Abstract
Purpose of Review
Psychological stress can impact memory systems in several different ways. In individuals with healthy defense and coping systems, stress results in the formation of negatively valenced memories whose ability to induce emotional and somatic distress subsides with time. Vulnerable individuals, however, go on to develop stress-related disorders such as post-traumatic stress disorder (PTSD) and suffer from significant memory abnormalities. Whether expressed as intrusive trauma memories, partial amnesia, or dissociative amnesia, such abnormalities are thought to be the core source of patients’ symptoms, which are often debilitating and implicate an entire socio-cognitive-affective spectrum.
Recent Findings
With this in mind, and focusing on stress-responsive hippocampal microcircuits, this article highlights recent advances in the neurobiology of memory that allow us to (1) isolate and visualize memory circuits, (2) change their activity using genetic tools and state-dependent manipulations, and (3) directly examine their impact on socio-affective circuits and global network connectivity. By integrating these approaches, we are now in a position to address important questions that have troubled psychiatry for a long time—questions such as are traumatic memories special, and why are stress effects on memory diverse.
Summary
Furthering our fundamental understanding of memory in the framework of adaptive and maladaptive stress responses has the potential to boost the development of new treatments that can benefit patients suffering from psychological trauma.
Keywords: Traumatic amnesia, Stress, Memory, Neurobiology, Circuits, Optogenetic, Chemogenetic
Introduction
In susceptible individuals, the experience of intense stress often exposes physical or mental vulnerabilities, sometimes causing life-long suffering. Although virtually every psychiatric disorder can be triggered or exacerbated by stress, for a cluster of psychological illnesses, stress is thought to play a central role (Trauma and Stressor-Related Disorders in DSM-5). What causes psychiatric symptoms to persist long after initial stress has ended remains an open question, nevertheless the impact of stress on memory systems is clearly relevant. This area of research has not been without controversy with most disagreements revolving around two key sets of questions.
The first is whether there is anything special about traumatic memories, and why, unlike other memories (including negatively valenced non-traumatic memories), traumatic memories are accompanied by debilitating affective and behavioral symptoms. Speculation on this topic has ranged from denying that there is anything special about traumatic memories [1] to arguing that the difference lies in abnormal activity of discrete brain circuits [2••, 3]. Both human imaging and preclinical rodent studies have provided support for the latter view [4–6], although direct evidence for a trauma-specific memory circuit is still lacking.
The second set of questions relates to the impact of stress on memory. The prevailing view is that in response to trauma, some aspects of the trauma-related memory are enhanced, leading to intrusive memories, while others, such the ability to recall them, are impaired [7]. The latter phenomenon, also known as traumatic amnesia, is thought to occur because traumatic memories are encoded in a way that makes them difficult to retrieve as coherent narratives but instead are represented as sensory, motor, and emotional fragments resulting in non-coherent and fragmented memories [8–11]. Several mechanisms have been proposed to explain these enhancing and impairing effects of stress on memory. Horowitz [12] hypothesized that the voluntary recall of trauma-related memories is impaired while their involuntary retrieval is enhanced, resulting in intrusive, persistent, and vivid memories. Along similar lines, it has been proposed that traumatic stress weakens memory processing through explicit (conscious) memory systems but enhances processing through implicit (automatic, often nonconscious) memory systems [9]. It should be mentioned, however, that, while there is a general agreement that stress and trauma induce strong, emotion-laden memories, evidence and views on impaired trauma memory are still controversial [1, 13, 14]. Ironically, those who doubt that stress can impair memory often refer to preclinical research in support of their criticism [1, 13] while overlooking the fact that in rodents, stress can both enhance and compromise memory [4, 15–18].
Dissociative Amnesia: a Special Case of Stress-Related Memory Deficit
In addition to trauma and stress-related disorders, overwhelming stressful experiences can also trigger a class of mental illnesses known as dissociative disorders. They are thought to arise when normally integrated functions of consciousness, such as memory, perception, and identity awareness become disrupted by overwhelming stressful experiences. They have a lifetime prevalence rate of about 10% in the overall population [19]. In addition, dissociative symptoms accompany most psychiatric disorders [20], including PTSD [21] and schizophrenia [22]. These symptoms, which are often debilitating, include partial or complete amnesia, anxiety, depression, suicidality, and severely compromised social functioning [23–25]. At a fundamental level, dissociative amnesia is thought to be rooted in state-dependent learning, wherein memories formed under a certain affective, stress- or drug-induced state are best retrieved under similar conditions [26–28]. The earliest theoretical accounts of such memory failure held that overwhelming stress prevents the adequate integration of traumatic and normal conscious experiences, causing a state of dissociation [26]. Importantly, it was also recognized that the amnesia only reflects a failure to recall but not to encode the memories [26, 29]. The state dependency of these memories was shown by successful memory retrieval when patients were in a similar psychological or physiological state as they were when the trauma occurred [11, 30, 31]. State-dependent learning may have important evolutionary benefits, by enabling quick decision-making under similar conditions when time or neural capabilities to process multiple options are limited [32]. It may also provide temporary relief from overwhelming stress by delaying coping and reducing negative affect. However, as a chronic coping strategy, it is thought to cause cognitive and affective incoherence, placing individuals at risk for many psychiatric disorders including dissociative amnesia [33]. Whereas significant advances have been made in our understanding of memory processing under normal states of consciousness, state-dependent memories, and their influence on behavior are not well understood at the neurobiological level.
Learning About Stress from Rodents: Translational Impact and Recent Advances in Memory Research
To get the obvious out of the way, there are clearly limits to the applicability of rodent data for understanding higher cognitive functions of humans in general, and of psychiatric patients in particular. Rodents have a much simpler central nervous system, we have no access to their subjective experiences, and no matter how we manipulate their neurons, we can never be sure that the changes we see in behavior will be relevant for human psychology or psychopathology. Nevertheless, even with these constraints, the degree to which rodent work on the stress response has translated to humans is quite striking. It has led to the discovery of the stress response itself [34], our understanding of its neuroendocrine, autonomic, and neuro-anatomical organization, and its modulation by individual molecules (genes, neurotransmitters, and signaling pathways), as well as its impact on discrete behaviors. Many mechanistic concepts relating to gene and environment interactions [16, 35, 36], developmental vulnerability [37, 38], and sex differences [39] have also been translated from rodent to human research. Especially given this track record, recent advances in our ability to study memory networks in rodents could bring far more depth to our understanding of the most fundamental principles of the organization of human memory systems, their responses to trauma, and their impact on social and affective processes.
