Supplemental Digital Content is available in the text.
Keywords: cholesterol, HDL; cholesterol, LDL; polymorphism, single nucleotide; stroke; triglycerides
Abstract
Background and Purpose—
Statin therapy is associated with a lower risk of ischemic stroke supporting a causal role of low-density lipoprotein (LDL) cholesterol. However, more evidence is needed to answer the question whether LDL cholesterol plays a causal role in ischemic stroke subtypes. In addition, it is unknown whether high-density lipoprotein cholesterol and triglycerides have a causal relationship to ischemic stroke and its subtypes. Our aim was to investigate the causal role of LDL cholesterol, high-density lipoprotein cholesterol, and triglycerides in ischemic stroke and its subtypes through Mendelian randomization (MR).
Methods—
Summary data on 185 genome-wide lipids-associated single nucleotide polymorphisms were obtained from the Global Lipids Genetics Consortium and the Stroke Genetics Network for their association with ischemic stroke (n=16 851 cases and 32 473 controls) and its subtypes, including large artery atherosclerosis (n=2410), small artery occlusion (n=3186), and cardioembolic (n=3427) stroke. Inverse-variance–weighted MR was used to obtain the causal estimates. Inverse-variance–weighted multivariable MR, MR-Egger, and sensitivity exclusion of pleiotropic single nucleotide polymorphisms after Steiger filtering and MR-Pleiotropy Residual Sum and Outlier test were used to adjust for pleiotropic bias.
Results—
A 1-SD genetically elevated LDL cholesterol was associated with an increased risk of ischemic stroke (odds ratio: 1.12; 95% confidence interval: 1.04–1.20) and large artery atherosclerosis stroke (odds ratio: 1.28; 95% confidence interval: 1.10–1.49) but not with small artery occlusion or cardioembolic stroke in multivariable MR. A 1-SD genetically elevated high-density lipoprotein cholesterol was associated with a decreased risk of small artery occlusion stroke (odds ratio: 0.79; 95% confidence interval: 0.67–0.90) in multivariable MR. MR-Egger indicated no pleiotropic bias, and results did not markedly change after sensitivity exclusion of pleiotropic single nucleotide polymorphisms. Genetically elevated triglycerides did not associate with ischemic stroke or its subtypes.
Conclusions—
LDL cholesterol lowering is likely to prevent large artery atherosclerosis but may not prevent small artery occlusion nor cardioembolic strokes. High-density lipoprotein cholesterol elevation may lead to benefits in small artery disease prevention. Finally, triglyceride lowering may not yield benefits in ischemic stroke and its subtypes.
Circulating lipid and lipoprotein biomarkers have consistently been associated with cardiovascular diseases as myocardial infarction and stroke.1 A previous meta-analysis of low-density lipoprotein cholesterol (LDLC)–lowering trials has shown risk reduction for ischemic stroke.2 In addition, the SPARCL trial (Stroke Prevention by Aggressive Reduction in Cholesterol Levels) in secondary stroke prevention demonstrated a significant reduction in recurrent stroke with atorvastatin.3 Importantly, it is unclear as to whether lipid lowering with statins is beneficial across different ischemic stroke subtypes. Although lipid lowering is likely to be effective in large artery atherosclerosis stroke, the evidence implicating elevated lipids in the small artery occlusion stroke is scant. In the recent J-STARS (Japan Statin Treatment Against Recurrent Stroke) stroke secondary prevention trial, pravastatin reduced large artery atherosclerosis recurrent stroke risk, but it had no effect on small artery occlusion risk.4 In addition, there is insufficient evidence whether high-density lipoprotein cholesterol (HDLC) and triglycerides may be causally involved in the development of ischemic stroke.5
Genetic variants are randomly distributed at conception and thus can be used to overcome 2 of the major problems of observational studies, biases because of confounding and reverse causation. Mendelian randomization (MR) is an analytic method that leverages genetic variants associated with heritable risk factors to generate causal estimates between such factors and diseases. This method has recently been used to provide evidence for a causal relationship of LDLC and triglycerides with coronary artery disease (CAD). However, MR studies have not indicated any causality between HDLC and CAD.6–10
All MR studies rely on 3 basic assumptions: the genetic instrument (1) should be reliably associated with the exposure, (2) should be associated with the outcome only through the exposure, and (3) should not be associated with other factors that affect the outcome. This means that the genetic variants used as instruments must exert their effects on the outcome exclusively through the exposure of interest and not through alternative pathways. Single genetic variants, as single nucleotide polymorphisms (SNPs), that fulfill the MR assumptions, can be used, but their use may only be meaningful in large studies powerful enough to study the effect of the small proportion of exposures explained by the single variants. However, summary-level data of large meta-analyses of genome-wide association studies (GWAS) are increasingly available and allow the use of combinations of SNPs in MR analyses. However, SNPs may not fulfill the MR assumptions and may lead to bias through pleiotropic effects. Several methods have been recently developed to correct for such bias.11,12
We conducted an MR study, using summary-level data from publicly available GWAS of lipids and other cardiometabolic traits, to investigate the causal relationship of LDLC, HDLC, and triglycerides in the development of ischemic stroke as a whole and its 3 main subtypes: cardioembolic, large artery atherosclerosis stroke, and small artery occlusion.
