Abstract
The development of a general catalyst system for the direct anti-Markovnikov hydrofunctionalization of alkenes is presented. A unique catalyst system comprised of an acridinium photooxidant and a hydrogen atom transfer reagent allows for a range of alkene anti-Markovnikov hydrofunctionalization reactions including hydroalkoxylation, hydroamination, and hydroacetoxylation.
Keywords: hydroalkoxylation, hydroamination, alkenes, photoredox, organocatalysis
Markovnikov alkene reactivity occupies a significant portion of chemical reaction space for unsaturated hydrocarbons and provides a direct method for the synthesis of some of the most important commodity and fine chemicals.1 Illustrative examples include the production of isopropanol, manufactured on a 1.5 million ton scale annually through the sulfuric acid promoted addition of water to propene, as well as the synthesis of tert-butyl amine, made through the direct addition of ammonia to isobutylene using a zeolite promoter.2 In the context of modern synthetic methods, these processes approach ideal, as they adhere to the principles of atom economy3 and employ readily available chemical feedstocks.
Of equal importance is ready access to the complimentary anti-Markovnikov addition adducts, but this has proven to be a challenging prospect for the field of catalysis. In the past several decades, synthetic chemists have devoted considerable effort towards the identification of a broadly applicable direct catalytic method for the reversal of Markovnikov selectivity. Efforts to date in this area have focused mainly on employing transition metal-based catalytic platforms to realize this goal, with notable achievements in anti-Markovnikov hydroamination made by Hartwig4 and Buchwald,5 and an anti-Markovnikov hydration protocol disclosed by Grubbs.6 However, a catalytic system that enables the addition of a variety of heteroatom nucleophiles across a broad range of activated and unactivated alkenes has remained elusive.7
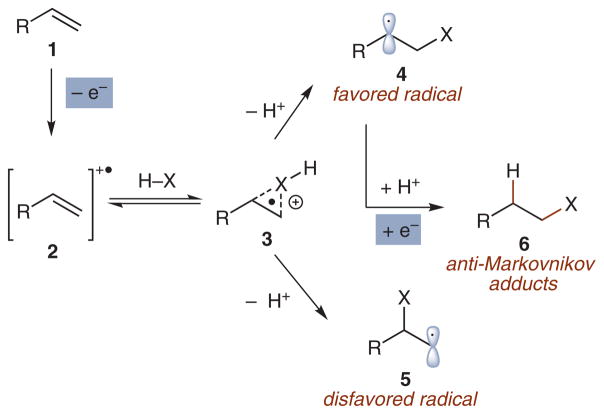
Prior to our efforts, a significant body of work from the laboratories of Arnold,8 Gassman,9 Inoue,10 and Mizuno11 demonstrated that heteroatom nucleophiles add with predictable anti-Markovnikov regioselectivity to cation radicals (2) derived from single electron oxidation of alkenes (Scheme 1).12,13 Aided by computational analysis, Arnold proposed that the observed regioselectivity in these processes could be attributed to selective homolytic bond cleavage of an initial three-membered adduct 3. The resultant, more stabilized radical 4 that is formed in lieu of less stable radical 5, can proceed to a variety of reaction events including cyclization, arylation by the photooxidant, or secondary oxidation followed by a second nucleophilic capture event.
Scheme 1.
Anti-Markovnikov selectivity for nucleophilic addition to cation radicals
Despite these reports, the use of cation radical-mediated strategies for anti-Markovnikov selective alkene hydrofunctionalizations to give products of type 6 in synthetic applications remains scarce, perhaps due to a dearth of catalytic variants. In addition, most olefins investigated in this context have been largely limited to 1,1-diarylethylene derivatives, which have been postulated to undergo nucleophilic addition through the formation of exciplex intermediates rather than discrete cation radicals.10,11 To be adopted as a general synthetic strategy, we felt that access to a broader range of alkene substrates was required as well as the identification of a more generally applicable catalyst system. As a starting point to the development of this catalysis program, we elected to investigate the intra-molecular anti-Markovnikov addition of alcohols to olefins, a reaction that had limited literature precedent.11 Key to the implementation of this strategy was the identification of a suitable catalyst that was capable of single electron oxidation of a number of alkene substrates.
Why Organic Photoredox Catalysis?
