Abstract
BACKGROUND
Pregnant women with an elevated viral load of hepatitis B virus (HBV) have a risk of transmitting infection to their infants, despite the infants’ receiving hepatitis B immune globulin.
METHODS
In this multicenter, double-blind clinical trial performed in Thailand, we randomly assigned hepatitis B e antigen (HBeAg)–positive pregnant women with an alanine aminotransferase level of 60 IU or less per liter to receive tenofovir disoproxil fumarate (TDF) or placebo from 28 weeks of gestation to 2 months post partum. Infants received hepatitis B immune globulin at birth and hepatitis B vaccine at birth and at 1, 2, 4, and 6 months. The primary end point was a hepatitis B surface antigen (HBsAg)–positive status in the infant, confirmed by the HBV DNA level at 6 months of age. We calculated that a sample of 328 women would provide the trial with 90% power to detect a difference of at least 9 percentage points in the transmission rate (expected rate, 3% in the TDF group vs. 12% in the placebo group).
RESULTS
From January 2013 to August 2015, we enrolled 331 women; 168 women were randomly assigned to the TDF group and 163 to the placebo group. At enrollment, the median gestational age was 28.3 weeks, and the median HBV DNA level was 8.0 log10 IU per milliliter. Among 322 deliveries (97% of the participants), there were 319 singleton births, two twin pairs, and one stillborn infant. The median time from birth to administration of hepatitis B immune globulin was 1.3 hours, and the median time from birth to administration of hepatitis B vaccine was 1.2 hours. In the primary analysis, none of the 147 infants (0%; 95% confidence interval [CI], 0 to 2) in the TDF group were infected, as compared with 3 of 147 (2%; 95% CI, 0 to 6) in the placebo group (P = 0.12). The rate of adverse events did not differ significantly between groups. The incidence of a maternal alanine aminotransferase level of more than 300 IU per liter after discontinuation of the trial regimen was 6% in the TDF group and 3% in the placebo group (P = 0.29).
CONCLUSIONS
In a setting in which the rate of mother-to-child HBV transmission was low with the administration of hepatitis B immune globulin and hepatitis B vaccine in infants born to HBeAg-positive mothers, the additional maternal use of TDF did not result in a significantly lower rate of transmission. (Funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development; ClinicalTrials.gov number, NCT01745822.)
Mother-to-child transmission of hepatitis B virus (HBV) accounts for the majority of cases of chronic HBV infection,1,2 a leading cause of cirrhosis and hepatocellular carcinoma worldwide.3 Without immunization, 30 to 42% of the infants born to HBV-infected mothers, depending on the maternal status for hepatitis B e antigen (HBeAg), a marker of high HBV replication, may be infected in utero, during delivery, or in infancy because of close contact.4 Chronic infection develops in 65 to 90% of infected infants.5
Universal immunization against HBV, starting at birth with the administration of at least three doses of vaccine by 6 months of age, has reduced the prevalence of infection.6,7 The additional administration of hepatitis B immune globulin at birth to infants born to HBV-infected mothers further reduces the risk of mother-to-child transmission.8 However, mother-to-child transmission still occurs in infants born to women with a high HBV viral load (>200,000 IU per milliliter)9–13 or with HBeAg.14 HBV escape mutants to hepatitis B vaccine or hepatitis B immune globulin have been detected in infants born to HBV-infected mothers.15,16
Antiviral agents that inhibit HBV replication, such as lamivudine, tenofovir disoproxil fumarate (TDF), and telbivudine, which have been administered to pregnant women with a high HBV viral load, may reduce the risk of mother-to-child transmission.9–11 The 2015 World Health Organization (WHO) guidelines for the management of chronic HBV infection did not recommend this approach, citing limited and low-quality evidence on the relative benefits or harm.3 In 2016, the American Association for the Study of Liver Diseases recommended antiviral therapy in hepatitis B surface antigen (HBsAg)–positive pregnant women who had an HBV DNA level of more than 200,000 IU per milliliter, despite a low quality or certainty of evidence.12
We present the results of a clinical trial in which we assessed the efficacy and safety of TDF, to prevent mother-to-child HBV transmission, in pregnant women with HBeAg. The risk of hepatic disease exacerbation after the discontinuation of the trial regimen was also assessed. A recent randomized, clinical trial that was conducted in China and funded by Gilead Sciences showed a significantly lower rate of mother-to- child HBV transmission among mothers who received TDF than among those who received standard care.13 Our design differed from the design of that trial, which was not placebo-controlled and not double-blind, which enrolled 200 women with an HBV DNA level of more than 200,000 IU per milliliter, and in which non–breast-fed infants received hepatitis B immune globulin at birth and at week 4 and HBV vaccine at birth and at weeks 4 and 24.13
METHODS
TRIAL DESIGN AND POPULATION
This phase 3, multicenter, randomized, double-blind, placebo-controlled, parallel-group clinical trial was conducted at 17 public hospitals in Thailand. The double-blind, placebo-controlled design was selected because limited work had been done regarding the efficacy of TDF in this context and because we wanted to ensure the reliable assessment of safety variables. Pregnant women were enrolled at 28 weeks of gestational age (within a window of ±10 days); the gestational age was based on the date of the last menstrual period and on sonogram data. Eligible women were 18 years of age or older, had positive HBsAg and HBeAg tests, and had an alanine aminotransferase level of 30 IU or less per liter at screening and a level of 60 IU or less per liter at trial entry. Women were excluded if they had a positive serologic test for human immunodeficiency virus (HIV) or hepatitis C virus, had received TDF at any time, had received any other anti-HBV treatment during the current pregnancy, had a creatinine clearance of less than 50 ml per minute (according to the Cock-croft–Gault formula), had confirmed proteinuria (>30 mg per deciliter), had confirmed normoglycemic glycosuria, or had evidence of a fetal anomaly that was incompatible with life. The protocol, along with the statistical analysis plan, is available with the full text of this article at NEJM.org. A list of the Program for Health, Prevention, and Treatment Study Coordination Center team and trial-site personnel is provided in the Supplementary Appendix, available at NEJM.org.
After written informed consent was obtained, participants were randomly assigned in a 1:1 ratio to receive either 300 mg of TDF or matching placebo (similar to active tablets minus the active pharmaceutical ingredient), administered once daily from 28 weeks of gestation to 2 months post partum. Randomization was performed with the use of permuted blocks and stratified according to trial site. The participants, the trial staff on site and at the coordination center, the investigators, and the laboratory personnel were unaware of the trial-group assignments.