Methods
Data Sources
Summary-level data for 185 genome-wide lipids-associated SNPs were obtained from the publicly available data through the Global Lipids Genetics Consortium.13 The Global Lipids Genetics Consortium GWAS included 188 577 individuals of primarily European ancestry. The summary-level data for ischemic stroke and its subtypes were obtained from the National Institute of Neurological Disorders and Stroke−Stroke Genetics Network.14 The Stroke Genetics Network GWAS included 16 851 ischemic stroke cases and 32 473 controls of predominantly European ancestry. Of the ischemic stroke cases, 2410 were subclassified as large artery atherosclerosis stroke, 3186 as small artery occlusion stroke, and 3427 as cardioembolic stroke using the Trial of Org 10172 in Acute Stroke Treatment criteria.15 The Global Lipids Genetics Consortium summary data were available in the public domain, and an ethics approval was obtained from each contributing study in the original publication.13 Each study included in the Stroke Genetics Network was approved by the local institutional review board and ethics committee, and all participants provided written informed consent.14 In line with the Transparency and Openness Promotion Guidelines, data and analytic methods used have been appropriately cited, and the data used for the main analyses are provided in Tables I and II in the online-only Data Supplement.
SNP Selection
We obtained summary estimates for 185 SNPs reported in the most recent lipids GWAS by Willer et al13 to be associated with LDLC, HDLC, and triglycerides. The 185 SNPs can be considered as independent because of low linkage disequilibrium (maximum r2<0.2 between any SNPs). Each instrumental variable was constructed from SNPs showing GWAS significant association (P<5×10–8) with the respective trait. The instrumental variables included 76 SNPs for LDLC, 86 SNPs for HDLC, and 51 SNPs for triglycerides. The LDLC, HDLC, and triglycerides instruments explained 6.4%, 5.9%, and 4.6% of the variances in LDLC, HDLC, and triglycerides, respectively, as estimated by the gtx package in R. We then obtained summary estimates for the same set of 185 SNPs from the Stroke Genetics Network GWAS for ischemic stroke and its subtypes, and the effect alleles were matched with all lipid and stroke summary data. Summary data on 2 SNPs (rs1998013 and rs7422339) were missing.
Statistical Analysis
We performed 3 different MR analyses: (1) conventional inverse-variance–weighted MR; (2) multivariable MR to adjust for pleiotropy using summary-level data of other known lipid- and cardiometabolic traits; and (3) MR-Egger to account for all pleiotropic bias from known and unknown factors.