The Hippocampal Memory System as a Stress Target
Multiple abnormalities of the hippocampal brain area have been found in patients suffering from stress-related disorders. These include volume reduction of the posterior (corresponding to the rodent dorsal) but not anterior (corresponding to the rodent ventral) hippocampus [40] in PTSD patients, multiple abnormalities of hippocampal connectivity to cortical and subcortical areas along its dorsoventral axis in patients with generalized anxiety disorder and PTSD [41], and disrupted hippocampal function in patients suffering from dissociative amnesia [42]. In light of this, two features of the hippocampus are particularly relevant—its key involvement in the formation of episodic memories [[43–46], memories for personally experienced events set in a spatio-temporal context [47]], and its unusually high susceptibility to stress [35, 48, 49].
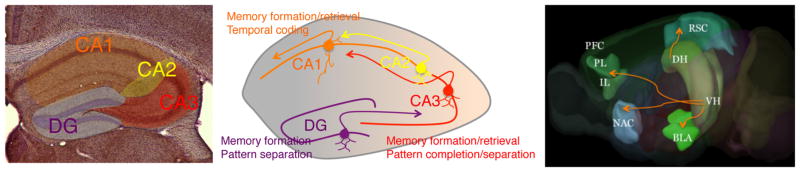
In rodents, negatively valenced episodic memories are commonly investigated using contextual fear conditioning, a form of associative learning wherein subjects learn to associate environmental contexts with stressful experiences such as footshocks [50]. As with human episodic memories, context fear memories require intact hippocampal [51–53] and cortical functions [54], for which reason they are considered to be similar to human explicit (consciously accessible) memories [55]. Much progress has been made in our understanding of the cellular and functional heterogeneity of hippocampal microcircuits as they encode and retrieve episodic memories. This includes organization of the trisynaptic microcircuit among the dentate gyrus (DG), CA3, and CA1 hippocampal subfields (Fig. 1), in which each subfield plays a distinct role in coding spatial (DG, CA3) and temporal (CA1) aspects of context memories [56, 57]. Although the main information flow is directed from DG and CA3 (sites of subcortical and cortical inputs) toward CA1 (main subcortical and cortical outputs), there are also afferents and efferents specific for individual subfields with distinct functional roles [58]. In addition to these subfields, it has become increasingly recognized that dorsoventral hippocampal subdivisions make unique genetic and anatomical contributions to distinct functions. The dorsal hippocampus (DH) has been implicated in memory, ventral hippocampus (VH) in fear and anxiety [59–61], and both in the regulation of social behaviors [62, 63]. Thus, the organization of hippocampal microcircuits enables both the processing of the spatio-temporal aspects of episodic memories, and their interaction with social and affective processes (Fig. 1).
Fig. 1.
Organization and function of hippocampal microcircuits. Hippocampal DG/CA1-CA3 subfields (left), and their roles in memory coding and retrieval (middle). Some of the connections of the dorsal (DH) and ventral (VH) hippocampus with neuronal networks processing negative and positive valence, social behavior, and executive function (right). BLA basolateral amygdala, NAC nucleus accumbens, PFC prefrontal cortex, IL infralimbic cortex, PL prelimbic cortex, RSC retrosplenial cortex. Adapted from the Allen Mouse Brain Atlas
The hippocampus is also known for its high susceptibility to stress, due, among other things, to the levels of glucocorticoid and mineralocorticoid receptors that mediate actions of stress hormones [64]. On a cellular level, this susceptibility is manifested through structural remodeling of dendritic spines [65], which has been found both in the CA3 [66, 67] and CA1 [48, 49, 68] subfields. In addition, stress targets synaptic plasticity, local circuit activity, and neurogenesis in the DG subfield [69]. It is not surprising, therefore, that stress effects on the hippocampus influence cognitive, affective, and social behaviors.
How Can We Study Stress-Enhanced Memories?
Studying the enhancement of memory by stress has been relatively easy both in rodents and in humans because there are clear manifestations of behavior indicative of memory strength. While it is recognized that many neurotransmitter systems shape memory formation, one system clearly stands out—the excitatory glutamate system. Glutamate, through ionotropic glutamate receptors in particular, provides the essential signal for neuronal activity required for the encoding, retrieval, and extinction of stress-induced memories that usually trigger fear and anxiety [70]. Actions of glutamate on hippocampal long-term synaptic plasticity and gene expression are well established, and include, depending on the specific glutamate receptor, long-term potentiation, long-term depression, and the expression of discrete transcription factors [71]. Some of these transcription factors are robustly induced by learning, a feature that has recently been exploited to genetically tag, visualize, and manipulate memory networks, as described below.
Labeling Memory Networks
Some of the first attempts to label memory networks were performed by measuring the co-expression of the immediate early gene activity-regulated cytoskeleton-associated protein (Arc) and Homer1a using the fluorescent in situ hybridization method [72, 73]. This method allows the visualization of neuronal populations that respond to similar or different environmental contexts presented about 30–40 min apart, because neurons activated by the first context show signals for Homer1a messenger RNA (mRNA), whereas those activated by the second context show signals for Arc mRNA. This approach successfully captured some of the functional differences of CA1 and CA3 neurons. Specifically, CA1 neurons showed a graded response (i.e., the number of overlapping neurons positively correlated with the contextual similarity), whereas the CA3 response was sparse and discontinuous [73]. Currently, the most commonly used genetic system for tagging active neurons takes advantage of the promoter of the cFos gene, which is highly responsive to glutamate signaling activated by environmental stimuli and can be used to drive neuronal expression of fluorescent proteins (e.g., green fluorescent protein, mCherry, etc.) [74, 75] or enzymes (e.g., lacZ) [76]. These “tagged” neurons can be visualized using specific antibodies or substrates, resulting in fluorescent signals. Importantly, initially activated neurons remain labeled for a long time, so that one can study the degree of overlap between neuronal populations involved in learning about different contexts [77•, 78, 79], or in learning versus retrieval [77•, 79–81], or in learning versus extinction of context-specific fear [76] (Fig. 2). These various approaches to isolate and tag memory networks have enabled us to advance our understanding of the cellular basis of context-specific memories, but more importantly, they can now be combined with functional manipulations of memory-coding neurons to directly test their roles in processing specific types of information.