Maternal visits were at 28, 32, and 36 weeks of gestation, at delivery, and at 1, 2, 3, 4, 6, and 12 months post partum. Infant visits were at birth and at 1, 2, 4, 6, 9, and 12 months of age for physical examination, for the recording of vaccinations (until 6 months of age), and for added scrutiny for signs or conditions suggesting possible mitochondrial dysfunction (at 6 and 12 months of age) (see the Supplementary Appendix).17 All the infants received hepatitis B immune globulin (Grifols) at birth and hepatitis B vaccine (at a dose 10 μg) at birth and at 1, 2, 4, and 6 months through the Thai immunization program. Infants were breast-fed.3,18 Infants had blood samples obtained for the determination of HBV infection status before vaccination and at 1, 2, 4, 6, 9, and 12 months of age. Details of the protocol have been published previously.19 All the analyses presented here are based on primary end-point data through 6 months post partum for women and through 6 months of age for infants.
INFECTION AND SAFETY ASSESSMENTS
HBV infection status at 6 months of age was determined with the Monolisa HBsAg Ultra kit (Bio-Rad). If the results were positive, plasma HBV DNA levels were measured at all visits by a polymerase-chain-reaction assay (RealTime HBV assay, Abbott Molecular). The level of antibody to HBsAg was measured at 2, 4, and 6 months with the Monolisa Anti-HBs Plus (Bio-Rad).
Women with an elevated alanine aminotransferase level (>60 IU per liter) at any trial visit had their level tested again within 2 weeks. If an elevation after 2 months post partum was confirmed, the reintroduction of the double-blind trial regimen was considered. Serious adverse events20 and signs and symptoms that were graded, according to the Division of AIDS tables,21 as being severe (grade 3) or potentially life-threatening (grade 4) were coded according to the Medical Dictionary for Regulatory Activities, version 19.0.22
END POINTS
The primary efficacy end point was the infant’s HBV infection status at 6 months of age (HBsAg-positive status confirmed by the detection of HBV DNA). Infants with the HBV infection status available at 6 months were included if their mother received at least one dose of TDF or placebo, and they were included in the analysis according to the randomized group assignment (i.e., intention-to-treat analyses). Pregnancies that resulted in multiple births were counted as one mother–infant pair and were counted as having HBV infection if at least one infant was infected. Three sensitivity analyses were conducted: one analysis considered infants from multiple pregnancies separately, one analysis imputed the last available HBV-infection status for infants who did not have 6-month status data, and one analysis considered infants with missing data as having HBV infection.
Secondary end points were the occurrence of maternal or infant adverse events (serious adverse events and grade 3 or 4 signs and symptoms), maternal hepatic flares (alanine aminotransferase level of >300 IU per liter) after the discontinuation of the trial regimen, and infant growth as measured by the WHO z scores for age for weight, height, and head circumference at 6 months. Exploratory end points were an anti–hepatitis B antibody titer of at least 10 IU per liter at 6 months in infants and the maternal HBV DNA level at delivery.
TRIAL OVERSIGHT
The protocol was approved by the ethics committees of the Institute for the Development of Human Research Protections at the Thailand Ministry of Public Health, the Faculty of Associated Medical Sciences, Chiang Mai University, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development and by the ethics committees at the clinical sites.19 The protocol was also approved as “nonengaged human subjects research” by the Centers for Disease Control and Prevention. A data and safety monitoring board reviewed the trial progress and safety data annually and the interim efficacy results when 50% of the primary end points in infants were available. TDF and matching placebo were manufactured and donated by Gilead Sciences. Abbott Molecular (Singapore) donated kits for the measurement of HBV DNA at delivery. Immune globulin and the Monolisa HBsAg Ultra kit (Bio-Rad) were purchased at full cost.
The authors, who are academic investigators, collected and analyzed the data and made the decision to submit the manuscript for publication. They guarantee the accuracy and completeness of the data and analyses and the fidelity of the trial to the protocol. None of the authors were employed by Gilead Sciences, Grifols, or Abbott Molecular, and these companies were not involved in the trial design, data collection or analysis, or manuscript preparation or review.
STATISTICAL ANALYSIS
We estimated that 328 pregnant women, 164 in each group, would need to be enrolled for the trial to have 90% power to detect a difference of at least 9 percentage points in the rate of HBV transmission, assuming rates of 3% in the TDF group and 12% in the placebo group, as assessed by a one-sided Fisher’s exact test with a 0.049 significance level accounting for one interim efficacy review (Haybittle–Peto rule, with a 0.001 significance level), and assuming a 5% loss to follow-up. P values were one-sided for efficacy analyses and two-sided for safety analyses and for the exploratory end point of the HBV DNA level at delivery. Fisher’s exact test was used for the analyses of the efficacy end points, adverse events, and hepatic flares; Student’s t-tests were used for the analyses of HBV DNA level and infant growth; and a log-rank test was used for the time-to-event analysis of the first adverse event. Proportions are presented along with 95% exact binomial confidence intervals.
RESULTS
PARTICIPANTS
Among 2512 HBsAg-positive pregnant women who were assessed for eligibility, 1470 (59%) were HBeAg-negative and 711 (28%) were excluded for other reasons (Fig. 1). Between January 8, 2013, and August 19, 2015, a total of 331 women underwent randomization, of whom 168 were assigned to the TDF group and 163 to the placebo group. A total of 5 women (2 in the TDF group and 3 in the placebo group) were incorrectly enrolled with minor eligibility violations (2 women had normoglycemic glycosuria, 2 had anti–hepatitis C virus antibodies, and 1 had an alanine aminotransferase level of >30 IU per liter at screening but <60 IU per liter at enrollment) but were included in all the analyses. The characteristics of the women in the two groups were similar (Table 1). No woman underwent amniocentesis. A total of 322 women (97% of the participants) delivered 324 infants, including 319 singleton births, two pairs of twins, and one stillborn infant (the twins and stillborn infant were in the TDF group). A total of 294 live-born infants (147 in the TDF group and 147 in the placebo group) were followed through 6 months of age (Fig. 1).
Figure 1. Enrollment, Randomization, and Follow-up of the Participants.