In organic chemistry, alkene oxidation often implies processes that incorporate oxygen atoms in the final ‘oxidized’ molecule. However, identifying chemical oxidants that do not deliver oxygen atoms to the olefin, yet are powerful enough to remove electrons from the parent molecule, is a significant challenge. Electrochemical oxidation is often employed in this context to access reactive radical ion intermediates,14 however, this reaction manifold leads to products of net two-electron oxidation processes, which we hoped to avoid in order to access a net redox-neutral anti-Markovnikov addition reaction of alkenes.
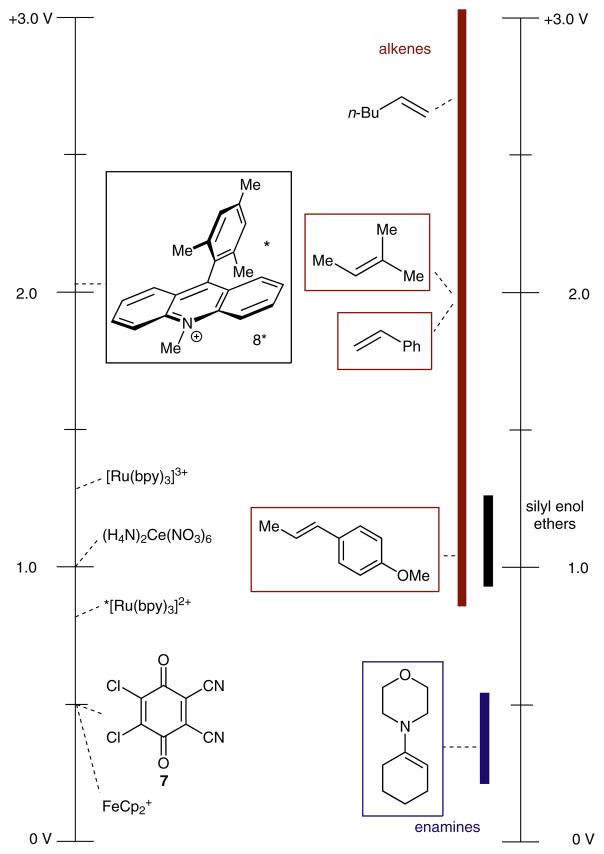
To put the challenge of alkene oxidation into perspective, Scheme 2 depicts the oxidation potentials of some prevalent alkene classes. Electron-rich alkenes such as en-amines and silyl enol ethers have relatively low oxidation potentials (+0.25–1.25 V vs. SCE), and can be easily oxidized by common ground state oxidants such as ferrocenium ion (FeCp2+) (+0.4 V vs. SCE), 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ, 7; +0.5 V) or cerium(IV) ammonium nitrate (CAN; +1.0 V vs. SCE). However, simple alkenes such as terminal styrenes, and mono-, di- and tri-substituted aliphatic alkenes generally have redox potentials that exceed +1.7 V vs. SCE. To our knowledge, there are no ground state oxidants that can approach this oxidizing capability.15 For this reason, we chose to explore excited-state oxidants as catalysts for this purpose. Recently, the venerable [Ru(bpy)3]2+ and derivatives thereof have been used extensively as photoredox catalysts to oxidize a range of electron-rich alkenes.16 Unfortunately, neither the excited state of [Ru(bpy)3]2+, nor its tricationic oxidation state ([Ru(bpy)3]3+) is capable of single electron oxidation of alkenes with potentials higher than +1.3 V vs. SCE, limiting the potential scope of alkenes to only the most electron rich.
Scheme 2.
Comparison of some common oxidant reduction potentials (left) to some common alkene oxidation potentials (right). All values are vs. SCE in MeCN
Conversely, some organic photooxidants have high reduction potentials in the excited state. Cyanoarene oxidants, such as 9,10-dicyanoanthracene, have excited state reduction potentials ranging beyond +2.0 V vs. SCE. However, this class of photooxidant has been shown to undergo radical-ion pair recombination reactions to afford net arylated products, and tend to suffer from short excited state lifetimes.17 Recently, however, Fukuzumi introduced a cationic organic oxidant, 9-mesityl-10-methylacridinium perchlorate (λ = 430 nm), that possesses an excited state reduction potential in excess of +2.06 V and has been proposed to possess a long-lived charge-transfer excited state (8*).18–20 Given this report, we felt that the Fukuzumi catalyst was an excellent starting point for further investigation.
