Supplemental Digital Content is Available in the Text.
Gouty pain nocifensive signs and enhanced joint nociceptor nerve activity in urate-injected rats develop in parallel and are decreased by intra-articular injection of hyaluronan.
Keywords: Gout, Arthritis, Hyaluronan, Nociceptors, Knee-joint inflammation, TRP channels
Abstract
The mechanisms whereby deposition of monosodium urate (MSU) crystals in gout activates nociceptors to induce joint pain are incompletely understood. We tried to reproduce the signs of painful gouty arthritis, injecting into the knee joint of rats suspensions containing amorphous or triclinic, needle MSU crystals. The magnitude of MSU-induced inflammation and pain behavior signs were correlated with the changes in firing frequency of spontaneous and movement-evoked nerve impulse activity recorded in single knee joint nociceptor saphenous nerve fibers. Joint swelling, mechanical and cold allodynia, and hyperalgesia appeared 3 hours after joint injection of MSU crystals. In parallel, spontaneous and movement-evoked joint nociceptor impulse activity raised significantly. Solutions containing amorphous or needle-shaped MSU crystals had similar inflammatory and electrophysiological effects. Intra-articular injection of hyaluronan (HA, Synvisc), a high-MW glycosaminoglycan present in the synovial fluid with analgesic effects in osteoarthritis, significantly reduced MSU-induced behavioral signs of pain and decreased the enhanced joint nociceptor activity. Our results support the interpretation that pain and nociceptor activation are not triggered by direct mechanical stimulation of nociceptors by MSU crystals, but are primarily caused by the release of excitatory mediators by inflammatory cells activated by MSU crystals. Intra-articular HA decreased behavioral and electrophysiological signs of pain, possibly through its viscoelastic filtering effect on the mechanical forces acting over sensitized joint sensory endings and probably also by a direct interaction of HA molecules with the transducing channels expressed in joint nociceptor terminals.
1. Introduction
Gout is a disease caused by the deposition of monosodium urate (MSU) crystals on the surface of the joint cartilage and periarticular structures, leading to acute attacks of usually very painful arthritis. Notably, at intercritical periods, the disease is asymptomatic, despite the regular presence of needle-shaped urate crystals in the joint structures and synovial fluid (SF).38,40 This observation raises questions about the mechanisms whereby sodium urate induces the intense joint pain observed during gout flares and the contribution to this pain of a direct mechanical stimulation of synovial nociceptor sensory nerve terminals by the MSU crystals, besides the well-established interactions of MSU crystals with mononuclear phagocytes and neutrophils.10,45 These interactions activate the inflammasome, with the production of multiple proinflammatory products such as lysosomal enzymes, oxygen-derived free radicals, eicosanoids and cytokines, as well as oxidative stress and cell damage.29,31,53 Proinflammatory substances act on the various ion channel types involved in stimulus transduction and impulse coding by joint nociceptors,22 either opening them directly or modulating their opening probability.21,24,32,54,55 The final consequence is augmented impulse firing by joint nociceptors leading to spontaneous pain and mechanical and thermal hyperalgesia.33,49,50 However, the translation of the complex response triggered by MSU crystals in the joint into an excitation of joint nociceptor nerve terminals evoking gouty pain is still incompletely understood.
Hyaluronan (sodium hyaluronate, HA), a glycosaminoglycan polymer present in synovial membranes and SF, has been postulated as a lubricating agent and protective rheological buffer, reducing the force transmitted by movements to joint nociceptor nerve endings.3,19,20,46 In osteoarthritis and in joint inflammatory processes, the viscosity, concentration, and molecular weight (MW) of HA contained in the SF seem to be markedly reduced.3 This is also the case for the HA present in the SF of symptomatic gout patients.47 Notably, restoration in osteoarthritic patients of SF rheological properties by intra-articular injection of high molecular weight hyaluronan (HMW-HA) alleviates pain.1,4,12 This pain reduction is apparently due to the attenuation by HA of the augmented nerve impulse discharges generated at sensitized joint nociceptor fibers.3,19,20,46 There is experimental evidence that HMW-HA molecules dampen the transmission of external mechanical forces to stretch-activated channels of the cell membrane,43 but recent data also indicate that HA reduces directly the opening probability of TRPV1 channels of joint nociceptor fibers,8 thus acting on the main final molecular target for many of the inflammatory mediators released in gout.9,14,26,27
In this study, we analyzed the time course and characteristics of knee joint pain behavior associated with knee-joint inflammation evoked by intra-articular injection of MSU crystals of different size and shape in rats, and studied the correlation between inflammatory signs and the changes in spontaneous and movement-evoked nerve impulse activity in knee-joint nociceptor fibers. We also used this experimental model of gouty arthritis to explore whether intra-articular injection of HMW-HA attenuates MSU-induced enhancement of joint nociceptor activity as occurs in osteoarthritis.
2. Methods
2.1. Animals
The study was performed in adult male Wistar rats weighing 300 to 350 g, housed singly in cages in sanitary ventilated animal rooms with controlled temperature (20°C), humidity (45%), and maintained on ad libitum food and water supply with 12 hours of light/dark cycles. All experimental procedures were performed according to the Spanish Royal Decree 53/2013 and the European Community Council directive 2010/63/EU. The Ethics Committee from Universidad Miguel Hernández, Alicante, Spain, approved this study.
2.2. Reagents
4.5 mg of MSU, purchased from Sigma (C5H3N4O3Na, MW = 190.1 g/mol; St Louis, MO), was sterilized by UV light exposition during 1 hour and suspended afterward in sterile phosphate buffered saline (PBS) from Sigma (St Louis, MO). Monosodium urate suspensions prepared using Sigma's product appeared under the microscope as aggregates of amorphous, small crystals.
Monosodium urate triclinic crystals were purchased from InvivoGen (C5H3N4O3Na, MW = 190.1 g/mol; San Diego, CA). Suspensions of these crystals from InvivoGen were prepared in sterile PBS. Under the microscope, the appearance of these crystals is, as the conventional, large needle-shaped crystals seen in gouty SF.
A sterile HMW-HA solution, commercially manufactured for human use as Synvisc (Sanofi Genzyme Biosurgery, Cambridge, MA) was purchased. Synvisc contains 80% (vol/vol) of Hylan A (MW ∼6 × 106) and 20% (vol/vol) of Hylan B (vinyl sulfone cross-linked Hylan A) in 0.9% NaCl.
2.3. Inflammation of the knee joint
Sterile suspensions of MSU (4.5 mg in 50 μL PBS) containing either small and amorphous crystals (Sigma) or needle, aciculate, triclinic crystals (InvivoGen) were injected into the right knee joint of isoflurane-anesthetized rats. Rats of control groups received an intra-articular injection of the vehicle alone (50 μL of PBS).
2.4. Measurement of knee diameter
The mediolateral diameter of the knee joints was measured using an electronic digital micrometer. The relationship between the diameter values of treated joints, related to their value before treatment was used as an index of knee-joint edema.
2.5. Behavioral assays
Rats were habituated to the behavior room for a minimum of 3 hours before testing, and to the testing chamber for at least 1 hour before testing. The same investigator performed the scoring in all behavioral tests, which were blind in respect to the type of intra-articular solution injected. Animal's groups were also randomized.