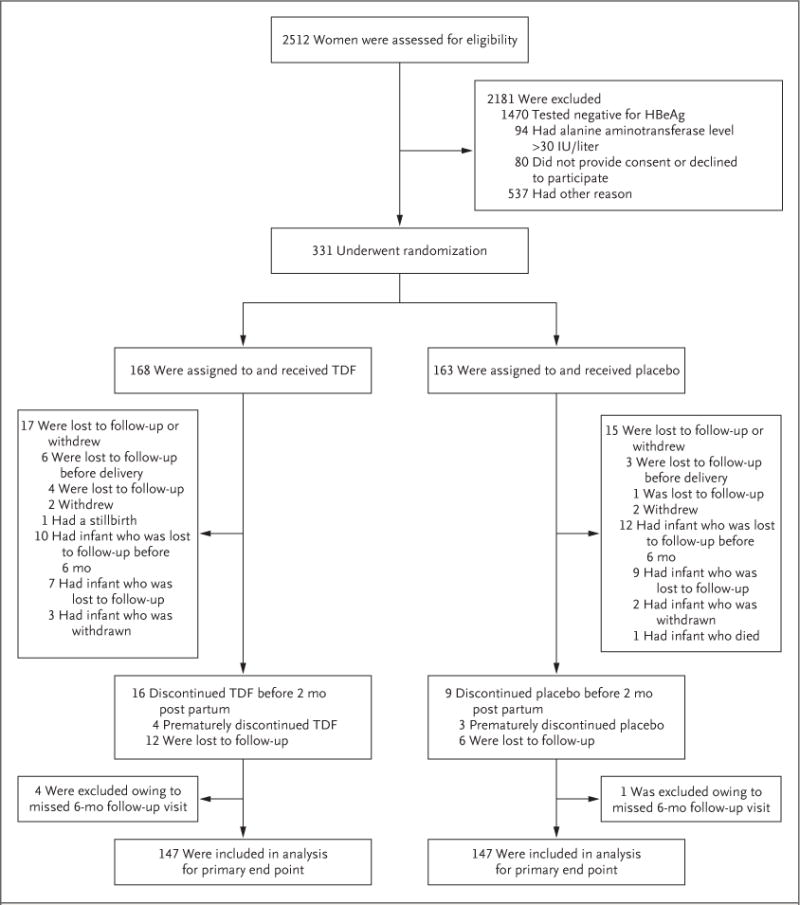
Other reasons for exclusion from the trial included the following: had an infant with a gestational age of more than 28 weeks at the screening or enrollment visit; planned to be followed at a nontrial site; did not return after screening visit; was younger than 18 years of age; had a positive test for hepatitis C virus or the human immunodeficiency virus; terminated the pregnancy; had a delivery, miscarriage, fetal death, or molar pregnancy; was receiving antiviral agents; had proteinuria or another contraindication; or presented after enrollment in the trial was already complete. HBeAg denotes hepatitis B e antigen, and TDF tenofovir disoproxil fumarate.
Table 1.
Maternal, Delivery, and Infant Characteristics.*
| Characteristic | TDF Group | Placebo Group | Total |
|---|---|---|---|
| Women at baseline | |||
| No. of women with data | 168 | 163 | 331 |
| Age — yr | |||
| Median (IQR) | 25.5 (22.6-29.1) | 26.7 (23.5-30.5) | 26.1 (22.9-30.0) |
| Range | 18.3-42.2 | 18.4-40.9 | 18.3-42.2 |
| Gestational age at enrollment — wk | |||
| Median (IQR) | 28.3 (27.9-28.6) | 28.1 (27.9-28.6) | 28.3 (27.9-28.6) |
| Range | 26.7-29.4 | 26.6-29.6 | 26.6-29.6 |
| Height — m | |||
| Median (IQR) | 1.58 (1.54-1.62) | 1.57 (1.52-1.60) | 1.57 (1.53-1.60) |
| Range | 1.40-1.73 | 1.40-1.74 | 1.40-1.74 |
| Weight — kg | |||
| Median (IQR) | 61.8 (56.0-70.5) | 60.5 (54.5-68.2) | 61.2 (55.5-69.7) |
| Range | 42.0-108.5 | 39.4-87.1 | 39.4-108.5 |
| HBV DNA | |||
| Mean — log10 IU/ml | 7.6±1.5 | 7.3±1.7 | 7.5±1.6 |
| Median (IQR) — log10 IU/ml | 8.1 (7.3-8.5) | 7.9 (6.8-8.5) | 8.0 (7.1-8.5) |
| Range — log10 IU/ml | 1.2-9.1 | 1.5-9.5 | 1.2-9.5 |
| >200,000 IU/ml — no. (%) | 152 (90) | 142 (87) | 294 (89) |
| Delivery | |||
| No. of women with data | 162 | 160 | 322 |
| No. of infants — no. (%) | |||
| 1 | 160 (99) | 160 (100) | 320 (99) |
| 2 | 2 (1) | 0 | 2 (1) |
| Delivery outcome — no. (%) | |||
| Live birth | 161 (99) | 160 (100) | 321 (100) |
| Stillbirth ante partum | 1 (1) | 0 | 1 (<1) |
| Type of delivery — no. (%) | |||
| Vaginal | 124 (77) | 113 (71) | 237 (74) |
| Cesarean section | 38 (23) | 47 (29) | 85 (26) |
| Cesarean section before labor onset — no. (%) | |||
| No | 146 (90) | 135 (84) | 281 (87) |
| Yes | 16 (10) | 25 (16) | 41 (13) |
| Gestational age | |||
| Median (IQR) — wk | 39.0 (38.3-39.7) | 38.9 (38.1-40.0) | 38.9 (38.1-39.9) |
| Range — wk | 35.3-42.1 | 32.7-42.4 | 32.7-42.4 |
| Distribution — no. (%) | |||
| ≥32 to <35 wk | 0 | 3 (2) | 3 (1) |
| ≥35 to <37 wk | 8 (5) | 10 (6) | 18 (6) |
| ≥37 wk | 154 (95) | 147 (92) | 301 (93) |
| HBV DNA — no. (%) | |||
| >200,000 IU/ml | 19 (12) | 143 (90) | 162 (51) |
| <15 IU/ml | 10 (6) | 1 (1) | 11 (3) |
| Infants | |||
| No. of infants with data | 163 | 160 | 323 |
| Sex — no. (%) | |||
| Male | 86 (53) | 84 (52) | 170 (53) |
| Female | 77 (47) | 76 (48) | 153 (47) |
| Apgar score at 1 min — no./total no. (%) | |||
| 0-3 | 2/163 (1) | 1/159 (1) | 3/322 (1) |
| 4-6 | 0/163 | 2/159 (1) | 2/322 (1) |
| 7-10 | 161/163 (99) | 156/159 (98) | 317/322 (98) |
| Birth weight — g | |||
| Median (IQR) | 3028 (2766-3310) | 3061 (2845-3395) | 3050 (2796-3352) |
| Range | 2250-4174 | 1935-4240 | 1935-4240 |
| Length — cm | |||
| Median (IQR) | 50 (49-52) | 51 (49-52) | 50 (49-52) |
| Range | 45-57 | 40-57 | 40-57 |
| Head circumference — cm | |||
| Median (IQR) | 33 (32-34) | 33 (32-34) | 33 (32-34) |
| Range | 30-36 | 29-37 | 29-37 |
| Hepatitis B immune globulin administered | |||
| No | 2 (1) | 1 (1) | 3 (1) |
| Yes | 161 (99) | 159 (99) | 320 (99) |
| Time from birth to administration of hepatitis B immune globulin — hr | |||
| Median (IQR) | 1.3 (0.8-2.3) | 1.2 (0.8-2.5) | 1.3 (0.8-2.5) |
| Range | 0.2-51.8 | 0.0-64.5 | 0.0-64.5 |
| HBV vaccine administered at birth | |||
| No | 0 | 1 (1) | 1 (<1) |
| Yes | 163 (100) | 159 (99) | 322 (100) |
| Time from birth to HBV vaccine — hr | |||
| Median (IQR) | 1.2 (0.8-2.0) | 1.2 (0.7-2.3) | 1.2 (0.7-2.2) |
| Range | 0.1-7.2 | 0.0-7.7 | 0.0-7.7 |
| No. of HBV vaccines administered in infants followed to 6 mo — no./total no. (%) | |||
| 1 | 1/155 (1) | 0/148 | 1/303 (<1) |
| ≥3 | 154/155 (99) | 148/148 (100) | 302/303 (>99) |
Plus–minus values are means ±SD. The characteristics of the women in the two groups were similar. Data on the hepatitis B virus (HBV) DNA level at delivery were missing for one woman in each group. Data on length and head circumference were missing for one infant in the placebo group, data on the time from birth to administration of hepatitis B immune globulin for three in the tenofovir disoproxil fumarate (TDF) group and for one in the placebo group, and data on the time from birth to administration of HBV vaccine for one in the TDF group and for three in the placebo group. IQR denotes interquartile range.