Fig. 2.
Labeling neuronal populations that respond to temporally separated events. a Neurons responding to different contexts presented 40 min apart (left), to fear conditioning (green) versus fear extinction (red, middle), and to memory formation (red) versus memory retrieval (green). Neurons responding to both events are labeled yellow. b Simplified schematic of the main principle of neuronal labeling, based on genetic insertion of a fluorescent tag. Both the tag (green) and the endogenous immediate early gene (cFos, red), are driven by the cFos promoter. Unlike cFos, whose expression is transient and subsides within several hours, the fluorescent tag lastingly labels the memory processing neurons. Vazdarjanova et al., reprinted by permission the Society for Neuroscience: J Neurosci. 24(29): 6489–96, copyright 2004) and Ramirez et al., reprinted by permission from Springer: Science. 341(6144): 387–91, copyright 2013
Manipulating Memory Networks
Recent advances with genetic [82], optogenetic [83], and chemogenetic [84] approaches have enabled us activate or inhibit discrete neuronal networks with unprecedented cellular resolution. These approaches include the expression of artificial receptors in nerve cells followed by their activation or inhibition either by drugs or by light. The most commonly employed receptor-activator systems so far are “designer receptors exclusively activated by designer drugs”—clozapine-N-oxide (DREADD-CNO), opsins, such as channelrhodopsin-2–light (ChR2-light), and “pharmacologically selective actuator molecules”—“pharmacologically selective effector molecules” (PSAM-PSEM). The use of these systems has already advanced our knowledge about the organization of neuronal circuits and microcircuits mediating contextual fear conditioning, including the role of the hippocampus and its cortical target, retrosplenial cortex [85, 86•, 87••]. Furthermore, in combination with the neuronal tagging techniques described above, it has become possible to manipulate the activity of specific memory-processing neuronal networks (Fig. 3) a nd thus change various features of selected memories. For example, the reactivation of memory coding neurons under defined experimental conditions can disrupt the context specificity [77•] or valence [88••] of memories, and even improve affective behavior [89]. By applying similar approaches to study in depth the impact of traumatic stress on memory and behavior, we will be able to address important issues related to the features of traumatic memories.
Fig. 3.
Manipulations of memory networks. Neurons within DG or CA1 involved in memory coding (green) can also be genetically engineered to express artificial receptors, which can either enhance or impair neuronal activity in response to artificial drugs or light. The most common systems are indicated on the right
Stress-Induced Amnesia: How Can We Study What Cannot Be Remembered?
Studying memory impairments has always been challenging because of the difficulty of knowing whether a memory was formed but cannot be retrieved or whether it was never formed in the first place [90]. Nevertheless, there are experimental conditions and approaches that allow us to study inaccessible memories, such as models of state-dependent learning. Girden and Culler (1937) were the first to demonstrate that a simple muscle reflex conditioned under curare disappears when the effect of the drug wears off and reappears when the animals are on drug again [91]. They termed the phenomenon “dissociation of learning” or “state-dependent learning”. This accidental observation did not gain much attention until thirty years later when highly visible publications provided evidence for complete or partial state-dependent learning in humans [92–94] and animals (reviewed in [95]), including invertebrates [32]. The findings provided a basis for important theoretical and experimental work on unconscious (i.e., implicit) memory processing and its effects on consciously executed behaviors [96].
Some of the earliest evidence for state-dependent learning comes from studies with compounds that are now known to be GABAergic agonists or positive allosteric modulators, such as alcohol [humans [92, 97, 98] and rats [95, 99]], amobarbital [93, 100], and diazepam [101]. We attempted to induce state-dependent memories by using the compound gaboxadol (4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol; also known as THIP [102], which acts as a superagonist of extrasynaptic GABAAR. We chose to focus on these receptors for three main reasons: first, alcohol typically induces state-dependent learning at low doses [103] at which it preferentially activates extrasynaptic GABAAR [104]; second, these GABAAR generate tonic inhibition in DG, which is important for processing episodic memories [105, 106]; and third, these receptors control brain states ranging from sleep to heightened affect and psychosis [107], making them good candidate mediators of state-dependent processes. After intrahippocampal infusion of gaboxadol, mice formed strong context memories. However, this was only evident when they were on drug again before the retrieval test [87••]. We also found that gaboxadol induced a significant increase of Egr-1 levels in the dentate gyrus granule cells (Fig. 4). Notably, analyses of the immediate early gene early growth response gene-1 (Egr-1) in hippocampal projection targets revealed that the drug completely changed the “routing” of hippocampally processed context information, with activation in DG/CA3 and their subcortical targets, and deactivation in cortical targets such as the retrosplenial and entorhinal cortices. Accordingly, we found that unlike normally encoded fear-inducing context memories, which require cortical processing, state-dependent context memories were actually enhanced following cortical inactivation [87••]. Together, these findings demonstrate that state-dependent memories can be studied at a neurobiological level, and, combined with the approaches described above, can be manipulated in a controlled fashion to better understand their impact on other processes and systems. Consistent with this possibility, the Tonegawa lab used optogenetic approaches to activate memories that are normally not retrievable. Challenging some of the most widely accepted views in the neurobiology of memory, the authors found that context-specific memories can be formed in the absence of protein synthesis or changes of synaptic plasticity and dendritic remodeling [108••]. Although not accessible for retrieval, such memories could be retrieved with artificial receptor-activator systems applied to DG neurons.
Fig. 4.
