Abstract
Human monkeypox is a zoonotic Orthopoxvirus with a presentation similar to smallpox. Clinical differentiation of the disease from smallpox and varicella is difficult. Laboratory diagnostics are principal components to identification and surveillance of disease, and new tests are needed for a more precise and rapid diagnosis. The majority of human infections occur in Central Africa, where surveillance in rural areas with poor infrastructure is difficult but can be accomplished with evidence-guided tools and educational materials to inform public health workers of important principles. Contemporary epidemiological studies are needed now that populations do not receive routine smallpox vaccination. New therapeutics and vaccines offer hope for the treatment and prevention of monkeypox; however, more research must be done before they are ready to be deployed in an endemic setting. There is a need for more research in the epidemiology, ecology, and biology of the virus in endemic areas to better understand and prevent human infections.
Keywords: monkeypox, Orthopoxvirus, smallpox
Monkeypox virus is an Orthopoxvirus, a genus that includes camelpox, cowpox, vaccinia, and variola viruses. The virus is the foremost Orthopoxvirus affecting human populations since smallpox eradication, confirmed by the World Health Organization in 1980. Clinical recognition, diagnosis, and prevention still remain challenges in the resource-poor endemic areas where monkeypox is found. Monkeypox epidemiology is informed by studies conducted at the end of smallpox eradication, but new assessments are needed now that routine smallpox vaccination has ended and there is associated waning herd immunity. Additionally, foundational ecological studies are necessary to better understand the animal species involved in transmission and maintenance of the virus, and to further inform prevention measures.
CLINICAL PICTURE
Human monkeypox was not recognized as a distinct infection in humans until 1970 during efforts to eradicate smallpox, when the virus was isolated from a patient with suspected smallpox infection in The Democratic Republic of the Congo (DRC) [1]. The majority of the clinical characteristics of human monkeypox infection mirror those of smallpox (discrete ordinary type or modified type, Table 1) [2–4]. An initial febrile prodrome is accompanied by generalized headache and fatigue. Prior to, and concomitant with, rash development is the presence of maxillary, cervical, or inguinal lymphadenopathy (1– 4 cm in diameter) in many patients (Figure 1). Enlarged lymph nodes are firm, tender, and sometimes painful. Lymphadenopathy was not characteristic of smallpox. The presence of lymphadenopathy may be an indication that there is a more effective immune recognition and response to infection by monkeypox virus vs variola virus, but this hypothesis requires further study [5].
Table 1.
Key Clinical Characteristics of Smallpox, Monkeypox, and Varicella
| Characteristic | Smallpox | Monkeypox | Varicella |
|---|---|---|---|
| Time period | |||
| Incubation period | 7–17 d | 7–17 d | 10–21 d |
| Prodromal period | 1–4 d | 1–4 d | 0–2 d |
| Rash period (from the appearance of lesions to desquamation) | 14–28 d | 14–28 d | 10–21 d |
| Symptoms | |||
| Prodromal fever | Yes | Yes | Uncommon, mild fever if present |
| Fever | Yes, often >40°C | Yes, often between 38.5°C and 40.5°C | Yes, up to 38.8°C |
| Malaise | Yes | Yes | Yes |
| Headache | Yes | Yes | Yes |
| Lymphadenopathy | No | Yes | No |
| Lesions on palms | or soles Yes | Yes | Rare |
| Lesion distribution | Centrifugal | Centrifugala | Centripetal |
| Lesion appearance | Hard and deep, well-circumscribed, umbilicated | Hard and deep, well- circumscribed, umbilicateda | Superficial, irregular borders, “dew drop on a rose petal” |
| Lesion progression | Lesions are often in one stage of development on the body; slow progression with each stage lasting 1–2 d | Lesions are often in one stage of development on the body; slow progression with each stage lasting 1–2 da | Lesions are often in multiple stages of development on the body; fast progression |
Differences in the appearance of rash have been noted in vaccinated (vaccination <20 years prior to illness) vs unvaccinated individuals. Vaccinated individuals were noted to have fewer lesions, smaller lesions, and better presentation of regional monomorphism and centrifugal distribution of rash.
Figure 1.

Cervical lymphadenopathy in a patient with active monkeypox during a monkeypox outbreak in Zaire, 1996–1997. Photograph credit: Dr Brian W. J. Mahy; provided by the Public Health Image Library, Centers for Disease Control and Prevention.
Fever often declines on the day of or up to 3 days after rash onset. Often, the rash first appears on the face and quickly appears in a centrifugal distribution on the body. The distinctive lesions (Figure 2) often present as first macular, then papular, then vesicular and pustular [6]. The number of lesions on a given patient may range from a few to thousands [7]. Lesions are often noted in the oral cavity and can cause difficulties with drinking and eating. Given the distinctive presentation of lesions, digital photographs and the Internet are 21st-century tools for clinical consultation.
Figure 2.