2.5.1 Weight-bearing measurements
Pain related to knee-joint inflammation was assessed measuring right and left hind limb weight distribution using an incapacitance tester device (Bioseb In vivo Research Instruments, Vitrolles, France). Rats were placed in an angled chamber positioned so that each hind paw rested on a horizontal force-transducing plate. The weight (g) borne by each hind limb was averaged over 5 seconds; 3 readings were taken and mean values then calculated. The hind limb weight-bearing behavior was measured on the same animals repeatedly along the course of the study. Results are expressed as % of asymmetry = weight in contralateral limb − weight in ipsilateral limb/total weight on both limbs × 100. Presence of asymmetry was considered a sign of hyperalgesia.
2.5.2. Mechanical threshold
Changes in hind paw withdrawal thresholds were assessed using mechanical stimulation with calibrated von Frey filaments BIO-VF-M model from Bioseb (Bioseb In vivo Research Instruments, France). Thresholds were determined using a modified version of Dixon up–down method.11 Rats were placed in transparent plastic cylinders on a metal mesh platform (2 × 1.5 mm) and von Frey filaments were applied to the plantar surface of a hind paw for up to 1 second. Brisk withdrawal of the hind paw during or immediately after application was considered a positive response. The threshold force required to elicit withdrawal (50% hind paw withdrawals) was determined for left and right hind paws. The threshold force data are presented as the difference with baseline values, with negative value indicating mechanical allodynia. Threshold values were assessed in rats before (baseline) and 3, 5, and 8 hours after injection in the right knee joint of MSU or vehicle (PBS) solutions. In a different group of rats, after baseline values were obtained, the corresponding MSU solution was injected in the right knee joint and mechanical threshold evaluated 3 hours later. Immediately afterward, an injection of Synvisc was performed and mechanical threshold evaluated again 2 and 5 hours later. In these animals, change in the von Frey threshold is expressed as the difference between threshold values after treatment minus baseline threshold.
2.5.3. Acetone testing
To test acetone-evoked evaporative cooling-induced sensations, rats were placed within a round plastic chamber on a metal mesh platform. A drop (100 μL) of acetone was applied sequentially onto the plantar surface of both left and right hind paws, and the number of lifting, licking, biting, shaking, and guarding nocifensive events was counted over the next 60 seconds. The number of events counted after treatment was divided by the number before treatment (baseline value); thus, a quotient value close to 1 indicates no difference with the baseline situation, whereas a higher value reflected cold allodynia.
2.6. Air pouch model
The rat air pouch model of synovial cavity was used.6 Male Wistar rats (300 g) were anaesthetized using isoflurane and injected subcutaneously with 20 mL of sterile air (gauge syringe with 0.22-μm filter) on the dorsal surface, just behind the scapula. The procedure was repeated 3 and 6 days after the first air injection. Then, rats with air pouches were anaesthetized using isoflurane and an 18-G cannula was inserted into the air pouch to inject 5 mL of a 1-mg/mL solution of amorphous or needle-shaped MSU crystals, or PBS alone. A 400-μL sample of air pouch fluid was removed immediately and 5 hours after injection, and collected into ethylenediaminetetraacetic acid (10 mM final concentration). Fifty microliters of this sample were used for flow cytometry.
2.7. Analysis of exudate cells by flow cytometry
Flow cytometry was performed using a FACSAria II flow cytometer. CD45+ antibody (FITC Mouse Anti-Rat CD45; BD Pharmingen, San Jose, CA) was used to identify leukocyte population. From samples collected at 0 and 5 hours, 50 μL were centrifuged for 10 minutes at 330 × g and then resuspended in 99 μL of FACS buffer (PBS+ 0.5% bovine serum albumin+ 2-mM ethylenediaminetetraacetic acid). Cells were treated for 10 minutes at 4°C with 1-μL CD45-antibody (0.5 mg/mL). Then, 1 mL of FACS buffer was added and the suspension centrifuged for 10 minutes at 330 × g. The supernatant was removed and cells resuspended in 200 μL of FACS buffer for the analysis by flow cytometry. CD45 (leukocyte common antigen) is ubiquitously expressed in all nucleated hematopoietic cells excluding erythrocytes.
2.8. Electrophysiological recordings in rat knee-joint afferent fibers
The detailed procedure for recording of joint nociceptor fibers in vivo has been reported previously.18 Animals were initially anaesthetized using ketamine (75 mg/kg) and xylazine (10 mg/kg) (intraperitoneally) followed by an injection of 40 mg/kg (i.p.) of sodium pentobarbital for deep anesthesia. Supplementary doses of sodium pentobarbital were injected intraperitoneally when required. The trachea, the left femoral vein, and femoral artery were cannulated. Body temperature was maintained at physiological levels. Heart frequency and blood pressure values were continuously monitored to evaluate the anesthesia level. The right femur was fixed by a special grip, and a pool was formed by skin flaps and filled with warm paraffin oil. The saphenous nerve from the right leg was cut and dissected. Fine filaments were split from the peripheral end and placed over a silver wire electrode for extracellular recording, until a functional single unit was obtained. The receptive fields of knee-joint afferent units were identified and located by probing the tissue over the knee joint and its surroundings with a hand-held glass rod. Thereafter, the receptive field of the nerve fiber was electrically stimulated (5-15 V for 0.5 ms) to calculate the conduction velocity from the latency of the evoked impulse and the distance measured between the stimulating and recording electrodes.
Mechanical stimulation was performed every 5 minutes and started from the middle flexed (resting) position of the joint and consisted of a passive, manually performed 10-second outward rotation (OR) within the working range of the joint (10-20 mNm, innocuous OR) followed by an OR of 10-second duration, which exceeded the normal working range of the joint (40-60 mNm, noxious outward rotation, NOR). Thereafter, the joint was returned to the resting position for 10 seconds and the same 2 steps were performed using inward rotations (innocuous, IR and noxious, NIR). Rotation to 10 to 20 mN was considered innocuous because at this intensity, the hind limb could be rotated to the end of the normal movement range without appreciable force. Rotation to 40 mNm was considered noxious because these rotations were performed against the resistance of the joint structures.18–20,51,52
The discharges recorded during the movements were analyzed by counting the total number of impulses obtained during the complete cycle ie, OR + NOR + IR + NIR. Also, the number of impulses evoked by each of these 4 movements was counted separately. The impulses recorded during the nonnoxious and noxious movements (outward and inward movements) were added separately (OR + IR and NOR + NIR).
Movement-evoked activity varied widely among individual fibers because of their different exposure to mechanical stress depending on the location of the receptive field in the joint. For this reason, impulse responses were normalized taking the preinjection values of activity as 100% response (control) and expressing the effects of experimental maneuvers as percentage of this value.
2.9. Statistical analysis
Statistical comparisons were made using GraphPad Prism. We used the paired t test to compare changes in the animals before and after treatments and unpaired t test for comparison between animal groups, as indicated.