All the enrolled women received at least one dose of TDF or placebo. The median duration from enrollment to delivery was 10.7 weeks (interquartile range, 10.0 to 11.6). A total of 306 women (92%), including 152 of 168 women (90%) in the TDF group and 154 of 163 (94%) in the placebo group, completed the trial regimen. More than 81% of the women returned the trial-regimen bottles at scheduled visits. The percentage of women who adhered to the regimen, with adherence defined as their having taken at least 80% of the pills in the previous 4-week period, was 93% at 32 weeks of gestation, 94% at 36 weeks of gestation, 86% at delivery, 92% at 1 month post partum, and 84% at 2 months post partum. The percentages for adherence ranged from 83 to 94% in the TDF group and from 85 to 95% in the placebo group.
The median duration from birth to the administration of the hepatitis B vaccine was 1.2 hours (interquartile range, 0.7 to 2.2). Only 12 infants (4%) received the vaccine more than 4 hours after birth. The median duration from birth to the administration of hepatitis B immune globulin was 1.3 hours (interquartile range, 0.8 to 2.5). A total of 3 infants did not receive hepatitis B immune globulin, including 1 infant in the placebo group, who was born with gross abnormalities and died soon after birth with no HBV assessment, and 2 infants in the TDF group (in the delivery room, the mothers did not identify themselves as participants in the trial).
EFFICACY
In the primary analysis, none of the 147 infants (0%; 95% exact binomial confidence interval [CI], 0 to 2) in the TDF group and 3 of 147 (2%; 95% CI, 0 to 6) in the placebo group had HBV infection at 6 months (P = 0.12) (Table 2). The sensitivity analyses provided similar results. The mothers of all 3 infected infants had an HBV DNA level of more than 7.8 log10 IU per milliliter at delivery. In 2 infected infants, the levels of HBsAg and HBV DNA were consistently detected from birth. In the third infected infant, who was delivered by means of cesarean section and was breast-fed, the levels of HBsAg and HBV DNA were first detected at 6 months of age. A sequence analysis showed that the mother and infant were infected with closely related viruses, both genotype B, that had no TDF-resistance mutations.
Table 2.
Primary, Secondary, and Exploratory End Points.*
| End Point | TDF Group | Placebo Group | P Value† | ||||
|---|---|---|---|---|---|---|---|
| No. of Participants | No. of Events | Value | No. of Participants | No. of Events | Value | ||
| Efficacy end points in infants at 6 mo | |||||||
| HBV infection — % (95% Cl) | |||||||
| Primary analysis | 147 | 0 | 0 (0-2) | 147 | 3 | 2 (0-6) | 0.12 |
| Analysis with twins considered separately | 149 | 0 | 0 (0-2) | 147 | 3 | 2 (0-6) | 0.12 |
| Analysis with last available infection status imputed | 160 | 0 | 0 (0-2) | 159 | 3 | 2 (0-5) | 0.12 |
| Analysis with missing data imputed as infected | 167 | 20 | 12 (8-18) | 163 | 19 | 12 (7-18) | 0.60 |
| Anti-HBV antibodies ≥10 IU/Iiter — % (95% Cl) | 147 | 147 | 100 (98-100) | 147 | 145 | 99 (95-100) | 0.25 |
| Safety end points at 6 mo | |||||||
| ALT >300 IU/1 iter in women after trial-regimen discontinuation — % (95% Cl) | 154 | 9 | 6 (3-11) | 157 | 5 | 3 (1-7) | 0.29 |
| Adverse event of grade 3 or 4 or serious adverse event — % (95% Cl) ‡ | |||||||
| In women | 168 | 41 | 24 (18-32) | 163 | 44 | 27 (20-34) | 0.62 |
| In infants | 161 | 43 | 27 (20-34) | 160 | 38 | 24 (17-31) | 0.61 |
| WHO z scores among infants at 6 mo | 148 | — | 146 | — | |||
| Weight for age | −0.4±1.1 | −0.2±1.1 | 0.09 | ||||
| Length for age | −0.2±1.2 | −0.2±1.2 | 0.67 | ||||
| Head circumference for age | −0.6±1.1 | −0.6±0.9 | 0.76 | ||||
| Exploratory end point | |||||||
| HBV DNA level among women at delivery — log10 lU/ml | 161 | — | 4.0±1.6 | 159 | — | 7.3±1.7 | <0.001 |
Plus–minus values are means ±SD. ALT denotes alanine aminotransferase, and WHO World Health Organization.
P values were calculated by Fisher’s exact test for binary outcomes and by Student’s t-test for continuous outcomes. They were one-sided for efficacy analyses and two-sided for safety analyses and for the exploratory end point of the HBV DNA level at delivery.