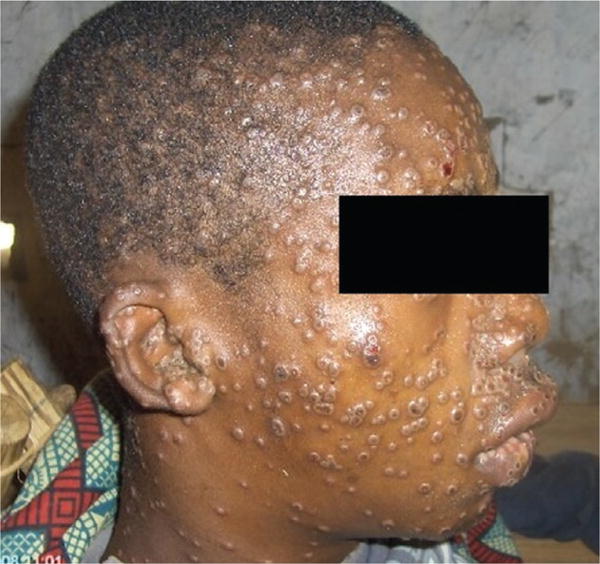
A patient with monkeypox showing characteristic lesions. Photograph credit: Dr Marcel Pie Balilo.
The extensive perturbation of the skin raises concerns about secondary bacterial infections of the skin, and this has been observed to be present in 19% of unvaccinated monkeypox patients [7]. The skin of patients has been noted being swollen, stiff, and painful until crusts appeared [4]. The occurrence of a second febrile period occurring when skin lesions become pustular has been associated with deterioration in the patient’s general condition [4].
Severe complications and sequelae were found to be more common among unvaccinated (74%) than vaccinated patients (39.5%). Patients have been observed with pulmonary distress or bronchopneumonia, often late in the course of illness, suggestive of secondary infection of the lungs. Vomiting or diarrhea can occur by the second week of illness and can contribute to severe dehydration. Encephalitis was observed in one patient and septicemia in another patient with > 4500 lesions [7]. Ocular infections can occur and may result in corneal scarring and permanent vision loss [8]. Pitted scarring is the most common long-term sequelae of those who survive an infection. The average case-fatality rate of unvaccinated patients has been recorded as high as 11%; children are often more prone to severe forms of disease [7]. In these clinical studies, prior vaccination was 3–19 years preceding monkeypox disease.
Varicella, caused by the varicella zoster virus (VZV) in the Herpesviridae family, is another febrile rash illness that is often confused with monkeypox, but several features help distinguish the 2 illnesses (Table 1). Varicella rarely has a prolonged febrile prodrome (1–2 days if present) and the fever is generally mild during this phase. The rash exhibited by VZV generally progresses more quickly than monkeypox and smallpox, and the lesion presentation can be quite different [2]. Additionally, although varicella patients rarely present with lesions on the palms and/or soles, lesions have been noted on the palms and/or soles of 5 household contacts initially thought to have had monkeypox infections, but who tested positive for VZV, in the Republic of the Congo (ROC) [9]. The lymphadenopathy in monkeypox patients has been noted to be a defining differentiating characteristic of the disease from varicella [7]. Additional vesiculopustular rash illnesses included on the differential are other herpetic infections, drug-associated eruptions, syphilis, yaws, scabies, and, more rarely, rickettsialpox.
Clinical distinction between rash illnesses is difficult in the absence of a diagnostic test. Given the similarities between smallpox and monkeypox, an existing smallpox algorithm (http:// www.bt.cdc.gov/agent/smallpox/diagnosis/riskalgorithm/) that takes into account major smallpox criteria (febrile prodrome, classic lesions, lesions in the same stage of development) and minor criteria [10] could be modified for monkeypox and used for diagnostic management. Namely, the inclusion of lymphadenopathy as a major criteria would allow for the addition of monkeypox in the algorithm, retaining smallpox in the differential. This will be an important consideration in the light of biosecurity concerns and the need to consistently rule out suspect smallpox disease. The implementation of such a protocol will be possible with the analysis of clinical and surveillance data from an endemic area. Public health officials should be contacted immediately upon clinical suspicion of an Orthopoxvirus infection. State health departments and the US Centers for Disease Control and Prevention offer consultation and diagnostic testing.
DIAGNOSTIC CONFIRMATION
Diagnostic assays are important components to the identification of an Orthopoxvirus infection. Table 2 lists the diagnostic assays that may be used to classify monkeypox or Orthopoxvirus from clinical specimens. These tests are most powerful when they are combined with clinical and epidemiological information, including a patient’s vaccination history. Given the limited cold chain and diminished resources for sample collection and storage, lesion exudate on a swab or crust specimens still remain some of the best and least invasive acute patient specimens. Viral DNA present in lesion material is stable for a long period of time if kept in a relatively dark, cool environment, an important factor to consider when cold chain is not readily available. Conventional tests such as viral isolation from a clinical specimen, electron microscopy, and immunohistochemistry remain valid techniques but require advanced technical skills and training, as well as a sophisticated laboratory. Specimens can be analyzed using real-time polymerase chain reaction (PCR) to assess the presence of Orthopoxvirus or monkeypox virus in a lesion sample [11–14]. These assays are highly sensitive and can efficiently detect viral DNA. Real-time PCR is currently best used in a major laboratory, thus limiting its use as a real-time diagnostic in rural, resource-poor areas. Advances in technologies may make diagnostic use of real-time PCR more feasible outside of major laboratories.