Effect of gaboxadol on hippocampal activity and state-dependent fear-inducing context memory. Infusion of gaboxadol into the hippocampus hyperactivated DG but not CA1 neurons. Under these conditions, mice acquired context fear (expressed as freezing behavior) only when they were tested on drug. Without the drug the mice did not show freezing. The drug did not elicit freezing in context 2 in which mice did not experience aversive footshocks, showing that this was a memory rather than a nonspecific drug effect. Adapted from Jovasevic et al. [87••], reprinted by permission from Macmillan Publishers Ltd: Nat Neurosci 18(9): 1265–71, copyright 2015
Assessing the Impact of Stress-Related Memories on Socio-affective Processes and Global Network Activity and Connectivity
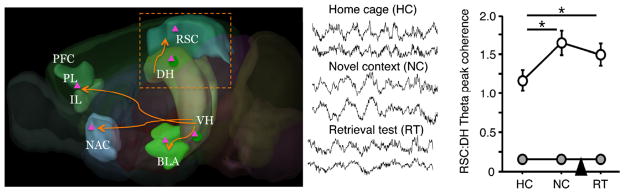
Until recently, it has been difficult to study interactions between different cognitive, social, and affective processes. Among other reasons, this has been because our main approaches were previously based on pharmacological manipulations that often target entire receptor populations. Increased specificity can be achieved by targeting specific scaffolding complexes of relevant receptors [71, 109, 110], or by targeting more specialized neurotransmitter systems. For example, we have found that antagonism of metabotropic mGluR5 and oxytocin receptors in the hippocampus and lateral septum disrupts specific aspects of social behavior without affecting the encoding or retrieval of context-specific fear [111]. Circuit approaches of well-defined neuronal populations that project to specific brain areas, allow for superior functional specificity [112]. By combining circuit labeling and manipulations, the resolution of our analyses is likely to further increase so that we can assess the impact of stress-related memories on neuronal networks that process socio-affective phenomena. In addition, by combining circuit manipulations with measurements of local field potentials (Fig. 5) or whole brain imaging [114], we can now determine how activation or inhibition of local memory networks affects global brain function and connectivity. This is of particular translational relevance, because similar measurements can be carried out and validated in humans.
Fig. 5.
Manipulating specific hippocampal projections to study the global impact of stress-related memories. In addition to labeling local hippocampal neurons, artificial receptors label their projections in various cortical and subcortical targets (left). If activators are applied into these targets, it is possible to manipulate individual projections coming from the hippocampus. Combined with analyses of oscillatory activity relevant for memory processing such as DH-RSC peak coherence during memory formation and memory retrieval (right), this approach can allow us to study interactions among selected systems. Adapted from Corcoran et al. [113•], reprinted by permission from Elsevier: Neurobiol Learn Mem 127:93–101, copyright 2016
Conclusion and Perspectives
It is generally believed that memories dependent on the hippocampus are explicit in that they require conscious access whereas those that are hippocampus-independent are implicit and have no such requirement [55, 96]. This idea has been challenged by the findings discussed above and is in line with human work showing that context-specific memories can be encoded both as explicit and implicit at the level of hippocampal microcircuits [115, 116]. Clarifying these issues is important because the DG/CA3 microcircuit seems to be able to code information in various ways––it is required for adequate context memory formation, but can also code inaccessible memories [87••, 108••], as well as stress-enhanced context memories, which can be formed even when CA3 dendritic remodeling is severely disrupted by chronic stress [117]. However, more research is needed to better understand the role of the tonic GABA system in stimulus processing by DG and CA3 neurons and in changing local and network activities that define stable brain states in which memories can be formed and retrieved.
Deepening our understanding of the DG/CA3 memory network might enable us to redefine neurobiological constraints on memory formation, storage, and retrieval, and allow us to gain new knowledge about the diverse stress effects on memory. Importantly, we can now directly test the views that “nothing about the clinical evidence suggests that traumatic memories are special, or that special techniques are required to recover them” [1] or, alternatively, that trauma memories are special and coded within a broader socio-cognitive-affective circuit [2••]. An obvious first step is to apply the described technologies to animal models that best reflect trauma-related memory deficits, either as persistent and extinction-resistant fear-provoking memories [118] or inaccessible, state-dependent memories [119]. While there are quite a few models to test the former, the latter will require the development of stress models resulting in state-dependent memory. The integration of these various approaches promises to improve our understanding of psychology and psychopathology and lead to the development of new ideas that can serve as a basis for more effective approaches to the treatment of traumatized patients.
Footnotes
Compliance with Ethical Standards
Conflict of Interest The work of Dr. Radulovic is supported by research grants from NIMH (MH078064 and MH108837).
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by the author.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Shobe KK, Kihlstrom J. Is traumatic memory special? Curr Dir Psychol Sci. 1997;6:70–4. [Google Scholar]
- 2••.Nadel L, Jacobs WJ. Traumatic memory is special. Curr Dir Psychol Sci. 1998;7(5):154–7. This is the first suggestion that trauma-related memories differ from nontraumatic memories at the circuit level. [Google Scholar]
- 3.McGaugh JL, Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain. Curr Opin Neurobiol. 2002;12(2):205–10. doi: 10.1016/s0959-4388(02)00306-9. [DOI] [PubMed] [Google Scholar]