Adverse events of grade 3 or 4 were defined as events that were severe and potentially life-threatening and were graded according to the Division of AIDS tables.21
At 6 months, 292 of 294 infants (99%) had a level of antibody to HBsAg of at least 10 IU per liter, including all 147 infants (100%) in the TDF group and 145 of 147 (99%) in the placebo group (P = 0.25). The 2 infants with a low level of antibody to HBsAg had HBV infection. The level of antibody to HBsAg in the third infected infant decreased from 76 IU per liter at 1 month to 27 IU per liter at 2 months and to 12 IU per liter at 6 months, which probably reflected the elimination of hepatitis B immune globulin injected at birth with no response to vaccine. None of the 3 infected infants had HBV cleared at 12 months.
The maternal HBV DNA level declined in the TDF group, from a mean of 7.6 log10 IU per milliliter at baseline to a mean of 4.0 log10 IU per milliliter at delivery, whereas there was no change in the HBV DNA level in the placebo group (P<0.001 for the between-group comparison at delivery) (Table 2). At delivery, 19 of 161 women (12%) in the TDF group had an HBV DNA level of more than 200,000 IU per milliliter, as compared with 143 of 159 (90%) in the placebo group. A total of 10 women (6%) in the TDF group and 1 (1%) in the placebo group had an HBV DNA level below the limit of detection (<15 IU per milliliter) (Table 1). The 19 women in the TDF group who had an HBV DNA level of more than 200,000 IU per milliliter at delivery had a mean HBV DNA level of 8.5 log10 IU per milliliter at baseline, and 5 had poor adherence to the trial regimen (had taken <80% of pills in the previous 4-week period) according to pill counts at one trial visit between entry and delivery.
SAFETY
After the discontinuation of the trial regimen, an acute hepatic exacerbation (defined as an alanine aminotransferase level of >300 IU per liter) occurred in 9 of 154 women (6%; 95% CI, 3 to 11) in the TDF group and in 5 of 157 (3%; 95% CI, 1 to 7) in the placebo group (P = 0.29) (Table 2). All the exacerbations were asymptomatic. Figure 2 shows the longitudinal levels of alanine aminotransferase among 17 women (9 in the TDF group and 8 in the placebo group) who had flares while taking the trial regimen and after discontinuation. The flares in women in the TDF group occurred after the discontinuation of TDF. No women started or restarted TDF after flares that occurred within 6 months post partum. No assessment of the potential development of TDF resistance was performed.
Figure 2. Alanine Aminotransferase Level over Time, According to Randomized Group, in Women Who Had a Level of More than 300 IU per Liter during the Trial.
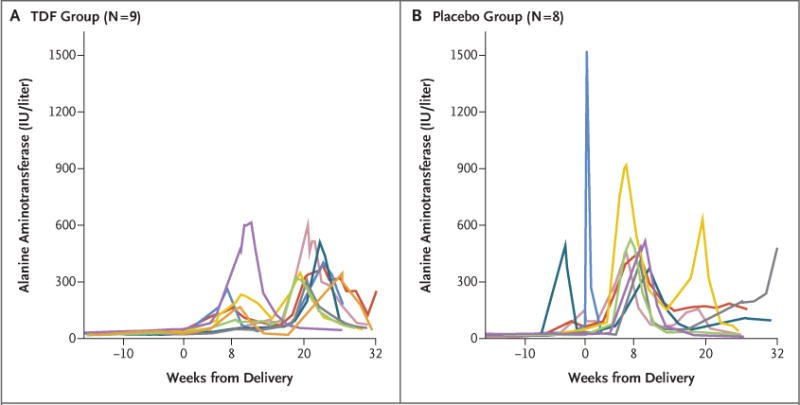
Shown are the longitudinal levels of alanine aminotransferase among 17 women (9 in the TDF group [Panel A] and 8 in the placebo group [Panel B]) who had an acute hepatic exacerbation (defined as an alanine aminotransferase level of >300 IU per liter) while taking the trial regimen and after discontinuation. Per-protocol TDF or placebo was taken until 2 months (8.6 weeks) post partum.
A total of 41 of 168 women (24%) in the TDF group and 44 of 163 (27%) in the placebo group had at least one adverse event (P = 0.62) (Table 2). The most frequent event was elevation of the alanine aminotransferase level (in 31 women [18%] in the TDF group and 24 [15%] in the placebo group), followed by peripartum conditions (in 6 [4%] and 13 [8%], respectively), including one stillbirth (in the TDF group). Women in the placebo group tended to have a first adverse event earlier than those in the TDF group, but by 6 months post partum, there was no significant difference between the trial groups (P = 0.50) (Fig. 3A).
Figure 3. Time to the First Adverse Event.
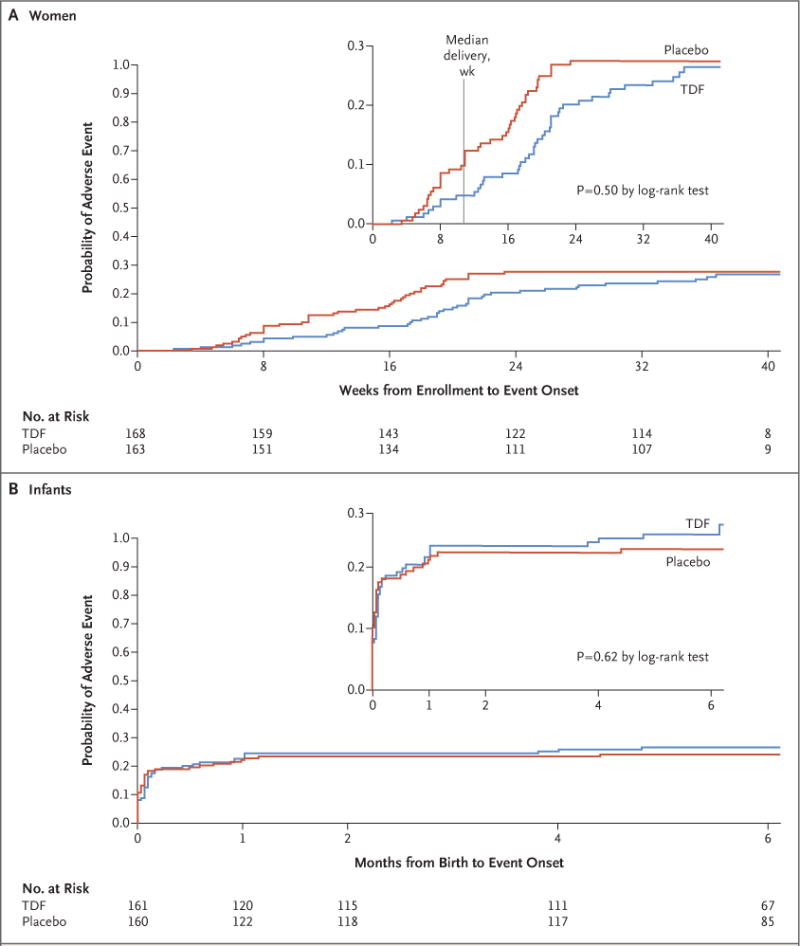
Insets show the same data on an enlarged y axis.