3. Results
3.1. Intra-articular injection of monosodium urate crystals induces knee-joint inflammation and behavioral signs of pain
Three hours after intra-articular injection of MSU solutions containing either amorphous (n = 10) or needled crystals (n = 7), the joint volume had increased, as reflected in the significantly larger joint diameter that persisted 5 and 8 hours later, in contrast with rats injected with PBS (n = 12) where no joint diameter change was observed (Figs. 1B and C). Amorphous crystals produced a significantly larger diameter increase than needle crystals (Δ = 1.9 ± 0.3, n = 10 and Δ = 1.01 ± 0.3, n = 8, respectively, Student t test P = 0.04*). Weight-bearing asymmetry between hind limbs was significantly larger in MSU-traded animals but did not differ with the type of crystals injected (Fig. 1D). Before MSU injection, body weight distribution between legs was very similar (mean baseline asymmetry 2 ± 1% n = 10), whereas 3 hours after injection of both types of MSU crystals, asymmetry raised around 30%, ie, approximately 70% of the weight was now supported by the noninjected hind limb (see methods); a modest recovery was observed 5 and 8 hours later (Fig. 1D). In Supplementary Figure 1, results were represented using the raw data (available online as supplemental digital content at http://links.lww.com/PAIN/A524).
Figure 1.
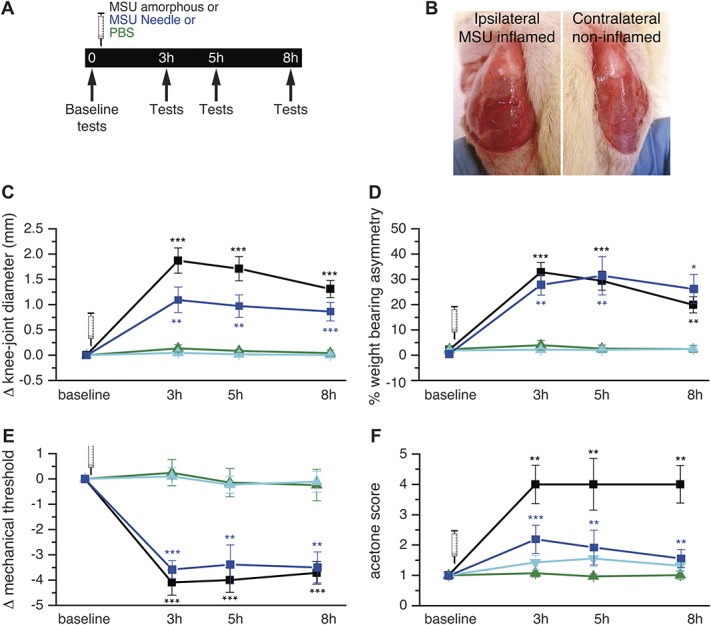
Nocifensive responses in rats evoked by intra-articular injection of MSU crystals. (A) Timeline of behavioral experiments after injection of amorphous or needle MSU crystals or PBS into the right knee joint; arrows signal the time at which experimental measures were performed. (B) Images of the knee joints exposed by removal of the skin, in the MSU-injected (left panel) and the contralateral, non-injected (right panel) hind limb. (C-F) Change in knee-joint diameter (C), % weight-bearing asymmetry (D), von Frey mechanical threshold (E) and cold (acetone) sensitivity (F), measured 3, 5, and 8 hours after ipsilateral injection of: amorphous MSU (black, n = 10); needle MSU (blue, n = 7); PBS (green, n = 12), and in untreated joint (cyan, n = 12). Paired t test ***P < 0.001, **P < 0.01 with baseline values. MSU, monosodium urate; PBS, phosphate buffered saline.
In the same groups of rats, von Frey mechanical threshold was measured in the paw of the injected limb. Three hours after intra-articular injection of urate crystals, mechanical threshold was significantly lower, but not influenced by the type of crystal (amorphous crystals 1.4 ± 0.5 g, baseline value = 5.5 ± 1.1 g, n = 7, needle crystals 1.0 ± 0.2, baseline value = 4.8 ± 0.5, n = 7), and it was smaller than in rats injected with PBS alone (Fig. 1E). The reduction in mechanical threshold reflecting mechanical allodynia persisted 5 and 8 hours after MSU injection. These rats also exhibited an enhanced sensibility to acetone-induced cooling evoked in the paw of the injected side (cold allodynia). Three hours after MSU injection, the response was 2 (needle) and 4 (amorphous) times higher than before MSU, or than after injection of PBS (Fig. 1F). Sensitization to cold persisted 8 hours after injection of MSU amorphous crystals, whereas when needle crystals were injected, a modest recovery was observed 5 and 8 hours later. Mechanical and thermal sensitivity at the untreated, contralateral hind limb did not change after the ipsilateral MSU injection (data not shown).
The level of inflammation induced by MSU amorphous and needle crystals was evaluated as the number and profile of leukocytes measured by flow cytometry in the exudate recruited in the air pouch gout model. Five hours after injection of either amorphous or needle-shaped MSU crystals, forward scatter and side scatter revealed the appearance of the 3 CD45 positive cell populations corresponding to lymphocyte, macrophage, and granulocyte. PBS evoked a much smaller cellular recruitment response, in which granulocytes were absent (Supplementary Figure 2, available online as supplemental digital content at http://links.lww.com/PAIN/A524).
3.2. Movement-evoked nerve impulse activity in knee-joint sensory fibers increases after monosodium urate injection
Activity in 25 single units obtained from saphenous nerve filaments of 24 rats was recorded. All units included in this study responded in a variable degree to both nonnoxious and noxious movements of the knee joint (see methods). Conduction velocity measured at the end of the experiment in 10 units ranged between 0.7 and 5.25 m/s, (average 2.16 ± 0.39 m/s).
Figure 2A shows an example of the nerve impulse firing and the corresponding instantaneous frequency change in a single joint nerve fiber, evoked by mechanical stimulation before (control) and 3 hours after intra-articular injection of a MSU amorphous crystals solution (intra-articular MSU). Rotations within the normal working range (nonnoxious OR and IR) generated an impulse activity that increased markedly when rotation exceeded the working range (noxious outward rotation, NOR and noxious inward rotation, NIR). The firing responses to movement were clearly enhanced 3 hours after intra-articular MSU crystals injection.
Figure 2.

Sensitization of knee-joint sensory fibers by MSU-evoked inflammation. (A) Sample recordings of nerve impulse activity produced by a single fiber innervating the knee joint, before (upper panel) and after (middle panel) intra-articular amorphous MSU injection. Impulse activity is displayed as instantaneous frequency (dots, top) and as the original nerve impulse records (spikes, bottom). The lower panel depicts the sequence of nonnoxious (OR and IR) and noxious (NOR and NIR) knee-joint rotations. (B) Mean value of the total number of movement-evoked impulses (OR + NOR + IR + NIR) measured before and after intra-articular injection of amorphous MSU (black symbols, n = 5), MSU needle crystals (blue symbols, n = 8), or PBS (green symbols, n = 7). Values are expressed as percentage of the average control response (mean of the 6 movements, before injection, indicated by the arrows). (C) Mean values of movement-evoked impulse activity in joints injected with PBS (green columns), needle MSU (blue columns), or amorphous MSU (black columns). The first column (control) shows the mean value of the 6 consecutive movements preceding intra-articular injections; the remaining columns show the mean value of impulse discharges evoked by 12 consecutive movements measured 1, 2, and 3 hours after intra-articular injection of PBS or MSU. Paired and unpaired t test, *P < 0.05, **P < 0.01, ***P < 0.001. IR, inward rotation; MSU, monosodium urate; NIR, noxious inward rotation; NOR, noxious outward rotation; OR, outward rotation; PBS, phosphate buffered saline.