- 4.Desmedt A, Marighetto A, Piazza PV. Abnormal fear memory as a model for posttraumatic stress disorder. Biol Psychiatry. 2015;78(5):290–7. doi: 10.1016/j.biopsych.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 5.Dejean C, Courtin J, Rozeske RR, Bonnet MC, Dousset V, Michelet T, et al. Neuronal circuits for fear expression and recovery: recent advances and potential therapeutic strategies. Biol Psychiatry. 2015;78(5):298–306. doi: 10.1016/j.biopsych.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of post-traumatic stress disorder and extinction: human neuroimaging research—past, present, and future. Biol Psychiatry. 2006;60(4):376–82. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 7.McNally RJ. Implicit and explicit memory for trauma-related information in PTSD. Ann N Y Acad Sci. 1997;821:219–24. doi: 10.1111/j.1749-6632.1997.tb48281.x. [DOI] [PubMed] [Google Scholar]
- 8.Terr LC. Childhood traumas: an outline and overview. Am J Psychiatry. 1991;148(1):10–20. doi: 10.1176/ajp.148.1.10. [DOI] [PubMed] [Google Scholar]
- 9.van der Kolk BA. The body keeps the score: memory and the evolving psychobiology of posttraumatic stress. Harv Rev Psychiatry. 1994;1(5):253–65. doi: 10.3109/10673229409017088. [DOI] [PubMed] [Google Scholar]
- 10.Freyd J. Betrayal trauma: The logic of forgetting childhood abuse. Harvard University Press; 1996. [Google Scholar]
- 11.Whitfield CL. Health Commun. 1995. Memory and abuse: remembering and healing the effects of trauma. [Google Scholar]
- 12.Horowitz MJ. Stress response syndromes. Jason Aronson; 1976. [Google Scholar]
- 13.Berntsen D, Rubin DC. Involuntary memories and dissociative amnesia: assessing key assumptions in PTSD research. Clin Psychol Sci. 2014;2(2):174–86. doi: 10.1177/2167702613496241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wittekind CE, Jelinek L, Kleim B, Muhtz C, Moritz S, Berna F. Age effect on autobiographical memory specificity: A study on autobiographical memory specificity in elderly survivors of childhood trauma. J Behav Ther Exp Psychiatry. 2016;54:247–53. doi: 10.1016/j.jbtep.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Finsterwald C, Steinmetz AB, Travaglia A, Alberini CM. From memory impairment to posttraumatic stress disorder-like phenotypes: the critical role of an unpredictable second traumatic experience. J Neurosci. 2015;35(48):15903–15. doi: 10.1523/JNEUROSCI.0771-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joels M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10(6):459–66. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Revest JM, Di Blasi F, Kitchener P, Rouge-Pont F, Desmedt A, Turiault M, et al. The MAPK pathway and Egr-1 mediate stress-related behavioral effects of glucocorticoids. Nat Neurosci. 2005;8(5):664–72. doi: 10.1038/nn1441. [DOI] [PubMed] [Google Scholar]
- 18.Radulovic J, Ruhmann A, Liepold T, Spiess J. Modulation of learning and anxiety by corticotropin-releasing factor (CRF) and stress: differential roles of CRF receptors 1 and 2. J Neurosci. 1999;19(12):5016–25. doi: 10.1523/JNEUROSCI.19-12-05016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sar V. Developmental trauma, complex PTSD, and the current proposal of DSM-5. Eur J Psychotraumatol. 2011:2. doi: 10.3402/ejpt.v2i0.5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sar V, Ross C. Dissociative disorders as a confounding factor in psychiatric research. Psychiatr Clin N Am. 2006;29(1):129–44. ix. doi: 10.1016/j.psc.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Lanius RA, Vermetten E, Loewenstein RJ, Brand B, Schmahl C, Bremner JD, et al. Emotion modulation in PTSD: clinical and neurobiological evidence for a dissociative subtype. Am J Psychiatry. 2010;167(6):640–7. doi: 10.1176/appi.ajp.2009.09081168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ross CA, BBK Dissociation and schizophrenia. J Trauma Dissociation. 2004;5:69–83. [Google Scholar]
- 23.Renard SB, Pijnenborg M, Lysaker PH. Dissociation and social cognition in schizophrenia spectrum disorder. Schizophr Res. 2012;137(1–3):219–23. doi: 10.1016/j.schres.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Dorahy MJ, Corry M, Shannon M, Webb K, McDermott B, Ryan M, et al. Complex trauma and intimate relationships: the impact of shame, guilt and dissociation. J Affect Disord. 2013;147(1–3):72–9. doi: 10.1016/j.jad.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Krammer S, Kleim B, Simmen-Janevska K, Maercker A. Childhood trauma and complex posttraumatic stress disorder symptoms in older adults: a study of direct effects and social-interpersonal factors as potential mediators. J Trauma Dissociation. 2015:1–16. doi: 10.1080/15299732.2014.991861. [DOI] [PubMed] [Google Scholar]
- 26.Janet P. L’Automtisme psychologique. Alcan; 1889. [Google Scholar]
- 27.Braun BG. Towards a theory of multiple personality and other dissociative phenomena. Psychiatr Clin N Am. 1984;7(1):171–93. [PubMed] [Google Scholar]
- 28.Spiegel D, Loewenstein RJ, Lewis-Fernandez R, Sar V, Simeon D, Vermetten E, et al. Dissociative disorders in DSM-5. Depress Anxiety. 2011;28(9):824–52. doi: 10.1002/da.20874. [DOI] [PubMed] [Google Scholar]
- 29.Breuer J, Freud S. Studies on hysteria. Hogarth Press; 1955. [Google Scholar]
- 30.van der Kolk BA, Fisler R. Dissociation and the fragmentary nature of traumatic memories: overview and exploratory study. J Trauma Stress. 1995;8(4):505–25. doi: 10.1007/BF02102887. [DOI] [PubMed] [Google Scholar]
- 31.Eich E. Mood as a mediator of place dependent memory. J Exp Psychol Gen. 1995;124(3):293–308. doi: 10.1037//0096-3445.124.3.293. [DOI] [PubMed] [Google Scholar]
- 32.Pompilio L, Kacelnik A, Behmer ST. State-dependent learned valuation drives choice in an invertebrate. Science. 2006;311(5767):1613–5. doi: 10.1126/science.1123924. [DOI] [PubMed] [Google Scholar]
- 33.John OP, Gross JJ. Healthy and unhealthy emotion regulation: personality processes, individual differences, and life span development. J Pers. 2004;72(6):1301–33. doi: 10.1111/j.1467-6494.2004.00298.x. [DOI] [PubMed] [Google Scholar]
- 34.Selye H. The general adaptation syndrome and the diseases of adaptation. J Clin Endocrinol. 1946;6:117–230. doi: 10.1210/jcem-6-2-117. [DOI] [PubMed] [Google Scholar]