Table 2.
Diagnostic Tests for Monkeypox or Orthopoxvirus
| Test | Pros | Cons |
|---|---|---|
| Viral culture/isolation: Live virus is grown and characterized from a patient specimen. | Can yield a pure, live culture of virus for definitive classification of the species. Orthopoxviruses produce distinctive “pocks” on chorioallantoic membranes; and other cell-based viral culture methods can be used. Patient specimens from lesions are the most reliable for this method, as viremia is not present the entire duration of illness. | The assay takes several days to complete. Patient specimens may contain bacteria, hampering culture attempts. Further characterization must be done for viral identification. Must be performed at a major laboratory with skilled technicians. |
| Electron microscopy: Negative staining produces a clear image of a brick-shaped particle, allowing for visual classification of a poxvirus, other than Parapoxvirus | Can be used to identify viral particles in a biopsy specimen, scab material, vesicular fluid, or viral culture. Can differentiate an Orthopoxvirus from Herpesviridae. | Orthopoxviruses are morphologically indistinguishable from each other. Must be performed at a major laboratory with skilled technicians and an electron microscope. |
| Immunohistochemistry: Tests for the presence of Orthopoxvirusspecific antigens. | Can be used to identify antigens in biopsy specimens. This technique can be used to rule out or identify other suspect agents | Not specific for monkeypox virus. Must be performed at a major laboratory with skilled technicians |
| PCR, including real-time PCR: Tests for the presence of monkeypox-specific DNA signatures. | Can diagnose an active case using lesion material from a patient. The assay uses viral DNA, which is stable if a specimen is kept in dark, cool conditions. Designed to be specific for monkeypox virus. | Highly sensitive assays where concerns about contamination are warranted. These assays require expensive equipment and reagents. Must be performed at a major laboratory with skilled technicians. |
| Anti-Orthopoxvirus IgG: Tests for the presence of Orthopoxvirus antibodies. | Can be used to assess a previous exposure to an Orthopoxvirus, including a pathogen or smallpox vaccination. | Requires the collection of blood (serum) and a cold chain. This assay is not specific for monkeypox virus. Results will be affected by prior smallpox vaccination. The duration of response is variable. Must be performed at a major laboratory with skilled technicians. |
| Anti-Orthopoxvirus IgM: Tests for the presence of Orthopoxvirus antibodies. | Can be used to assess a recent exposure to an Orthopoxvirus, including a pathogen or smallpox vaccination. This assay could be used as a diagnostic for suspect Orthopoxvirus patients with prior smallpox vaccination. | Requires the collection of blood (serum) and a cold chain. This assay is not specific for monkeypox virus. Must be performed at a major laboratory with skilled technicians. |
| Tetracore Orthopox BioThreat Alert: Tests for the presence of Orthopoxvirus antigens. | Can rapidly diagnose an active case using lesion material from a patient; a point-of-care diagnostic test. Can be performed at ambient temperature with little expertise. | This assay is not specific for monkeypox virus. Needs to be tested in endemic settings. Less sensitive than PCR. |
Abbreviations: IgG, immunoglobulin G; IgM, immunoglobulin M; PCR, polymerase chain reaction.
Determining the cause of cases identified retrospectively requires antibody-based diagnostics. Anti-Orthopoxvirus immunological assays have cross-reactivity to a variety of Orthopoxviruses, and these assays may be useful in areas where there is prior evidence as to what virus is causing illness. Anti-Orthopoxvirus immunoglobulin G (IgG) alone will not provide a definitive diagnosis for retrospective patients who have been exposed to an Orthopoxvirus, including by vaccination, during their lifetime. Alternatively, serological assays that assess anti-Orthopoxvirus immunoglobulin M (IgM) are more applicable to diagnose recent retrospective infections, including in individuals with prior vaccination [15].
A field-deployable point-of-care test is ideal, but there are few developments in this area. A recent pilot of the Tetracore Orthopox BioThreat Alert provided promising results using lesion specimens from acute Orthopoxvirus infections. This assay reliably detected vaccinia and monkeypox viruses in preparations with 107 plaque-forming units/mL, and correct identification of clinical specimens occurred in 5 of 6 specimens tested [16]. Although not specific for monkeypox virus, this assay could be used in monkeypoxendemic areas for Orthopoxvirus confirmation by proxy, and it will be important to test this in endemic settings. Patients with monkeypox virus often seek diagnosis and care at rural clinics or hospitals without electricity; thus, there is a need for the development of assays that can be tested in very basic environments with limited training of personnel.