A total of 43 infants (27%) in 161 mother–infant pairs in the TDF group and 38 infants (24%) in 160 mother–infant pairs in the placebo group had at least one adverse event of grade 3 or 4 or a serious adverse event (P = 0.61) (Table 2). The most frequent adverse event was jaundice or hyperbilirubinemia (in 24 infants [15%] in the TDF group and 17 [11%] in the placebo group). As mentioned earlier, one infant in the placebo group was born with gross abnormalities and died soon after birth. The time to the first adverse event did not differ significantly between groups (P = 0.62) (Fig. 3B). The measurements of infant growth at 6 months did not differ significantly between groups (P≥0.09 for all comparisons) (Table 2), and similar growth over time was observed in the two groups (Fig. S1 in the Supplementary Appendix).
DISCUSSION
In this trial, HBV infection at 6 months of age was not detected among infants in the TDF group, and infection was detected in three infants (2%) in the placebo group. These differences did not reach statistical significance, and thus the trial is considered to be a negative one. Although the prespecified statistical significance was not reached, the observed 0% rate of mother-to-child transmission in the TDF group, considered along with the sample size, indicates, with 95% confidence, that the long-term risk of HBV infection among these infants would be less than 2.5%.23 No maternal or infant safety concerns that were considered by the investigators to be related to the maternal use of TDF or that occurred after the discontinuation of TDF were identified, but the sample size was small. No significant differences in infant growth were detected between the two groups.
Our trial provides data on the role of antiviral agents for the prevention of in utero and intra-partum transmission of HBV.10,13 A low rate of transmission with an antiviral agent may relate to the reduction in maternal viral load as well as to preexposure prophylaxis for the fetus. The appropriate duration of antiviral treatment is unknown. In our trial, an HBV DNA level of more than 200,000 IU per milliliter was observed at delivery in 12% of the women in the TDF group after 10.7 weeks of the trial regimen — a rate that is somewhat less than the 25% rate that was observed among women who received TDF for a mean duration of 8.6 weeks in an earlier study.13 The use of telbivudine for a median of 15.5 weeks resulted in no women with an HBV DNA level of more than 200,000 IU per milliliter at delivery.10 Nevertheless, no perinatal transmission was observed in these three studies.
The percentage of HBV-infected infants in the placebo group (2%) was lower than was originally assumed (12%) on the basis of published data, mostly from China.9,24 Since the beginning of our trial, studies have shown HBV transmission rates of 7% or more among infants who received HBV vaccine and hepatitis B immune globulin and who had been born to mothers with higher HBV DNA levels.9–11,13,14 Although the discrepancy is possibly related to sampling variations, it could be related to the duration between birth and the first administration of the hepatitis B vaccine. Maternal use of TDF may prevent transmissions that would occur when the birth dose is delayed, but its exact timing has not been reported consistently in previous perinatal studies. Early studies involving humans and chimpanzees showed that the timing of vaccination after exposure may be essential.25–27 In our trial, 96% of the infants received the vaccination within 4 hours after birth. It remains unclear whether the administration of more vaccine doses is more efficacious than the administration of the three vaccine doses that is recommended in the United States (at birth and at 2 and 4 months) and by the WHO. The hepatitis B immunization schedule in Thailand (at birth and at 1, 2, 4, and 6 months in infants born to HBsAg-positive women),28 which was strictly adhered to in our trial, may have contributed to the low rate of HBV transmission that was observed.
Amniocentesis14 and mode of delivery29 may also be associated with the risk of HBV transmission. In our trial, 26% of the deliveries were cesarean sections (none of which were elective), and no woman underwent amniocentesis. The previous studies of TDF and telbivudine that showed rates of transmission in the control groups that were higher than in our control group10,13 were conducted in academic hospitals in China where amniocentesis may have been more frequent and the rate of cesarean sections (50%) was higher than in our trial. Finally, the relatively higher prevalence of circulating escape mutants in China (approximately 10%) could explain a higher risk of failure of vaccine or immune globulin, but data from Thailand for comparison are limited.30
Antiviral treatment was systematically discontinued in the postpartum period, and hepatic disease exacerbation was not rare among these women with chronic HBV infection and a high HBV viral load, as observed in the placebo group. All the hepatic exacerbations were asymptomatic and, in the TDF group, did not occur until antiviral treatment was discontinued. The risks of maternal adverse events other than asymptomatic flares in the alanine aminotransferase level during pregnancy were similar in the two groups.
In our trial, no significant safety concerns associated with TDF were observed in the pregnant women and infants, which is also relevant for pregnant women who are receiving TDF as part of HIV treatment or as HIV preexposure prophylaxis (if they are not infected with HIV). Infant growth did not differ significantly between groups, although the infants in the TDF group had somewhat lower mean weight-for-age z scores at 6 months than those in the placebo group. In a recent perinatal HIV study,31 a higher proportion of infants had low birth weight when mothers received TDF-containing triple anti-retroviral therapy than when they received zidovudine.
A limitation of recent perinatal HBV infection– prevention studies has been the assumptions that were used to calculate the sample size. We calculated the sample size to provide the trial with more than 90% power to detect a difference of 9 percentage points in the rate of transmission (expected rate, 3% in the TDF group vs. 12% in the placebo group). Pan et al.13 assumed a difference of 18 percentage points (expected rate, 2% in the TDF group vs. 20% in the control group). A superiority trial assessing a difference of 1.9 percentage points (0.1% vs. 2.0%) with 90% power would require a sample of more than 1600 mother–infant pairs, but feasibility might be limited as the use of antiviral treatment in this context increases.12,32
In conclusion, in the context of a low rate of HBV transmission, this trial did not show that maternal use of TDF, in addition to the administration of hepatitis B immune globulin and vaccine to infants, resulted in a lower rate of mother-to-child HBV transmission than placebo.