- 35.McEwen BS. Glucocorticoids, depression, and mood disorders: structural remodeling in the brain. Metab Clin Exp. 2005;54(5 Suppl 1):20–3. doi: 10.1016/j.metabol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 36.de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6(6):463–75. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 37.McClelland S, Korosi A, Cope J, Ivy A, Baram TZ. Emerging roles of epigenetic mechanisms in the enduring effects of early-life stress and experience on learning and memory. Neurobiol Learn Mem. 2011;96(1):79–88. doi: 10.1016/j.nlm.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen H, Kaplan Z, Matar MA, Loewenthal U, Zohar J, Richter-Levin G. Long-lasting behavioral effects of juvenile trauma in an animal model of PTSD associated with a failure of the autonomic nervous system to recover. Eur Neuropsychopharmacol. 2007;17(6–7):464–77. doi: 10.1016/j.euroneuro.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 39.Valentino RJ, Bangasser D, Van Bockstaele EJ. Sex-biased stress signaling: the corticotropin-releasing factor receptor as a model. Mol Pharmacol. 2013;83(4):737–45. doi: 10.1124/mol.112.083550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonne O, Vythilingam M, Inagaki M, Wood S, Neumeister A, Nugent AC, et al. Reduced posterior hippocampal volume in post-traumatic stress disorder. J Clin Psychiatry. 2008;69(7):1087–91. doi: 10.4088/jcp.v69n0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen AC, Etkin A. Hippocampal network connectivity and activation differentiates post-traumatic stress disorder from generalized anxiety disorder. Neuropsychopharmacology. 2013;38(10):1889–98. doi: 10.1038/npp.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kikuchi H, Fujii T, Abe N, Suzuki M, Takagi M, Mugikura S, et al. Memory repression: brain mechanisms underlying dissociative amnesia. J Cogn Neurosci. 2010;22(3):602–13. doi: 10.1162/jocn.2009.21212. [DOI] [PubMed] [Google Scholar]
- 43.O’Keefe J, Nadel L. The hippocampus as a cognitive map. Clarendon Press; 1978. [Google Scholar]
- 44.Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99(2):195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 45.Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35(4):625–41. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- 46.Eichenbaum H, Cohen NJ. Representation in the hippocampus: what do hippocampal neurons code? Trends Neurosci. 1988;11(6):244–8. doi: 10.1016/0166-2236(88)90100-2. [DOI] [PubMed] [Google Scholar]
- 47.Tulving E. Ecphoric processes in episodic memory. Philos T Roy Soc B. 1983;302(1110):361–71. [Google Scholar]
- 48.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22(15):6810–8. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maras PM, Molet J, Chen Y, Rice C, Ji SG, Solodkin A, et al. Preferential loss of dorsal-hippocampus synapses underlies memory impairments provoked by short, multimodal stress. Mol Psychiatry. 2014;19(7):811–22. doi: 10.1038/mp.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fanselow MS. Factors governing one-trial contextual conditioning. Anim Learn Behav. 1990;18(3):264–70. [Google Scholar]
- 51.Selden NR, Everitt BJ, Jarrard LE, Robbins TW. Complementary roles for the amygdala and hippocampus in aversive conditioning to explicit and contextual cues. Neuroscience. 1991;42(2):335–50. doi: 10.1016/0306-4522(91)90379-3. [DOI] [PubMed] [Google Scholar]
- 52.Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256(5057):675–7. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- 53.Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106(2):274–85. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 54.Burwell RD, Saddoris MP, Bucci DJ, Wiig KA. Corticohippocampal contributions to spatial and contextual learning. J Neurosci. 2004;24(15):3826–36. doi: 10.1523/JNEUROSCI.0410-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barco A, Bailey CH, Kandel ER. Common molecular mechanisms in explicit and implicit memory. J Neurochem. 2006;97(6):1520–33. doi: 10.1111/j.1471-4159.2006.03870.x. [DOI] [PubMed] [Google Scholar]
- 56.Rolls ET. An attractor network in the hippocampus: theory and neurophysiology. Learn Mem. 2007;14(11):714–31. doi: 10.1101/lm.631207. [DOI] [PubMed] [Google Scholar]
- 57.Kesner RP, Rolls ET. A computational theory of hippocampal function, and tests of the theory: new developments. Neurosci Biobehav Rev. 2015;48:92–147. doi: 10.1016/j.neubiorev.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 58.Hunsaker MR, Tran GT, Kesner RP. A double dissociation of subcortical hippocampal efferents for encoding and consolidation/retrieval of spatial information. Hippocampus. 2008;18(7):699–709. doi: 10.1002/hipo.20429. [DOI] [PubMed] [Google Scholar]
- 59.Strange BA, Witter MP, Lein ES, Moser EI. Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci. 2014;15(10):655–69. doi: 10.1038/nrn3785. [DOI] [PubMed] [Google Scholar]
- 60.Moser MB, Moser EI. Functional differentiation in the hippocampus. Hippocampus. 1998;8(6):608–19. doi: 10.1002/(SICI)1098-1063(1998)8:6<608::AID-HIPO3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 61.Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65(1):7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Allsop SA, Vander Weele CM, Wichmann R, Tye KM. Optogenetic insights on the relationship between anxiety-related behaviors and social deficits. Front Behav Neurosci. 2014;8:241. doi: 10.3389/fnbeh.2014.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Finlay JM, Dunham GA, Isherwood AM, Newton CJ, Nguyen TV, Reppar PC, et al. Effects of prefrontal cortex and hippocampal NMDA NR1-subunit deletion on complex cognitive and social behaviors. Brain Res. 2015;1600:70–83. doi: 10.1016/j.brainres.2014.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Groeneweg FL, Karst H, de Kloet ER, Joels M. Mineralocorticoid and glucocorticoid receptors at the neuronal membrane, regulators of nongenomic corticosteroid signalling. Mol Cell Endocrinol. 2011 doi: 10.1016/j.mce.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 65.Ammassari-Teule M. Is structural remodeling in regions governing memory an univocal correlate of memory? Neurobiol Learn Mem. 2016;136:28–33. doi: 10.1016/j.nlm.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 66.Sandi C, Davies HA, Cordero MI, Rodriguez JJ, Popov VI, Stewart MG. Rapid reversal of stress induced loss of synapses in CA3 of rat hippocampus following water maze training. Eur J Neurosci. 2003;17(11):2447–56. doi: 10.1046/j.1460-9568.2003.02675.x. [DOI] [PubMed] [Google Scholar]