Figure 2B represents the values of movement-evoked impulse responses, obtained by pooling the data of all the explored units, after injection of amorphous (n = 5, black symbols) and needle (n = 8, blue symbols) crystals or PBS (n = 7, green symbols), to illustrate the gradual rise with time of the movement-evoked mean impulse activity in MSU-treated rats in contrast with PBS-injected animals. In Figure 2C, we represented the mean values of the impulse activity evoked by 12 movements (60 minutes), measured 1, 2, and 3 hours after PBS (green bars), amorphous (black bars), and needle MSU crystals (blue bars), and expressed as percent of the response obtained during the respective preinjection, control periods. The actual values in number of impulses/movement are presented in Supplementary Table 1 (available online as supplemental digital content at http://links.lww.com/PAIN/A524). Comparison between the effects on the firing response of MSU crystals and PBS 3 hours after injection confirmed that the impulse activity was significantly higher (+55% in average) in MSU-treated rats (Fig. 2C), whereas the morphology of the injected crystals did not affect the magnitude of the nerve impulse discharges.
A similar analysis was applied, separating the firing discharges that occur during the movements performed within the working range (OR and IR), from those obtained during movements into the noxious range (NOR and NIR, Fig. 2C). As illustrated in Figures 3A and B, the gradual increase in movement-evoked firing after injection of MSU amorphous (black symbols) or needled (blue symbols) crystal solutions was present both in the response to nonnoxious and to noxious stimuli. In both cases, this increase developed gradually along the time of recording (3 hours). By contrast, changes observed in the group of rats where PBS was injected were negligible (green symbols). In Figures 3C and D, we summarized the mean increase in movement-evoked responses to nonnoxious and noxious movements, at different times after the injection 3 hours earlier of both types of MSU crystals or PBS. The data evidence that 3 hours after MSU amorphous and needle crystals injection, the mean impulse response to nonnoxious movements had increased strikingly, being respectively 136% and 110% of the response obtained with PBS injection alone. For noxious movements, firing was respectively 43% and 52% higher than after PBS; differences were, in all cases, significant. The actual values of number of impulses/movement during nonnoxious and noxious movements under the different treatments are presented in Supplementary Table 2 (available online as supplemental digital content at http://links.lww.com/PAIN/A524).
Figure 3.
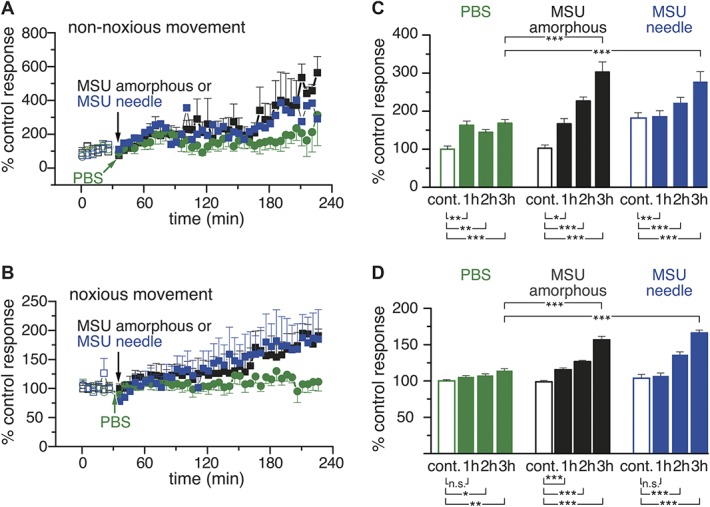
Sensitization by MSU-driven inflammation of the response of knee-joint sensory fibers to nonnoxious and noxious rotations. (A and B) Time course of the change in mean number of movement-evoked impulses evoked by nonnoxious (OR + IR) and noxious (NOR + NIR) rotations after intra-articular injection of amorphous MSU (black symbols, n = 5), MSU needle crystals (blue symbols, n = 8), or PBS (green symbols, n = 7); values are expressed as percentage of the average control response (mean of the 6 movements before intra-articular injection of the tested solution, which is indicated by the arrows). (C and D) Mean values of nonnoxious (C) and noxious (D) movement-evoked impulse activity in joints injected with PBS (green columns), needle MSU crystals (blue columns), or amorphous MSU (black columns). The first column (control) shows the mean value of the 6 consecutive movements preceding intra-articular injections; the remaining columns show the mean value of impulse discharges evoked by 12 consecutive movements measured 1, 2, and 3 hours after intra-articular injection of PBS or MSU. Paired and unpaired t test, *P < 0.05, **P < 0.01, ***P < 0.001. IR, inward rotation; MSU, monosodium urate; NIR, noxious inward rotation; NOR, noxious outward rotation; OR, outward rotation; PBS, phosphate buffered saline.
3.3. Background impulse activity on knee-joint sensory fibers at rest also increases during MSU-evoked inflammation
We also measured whether the incidence of spontaneous impulses, occasionally fired during the intervals between joint rotations, changed after intra-articular injection of amorphous or aciculate crystals. During the control recording period (first 6 movements in 30 min) detectable ongoing activity (defined as a mean firing frequency value >0.01 imps/s) explored in 12 fibers was detected in 5 of them, with an average value of 0.03 ± 0.02 imps/s (n = 5). In a group of rats that had received 3 hours earlier an injection of solution of amorphous MSU crystals, ongoing activity was present in 7 of 14 fibers, with a mean firing frequency of 0.3 ± 0.1 imps/s (n = 7), a value that was 10 times higher than before MSU treatment (Student t test **P < 0.01). When MSU needle crystals were intra-articularly injected at 3 hours, the ongoing activity was present in 4 of 9 fibers, with a mean frequency of 0.12 ± 0.01 imps/s (n = 4), 4 times higher than in control conditions in this occasion (Student t test **P < 0.01). There were no statistically significant differences between amorphous and needle crystals. Eight hours after injection of MSU amorphous crystals, ongoing activity, measured in 7 fibers during the second and fourth hour recording periods, was present in 5 fibers (mean firing frequency = 0.5 ± 0.1 imps/s; n = 5, P < 0.01). Altogether, these data confirm that MSU-evoked inflammation produced sensitization in a significant number of movement-sensitive joint sensory nerve fibers.
3.4. High molecular weight hyaluronan (Synvisc) attenuates the behavioral signs of pain and the increased impulse firing caused by monosodium urate–evoked inflammation
Ten rats treated with MSU amorphous and 8 treated with MSU needle crystals received 3 hours later an injection (50 μL) of Synvisc within the inflamed knee joint. Pain behavioral tests were performed 5 hours and 8 hours after the initial MSU injection. Figure 4 shows the changes in knee-joint diameter, weight-bearing asymmetry, and mechanical and cold sensitivity expressed as percent of the peak values measured at various times after injection of amorphous (black, n = 10) or needle MSU crystals (blue, n = 8), in rats additionally injected with Synvisc (red bars) and in untreated rats (black, n = 7 or blue bars, n = 7). Five hours after Synvisc treatment, pain behavior parameters were significantly lower in comparison with Synvisc-untreated animals (black bars for amorphous or blue bars for needle MSU); weight-bearing asymmetry was respectively 53% and 79% lower, mechanical allodynia 52% and 62%, and cold allodynia 30% and 50%.
Figure 4.