Supplementary Material
Acknowledgments
Supported by a grant (U01HD071889) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) under a cooperative agreement among the NICHD, the CDC, and the Institut de Recherche pour le Développement. The trial was conducted under an agreement between the Thailand International Cooperation Agency and the French Embassy to Thailand.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We thank the members of the data and safety monitoring board: Kenneth McIntosh (chair; Harvard University, Cambridge, MA), Marc Bulterys (CDC), Suwachai Intaraprasert (Mahidol University, Bangkok, Thailand), Jean-Yves Mary (INSERM, Paris), Mark Mirochnick (Boston University, Boston), Yong Poovorawan (Chulalongkorn University, Bangkok, Thailand), and Tawesak Tanwandee (Mahidol University); Lynne Mofenson (originally of the NICHD and then of the Elizabeth Glaser Pediatric AIDS Foundation, Bethesda, MD); Jacques Berger (Institut de Recherche pour le Développement representative in Bangkok, Thailand); the trial teams; and the participants and their families, who made possible the successful conduct of the trial.
APPENDIX
The authors’ full names and academic degrees are as follows: Gonzague Jourdain, M.D., Ph.D., Nicole Ngo-Giang-Huong, Pharm.D., Ph.D., Linda Harrison, M.Sc., Luc Decker, Ph.D., Woottichai Khamduang, Ph.D., Camlin Tierney, Ph.D., Nicolas Salvadori, M.Sc., Tim R. Cressey, Ph.D., Wasna Sirirungsi, Ph.D., Jullapong Achalapong, M.D., Prapap Yuthavisuthi, M.D., Prateep Kanjanavikai, M.D., Orada P. Na Ayudhaya, M.D., Thitiporn Siriwachirachai, M.D., Sinart Prommas, M.D., Prapan Sabsanong, M.D., Aram Limtrakul, M.D., Supang Varadisai, M.D., Chaiwat Putiyanun, M.D., Pornnapa Suriyachai, M.D., Prateung Liampongsabuddhi, M.D., Suraphan Sangsawang, M.D., Wanmanee Matanasarawut, M.D., Sudanee Buranabanjasatean, M.D., Pichit Puernngooluerm, M.D., Chureeratana Bowon-watanuwong, M.D., Thanyawee Puthanakit, M.D., Virat Klinbuayaem, M.D., Satawat Thongsawat, M.D., Sombat Thanprasertsuk, M.D., George K. Siberry, M.D., Diane H. Watts, M.D., Nahida Chakhtoura, M.D., Ms.G.H., Trudy V. Murphy, M.D., Noele P. Nelson, M.D., Ph.D., M.P.H., Raymond T. Chung, M.D., Stanislas Pol, M.D., Ph.D., and Nantasak Chotivanich, M.D.
The authors’ affiliations are as follows: the Institut de Recherche pour le Développement Unité Mixte Internationale 174–Program for Health, Prevention, and Treatment (PHPT) (G.J., N.N.-G.-H., L.D., N.S., T.R.C.), the Faculty of Associated Medical Sciences, Chiang Mai University (G.J., N.N.-G.-H., L.D., W.K., N.S., T.R.C., W.S.), Nakornping Hospital (A.L.), Health Promotion Center Region 1 (S.S.), the Medical Department, Sanpatong Hospital (V.K.), and the Department of Internal Medicine, Faculty of Medicine, Chiang Mai University (S. Thongsawat), Chiang Mai, the Obstetrics and Gynecology Department, Chiangrai Prachanukroh Hospital (J.A.), and Mae Chan Hospital (S.B.), Chiang Rai, Prapokklao Hospital, Chantaburi (P.Y.), Banglamung Hospital (P.K.) and Chonburi Hospital (C.B., N. Chotivanich), Chonburi, Nopparat Rajathanee Hospital (O.P.N.A.), Bhumibol Adulyadej Hospital (S. Prommas), and the Faculty of Medicine, Chulalongkorn University (T.P.), Bangkok, Khon Kaen Hospital, Khon Kaen (T.S.), Samutprakarn Hospital, Samutprakarn (P. Sabsanong), Samutsakhon Hospital, Samutsakorn (S.V.), Chiang Kham Hospital (C.P.) and Phayao Hospital (P. Suriyachai), Phayao, Lampang Hospital, Lampang (P.L.), Lamphun Hospital, Lamphun (W.M.), Maharaj Nakornratchasrima Hospital, Nakornratchasrima (P.P.), and the Department of Disease Control, Ministry of Public Health, Nonthaburi (S. Thanprasertsuk) — all in Thailand; the Department of Immunology and Infectious Diseases (G.J., N.N.-G.-H., T.R.C.) and the Center for Biostatistics in AIDS Research (L.H., C.T.), Harvard T.H. Chan School of Public Health, and the Gastrointestinal Unit, Massachusetts General Hospital (R.T.C.) — both in Boston; the Department of Molecular and Clinical Pharmacology, Institute of Translational Medicine, University of Liverpool, Liverpool, United Kingdom (T.R.C.); the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD (G.K.S., N. Chakhtoura); the Office of the Global AIDS Coordinator, Department of State, Washington, DC (D.H.W.); the Centers for Disease Control and Prevention, Atlanta (T.V.M., N.P.N.); and Université Paris Descartes, INSERM Unité 1223, Institut Pasteur, the Department of Hepato-Gastroenterology, Cochin University Hospital, Paris (S. Pol).
Footnotes
The views expressed in this article are those of the authors and do not necessarily represent the official position of the National Institutes of Health or the Centers for Disease Control and Prevention (CDC).