- 67.Sebastian V, Estil JB, Chen D, Schrott LM, Serrano PA. Acute physiological stress promotes clustering of synaptic markers and alters spine morphology in the hippocampus. PLoS One. 2013;8(10):e79077. doi: 10.1371/journal.pone.0079077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pawlak R, Rao BS, Melchor JP, Chattarji S, McEwen B, Strickland S. Tissue plasminogen activator and plasminogen mediate stress-induced decline of neuronal and cognitive functions in the mouse hippocampus. Proc Natl Acad Sci U S A. 2005;102(50):18201–6. doi: 10.1073/pnas.0509232102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fa M, Xia L, Anunu R, Kehat O, Kriebel M, Volkmer H, et al. Stress modulation of hippocampal activity—spotlight on the dentate gyrus. Neurobiol Learn Mem. 2014;112:53–60. doi: 10.1016/j.nlm.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 70.Davis M, Myers KM. The role of glutamate and gamma-aminobutyric acid in fear extinction: clinical implications for exposure therapy. Biol Psychiatry. 2002;52(10):998–1007. doi: 10.1016/s0006-3223(02)01507-x. [DOI] [PubMed] [Google Scholar]
- 71.Grant SG, O’Dell TJ. Multiprotein complex signaling and the plasticity problem. Curr Opin Neurobiol. 2001;11(3):363–8. doi: 10.1016/s0959-4388(00)00220-8. [DOI] [PubMed] [Google Scholar]
- 72.Vazdarjanova A, McNaughton BL, Barnes CA, Worley PF, Guzowski JF. Experience-dependent coincident expression of the effector immediate-early genes arc and Homer 1a in hippocampal and neocortical neuronal networks. J Neurosci. 2002;22(23):10067–71. doi: 10.1523/JNEUROSCI.22-23-10067.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vazdarjanova A, Guzowski JF. Differences in hippocampal neuronal population responses to modifications of an environmental context: evidence for distinct, yet complementary, functions of CA3 and CA1 ensembles. J Neurosci. 2004;24(29):6489–96. doi: 10.1523/JNEUROSCI.0350-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barth AL. Visualizing circuits and systems using transgenic reporters of neural activity. Curr Opin Neurobiol. 2007;17(5):567–71. doi: 10.1016/j.conb.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garner A, Mayford M. New approaches to neural circuits in behavior. Learn Mem. 2012;19(9):385–90. doi: 10.1101/lm.025049.111. [DOI] [PubMed] [Google Scholar]
- 76.Tronson NC, Schrick C, Guzman YF, Huh KH, Srivastava DP, Penzes P, et al. Segregated populations of hippocampal principal CA1 neurons mediating conditioning and extinction of contextual fear. J Neurosci. 2009;29(11):3387–94. doi: 10.1523/JNEUROSCI.5619-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77•.Ramirez S, Liu X, Lin PA, Suh J, Pignatelli M, Redondo RL, et al. Creating a false memory in the hippocampus. Science. 2013;341(6144):387–91. doi: 10.1126/science.1239073. This is the first use of optogenetics to manipulate a memory. [DOI] [PubMed] [Google Scholar]
- 78.Cai DJ, Aharoni D, Shuman T, Shobe J, Biane J, Song W, et al. A shared neural ensemble links distinct contextual memories encoded close in time. Nature. 2016;534(7605):115–8. doi: 10.1038/nature17955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Deng W, Mayford M, Gage FH. Selection of distinct populations of dentate granule cells in response to inputs as a mechanism for pattern separation in mice. eLife. 2013;2:e00312. doi: 10.7554/eLife.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reijmers LG, Perkins BL, Matsuo N, Mayford M. Localization of a stable neural correlate of associative memory. Science. 2007;317(5842):1230–3. doi: 10.1126/science.1143839. [DOI] [PubMed] [Google Scholar]
- 81.Tayler KK, Tanaka KZ, Reijmers LG, Wiltgen BJ. Reactivation of neural ensembles during the retrieval of recent and remote memory. Curr Biol. 2013;23(2):99–106. doi: 10.1016/j.cub.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 82.Xu W, Sudhof TC. A neural circuit for memory specificity and generalization. Science. 2013;339(6125):1290–5. doi: 10.1126/science.1229534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tye KM, Deisseroth K. Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat Rev Neurosci. 2012;13(4):251–66. doi: 10.1038/nrn3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sternson SM, Roth BL. Chemogenetic tools to interrogate brain functions. Annu Rev Neurosci. 2014;37:387–407. doi: 10.1146/annurev-neuro-071013-014048. [DOI] [PubMed] [Google Scholar]
- 85.Zhu H, Pleil KE, Urban DJ, Moy SS, Kash TL, Roth BL. Chemogenetic inactivation of ventral hippocampal glutamatergic neurons disrupts consolidation of contextual fear memory. Neuropsychopharmacology. 2014;39(8):1880–92. doi: 10.1038/npp.2014.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86•.Cowansage KK, Shuman T, Dillingham BC, Chang A, Golshani P, Mayford M. Direct reactivation of a coherent neocortical memory of context. Neuron. 2014;84(2):432–41. doi: 10.1016/j.neuron.2014.09.022. This is the first successful use of a chemogenetic approach to reactivate a cortical memory. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87••.Jovasevic V, Corcoran KA, Leaderbrand K, Yamawaki N, Guedea AL, Chen HJ, et al. GABAergic mechanisms regulated by miR-33 encode state-dependent fear. Nat Neurosci. 2015;18(9):1265–71. doi: 10.1038/nn.4084. This study shows that state-dependent fear memories can be formed under heightened activation of extrasynaptic GABAAR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88••.Redondo RL, Kim J, Arons AL, Ramirez S, Liu X, Tonegawa S. Bidirectional switch of the valence associated with a hippocampal contextual memory engram. Nature. 2014;513(7518):426–30. doi: 10.1038/nature13725. An important paper demonstrating how the valence of memories can be changed from negative to positive during reactivation of memory coding circuits. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ramirez S, Liu X, MacDonald CJ, Moffa A, Zhou J, Redondo RL, et al. Activating positive memory engrams suppresses depression-like behaviour. Nature. 2015;522(7556):335–9. doi: 10.1038/nature14514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Squire LR. Lost forever or temporarily misplaced? The long debate about the nature of memory impairment. Learn Mem. 2006;13(5):522–9. doi: 10.1101/lm.310306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Girden E, Culler E. Conditioned responses in curarized striate muscle in dogs. J Comp Psychol. 1937;23(3):261–74. [Google Scholar]
- 92.Goodwin DW, Powell B, Bremer D, Hoine H, Stern J. Alcohol and recall: state-dependent effects in man. Science. 1969;163(3873):1358–60. doi: 10.1126/science.163.3873.1358. [DOI] [PubMed] [Google Scholar]