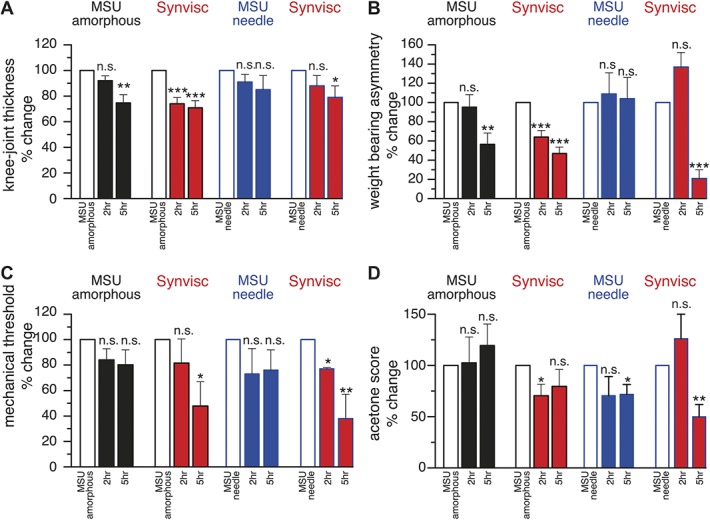
Effect of hyaluronan (Synvisc) on nocifensive responses developed after MSU-evoked inflammation. Data of all parameters are expressed as % of the values measured 3 hours after amorphous (black, n = 17) or needle MSU (blue, n = 15) injection, represented by the empty bars. (A) Joint diameter, (B) weight-bearing asymmetry, (C) mechanical threshold, and (D) acetone (cold) sensitivity. Values were measured 2 and 5 hours after Synvisc injection (red bars n = 10, after amorphous MSU, n = 7, after needle MSU). Paired t test ***P < 0.001, **P < 0.01, *P < 0.05. MSU, monosodium urate.
In a separate group of 23 rats, we also explored the possibility that hyaluronan influenced the increase in nociceptor impulse activity observed after MSU injection. For this purpose, an amorphous MSU crystal solution was injected intra-articularly and 3 hours later (the time to reach the inflammation peak, Figs. 1C–F), nerve impulse activity was recorded during 1 hour while performing complete movement cycles every 5 minutes. At the end of this period, Synvisc (n = 7) or NaCl 0.9% solution (the vehicle of Synvisc, n = 9) were injected into the MSU-inflamed joint, maintaining the recording of movement-evoked impulse activity along the following 4 hours and alternating 1 hour of movement application with a pause of 1 hour to reduce the risk of mechanical injury of the joint caused by the repeated stimulation. In an additional group of 7 rats, the same protocol was followed but no Synvisc injection was performed.
Figure 5A shows that the magnitude of the firing response to repeated, complete joint movement cycles raised gradually (black squares). In rats receiving Synvisc (whose control response values are represented by empty red circles), mean movement-evoked impulse activity was lower (solid red circles) 2 and 4 hours after hyaluronan treatment while activity did not change in rats receiving saline (purple triangles). The effect of Synvisc is illustrated in the sample recordings of the impulse firing of a single nociceptor unit previously sensitized by a MSU injection, before and 2 hours after Synvisc (Fig. 5B). The decrease produced by Synvisc in total movement-evoked firing activity affected the impulse discharge produced both by the innocuous (−30%, n = 6) and the noxious (−40%, n = 7) components of the movement (Figs. 5C and D, red bars). By contrast, impulse activity did not change significantly in MSU-inflamed animals that were not injected or that received an injection of saline instead of Synvisc (Figs. 5C and D, black and purple bars). Supplementary Table 3 presents the mean number of impulses/movement measured during the different recording periods shown in Figure 5 (available online as supplemental digital content at http://links.lww.com/PAIN/A524).
Figure 5.
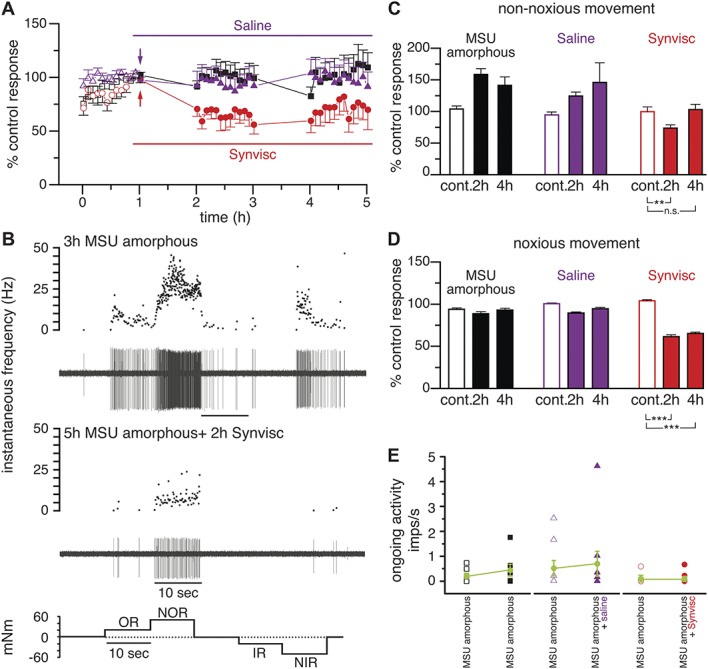
Effect of hyaluronan (Synvisc) on movement-evoked nerve impulse activity in MSU-inflamed joints. (A) Time course of the change in nerve impulse discharges evoked by complete movement cycles (OR + NOR + IR + NIR) measured in 23 single nerve fibers of joints injected with amorphous MSU 3 hours before, expressed as % of the mean ± SEM of the control response value. Black symbols show the data in MSU-inflamed knee joints that did not receive additional treatment (n = 7), red filled circles in MSU-inflamed knee joints that additionally received an intra-articular injection of Synvisc (red arrow, n = 7), and purple symbols represent the data in joints receiving an intra-articular injection of NaCl 0.9% (vehicle of Synvisc, purple arrow, n = 9). The response values of these fibers previous to treatments are represented as empty symbols. (B) Example of the impulse activity recorded in a single fiber innervating a knee joint inflamed 3 hours earlier by MSU injection (upper panel) to illustrate the firing frequency reduction observed 2 hours after intra-articular injection of Synvisc (middle panel). In both cases, the upper traces represent the instantaneous frequency and the lower traces the original recordings of nerve impulse activity. The bottom panel depicts the sequence of nonnoxious and noxious rotations applied as stimuli. (C and D) Mean nerve impulse activity in joints injected 3 hours earlier with MSU, expressed as percentage of the activity evoked by the movements performed during the first hour (control period, taken as 100%). Empty columns represent the mean values of the last 3 movements of the control period, before any experimental maneuver. The remaining columns show the mean value of the 12 movements performed during each of the indicated periods, for the nonnoxious (OR + IR) (C) and the noxious (NOR + NIR) (D) components of the movement. Unpaired t test, ***P < 0.001, **P < 0.01, *P < 0.05. (E) Ongoing activity frequency values of joint nerve fibers (n = 7) 3 hours after intra-articular injection of MSU (open black squares) and 8 hours after intra-articular injection of MSU (filled black squares); in nerve fibers (n = 8) 3 hours after intra-articular injection of MSU (empty purple triangles) and 8 hours after MSU injection but receiving also 4 hours after MSU an intra-articular injection of saline (NaCl 0.9%, filled purple triangles); and in joint nerve fibers (n = 8) 3 hours after i.a. injection of amorphous MSU (empty red circles) and 8 hours after amorphous MSU injection but receiving also 4 hours after MSU an i.a. injection of Synvisc (red filled circles). Green circles correspond to mean values in each case. IR, inward rotation; MSU, monosodium urate; NIR, noxious inward rotation; NOR, noxious outward rotation; OR, outward rotation.