THE CHANGING FACE OF MONKEYPOX EPIDEMIOLOGY
Historically, there have been reports of human monkeypox infections in West Africa, but since 1981 most reported infections have occurred in the Congo Basin of Central Africa [17]. DRC continues to report the majority of human monkeypox cases each year. Recently, infections also were noted in the Central African Republic, ROC, and Sudan [8, 18, 19], but it is unclear if these infections were the result of movement across the DRC border or the occurrence of indigenous disease. Improved phylogeography and georeferencing of human cases will aid in a better understanding of the distribution of cases, and these data can be used to develop more accurate ecological models of monkeypox distribution [20,21].Domestically, the United States experienced a monkeypox outbreak among humans and captive prairie dogs in 2003, and traceback studies identified a shipment of wild rodents from Ghana as the probable source [22, 23].
Monkeypox can infect a taxonomically wide variety of mammalian species; however, the virus has only been isolated once from a wild animal, a Funisciurus squirrel in DRC [24]. The extent of viral circulation in animal populations and the precise species that may harbor the virus is not entirely known, although several lines of evidence point to rodents as a likely reservoir [25]. Human infections have been linked to contact with animals, but the precise exposure of a human case can be difficult to pinpoint in areas where contact with animals via household rodent infestations and the hunting or preparation of bushmeat from a variety of species is common. Transmission is believed to occur via saliva/respiratory excretions or contact with lesion exudate or crust material [26, 27]. Viral shedding via feces may represent another exposure source [26]. Although human-to-human transmission of monkeypox is apparently less efficient than that observed in smallpox, it did occur in up to 11.7% of household contacts of patients who did not have prior smallpox vaccination; evidence indicates that household members or those who care for a monkeypox patient are at increased risk for acquiring an infection [27]. The longest uninterrupted chain or sequential transmission events of human-to-human spread is posited to be 6 individuals, and clusters of patients have been commonly noted [8, 18, 27]. Transmission in hospital settings has also been documented [8], and may be prevented with standard precautions, as well as vaccination of those at risk, including healthcare workers [28]. In the United States, vaccination is recommended for any persons who are at risk of exposure to an Orthopoxvirus species, including occupational exposures [29].
Surveillance for human monkeypox infections in endemic areas is a challenge. Poor infrastructure, scarce resources, inappropriate diagnostic specimens and/or lack of specimen collection, and clinical difficulties in recognizing monkeypox illness are some of the challenges encountered by surveillance systems. As more information is gained from contemporary monkeypox cases, together with the data from past efforts, it will be important to reassess the characteristics of the disease that help identify monkeypox from other rash illnesses. Current case definitions may be sensitive and broadly identify rash illnesses, but the refinement and use of a more specific case definition will provide better detection of actual monkeypox cases, aiding in patient care and isolation to prevent human-to-human transmission. Continued training of healthcare workers is needed to maintain knowledge, vigilance, and support for monkeypox surveillance. Ultimately, a broader laboratory-based surveillance network will augment our knowledge of disease burden.
Smallpox vaccination (using vaccinia virus) provides protection against Orthopoxvirus infections, including monkeypox. Smallpox vaccination ended around 1982 in DRC. As a result, (1) there is waning vaccine immunity in the individuals who were vaccinated by 1982, and (2) there are large numbers of people who have never been vaccinated and, in the absence of a previous exposure and development of immunity, are susceptible to an Orthopoxvirus infection. The question of how this changing Orthopoxvirus immunity via the absence of a vaccination will alter the incidence of human monkeypox is one that is difficult to answer but is nevertheless concerning based on the available data.
There is a wealth of human monkeypox epidemiological data from patients and their contacts in Equateur Province of DRC from 1981 to 1986, in the days following smallpox eradication. The attack rate of household members was significantly lower among those who had prior vaccination than those without vaccination. At the time of these studies, approximately 70% of all case contacts were vaccinated (3–19 years previously), and prior vaccination conferred 85% protection against monkeypox. The average annual incidence of monkeypox in the Bumba Health Zone was 0.63 per 10 000 persons [27, 30]. A more recent assessment of a cohort of patients from Sankuru District, DRC, showed a dramatic increase in average annual incidence to 5.53 per 10 000. An obvious hypothesized factor affecting this increase in incidence is the lack of vaccination; indeed, only 24% of the local population and 4% of the monkeypox patients had prior vaccination. These recent data suggest that vaccination >25 years prior may still protect individuals against an Orthopoxvirus infection and, also, that the lack of vaccination in these populations may contribute to an increased incidence of infection [31]. In the US outbreak, however, 24% (6/29) of the cases had received prior childhood smallpox vaccination, indicating that childhood vaccination was not entirely protective against disease [32]. These observations deserve further study, accounting for additional virologic, anthropologic, and ecological variables to more effectively parse the factors affecting this increase in incidence and the role of vaccination, or lack thereof.
