes of patients with mild to moderate obstructive sleep apnoea as confirmed by cluster analysis. Respirology. 2012;17:99–107. doi: 10.1111/j.1440-1843.2011.02037.x. [DOI] [PubMed] [Google Scholar]
- 4.Wellman A, Edwards BA, Sands SA, Owens RL, Nemati S, Butler JP, Passaglia CL, Jackson AC, Malhotra A, White DP. A simplified method for determining phenotypic traits in patients with obstructive sleep apnea. J Appl Physiol. 2013 doi: 10.1152/japplphysiol.00747.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joosten SA, Edwards BA, Wellman A, Turton A, Skuza EM, Berger PJ, Hamilton GS. The Effect of Body Position on Physiological Factors that Contribute to Obstructive Sleep Apnea. Sleep. 2015;38:1469–78. doi: 10.5665/sleep.4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moon IJ, Han DH, Kim JW, Rhee CS, Sung MW, Park JW, Kim DS, Lee CH. Sleep magnetic resonance imaging as a new diagnostic method in obstructive sleep apnea syndrome. Laryngoscope. 2010;120:2546–54. doi: 10.1002/lary.21112. [DOI] [PubMed] [Google Scholar]
- 7.Jordan AS, White DP, Owens RL, Eckert DJ, Rahangdale S, Yim-Yeh S, Malhotra A. The effect of increased genioglossus activity and end-expiratory lung volume on pharyngeal collapse. J Appl Physiol. 2010;109:469–75. doi: 10.1152/japplphysiol.00373.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Squier SB, Patil SP, Schneider H, Kirkness JP, Smith PL, Schwartz AR. Effect of end-expiratory lung volume on upper airway collapsibility in sleeping men and women. J Appl Physiol. 2010;109:977–85. doi: 10.1152/japplphysiol.00080.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stadler DL, McEvoy RD, Sprecher KE, Thomson KJ, Ryan MK, Thompson CC, Catcheside PG. Abdominal compression increases upper airway collapsibility during sleep in obese male obstructive sleep apnea patients. Sleep. 2009;32:1579–87. doi: 10.1093/sleep/32.12.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szollosi I, Roebuck T, Thompson B, Naughton MT. Lateral sleeping position reduces severity of central sleep apnea/Cheyne-Stokes respiration. Sleep. 2006;29:1045–51. doi: 10.1093/sleep/29.8.1045. [DOI] [PubMed] [Google Scholar]
- 11.Edwards BA, Sands SA, Feeney C, Skuza EM, Brodecky V, Wilkinson MH, Berger PJ. Continuous positive airway pressure reduces loop gain and resolves periodic central apneas in the lamb. Respir Physiol Neurobiol. 2009;168:239–49. doi: 10.1016/j.resp.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Khoo MC, Kronauer RE, Strohl KP, Slutsky AS. Factors inducing periodic breathing in humans: a general model. J Appl Physiol. 1982;53:644–59. doi: 10.1152/jappl.1982.53.3.644. [DOI] [PubMed] [Google Scholar]
- 13.Sands SA, Edwards BA, Kee K, Turton A, Skuza EM, Roebuck T, O’Driscoll DM, Hamilton GS, Naughton MT, Berger PJ. Loop gain as a means to predict a positive airway pressure suppression of Cheyne-Stokes respiration in patients with heart failure. Am J Respir Crit Care Med. 2011;184:1067–75. doi: 10.1164/rccm.201103-0577OC. [DOI] [PubMed] [Google Scholar]
- 14.Terrill PI, Edwards BA, Nemati S, Butler JP, Owens RL, Eckert DJ, White DP, Malhotra A, Wellman A, Sands SA. Quantifying the ventilatory control contribution to sleep apnoea using polysomnography. Eur Respir J. 2015;45:408–18. doi: 10.1183/09031936.00062914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wellman A, Malhotra A, Jordan AS, Stevenson KE, Gautam S, White DP. Effect of oxygen in obstructive sleep apnea: role of loop gain. Respir Physiol Neurobiol. 2008;162:144–51. doi: 10.1016/j.resp.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wellman A, Jordan AS, Malhotra A, Fogel RB, Katz ES, Schory K, Edwards JK, White DP. Ventilatory control and airway anatomy in obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:1225–32. doi: 10.1164/rccm.200404-510OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edwards BA, Sands SA, Eckert DJ, White DP, Butler JP, Owens RL, Malhotra A, Wellman A. Acetazolamide improves loop gain but not the other physiological traits causing obstructive sleep apnoea. J Physiol. 2012;590:1199–211. doi: 10.1113/jphysiol.2011.223925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Younes M. Role of respiratory control mechanisms in the pathogenesis of obstructive sleep disorders. J Appl Physiol. 2008;105:1389–405. doi: 10.1152/japplphysiol.90408.2008. [DOI] [PubMed] [Google Scholar]
- 19.Onal E, Lopata M. Periodic breathing and the pathogenesis of occlusive sleep apneas. Am Rev Respir Dis. 1982;126:676–80. doi: 10.1164/arrd.1982.126.4.676. [DOI] [PubMed] [Google Scholar]