We also analyzed the effect of Synvisc on the ongoing activity developing in nociceptor fibers of MSU-inflamed joints. In untreated animals, noticeable spontaneous impulse activity during the last recording period (4-5 hours in the experiments shown in Fig. 5A, 8 hours after amorphous MSU crystals injection) was present in 5 of 7 fibers (mean = 0.6 ± 0.3 imps/s; n = 5). Notably, after MSU injection, the firing frequency of spontaneously active fibers becomes more variable, with a tendency of mean frequency values to increase with time (Fig. 5E, black squares). By contrast, in Synvisc-injected joints, this tendency to display an increased ongoing activity 8 hours after MSU was absent (red circles), a neutralizing effect that did not appear when NaCl 0.9% instead of Synvisc was injected (purple triangles). These results suggest that spontaneous activity values stabilized after Synvisc, which also moderated the augmented impulse activity with time underlying the development of movement-evoked allodynia and hyperalgesia observed in MSU-injected rats.
4. Discussion
In this study, we describe for the first time in mammals that injection of MSU crystals in the rat knee joint evokes an augmented joint nociceptor activity that evolves in parallel with the local inflammation and behavioral signs of pain, closely resembling those observed in human gouty attacks and thus appearing as a potentially useful animal model of gout arthritis. Our data further show that intra-articular injection of sodium hyaluronate of HMW reduces joint nociceptor impulse activity elicited by movements that likely cause joint pain, thus demonstrating that the analgesic action of HMW-HA, previously observed in osteoarthritis, also occurs in gouty arthritis.
4.1. Mechanisms for augmented joint nociceptor activity, local inflammation, and pain induced by monosodium urate crystals
In human gout and pseudogout arthritis, it has been proposed that deposition of MSU or calcium pyrophosphate crystals in joint SF and tissues mechanically harms joint surfaces contributing to acute inflammation and pain.37 Several other mechanisms possibly determine the reaction to intra-articular crystals, and many lines of evidence support the central role of immune cell activation as the main triggering mechanism. Our observation in rats that larger, needle-shaped MSU crystals were not more effective than the small, amorphous ones in causing inflammation, nocifensive behavior, and enhancement of joint nociceptor activity speaks against direct mechanical injury by crystals as the mechanism for stimulation of sensory nerve terminals in gout pain. This confirms previous clinical data showing that signs of gout were not influenced by the size or shape of SF crystals.7,10 The apparently minor contribution to gout pain of mechanical stimulation of nociceptors by crystals also explains the absence of pain in patients during asymptomatic periods between acute gout attacks, despite the presence of abundant aciculate MSU crystals on the cartilage surface and SF.39–41
In our experimental model of gout in rats, we provide evidence that joint inflammatory signs evolved in parallel with the appearance of strong excitation of nociceptors and parallel behavioral signs of nociceptor sensitization. As with mechanical force, there is no experimental proof of direct chemical interaction of MSU molecules with membrane receptor proteins of nociceptor endings such as TRPV1 and TRPA1, the main ion channels mediating nociceptor's depolarization by endogenous or exogenous chemicals.22,57,58 In fact, it is well established that crystals are first detected by innate immune cells such as macrophages, monocytes, or neutrophils that respond and produce active IL-1 β. This ultimately activates NF-kB which turns on the transcription of cytokines and chemokines, initiating the release of inflammatory mediators, which finally act of nociceptor endings, opening TRPV1 and TRPA1 channels either directly or through intracellular signaling cascades. This leads to nociceptor's membrane depolarization, the canonical mechanism for their sensitization in joints and other tissues.16,22,24,42,57,58 Antidromic firing of articular nociceptors releases neuropeptides from peripheral nerve terminals,5 thereby potentiating the local inflammatory response (neurogenic inflammation).25
Collectively, our study provides new experimental evidence confirming that interaction with inflammatory mediators released by innate immune cells after their contact with MSU crystals is the principal mechanism whereby joint nociceptor terminals are excited in gout and develop the spontaneous ongoing activity and enhanced responsiveness to innocuous and noxious stimuli underlying the strong pain characteristic of gouty attacks.
4.2. Correlation between nocifensive behavior and joint nociceptor impulse activity in monosodium urate–injected rats
Previous studies in the rat had shown that intra-articular injection of MSU evokes nocifensive behaviors resembling painful acute gouty attacks in humans.33,34,36,57,58 In chicken, augmented joint nerve afferent activity evoked by intra-articular MSU crystals was also reported.17 However, this is the first evidence in mammals linking directly the time course and amplitude of increased spontaneous and movement-evoked nociceptor activity in joint nociceptors caused by MSU crystals, with the appearance of weight-bearing asymmetry, lower cutaneous mechanical threshold, and larger nocifensive responses to cold, all of them behavioral signs of peripheral and central sensitization and pain.28,50 The observed increases in spontaneous activity, albeit discrete, seem to be sufficient to evoke mechanical and cold allodynia, 2 typical behavioral indicators of sensitization.51 This also occurs in cutaneous nociceptors, where the rise of the proportion of spontaneously active nociceptor fibers from 7% to 38% during inflammation sustains spontaneous pain.13
4.3. Hylaluronan effects on monosodium urate–sensitized joint nociceptor nerve fibers
Hyaluronan is an important chemical component of the SF.3 Although HA does not contribute to urate crystal deposition in gout30,39,56, it regulates a variety of general cellular and molecular processes associated with inflammation, including expression of inflammatory genes23, immune cell infiltration,35 phagocytosis of urate crystals by macrophages,15 macrophage transition from proinflammatory to anti-inflammatory states, and release of inflammatory cytokines.44 These effects are critically dependent on HA's molecular weight, with HMW-HA having a predominant inhibitory effect and Low Molecular Weight Hylan (LMW-HA) fragments a proinflammatory action.48 Modulation by HMW-HA molecules of the production and release of inflammatory mediators by inflammatory cells is one of the potential mechanisms contributing to the attenuation by Synvisc of MSU-induced joint nociceptor activity and nocifensive behavior. However, direct reduction by HA molecules of nociceptor excitation possibly plays a more significant role. Synovial HMW-HA behaves as an elastoviscous filter that attenuates the transmission of external mechanical forces to nociceptor endings in joint tissues,2 thereby reducing the effective mechanical energy reaching the stretch-activated channels located in the membrane of these nerve terminals.43 This buffering action on nociceptor excitation is closely dependent on the rheological properties of HA and explains the efficacy of HMW-HA in decreasing the firing response of nociceptors in various experimental models of inflamed joints.19,20,46 But in addition, HMW-HA interacts directly with TRPV1 reducing its higher opening probability generated by natural stimuli, including endogenous inflammatory mediators.8 Both mechanisms may contribute to the inhibitory effects of Synvisc on nociceptor activation and nocifensive responses that we observed in experimental gout.
Whether HA molecules naturally present in joints counteract the onset of inflammation and pain in human gout has not been determined. Notably, the viscosity, concentration, and MW of HA in the SF of acute gout are low.47 This is also the case in OA, a form of arthritis that frequently co-occurs with gout in which injection of HMW-HA decreases pain.1,4,12 Although the pathogenic link between OA and human gout has not been established yet, it is tempting to speculate that in both diseases, endogenous SF HA may play a role in delimiting the intensity of joint inflammation and pain, depending on its concentration and viscosity.