Reaction Development
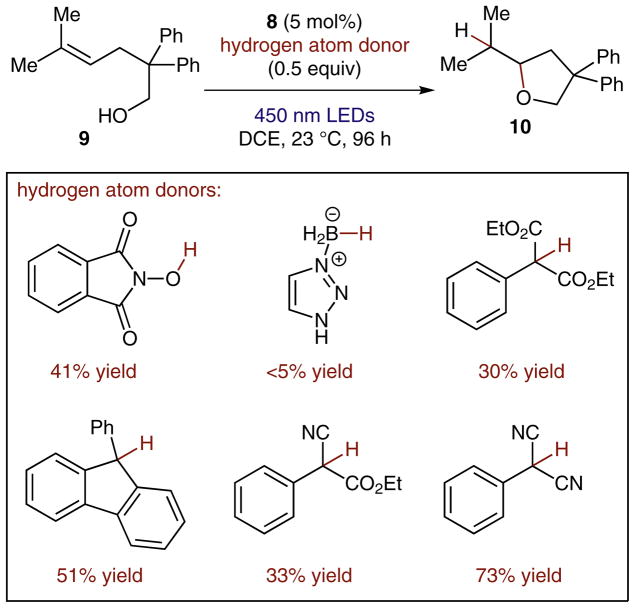
With the goal of hydrofunctionalization of a broad range of alkenes in mind, we set out to investigate the hydroetherification reaction of 9 (Scheme 3).21 We elected to investigate substrate 9 due to its high oxidation potential (Ep/2 = +1.95 V vs. SCE),21 which lies well outside the range of ground state oxidants, providing us with an excellent benchmark for the proposed reaction.
Scheme 3.
A selection of H-atom donors screened for anti-Markovnikov hydroalkoxylations
Unsurprisingly, no reaction was observed by using [Ru(bpy)3]2+ as the photooxidation catalyst, and reactions with cyanoarene photooxidants gave rise to complex product mixtures. Perhaps more surprising to us, however, was that the use of the Fukuzumi photocatalyst, despite prolonged irradiation and reaction times, gave only a modest yield of product with relatively poor mass balance. Importantly, however, the productive reaction proceeded with complete anti-Markovnikov selectivity, with major byproduct formation appearing to be the result of a net two-electron oxidation of the substrate.
Hypothesizing that the reduction of the concomitant cation-radical was the root cause of the divergent reactivity, we believed that shifting the necessary redox event to a more easily reduced radical species might better facilitate the desired reaction.
To accomplish this, we envisioned a hydrogen atom transfer event from a second catalyst that would complete the transformation and better facilitate the second redox event. A subset of the hydrogen atom donor catalysts that were screened in this context appears in Scheme 3. From this screen, 2-phenylmalononitrile was identified as uniquely suited to the promotion of the desired anti-Markovnikov heterofunctionalization reaction and, to our knowledge, had not been previously utilized as a hydrogen atom donor.
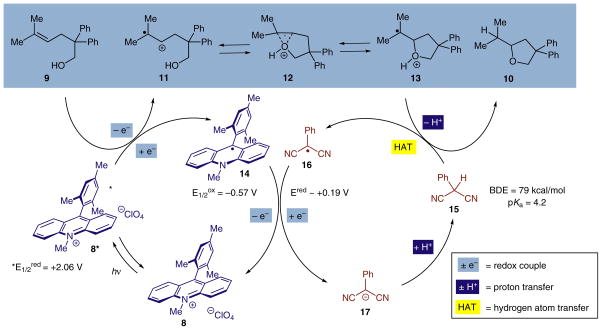
Our working hypothesis for this effect is that 2-phenylmalononitrile (15) acts as a redox-cycling source of atomic hydrogen (Scheme 4). The C–H bond has been estimated to have a homolytic bond dissociation energy of 79 kcal/mol,22 which is low enough to be abstracted by the generated tertiary aliphatic radical 13. Additionally, the resulting conjugate radical of 2-phenylmalononitrile 16 is highly electrophilic and acts as an oxidant (Ered ~ +0.19 V vs. SCE, MeCN)23 for acridine radical 14 (E1/2ox = −0.57 V vs. SCE, MeCN),18 which is returned to the ground state 8. The resulting phenylmalononitrile anion 17 could then plausibly act as a mild base to neutralize the acid generated during the course of the reaction and reset the hydrogen-atom donor catalyst 15.