VIRUS DIFFERENCES: WEST VS CENTRAL AFRICAN MONKEYPOX
There are 2 distinct phylogenetic clades of monkeypox viruses: those that exist in West Africa and those in Central Africa. Experience during the 2003 US outbreak with the West African clade suggested that disease severity also differed across clades [33]. There are very few documented cases of West African monkeypox: Liberia, Sierra Leone, Nigeria, and Côte d’Ivoire have each reported <10 cases between 1970 and 2005, and the US outbreak had 47 cases [17]. Generally, West African monkeypox infections exhibit a less severe illness in humans and nonhuman primates [5, 33, 34]. The US outbreak had a number of hospitalized patients and severe disease, but no fatalities [35].
Genome comparisons of West and Central African strains yielded a set of candidate genes that may be involved in the differentiating clade virulence. These open reading frames are predicted to be involved in alterations to the viral life cycle, host range, or immune evasion, or are virulence factors [17]. Central African monkeypox prevents T-cell receptor–mediated T-cell activation, prohibiting inflammatory cytokine production in human cells derived from previously infected monkeypox patients. These results suggest that monkeypox may produce a modulator that suppresses host T-cell responses [36]. Several immune evasion candidates have been identified in Central African monkeypox virus [17].
The monkeypox virus inhibitor of complement enzymes, a gene that inhibits complement enzymes and is absent in West African strains, has been implicated as an important immune-modulating factor contributing to the increased virulence of Central African strains [37, 38]. Additionally, Central African monkeypox strains selectively downregulate host responses compared to West African strains, specifically apoptosis in the host [39]. Multiple loci may be involved in the observed pathogenicity differences [17, 34, 38, 39]. Furthermore, transcriptional studies have shown that Central African monkeypox appears to selectively silence transcription of genes involved in host immunity during an infection [40]. Determining the range of effects produced with these different viruses will require a multifaceted effort.
THERAPEUTICS AND VACCINES
Several compounds have shown promise as antiviral therapeutics against Orthopoxvirus species; 3 of the most promising compounds are summarized in Table 3. Cidofovir has antiviral activity against a variety of viruses by inhibiting viral DNA polymerase. CMX-001 is a modified cidofovir compound that lacks the extent of nephrotoxicity seen with cidofovir. Antiviral activity of CMX-001 has been demonstrated with a variety of Orthopoxvirus species. The drug ST-246 blocks the release of the intracellular virus from the cell, and has shown promising results against a variety of Orthopoxvirus species, including variola virus [41]. These compounds have been used in varying combinations, also with vaccinia immune globulin, investigationally, to treat severe vaccine-associated adverse events [42, 43]. Development of strategies to use these drugs in endemic areas to treat disease will need to be considered.
Table 3.
Promising Therapeutics for the Treatment of Orthopoxvirus Infections
| Antiviral Therapeutic | Mechanism of Action | Clinical Considerations | Stage of Development or Use |
|---|---|---|---|
| Cidofovir | Inhibits DNA polymerase | Intravenous administration with hydration and probenecid; nephrotoxicity has been seen | Licensed for the use of cytomegalovirus retinitis in AIDS patients. Has been used to treat other poxvirus infections (molluscum contagiosum and orf virus). |
| CMX-001 | Modified cidofovir compound; inhibits DNA polymerase | Lacks nephrotoxicity seen with cidofovir; oral administration | In development. |
| ST-246 | Inhibits release of intracellular virus | Oral administration | Is maintained in the United States in the Strategic National Stockpile. Available for other Orthopoxvirus infections under an investigational protocol |
Smallpox vaccines, comprised of fully replicative vaccinia virus, are currently not in use in monkeypox-endemic areas given concerns about severe adverse events in a population with an uncertain immunocompromised profile. The risk of pathogenic monkeypox disease must be balanced with the risk of adverse events from replicative vaccines such as ACAM 2000 (Table 4) [29]. An ideal vaccine for use in monkeypox-endemic areas would be one that does not have these risk groups and could be administered readily to children, as well [17]. There is no vaccination that meets all of these criteria, but some next-generation vaccines take one step closer to reaching that goal (Table 4).
Table 4.