Conflict of interest statement
The authors have no conflicts of interest to declare.
This work was supported by funds from Matrix Biology Institute (EE.UU), and by grants from the Spanish Ministry of Economy and Competitiveness SAF2014-545.18-C3-2-R, SAF2013-45608-R, SAF2016-77233-R co-financed by the European Regional Development Fund (ERDF), and the “Severo Ochoa” Program for Centers of Excellence in R&D SEV-2013-0317 and a fellowship from the Generalitat Valenciana to A. Marcotti (GRISOLIA/2015/034).
Supplementary Material
Acknowledgments
The authors thank E. Quintero, A. Caler, and V. J. Rodriguez for technical assistance, S. Ingham for illustrations, and Dr. E. Caparrós and A. Caler for their advice on flow cytometry experiments.
Appendix A. Supplement digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/A524.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
References
- [1].Ayhan E, Kesmezacar H, Akgun I. Intraarticular injections (corticosteroid, hyaluronic acid, platelet rich plasma) for the knee osteoarthritis. World J Orthop 2014;5:351–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Balazs EA. The viscoelastic intercellular matrix and control of cell function by hyaluronan. In: Laurent T, editor. The chemistry, biology and medical applications of hyaluronan and its derivatives. London: Portland Press, 1998. p. 185–204. [Google Scholar]
- [3].Balazs EA, Watson D, Duff IF, Roseman S. Hyaluronic acid in synovial fluid. I. Molecular parameters of hyaluronic acid in normal and arthritis human fluids. Arthritis Rheum 1967;10:357–76. [DOI] [PubMed] [Google Scholar]
- [4].Bert JM, Waddell DD. Viscosupplementation with hylan g-f 20 in patients with osteoarthrosis of the knee. Ther Adv Musculoskelet Dis 2010;2:127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bjurholm A, Kreicbergs A, Ahmed M, Schultzberg M. Noradrenergic and peptidergic nerves in the synovial membrane of the Sprague-Dawley rat. Arthritis Rheum 1990;33:859–65. [DOI] [PubMed] [Google Scholar]
- [6].Brooks PM, Burton D, Forrest MJ. Crystal-induced inflammation in the rat subcutaneous air-pouch. Br J Pharmacol 1987;90:413–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Busso N, So A. Mechanisms of inflammation in gout. Arthritis Res Ther 2010;12:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Caires R, Luis E, Taberner FJ, Fernandez-Ballester G, Ferrer-Montiel A, Balazs EA, Gomis A, Belmonte C, de la Pena E. Hyaluronan modulates TRPV1 channel opening, reducing peripheral nociceptor activity and pain. Nat Commun 2015;6:8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Constantin CE, Mair N, Sailer CA, Andratsch M, Xu ZZ, Blumer MJ, Scherbakov N, Davis JB, Bluethmann H, Ji RR, Kress M. Endogenous tumor necrosis factor alpha (TNFalpha) requires TNF receptor type 2 to generate heat hyperalgesia in a mouse cancer model. J Neurosci 2008;28:5072–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Crisan TO, Cleophas MC, Oosting M, Lemmers H, Toenhake-Dijkstra H, Netea MG, Jansen TL, Joosten LA. Soluble uric acid primes TLR-induced proinflammatory cytokine production by human primary cells via inhibition of IL-1Ra. Ann Rheum Dis 2016;75:755–62. [DOI] [PubMed] [Google Scholar]
- [11].Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994;53:55–63. [DOI] [PubMed] [Google Scholar]
- [12].Chevalier X, Jerosch J, Goupille P, van Dijk N, Luyten FP, Scott DL, Bailleul F, Pavelka K. Single, intra-articular treatment with 6 ml hylan G-F 20 in patients with symptomatic primary osteoarthritis of the knee: a randomised, multicentre, double-blind, placebo controlled trial. Ann Rheum Dis 2010;69:113–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Djouhri L, Koutsikou S, Fang X, McMullan S, Lawson SN. Spontaneous pain, both neuropathic and inflammatory, is related to frequency of spontaneous firing in intact C-fiber nociceptors. J Neurosci 2006;26:1281–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fang D, Kong LY, Cai J, Li S, Liu XD, Han JS, Xing GG. Interleukin-6-mediated functional upregulation of TRPV1 receptors in dorsal root ganglion neurons through the activation of JAK/PI3K signaling pathway: roles in the development of bone cancer pain in a rat model. PAIN 2015;156:1124–44. [DOI] [PubMed] [Google Scholar]
- [15].Forrester JV, Balazs EA. Inhibition of phagocytosis by high molecular weight hyaluronate. Immunology 1980;40:435–46. [PMC free article] [PubMed] [Google Scholar]
- [16].Gangadharan V, Kuner R. Pain hypersensitivity mechanisms at a glance. Dis Model Mech 2013;6:889–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gentle MJ. Sodium urate arthritis: effects on the sensory properties of articular afferents in the chicken. PAIN 1997;70:245–51. [DOI] [PubMed] [Google Scholar]
- [18].Gomis A, Meini S, Miralles A, Valenti C, Giuliani S, Belmonte C, Maggi CA. Blockade of nociceptive sensory afferent activity of the rat knee joint by the bradykinin B2 receptor antagonist fasitibant. Osteoarthritis Cartilage 2013;21:1346–54. [DOI] [PubMed] [Google Scholar]
- [19].Gomis A, Miralles A, Schmidt RF, Belmonte C. Nociceptive nerve activity in an experimental model of knee joint osteoarthritis of the Guinea pig: effect of intra-articular hyaluronan application. PAIN 2007;130:126–36. [DOI] [PubMed] [Google Scholar]
- [20].Gomis A, Pawlak M, Balazs EA, Schmidt RF, Belmonte C. Effects of different molecular weight elastoviscous hyaluronan solutions on articular nociceptive afferents. Arthritis Rheum 2004;50:314–26. [DOI] [PubMed] [Google Scholar]
- [21].Gouin O, L'Herondelle K, Lebonvallet N, Le Gall-Ianotto C, Sakka M, Buhe V, Plee-Gautier E, Carre JL, Lefeuvre L, Misery L, Le Garrec R. TRPV1 and TRPA1 in cutaneous neurogenic and chronic inflammation: pro-inflammatory response induced by their activation and their sensitization. Protein Cell 2017;8:644–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hoffmeister C, Silva MA, Rossato MF, Trevisan G, Oliveira SM, Guerra GP, Silva CR, Ferreira J. Participation of the TRPV1 receptor in the development of acute gout attacks. Rheumatology (Oxford) 2014;53:240–9. [DOI] [PubMed] [Google Scholar]
- [23].Horton MR, Burdick MD, Strieter RM, Bao C, Noble PW. Regulation of hyaluronan-induced chemokine gene expression by IL-10 and IFN-gamma in mouse macrophages. J Immunol 1998;160:3023–30. [PubMed] [Google Scholar]
- [24].Huang J, Zhang X, McNaughton PA. Inflammatory pain: the cellular basis of heat hyperalgesia. Curr Neuropharmacol 2006;4:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov 2014;13:533–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jin X, Gereau RW., IV Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-alpha. J Neurosci 2006;26:246–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Julius D. TRP channels and pain. Annu Rev Cell Dev Biol 2013;29:355–84. [DOI] [PubMed] [Google Scholar]