Scheme 4.
Proposed mechanism of anti-Markovnikov hydroalkoxylation mediated by organic photoredox catalysis
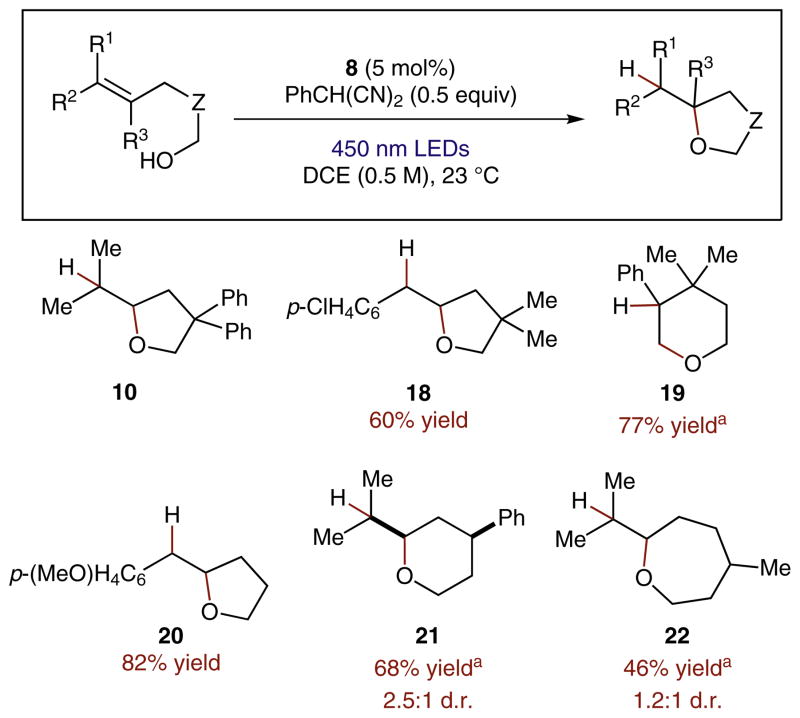
An array of alkenols was found to undergo the anti-Markovnikov hydroetherification reaction when subjected to the described photoredox reaction conditions (Scheme 5). Tertiary aliphatic olefins and styrenes were competent substrates in this transformation, forming cyclic ethers ranging in size from five to seven atoms (10, 21, 22). Notably, a challenging 6-endo cyclization was accomplished and furnished the expected anti-Markovnikov tetrahydropyran adduct 19 despite the presence of an alternative and more kinetically feasible 5-exo pathway. Although initially only intramolecular anti-Markovnikov hydroalkoxylation reactions were investigated, we also reported an example of the addition of methanol to a styrene with complete regioselectivity, and demonstrated that the anti-Markovnikov hydrolactonization of an alkenoic acid was also possible by employing this system.
Scheme 5.
Abbreviated scope of the hydroalkoxylation reaction mediated by organic photoredox catalysis. a2.0 Equivalents of 2-phenylmalononitrile were employed as H-atom source.