References
- 1.Beasley RP. Hepatitis B virus: the major etiology of hepatocellular carcinoma. Cancer. 1988;61:1942–56. doi: 10.1002/1097-0142(19880515)61:10<1942::aid-cncr2820611003>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 2.Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97–107. doi: 10.1046/j.1365-2893.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection. 2015 Mar; ( http://apps.who.int/iris/bitstream/10665/154590/1/9789241549059_eng.pdf) [PubMed]
- 4.Margolis HS, Coleman PJ, Brown RE, Mast EE, Sheingold SH, Arevalo JA. Prevention of hepatitis B virus transmission by immunization: an economic analysis of current recommendations. JAMA. 1995;274:1201–8. [PubMed] [Google Scholar]
- 5.Stevens CE, Beasley RP, Tsui J, Lee W-C. Vertical transmission of hepatitis B antigen in Taiwan. N Engl J Med. 1975;292:771–4. doi: 10.1056/NEJM197504102921503. [DOI] [PubMed] [Google Scholar]
- 6.Chan C-Y, Lee S-D, Lo K-J. Legend of hepatitis B vaccination: the Taiwan experience. J Gastroenterol Hepatol. 2004;19:121–6. doi: 10.1111/j.1440-1746.2004.03153.x. [DOI] [PubMed] [Google Scholar]
- 7.Posuwan N, Wanlapakorn N, Sa-Nguanmoo P, et al. The success of a universal hepatitis B immunization program as part of Thailand’s EPI after 22 years’ implementation. PLoS One. 2016;11(3):e0150499. doi: 10.1371/journal.pone.0150499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee C, Gong Y, Brok J, Boxall EH, Gluud C. Effect of hepatitis B immunisation in newborn infants of mothers positive for hepatitis B surface antigen: systematic review and meta-analysis. BMJ. 2006;332:328–36. doi: 10.1136/bmj.38719.435833.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu W-M, Cui Y-T, Wang L, et al. Lamivudine in late pregnancy to prevent perinatal transmission of hepatitis B virus infection: a multicentre, randomized, double-blind, placebo-controlled study. J Viral Hepat. 2009;16:94–103. doi: 10.1111/j.1365-2893.2008.01056.x. [DOI] [PubMed] [Google Scholar]
- 10.Han G-R, Cao M-K, Zhao W, et al. A prospective and open-label study for the efficacy and safety of telbivudine in pregnancy for the prevention of perinatal transmission of hepatitis B virus infection. J Hepatol. 2011;55:1215–21. doi: 10.1016/j.jhep.2011.02.032. [DOI] [PubMed] [Google Scholar]
- 11.Chen H-L, Lee C-N, Chang C-H, et al. Efficacy of maternal tenofovir disoproxil fumarate in interrupting mother-to-infant transmission of hepatitis B virus. Hepatology. 2015;62:375–86. doi: 10.1002/hep.27837. [DOI] [PubMed] [Google Scholar]
- 12.Terrault NA, Bzowej NH, Chang K-M, Hwang JP, Jonas MM, Murad MH. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63:261–83. doi: 10.1002/hep.28156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan CQ, Duan Z, Dai E, et al. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med. 2016;374:2324–34. doi: 10.1056/NEJMoa1508660. [DOI] [PubMed] [Google Scholar]
- 14.Wen W-H, Chang M-H, Zhao L-L, et al. Mother-to-infant transmission of hepatitis B virus infection: significance of maternal viral load and strategies for intervention. J Hepatol. 2013;59:24–30. doi: 10.1016/j.jhep.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Oon CJ, Chen WN. Current aspects of hepatitis B surface antigen mutants in Singapore. J Viral Hepat. 1998;5(Suppl 2):17–23. doi: 10.1046/j.1365-2893.1998.0050s2017.x. [DOI] [PubMed] [Google Scholar]
- 16.Carman WF, Zanetti AR, Karayiannis P, et al. Vaccine-induced escape mutant of hepatitis B virus. Lancet. 1990;336:325–9. doi: 10.1016/0140-6736(90)91874-a. [DOI] [PubMed] [Google Scholar]
- 17.Blanche S, Tardieu M, Benhammou V, Warszawski J, Rustin P. Mitochondrial dysfunction following perinatal exposure to nucleoside analogues. AIDS. 2006;20:1685–90. doi: 10.1097/01.aids.0000242814.42344.77. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. Hepatitis B and breastfeeding. 1996 Nov; ( http://www.who.int/maternal_child_adolescent/documents/pdfs/hepatitis_b_and_breastfeeding.pdf)
- 19.Jourdain G, Ngo-Giang-Huong N, Cressey TR, et al. Prevention of mother-to-child transmission of hepatitis B virus: a phase III, placebo-controlled, double-blind, randomized clinical trial to assess the efficacy and safety of a short course of tenofovir disoproxil fumarate in women with hepatitis B virus e-antigen. BMC Infect Dis. 2016;16:393. doi: 10.1186/s12879-016-1734-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH harmonised tripartite guideline: guideline for Good Clinical Practice E6(R1): current Step 4 version. 1996 Jun 10; ( https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf)
- 21.Division of AIDS table for grading the severity of adult and pediatric adverse events. 2004 ( http://rsc.tech-res.com/docs/default-source/safety/table_for_grading_severity_of_adult_pediatric_adverse_events.pdf?sfvrsn=6)
- 22.Brown EG, Wood L, Wood S. The Medical Dictionary for Regulatory Activities (MedDRA) Drug Saf. 1999;20:109–17. doi: 10.2165/00002018-199920020-00002. [DOI] [PubMed] [Google Scholar]
- 23.Hanley JA, Lippman-Hand A. If nothing goes wrong, is everything all right? Interpreting zero numerators. JAMA. 1983;249:1743–5. [PubMed] [Google Scholar]
- 24.Chen HL, Lin LH, Hu FC, et al. Effects of maternal screening and universal immunization to prevent mother-to-infant transmission of HBV. Gastroenterology. 2012;142(4):773–781.e2. doi: 10.1053/j.gastro.2011.12.035. [DOI] [PubMed] [Google Scholar]
- 25.Szmuness W, Stevens CE, Harley EJ, et al. Hepatitis B vaccine: demonstration of efficacy in a controlled clinical trial in a high-risk population in the United States. N Engl J Med. 1980;303:833–41. doi: 10.1056/NEJM198010093031501. [DOI] [PubMed] [Google Scholar]
- 26.Iwarson S, Wahl M, Ruttimann E, Snoy P, Seto B, Gerety RJ. Successful post-exposure vaccination against hepatitis B in chimpanzees. J Med Virol. 1988;25:433–9. doi: 10.1002/jmv.1890250407. [DOI] [PubMed] [Google Scholar]
- 27.Wahl M, Iwarson S, Snoy P, Gerety RJ. Failure of hepatitis B immune globulin to protect against exp infection in chimpanzees. J Hepatol. 1989;9:198–203. doi: 10.1016/0168-8278(89)90051-2. [DOI] [PubMed] [Google Scholar]
- 28.Expert Committee. Thailand practice guidelines for management of chronic hepatitis B and C 2012. Bangkok, Thailand: Thai Association for the Study of the Liver; 2012. (In Thai.) [Google Scholar]
- 29.Pan CQ, Zou H-B, Chen Y, et al. Cesarean section reduces perinatal transmission of hepatitis B virus infection from hepatitis B surface antigen-positive women to their infants. Clin Gastroenterol Hepatol. 2013;11:1349–55. doi: 10.1016/j.cgh.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 30.Bian T, Yan H, Shen L, et al. Change in hepatitis B virus large surface antigen variant prevalence 13 years after implementation of a universal vaccination program in China. J Virol. 2013;87:12196–206. doi: 10.1128/JVI.02127-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fowler MG, Qin M, Fiscus SA, et al. Benefits and risks of antiretroviral therapy for perinatal HIV prevention. N Engl J Med. 2016;375:1726–37. doi: 10.1056/NEJMoa1511691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1–98. doi: 10.1007/s12072-015-9675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