- 93.Bustamante JA, Jordan A, Vila M, Gonzalez A, Insua A. State dependent learning in humans. Physiol Behav. 1970;5(7):793–6. doi: 10.1016/0031-9384(70)90281-7. [DOI] [PubMed] [Google Scholar]
- 94.Lang AJ, Craske MG, Brown M, Ghaneian A. Fear-related state dependent memory. Cognit Emot. 2001;15:695–703. [Google Scholar]
- 95.Overton DA. Historical context of state dependent learning and discriminative drug effects. Behav Pharmacol. 1991;2(4 And 5):253–64. [PubMed] [Google Scholar]
- 96.Squire LR, Dede AJ. Conscious and unconscious memory systems. Cold Spring Harb Perspect Biol. 2015;7(3):a021667. doi: 10.1101/cshperspect.a021667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hinrichsen JJ, Katahn M, Levenson RW. Alcohol-induced state-dependent learning in non-alcoholics. Pharmacol Biochem Behav. 1974;2(3):293–6. doi: 10.1016/0091-3057(74)90072-0. [DOI] [PubMed] [Google Scholar]
- 98.Weingartner H, Adefris W, Eich JE, Murphy DL. Encoding-imagery specificity in alcohol state-dependent learning. J Exp Psychol Hum Learn. 1976;2(1):83–7. [PubMed] [Google Scholar]
- 99.Duncan PM. The effect of external stimulus change on ethanol-produced dissociation. Pharmacol Biochem Behav. 1979;11(4):377–81. doi: 10.1016/0091-3057(79)90110-2. [DOI] [PubMed] [Google Scholar]
- 100.Ley P, Jain VK, Swinson RP, Eaves D, Bradshaw PW, Kincey JA, et al. A state-dependent learning effect produced by amylobarbitone sodium. Br J Psychiatry. 1972;120(558):511–5. doi: 10.1192/bjp.120.558.511. [DOI] [PubMed] [Google Scholar]
- 101.Jensen HH, Hutchings B, Poulsen JC. Conditioned emotional responding under diazepam: a psychophysiological study of state dependent learning. Psychopharmacology (Berlin) 1989;98(3):392–7. doi: 10.1007/BF00451693. [DOI] [PubMed] [Google Scholar]
- 102.Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human alpha(4)beta(3)delta GABA(A) receptors. Br J Pharmacol. 2002;136(7):965–74. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hernandez LL, Valentine JD, Powell DA. Ethanol enhancement of Pavlovian conditioning. Behav Neurosci. 1986;100(4):494–503. doi: 10.1037//0735-7044.100.4.494. [DOI] [PubMed] [Google Scholar]
- 104.Wei W, Faria LC, Mody I. Low ethanol concentrations selectively augment the tonic inhibition mediated by delta subunit-containing GABAA receptors in hippocampal neurons. J Neurosci. 2004;24(38):8379–82. doi: 10.1523/JNEUROSCI.2040-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nusser Z, Mody I. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophysiol. 2002;87(5):2624–8. doi: 10.1152/jn.2002.87.5.2624. [DOI] [PubMed] [Google Scholar]
- 106.Mortensen M, Patel B, Smart TG. GABA potency at GABA(A) receptors found in synaptic and extrasynaptic zones. Front Cell Neurosci. 2011;6:1. doi: 10.3389/fncel.2012.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brickley SG, Mody I. Extrasynaptic GABA(A) receptors: their function in the CNS and implications for disease. Neuron. 2012;73(1):23–34. doi: 10.1016/j.neuron.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108••.Ryan TJ, Roy DS, Pignatelli M, Arons A, Tonegawa S. Memory. Engram cells retain memory under retrograde amnesia. Science. 2015;348(6238):1007–13. doi: 10.1126/science.aaa5542. This work shows that memories can be formed even when changes in synaptic plasticity, morphology, and protein synthesis do not occur, however, such memories are not accessible to recall. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Corcoran KA, Leaderbrand K, Jovasevic V, Guedea AL, Kassam F, Radulovic J. Regulation of fear extinction versus other affective behaviors by discrete cortical scaffolding complexes associated with NR2B and PKA signaling. Transl Psychiatry. 2015;5:e657. doi: 10.1038/tp.2015.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gao CTN, Radulovic J. Modulation of behavior by scaffolding proteins of the postsynaptic density. Neurobiol Learn Mem. 2013 doi: 10.1016/j.nlm.2013.04.014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mesic I, Guzman YF, Guedea AL, Jovasevic V, Corcoran KA, Leaderbrand K, et al. Double dissociation of the roles of metabotropic glutamate receptor 5 and oxytocin receptor in discrete social behaviors. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gradinaru V, Zhang F, Ramakrishnan C, Mattis J, Prakash R, Diester I, et al. Molecular and cellular approaches for diversifying and extending optogenetics. Cell. 2010;141(1):154–65. doi: 10.1016/j.cell.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113•.Corcoran KA, Frick BJ, Radulovic J, Kay LM. Analysis of coherent activity between retrosplenial cortex, hippocampus, thalamus, and anterior cingulate cortex during retrieval of recent and remote context fear memory. Neurobiol Learn Mem. 2016;127:93–101. doi: 10.1016/j.nlm.2015.11.019. The authors demonstrate that coherent oscillatory activity between the hippocampus and retrosplenial cortex, typically occurring during memory encoding and retrieval, can be assessed by LFP recordings in freely moving mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Michaelides M, Hurd YL. DREAMM: a biobehavioral imaging methodology for dynamic in vivo whole-brain mapping of cell type-specific functional networks. Neuropsychopharmacology. 2015;40(1):239–40. doi: 10.1038/npp.2014.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chun MM, Jiang Y. Implicit, long-term spatial contextual memory. J Exp Psychol Learn Mem Cogn. 2003;29(2):224–34. doi: 10.1037/0278-7393.29.2.224. [DOI] [PubMed] [Google Scholar]
- 116.Chun MM, Phelps EA. Memory deficits for implicit contextual information in amnesic subjects with hippocampal damage. Nat Neurosci. 1999;2(9):844–7. doi: 10.1038/12222. [DOI] [PubMed] [Google Scholar]
- 117.Conrad CD, LeDoux JE, Magarinos AM, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 1999;113(5):902–13. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- 118.Huh KH, Guzman YF, Tronson NC, Guedea AL, Gao C, Radulovic J. Hippocampal Erk mechanisms linking prediction error to fear extinction: roles of shock expectancy and contextual aversive valence. Learn Mem. 2009;16(4):273–8. doi: 10.1101/lm.1240109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Reus VI, Weingartner H, Post RM. Clinical implications of state-dependent learning. Am J Psychiatry. 1979;136(7):927–31. doi: 10.1176/ajp.136.7.927. [DOI] [PubMed] [Google Scholar]