A General Manifold for Catalysis
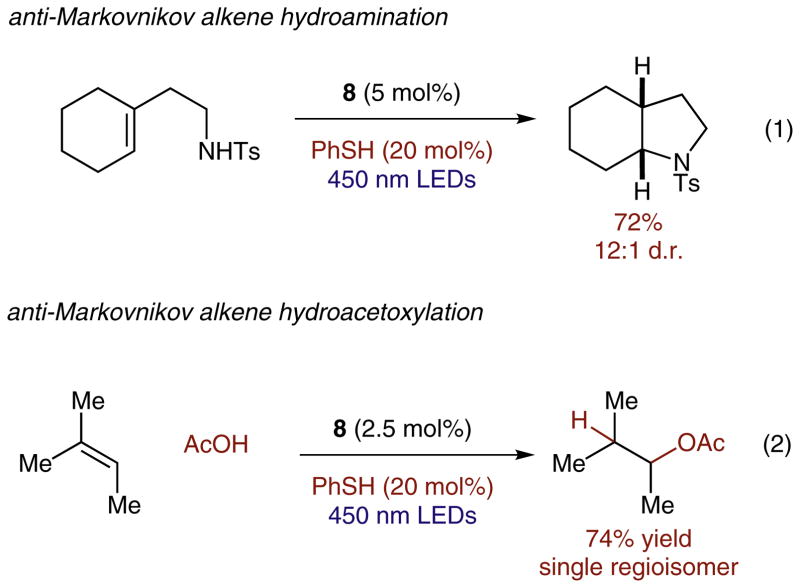
In the months following our initial communication, we have been able to demonstrate that this general catalytic system is applicable to a variety of other transformations (Scheme 6). Our laboratory recently reported an intramolecular anti-Markovnikov hydroamination reaction of unsaturated amines using this catalytic protocol (eq. 1).24 A major advance in this work was the use of thiophenol in truly catalytic quantities as the redox-active hydrogen atom donor in place of the 2-phenylmalononitrile. Additionally, this catalytic platform has been extended to achieve the intermolecular anti-Markovnikov addition of carboxylic acids to alkenes (eq. 2).25 In particular, this second transformation offers a convergent method for the synthesis of esters, or for the installation of an alcohol equivalent in anti-Markovnikov fashion without the use of transition metals or organoboron reagents.
Scheme 6.
Other transformations recently reported by the Nicewicz laboratory relying on variants of the reported hydroetherification reaction conditions
Future Outlook
Further research in our laboratory is targeted towards gaining a deeper understanding of the mechanism of these transformations, in an effort to identify more efficient and stereoselective catalysts. A clear limitation to our current system is the inability to employ terminal aliphatic olefins as substrates. To address this, we are designing catalysts with higher excited state oxidizing capabilities to access high-value targets such as styrenes and terminal aliphatic alkenes. Nevertheless, these initial disclosures have demonstrated the immense potential of electron-transfer catalysis in the context of new, anti-Markovnikov selective transformations and we envision that many opportunities lie in store for the implementation of this unique catalytic strategy.
Acknowledgments
The authors gratefully acknowledge The University of North Carolina at Chapel Hill, Award No. R01 GM098340 from the National Institute of General Medical Sciences and an Eli Lilly New Faculty Award for research support.
Biographies

David Nicewicz (left) earned a B.S. (2000) and M.S. in Chemistry (2002) from the University of North Carolina at Charlotte under the direction of Professor Craig A. Ogle. David then went on to the University of North Carolina at Chapel Hill for his graduate studies, where he completed his Ph.D. in the laboratory of Professor Jeffrey S. Johnson in 2006. From 2007 to 2009, Nicewicz was a Ruth L. Kirschstein National Institutes of Health Postdoctoral Fellow in the Laboratories of Professor David W. C. MacMillan at Princeton University. Nicewicz returned to the University of North Carolina at Chapel Hill in 2009, where he is currently an Assistant Professor in the Department of Chemistry. His research interests include catalysis via single electron manifolds and complex molecule synthesis.

Dave Hamilton (right) received a B.A. (magna cum laude, 2009) in Chemistry from Hamilton College (Clinton, NY), under the direction of Professor Ian J. Rosenstein. He then moved to the University of North Carolina at Chapel Hill for his graduate studies, and currently focuses on the development of olefin heterofunctionalization methods using photoredox catalysis in the Nicewicz laboratory.
References and Notes
- 1.(a) Beller M, Seavad J, Tillack A, Jiao H. Angew Chem Int Ed. 2004;43:3368. doi: 10.1002/anie.200300616. [DOI] [PubMed] [Google Scholar]; (b) Müller TE, Hultzsch KC, Yus M, Foubelo F, Tada M. Chem Rev. 2008;108:3795. doi: 10.1021/cr0306788. [DOI] [PubMed] [Google Scholar]; (c) Hintermann L. Top Organomet Chem. 2010;31:123. [Google Scholar]
- 2.Wissermel K, Arpe H-J. Industrial Organic Chemistry. 5. Wiley-VCH; Weinheim: 2010. [Google Scholar]
- 3.(a) Trost BM. Science. 1991;254:1471. doi: 10.1126/science.1962206. [DOI] [PubMed] [Google Scholar]; (b) Newhouse T, Baran PS, Hoffmann RW. Chem Soc Rev. 2009;38:3010. doi: 10.1039/b821200g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Utsunomiya M, Kuwano R, Kawatsura M, Hartwig JF. J Am Chem Soc. 2003;125:5608. doi: 10.1021/ja0293608. [DOI] [PubMed] [Google Scholar]; (b) Utsunomiya M, Kuwano R, Kawatsura M, Hartwig JF. J Am Chem Soc. 2004;126:2702. doi: 10.1021/ja031542u. [DOI] [PubMed] [Google Scholar]
- 5.Zhu S, Niljianskul N, Buchwald SL. J Am Chem Soc. 2013;135:15746. doi: 10.1021/ja4092819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong G, Teo P, Wickens ZK, Grubbs RH. Science. 2011;333:1609. doi: 10.1126/science.1208685. [DOI] [PubMed] [Google Scholar]
- 7.Catalytic, anti-Markovnikov addition reactions of nucleophiles to olefins has been described as a ‘top-10 challenge’ for catalysis, see: Haggin J. Chem Eng News. 1993;71:23.