- [28].Kelly S, Dunham JP, Murray F, Read S, Donaldson LF, Lawson SN. Spontaneous firing in C-fibers and increased mechanical sensitivity in A-fibers of knee joint-associated mechanoreceptive primary afferent neurones during MIA-induced osteoarthritis in the rat. Osteoarthritis Cartilage 2012;20:305–13. [DOI] [PubMed] [Google Scholar]
- [29].Malawista SE, de Boisfleury AC, Naccache PH. Inflammatory gout: observations over a half-century. FASEB J 2011;25:4073–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Martillo MA, Nazzal L, Crittenden DB. The crystallization of monosodium urate. Curr Rheumatol Rep 2014;16:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006;440:237–41. [DOI] [PubMed] [Google Scholar]
- [32].Meng J, Wang J, Steinhoff M, Dolly JO. TNFalpha induces co-trafficking of TRPV1/TRPA1 in VAMP1-containing vesicles to the plasmalemma via Munc18-1/syntaxin1/SNAP-25 mediated fusion. Sci Rep 2016;6:21226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Moilanen LJ, Hamalainen M, Lehtimaki L, Nieminen RM, Moilanen E. Urate crystal induced inflammation and joint pain are reduced in transient receptor potential ankyrin 1 deficient mice–potential role for transient receptor potential ankyrin 1 in gout. PLoS One 2015;10:e0117770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Moilanen LJ, Hamalainen M, Nummenmaa E, Ilmarinen P, Vuolteenaho K, Nieminen RM, Lehtimaki L, Moilanen E. Monosodium iodoacetate-induced inflammation and joint pain are reduced in TRPA1 deficient mice–potential role of TRPA1 in osteoarthritis. Osteoarthritis Cartilage 2015;23:2017–26. [DOI] [PubMed] [Google Scholar]
- [35].Monslow J, Govindaraju P, Pure E. Hyaluronan—a functional and structural sweet spot in the tissue microenvironment. Front Immunol 2015;6:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Otsuki T, Nakahama H, Niizuma H, Suzuki J. Evaluation of the analgesic effects of capsaicin using a new rat model for tonic pain. Brain Res 1986;365:235–40. [DOI] [PubMed] [Google Scholar]
- [37].Pang L, Hayes CP, Buac K, Yoo DG, Rada B. Pseudogout-associated inflammatory calcium pyrophosphate dihydrate microcrystals induce formation of neutrophil extracellular traps. J Immunol 2013;190:6488–500. [DOI] [PubMed] [Google Scholar]
- [38].Pascual E. Persistence of monosodium urate crystals and low-grade inflammation in the synovial fluid of patients with untreated gout. Arthritis Rheum 1991;34:141–5. [DOI] [PubMed] [Google Scholar]
- [39].Pascual E, Addadi L, Andres M, Sivera F. Mechanisms of crystal formation in gout-a structural approach. Nat Rev Rheumatol 2015;11:725–30. [DOI] [PubMed] [Google Scholar]
- [40].Pascual E, Batlle-Gualda E, Martinez A, Rosas J, Vela P. Synovial fluid analysis for diagnosis of intercritical gout. Ann Intern Med 1999;131:756–9. [DOI] [PubMed] [Google Scholar]
- [41].Pascual E, Doherty M. Aspiration of normal or asymptomatic pathological joints for diagnosis and research: indications, technique and success rate. Ann Rheum Dis 2009;68:3–7. [DOI] [PubMed] [Google Scholar]
- [42].Patapoutian A, Tate S, Woolf CJ. Transient receptor potential channels: targeting pain at the source. Nat Rev Drug Discov 2009;8:55–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Peña Ede L, Sala S, Rovira JC, Schmidt RF, Belmonte C. Elastoviscous substances with analgesic effects on joint pain reduce stretch-activated ion channel activity in vitro. PAIN 2002;99:501–8. [DOI] [PubMed] [Google Scholar]
- [44].Petrey AC, de la Motte CA. Hyaluronan, a crucial regulator of inflammation. Front Immunol 2014;5:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Popa-Nita O, Naccache PH. Crystal-induced neutrophil activation. Immunol Cell Biol 2010;88:32–40. [DOI] [PubMed] [Google Scholar]
- [46].Pozo MA, Balazs EA, Belmonte C. Reduction of sensory responses to passive movements of inflamed knee joints by hylan, a hyaluronan derivative. Exp Brain Res 1997;116:3–9. [DOI] [PubMed] [Google Scholar]
- [47].Praest BM, Greiling H, Kock R. Assay of synovial fluid parameters: hyaluronan concentration as a potential marker for joint diseases. Clin Chim Acta 1997;266:117–28. [DOI] [PubMed] [Google Scholar]
- [48].Rayahin JE, Buhrman JS, Zhang Y, Koh TJ, Gemeinhart RA. High and low molecular weight hyaluronic acid differentially influence macrophage activation. ACS Biomater Sci Eng 2015;1:481–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Richter F, Natura G, Loser S, Schmidt K, Viisanen H, Schaible HG. Tumor necrosis factor causes persistent sensitization of joint nociceptors to mechanical stimuli in rats. Arthritis Rheum 2010;62:3806–14. [DOI] [PubMed] [Google Scholar]
- [50].Schaible HG. Nociceptive neurons detect cytokines in arthritis. Arthritis Res Ther 2014;16:470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Schaible HG, Grubb BD. Afferent and spinal mechanisms of joint pain. PAIN 1993;55:5–54. [DOI] [PubMed] [Google Scholar]
- [52].Schaible HG, Schmidt RF. Effects of an experimental arthritis on the sensory properties of fine articular afferent units. J Neurophysiol 1985;54:1109–22. [DOI] [PubMed] [Google Scholar]
- [53].Schroder K, Tschopp J. The inflammasomes. Cell 2010;140:821–32. [DOI] [PubMed] [Google Scholar]
- [54].Spicarova D, Palecek J. Tumor necrosis factor alpha sensitizes spinal cord TRPV1 receptors to the endogenous agonist N-oleoyldopamine. J Neuroinflammation 2010;7:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Studer M, McNaughton PA. Modulation of single-channel properties of TRPV1 by phosphorylation. J Physiol 2010;588:3743–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Tak HK, Cooper SM, Wilcox WR. Studies on the nucleation of monosodium urate at 37 degrees c. Arthritis Rheum 1980;23:574–80. [DOI] [PubMed] [Google Scholar]
- [57].Trevisan G, Hoffmeister C, Rossato MF, Oliveira SM, Silva MA, Ineu RP, Guerra GP, Materazzi S, Fusi C, Nassini R, Geppetti P, Ferreira J. Transient receptor potential ankyrin 1 receptor stimulation by hydrogen peroxide is critical to trigger pain during monosodium urate-induced inflammation in rodents. Arthritis Rheum 2013;65:2984–95. [DOI] [PubMed] [Google Scholar]
- [58].Trevisan G, Hoffmeister C, Rossato MF, Oliveira SM, Silva MA, Silva CR, Fusi C, Tonello R, Minocci D, Guerra GP, Materazzi S, Nassini R, Geppetti P, Ferreira J. TRPA1 receptor stimulation by hydrogen peroxide is critical to trigger hyperalgesia and inflammation in a model of acute gout. Free Radic Biol Med 2014;72:200–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