Smallpox Vaccines
| Vaccine | Pros | Cons | Stage of Development or Use |
|---|---|---|---|
| ACAM2000: Live vaccinia virus | Single-dose administration. A successful take is noted by observation of a lesion at the vaccination site. Lyophilized preparation for long-term storage. | Live viral vaccine that replicates in mammalian cells; autoinoculation and contact transmission are risks. In low- disease-risk situations, should not be used for individuals with immunocompromising conditions, history of eczema or atopic dermatitis, or pregnant females. Cardiac events postvaccination have been noted to occur. | Licensed vaccination in the United States. Currently available to specific populations from the Strategic National Stockpile. |
| Modified vaccinia Ankara; IMVAMUNE (US); IMVANEX (Europe): Attenuated vaccinia virus | The virus has limited replication in mammalian cells. No lesion produced at the vaccination site. | Two-dose administration by injection. | European Commission has authorized marketing for immunization of the general adult population, including those who are immunocompromised. Maintained in the United States’ Strategic National Stockpile. |
| LC16m8: Attenuated vaccinia virus | Single-dose administration. Exhibits a safer profile and less adverse events than ACAM2000 in human and animal vaccinations. | Attenuated virus that can still replicate in mammalian cells. | Licensed for use in Japan. |
Modified vaccinia Ankara (MVA) is an attenuated vaccinia virus that cannot achieve complete replication in mammalian cells. MVA has shown protection in primate models challenged with lethal doses of monkeypox virus [44–46]. However, this vaccine has not conferred protection in primates with severely diminished T-cell function [47]. LC16m8 is another vaccine that has been altered to prevent viral replication and has shown protection against severe monkeypox illness in nonhuman primates [48]. LC16m8 was used to vaccinate >50 000 schoolchildren in Japan with few reported adverse events [49].
CONCLUSIONS
Human monkeypox has the potential for spread via zoonotic reservoirs, as was demonstrated by the US outbreak. Civil conflict and displacements cause concerns for movement of the virus into an area without monkeypox [50, 51], or movement of individuals to more heavily forested areas more prone for interaction with wildlife and a range of zoonoses. The documented rise in incidence of human disease needs further evaluation and consideration with additional studies to better understand the range of factors involved in disease transmission and spread. There are still many unanswered questions about human disease, animal reservoirs, and the virus itself—advances in our understanding of this important zoonosis will help better guide prevention strategies and mitigate human disease.
Acknowledgments
The authors appreciate comments by anonymous reviewers that improved this manuscript.
Financial support. This work was supported by the Centers for Disease Control and Prevention.
Footnotes
Clinical Infectious Diseases
Published by Oxford University Press on behalf of the Infectious Diseases Society of America 2013. This work is written by (a) US Government employee(s) and is in the public domain in the US.
Disclaimer. The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Potential conflicts of interest. Both authors: No reported conflicts.
Both authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Ladnyj ID, Ziegler P, Kima E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull World Health Organ. 1972;46:593–7. [PMC free article] [PubMed] [Google Scholar]
- 2.Breman JG, Henderson DA. Diagnosis and management of smallpox. N Engl J Med. 2002;346:1300–8. doi: 10.1056/NEJMra020025. [DOI] [PubMed] [Google Scholar]
- 3.Breman JG, Kalisa R, Steniowski MV, Zanotto E, Gromyko AI, Arita I. Human monkeypox, 1970–79. Bull World Health Organ. 1980;58:165–82. [PMC free article] [PubMed] [Google Scholar]
- 4.Jezek Z, Fenner F. Human monkeypox. New York: Karger; 1988. [Google Scholar]
- 5.Damon IK. Status of human monkeypox: clinical disease, epidemiology and research. Vaccine. 2011;29(suppl 4):D54–9. doi: 10.1016/j.vaccine.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Di Giulio DB, Eckburg PB. Human monkeypox: an emerging zoonosis. Lancet Infect Dis. 2004;4:15–25. doi: 10.1016/S1473-3099(03)00856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jezek Z, Szczeniowski M, Paluku KM, Mutombo M. Human monkeypox: clinical features of 282 patients. J Infect Dis. 1987;156:293–8. doi: 10.1093/infdis/156.2.293. [DOI] [PubMed] [Google Scholar]
- 8.Learned LA, Reynolds MG, Wassa DW, et al. Extended interhuman transmission of monkeypox in a hospital community in the Republic of the Congo, 2003. Am J Trop Med Hyg. 2005;73:428–34. [PubMed] [Google Scholar]
- 9.Macneil A, Reynolds MG, Braden Z, et al. Transmission of atypical varicella-zoster virus infections involving palm and sole manifestations in an area with monkeypox endemicity. Clin Infect Dis. 2009;48:e6–8. doi: 10.1086/595552. [DOI] [PubMed] [Google Scholar]