- 8.(a) Neunteufel RA, Arnold DR. J Am Chem Soc. 1973;95:4080. [Google Scholar]; (b) Arnold DR, Chan MSW, McManus KA. Can J Chem. 1995;74:2143. [Google Scholar]; (c) Mangion D, Arnold DR. Acc Chem Res. 2002;35:297. doi: 10.1021/ar010108z. [DOI] [PubMed] [Google Scholar]
- 9.(a) Gassman PG, Bottorff KJ. Tetrahedron Lett. 1987;28:5449. [Google Scholar]; (b) Gassman PG, Bottorff KJ. J Am Chem Soc. 1987;109:7547. [Google Scholar]
- 10.(a) Inoue Y, Okano T, Yamasaki N, Tai A. J Chem Soc, Chem Commun. 1993:718. [Google Scholar]; (b) Asaoka S, Kitazawa T, Wada T, Inoue YJ. J Am Chem Soc. 1999;121:8486. [Google Scholar]; (c) Takehara Y, Ohta N, Shiraishi S, Asaoka S, Wada T, Inoue Y. J Photochem Photobiol, A. 2001;145:53. [Google Scholar]; (d) Asaoka S, Wada T, Inoue Y. J Am Chem Soc. 2003;125:3008. doi: 10.1021/ja028680o. [DOI] [PubMed] [Google Scholar]
- 11.(a) Mizuno K, Nakanishi I, Ichinose N, Otsuji Y. Chem Lett. 1989;41:1095. [Google Scholar]; (b) Mizuno K, Tamai T, Nishiyama T, Tani K, Sawasaki M, Otsuji Y. Angew Chem Int Ed Engl. 1994;33:2113. [Google Scholar]
- 12.For a general review on the reactivity patterns of alkene cation-radicals, see: Schmittel M, Burghart A. Angew Chem Int Ed Engl. 1997;36:2550.
- 13.For hydrofunctionalizations using alkene cation-radical surrogates, see: Crich D, Ranganathan K, Neelamkavil S, Huang X. J Am Chem Soc. 2005;125:7942. doi: 10.1021/ja035639s.Crich D, Ranganathan K. J Am Chem Soc. 2005;127:9924. doi: 10.1021/ja051657t.Crich D, Shirai M, Brebion F, Rumthao S. Tetrahedron. 2006;62:6501.Crich D, Brebion F, Suk DH. Radicals in Synthesis I. Top Curr Chem. 2006;263:1.
- 14.(a) Sutterer A, Moeller KD. J Am Chem Soc. 2000;122:5636. [Google Scholar]; (b) Moeller KD. Synlett. 2009:1208. [Google Scholar]; (c) Campbell JM, Xu H, Moeller KD. J Am Chem Soc. 2012;134:18338. doi: 10.1021/ja307046j. [DOI] [PubMed] [Google Scholar]; (d) Perkins RJ, Xu H, Campbell JM, Moeller KD. Beilstein J Org Chem. 2013;9:1630. doi: 10.3762/bjoc.9.186. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Smith JA, Moeller KD. Org Lett. 2013;15:5818. doi: 10.1021/ol402826z. [DOI] [PubMed] [Google Scholar]
- 15.For a comprehensive accounting of reduction potentials of single-electron oxidants, see: Connelly NG, Geiger WE. Chem Rev. 1996;96:877. doi: 10.1021/cr940053x.