- 20.Wellman A, Edwards BA, Sands SA, Owens RL, Nemati S, Butler J, Passaglia CL, Jackson AC, Malhotra A, White DP. A simplified method for determining phenotypic traits in patients with obstructive sleep apnea. J Appl Physiol. 2013;114:911–22. doi: 10.1152/japplphysiol.00747.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188:996–1004. doi: 10.1164/rccm.201303-0448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edwards BA, Wellman A, Sands SA, Owens RL, Eckert DJ, White DP, Malhotra A. Obstructive sleep apnea in older adults is a distinctly different physiological phenotype. Sleep. 2014;37:1227–36. doi: 10.5665/sleep.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edwards BA, Andara C, Landry S, Sands SA, Joosten SA, Owens RL, White DP, Hamilton GS, Wellman A. Upper-airway Collapsibility and Loop Gain Predict the Response to Oral Appliance Therapy in Obstructive Sleep Apnea Patients. Am J Respir Crit Care Med. 2016 doi: 10.1164/rccm.201601-0099OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khoo MCK, Kronauer RE, Strohl KP, Slutsky AS. Factors inducing periodic breathing in humans: a general model. Journal of Applied Physiology. 1982;53:644–59. doi: 10.1152/jappl.1982.53.3.644. [DOI] [PubMed] [Google Scholar]
- 25.Francis DP, Willson K, Davies LC, Coats AJ, Piepoli M. Quantitative general theory for periodic breathing in chronic heart failure and its clinical implications. Circulation. 2000;102:2214–21. doi: 10.1161/01.cir.102.18.2214. [DOI] [PubMed] [Google Scholar]
- 26.Joosten SA, Sands SA, Edwards BA, Hamza K, Turton A, Lau KK, Crossett M, Berger PJ, Hamilton GS. Evaluation of the role of lung volume and airway size and shape in supine-predominant obstructive sleep apnoea patients. Respirology. 2015;20:819–27. doi: 10.1111/resp.12549. [DOI] [PubMed] [Google Scholar]
- 27.Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, Casaburi R, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Hankinson J, Jensen R, Johnson D, Macintyre N, McKay R, Miller MR, Navajas D, Pellegrino R, Viegi G. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26:511–22. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- 28.Iber C American Academy of Sleep Medicine. The AASM manual for the scoring of sleep and associated events : rules, terminology, and technical specifications. American Academy of Sleep Medicine; Westchester, IL: 2007. [Google Scholar]
- 29.Joosten SA, Edwards BA, Wellman A, Turton A, Samarasinghe T, Skuza E, Berger P, Hamilton GS. B110 UPPER AIRWAY AND RESPIRATORY CONTROL DURING SLEEP. American Thoracic Society; 2014. Obstructive Sleep Apnea Phenotypic Trait Changes From Supine To Lateral Position; p. A3909-A. [Google Scholar]
- 30.Behrakis PK, Baydur A, Jaeger MJ, Milic-Emili J. Lung mechanics in sitting and horizontal body positions. Chest. 1983;83:643–6. doi: 10.1378/chest.83.4.643. [DOI] [PubMed] [Google Scholar]
- 31.Barnas GM, Green MD, Mackenzie CF, Fletcher SJ, Campbell DN, Runcie C, Broderick GE. Effect of posture on lung and regional chest wall mechanics. Anesthesiology. 1993;78:251–9. doi: 10.1097/00000542-199302000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Stadler DL, McEvoy RD, Bradley J, Paul D, Catcheside PG. Changes in lung volume and diaphragm muscle activity at sleep onset in obese obstructive sleep apnea patients vs. healthy-weight controls. Journal of Applied Physiology. 2010;109:1027–36. doi: 10.1152/japplphysiol.01397.2009. [DOI] [PubMed] [Google Scholar]
- 33.Lange RA, Katz J, McBride W, Moore DM, Jr, Hillis LD. Effects of supine and lateral positions on cardiac output and intracardiac pressures. Am J Cardiol. 1988;62:330–3. doi: 10.1016/0002-9149(88)90240-8. [DOI] [PubMed] [Google Scholar]
- 34.Jones AY, Dean E. Body position change and its effect on hemodynamic and metabolic status. Heart & lung : the journal of critical care. 2004;33:281–90. doi: 10.1016/j.hrtlng.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 35.Clauss RH, Scalabrini BY, Ray JF, 3rd, Reed GE. Effects of changing body position upon improved ventilation-perfusion relationships. Circulation. 1968;37:II214–7. doi: 10.1161/01.cir.37.4s2.ii-214. [DOI] [PubMed] [Google Scholar]
- 36.Remolina C, Khan AU, Santiago TV, Edelman NH. Positional hypoxemia in unilateral lung disease. N Engl J Med. 1981;304:523–5. doi: 10.1056/NEJM198102263040906. [DOI] [PubMed] [Google Scholar]
- 37.van Kesteren ER, van Maanen JP, Hilgevoord AAJ, Laman DM, de Vries N. Quantitative effects of trunk and head position on the apnea hypopnea index in obstructive sleep apnea. Sleep. 2011;34:1075–81. doi: 10.5665/SLEEP.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Terrill PI, Edwards BA, Wellman A, Nemati S, Owens RL, Butler JP, Malhotra A, Sands SA. Estimating the respiratory arousal threshold from routine polysomnogram [Abstract] Sleep and Biological Rhythms. 2015;13(suppl 1):17–8. [Google Scholar]