- 10.Seward JF, Galil K, Damon I, et al. Development and experience with an algorithm to evaluate suspected smallpox cases in the United States, 2002–2004. Clin Infect Dis. 2004;39:1477–83. doi: 10.1086/425500. [DOI] [PubMed] [Google Scholar]
- 11.Kulesh DA, Loveless BM, Norwood D, et al. Monkeypox virus detection in rodents using real-time 3′-minor groove binder TaqMan assays on the Roche LightCycler. Lab Invest. 2004;84:1200–8. doi: 10.1038/labinvest.3700143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Olson VA, Laue T, Laker MT, Damon IK. Detection of monkeypox virus with real-time PCR assays. J Clin Virol. 2006;36:194–203. doi: 10.1016/j.jcv.2006.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olson VA, Laue T, Laker MT, et al. Real-time PCR system for detection of orthopoxviruses and simultaneous identification of smallpox virus. J Clin Microbiol. 2004;42:1940–6. doi: 10.1128/JCM.42.5.1940-1946.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shchelkunov SN, Shcherbakov DN, Maksyutov RA, Gavrilova EV. Species-specific identification of variola, monkeypox, cowpox, and vaccinia viruses by multiplex real-time PCR assay. J Virol Methods. 2011;175:163–9. doi: 10.1016/j.jviromet.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karem KL, Reynolds M, Braden Z, et al. Characterization of acutephase humoral immunity to monkeypox: use of immunoglobulin M enzyme-linked immunosorbent assay for detection of monkeypox infection during the 2003 North American outbreak. Clin Diagn Lab Immunol. 2005;12:867–72. doi: 10.1128/CDLI.12.7.867-872.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Townsend MB, Macneil A, Reynolds MG, et al. Evaluation of the Tetracore Orthopox BioThreat® antigen detection assay using laboratory grown orthopoxviruses and rash illness clinical specimens. J Virol Methods. 2013;187:37–42. doi: 10.1016/j.jviromet.2012.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reynolds MG, Damon IK. Outbreaks of human monkeypox after cessation of smallpox vaccination. Trends Microbiol. 2012;20:80–7. doi: 10.1016/j.tim.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Formenty P, Muntasir MO, Damon I, et al. Human monkeypox outbreak caused by novel virus belonging to Congo Basin clade, Sudan, 2005. Emerg Infect Dis. 2010;16:1539–45. doi: 10.3201/eid1610.100713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berthet N, Nakoune E, Whist E, et al. Maculopapular lesions in the Central African Republic. Lancet. 2011;378:1354. doi: 10.1016/S0140-6736(11)61142-2. [DOI] [PubMed] [Google Scholar]
- 20.Lash RR, Carroll DS, Hughes CM, et al. Effects of georeferencing effort on mapping monkeypox case distributions and transmission risk. Int J Health Geogr. 2012;11:23. doi: 10.1186/1476-072X-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellis CK, Carroll DS, Lash RR, et al. Ecology and geography of human monkeypox case occurrences across Africa. J Wildl Dis. 2012;48:335–47. doi: 10.7589/0090-3558-48.2.335. [DOI] [PubMed] [Google Scholar]
- 22.Reed KD, Melski JW, Graham MB, et al. The detection of monkeypox in humans in the Western Hemisphere. N Engl J Med. 2004;350:342–50. doi: 10.1056/NEJMoa032299. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease C, Prevention. Update: multistate outbreak of monkeypox—Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:642–6. [PubMed] [Google Scholar]
- 24.Khodakevich L, Jezek Z, Kinzanzka K. Isolation of monkeypox virus from wild squirrel infected in nature. Lancet. 1986;1:98–9. doi: 10.1016/S0140-6736(86)90748-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reynolds MG, Carroll DS, Karem KL. Factors affecting the likelihood of monkeypox’s emergence and spread in the post-smallpox era. Curr Opin Virol. 2012;2:335–43. doi: 10.1016/j.coviro.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hutson CL, Olson VA, Carroll DS, et al. A prairie dog animal model of systemic orthopoxvirus disease using West African and Congo Basin strains of monkeypox virus. J Gen Virol. 2009;90(Pt 2):323–33. doi: 10.1099/vir.0.005108-0. [DOI] [PubMed] [Google Scholar]
- 27.Jezek Z, Grab B, Szczeniowski MV, Paluku KM, Mutombo M. Human monkeypox: secondary attack rates. Bull World Health Organ. 1988;66:465–70. [PMC free article] [PubMed] [Google Scholar]
- 28.Fleischauer AT, Kile JC, Davidson M, et al. Evaluation of human-to-human transmission of monkeypox from infected patients to health care workers. Clin Infect Dis. 2005;40:689–94. doi: 10.1086/427805. [DOI] [PubMed] [Google Scholar]
- 29.Vaccinia (smallpox) vaccine recommendations of the Advisory Committee on Immunization Practices (ACIP), 2001. MMWR Morb Mortal Wkly Rep. 2001;50(RR10):1–25. [PubMed] [Google Scholar]
- 30.Fine PE, Jezek Z, Grab B, Dixon H. The transmission potential of mon-keypox virus in human populations. Int J Epidemiol. 1988;17:643–50. doi: 10.1093/ije/17.3.643. [DOI] [PubMed] [Google Scholar]