- 16.Several reviews have been written on transition-metal photoredox catalysis, see: Narayanam JMR, Stephenson CRJ. Chem Soc Rev. 2011;40:102. doi: 10.1039/b913880n.Yoon TP, Ischay MA, Du J. Nat Chem. 2012;2:527. doi: 10.1038/nchem.687.Prier CK, Rankic DA, MacMillan DWC. Chem Rev. 2013;113:5322. doi: 10.1021/cr300503r.
- 17.Such lifetimes tend to be on the order of nanoseconds; specific values can be found in the following references: Ohkubo K, Suga K, Morikawa K, Fukuzumi S. J Am Chem Soc. 2003;125:12850. doi: 10.1021/ja036645r.Chang YC, Chang PW, Wang CM. J Phys Chem B. 2003;107:1628.
- 18.For initial disclosure, see: Fukuzumi S, Kotani H, Ohkubo K, Ogo S, Tkachenko NV, Lemmetyinen H. J Am Chem Soc. 2004;126:1600. doi: 10.1021/ja038656q.
- 19.The existence of an exceedingly long-lived charge transfer state for 8 has been a matter of some contention. For a full perspective of this debate, see: Benniston AC, Harriman A, Li P, Rostron JP, van Ramesdonk HJ, Groeneveld MM, Zhang H, Verhoeven JW. J Am Chem Soc. 2005;127:16054. doi: 10.1021/ja052967e.Ohkubo K, Kotani H, Fukuzumi S. Chem Commun. 2005:4520. doi: 10.1039/b506479a.Verhoeven JW, van Ramesdonk HJ, Zhang H, Groeneveld MM, Benniston AC, Harriman A. Int J Photoenergy. 2005;7:103.Benniston AC, Harriman A, Li P, Rostron JP, Verhoeven JW. Chem Commun. 2005:2701. doi: 10.1039/b501262g.Benniston AC, Harriman A, Verhoeven JW. Phys Chem Chem Phys. 2008;10:5156. doi: 10.1039/b807893a.Fukuzumi S, Kotani H, Ohkubo K. Phys Chem Chem Phys. 2008;10:5159. doi: 10.1039/b809264h.Benniston AC, Elliott KJ, Harrington RW, Clegg W. Eur J Org Chem. 2009:253.Hoshino M, Uekusa H, Tomita A, Koshihara S, Sato T, Nozawa S, Adachi S, Ohkubo K, Kotani H, Fukuzumi S. J Am Chem Soc. 2012;134:4569. doi: 10.1021/ja300602h.
- 20.Regardless of their photophysical properties, acridinium-derived photocatalysts have proven useful as photooxidants capable of the catalysis of a multitude of organic transformations. For examples, see: Kotani H, Ohkubo K, Fukuzumi S. J Am Chem Soc. 2004;126:15999. doi: 10.1021/ja048353b.Ohkubo K, Nanjo T, Fukuzumi S. Bull Chem Soc Jpn. 2006;79:1489.Ohkubo K, Mizushima K, Iwata R, Souma K, Suzuki N, Fukuzumi S. Chem Commun. 2009;46:601. doi: 10.1039/b920606j.Ohkubo K, Fujimoto A, Fukuzumi S. Chem Commun. 2011;47:8515. doi: 10.1039/c1cc12534f.Ohkubo K, Mizushima K, Iwata R, Fukuzumi S. Chem Sci. 2011;2:715.Fukuzumi S, Ohkubo K. Chem Sci. 2013;4:561.
- 21.Hamilton DS, Nicewicz DA. J Am Chem Soc. 2012;134:18577. doi: 10.1021/ja309635w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bordwell FG, Cheng JP, Ji GZ, Satish AV, Zhang X. J Am Chem Soc. 1991;113:9790. [Google Scholar]
- 23.Estimated from the oxidation potential of 17, see: Ohkita M, Suzuki T, Tsuji T, Yamada M. Heterocycles. 2001;54:387.
- 24.Nguyen TM, Nicewicz DA. J Am Chem Soc. 2013;135:9588. doi: 10.1021/ja4031616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perkowski AJ, Nicewicz DA. J Am Chem Soc. 2013;135:10334. doi: 10.1021/ja4057294. [DOI] [PMC free article] [PubMed] [Google Scholar]