- 31.Rimoin AW, Mulembakani PM, Johnston SC, et al. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc Natl Acad Sci U S A. 2010;107:16262–7. doi: 10.1073/pnas.1005769107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karem KL, Reynolds M, Hughes C, et al. Monkeypox-induced immunity and failure of childhood smallpox vaccination to provide complete protection. Clin Vaccine Immunol. 2007;14:1318–27. doi: 10.1128/CVI.00148-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Likos AM, Sammons SA, Olson VA, et al. A tale of two clades: monkey-pox viruses. J Gen Virol. 2005;86(Pt 10):2661–72. doi: 10.1099/vir.0.81215-0. [DOI] [PubMed] [Google Scholar]
- 34.Saijo M, Ami Y, Suzaki Y, et al. Virulence and pathophysiology of the Congo Basin and West African strains of monkeypox virus in nonhuman primates. J Gen Virol. 2009;90(Pt 9):2266–71. doi: 10.1099/vir.0.010207-0. [DOI] [PubMed] [Google Scholar]
- 35.Reynolds MG, Yorita KL, Kuehnert MJ, et al. Clinical manifestations of human monkeypox influenced by route of infection. J Infect Dis. 2006;194:773–80. doi: 10.1086/505880. [DOI] [PubMed] [Google Scholar]
- 36.Hammarlund E, Dasgupta A, Pinilla C, Norori P, Fruh K, Slifka MK. Monkeypox virus evades antiviral CD4+ and CD8+ T cell responses by suppressing cognate T cell activation. Proc Natl Acad Sci U S A. 2008;105:14567–72. doi: 10.1073/pnas.0800589105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Estep RD, Messaoudi I, O’Connor MA, et al. Deletion of the monkey-pox virus inhibitor of complement enzymes locus impacts the adaptive immune response to monkeypox virus in a nonhuman primate model of infection. J Virol. 2011;85:9527–42. doi: 10.1128/JVI.00199-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hudson PN, Self J, Weiss S, et al. Elucidating the role of the complement control protein in monkeypox pathogenicity. PLoS One. 2012;7:e35086. doi: 10.1371/journal.pone.0035086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kindrachuk J, Arsenault R, Kusalik A, et al. Systems kinomics demonstrates Congo Basin monkeypox virus infection selectively modulates host cell signaling responses as compared to West African monkeypox virus. Mol Cell Proteomics. 2012;11:M111 015701. doi: 10.1074/mcp.M111.015701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rubins KH, Hensley LE, Relman DA, Brown PO. Stunned silence: gene expression programs in human cells infected with monkeypox or vaccinia virus. PLoS One. 2011;6:e15615. doi: 10.1371/journal.pone.0015615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parker S, Handley L, Buller RM. Therapeutic and prophylactic drugs to treat orthopoxvirus infections. Future Virol. 2008;3:595–612. doi: 10.2217/17460794.3.6.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lederman ER, Davidson W, Groff HL, et al. Progressive vaccinia: case description and laboratory-guided therapy with vaccinia immune globulin, ST-246, and CMX001. J Infect Dis. 2012;206:1372–85. doi: 10.1093/infdis/jis510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vora S, Damon I, Fulginiti V, et al. Severe eczema vaccinatum in a household contact of a smallpox vaccinee. Clin Infect Dis. 2008;46:1555–61. doi: 10.1086/587668. [DOI] [PubMed] [Google Scholar]
- 44.Earl PL, Americo JL, Wyatt LS, et al. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature. 2004;428:182–5. doi: 10.1038/nature02331. [DOI] [PubMed] [Google Scholar]
- 45.Earl PL, Americo JL, Wyatt LS, et al. Rapid protection in a monkeypox model by a single injection of a replication-deficient vaccinia virus. Proc Natl Acad Sci U S A. 2008;105:10889–94. doi: 10.1073/pnas.0804985105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stittelaar KJ, van Amerongen G, Kondova I, et al. Modified vaccinia virus Ankara protects macaques against respiratory challenge with monkeypox virus. J Virol. 2005;79:7845–51. doi: 10.1128/JVI.79.12.7845-7851.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Edghill-Smith Y, Bray M, Whitehouse CA, et al. Smallpox vaccine does not protect macaques with AIDS from a lethal monkeypox virus challenge. J Infect Dis. 2005;191:372–81. doi: 10.1086/427265. [DOI] [PubMed] [Google Scholar]
- 48.Saijo M, Ami Y, Suzaki Y, et al. LC16m8, a highly attenuated vaccinia virus vaccine lacking expression of the membrane protein B5R, protects monkeys from monkeypox. J Virol. 2006;80:5179–88. doi: 10.1128/JVI.02642-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kenner J, Cameron F, Empig C, Jobes DV, Gurwith M. LC16m8: an attenuated smallpox vaccine. Vaccine. 2006;24:7009–22. doi: 10.1016/j.vaccine.2006.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reynolds MG, Emerson GL, Pukuta E, et al. Detection of human monkeypox in the Republic of the Congo following intensive community education. Am J Trop Med Hyg. 2013;88:982–5. doi: 10.4269/ajtmh.12-0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakazawa Y, Emerson GL, Carroll DS, et al. Phylogenetic and ecologic perspectives of a monkeypox outbreak, southern Sudan, 2005. Emerg Infect Dis. 2013;19:237–45. doi: 10.3201/eid1902.121220. [DOI] [PMC free article] [PubMed] [Google Scholar]