First, we performed inverse-variance–weighted MR (hereafter referred to as conventional MR) using each set of SNPs for each trait as instrumental variables. This method is a weighted linear regression between the instrumental SNP-β estimates of each lipid trait as exposure variables and the stroke β estimates of the same SNPs as outcome variables. This regression is weighted by the inverse-variance of SNP–stroke association, and the regression line is fixed to zero. This method, however, does not correct for pleiotropic bias if present.16 To correct for that, we performed inverse-variance–weighted multivariable MR (hereafter referred to as multivariable MR) using all 185 SNPs. This method adjusts for pleiotropic effects across the included lipid traits in our analyses using β’s from SNP–stroke as outcome variables and β’s from SNP-LDLC, SNP-HDLC, and SNP-triglycerides as predictors in 1 multivariable model. The intercept was constrained to zero, and the regression was weighted by the inverse-variance of the SNP–stroke associations.17 We additionally performed multivariable MR using a total of 343 SNPs (r2<0.2). In addition to the 185 primarily lipid associated SNPs, we included SNPs that associate primarily with other cardiometabolic traits, including 97 SNPs for body mass index, 49 for waist-to-hip ratio adjusted for body mass index, 36 for fasting plasma glucose, and 26 for fasting plasma insulin, all obtained from publicly available data releases of the latest GWAS meta-analyses.13,18–20 We additionally performed MR-Egger as previously described.12 Egger regression was previously developed to detect small-study bias in meta-analyses and can be similarly used to detect bias because of unbalanced pleiotropy in MR studies. In contrast to conventional MR, the regression line is unconstrained, and the intercept represents the average pleiotropic effects across all SNPs, assuming that the distribution of pleiotropic effects is independent from the genetic associations with exposure, also known as the INstrument Strength Independent of Direct Effect assumption. In addition to MR-Egger, we performed sensitivity analyses to minimize biases because of pleiotropy by excluding SNPs exhibiting potential pleiotropy using Steiger filtering. Steiger filtering was performed using the 185 lipid-associated SNPs for each lipid trait. SNPs were excluded if they explained larger variance of any of the other 2 lipid traits compared with the trait of interest. We performed a 2-stage Steiger filtering. The first stage was based solely on the r2 values of each SNP with respect to the 3 lipid traits. For example, for the LDLC instrument, we included an SNP if it had a larger r2 value for LDLC compared with HDLC or triglycerides. In a stricter additional stage, SNPs were included only if r2 values were significantly larger for the trait compared with the other 2 traits (P<0.05), as described before.21 Finally, analyses were done using instruments that only included SNPs that exclusively associated with the trait of interest and not with the other traits (P>5×10–8). We analyzed all of these instruments for outlier pleiotropy using MR-Pleiotropy Residual Sum and Outlier test and performed conventional MR and MR-Egger after exclusion of outlier SNPs. Analyses were performed using the MendelianRandomization, TwoSampleMR, and MR-Pleiotropy Residual Sum and Outlier test packages in R version 3.2.22,23 Bonferroni-corrected 2-sided P values (P=0.004; 0.05/12) for 12 tests (3 exposures and 4 outcomes) were used.
We performed conventional MR analyses for LDLC using variants in genes encoding targets for LDLC lowering (HMGCR, PCSK9, and NPC1L1) or HDLC elevation (CETP). Variants in these genes were previously selected using r2<0.4, and thus we incorporated covariance matrices in all MR analyses.8,24
Odds ratio (OR) thresholds were calculated for all stroke subtypes given the case count, sample size, instrument strength, and 80% minimum power.25 For ischemic stroke, we had 80% power to detect associations with ORs as low as 1.11 with an instrument explaining 6.4% of the exposure and as high as 1.26 with instruments explaining 1.2% of the exposure. The OR range was 1.24 to 1.56 for large artery atherosclerosis stroke, 1.21 to 1.49 for small artery occlusion stroke, and 1.20 to 1.48 for cardioembolic stroke (Table III in the online-only Data Supplement).
Results
The associations between LDLC and ischemic stroke and subtypes are shown in Figure 1. Genetically predicted LDLC was associated with higher risk for ischemic stroke (OR: 1.12; 95% confidence interval [CI]: 1.01–1.24; per 1-SD elevation of LDLC) by conventional MR. MR-Egger showed a stronger association (OR: 1.22; 95% CI: 1.05–1.43), and the intercept did not indicate pleiotropic bias (P intercept=0.14). In addition, conventional MR suggested a direct association between genetically elevated LDLC and large artery atherosclerosis stroke (OR: 1.28; 95% CI: 1.07–1.53). Fully adjusted multivariable MR and MR-Egger showed stronger associations (OR: 1.36; 95% CI: 1.17–1.57 and OR: 1.40; 95% CI: 1.06–1.86, respectively), and MR-Egger intercept showed no pleiotropy (P=0.39). After Bonferroni correction, only multivariable MR analyses remained significant. Genetically predicted LDLC did not associate with small artery occlusion nor with cardioembolic stroke.
Figure 1.
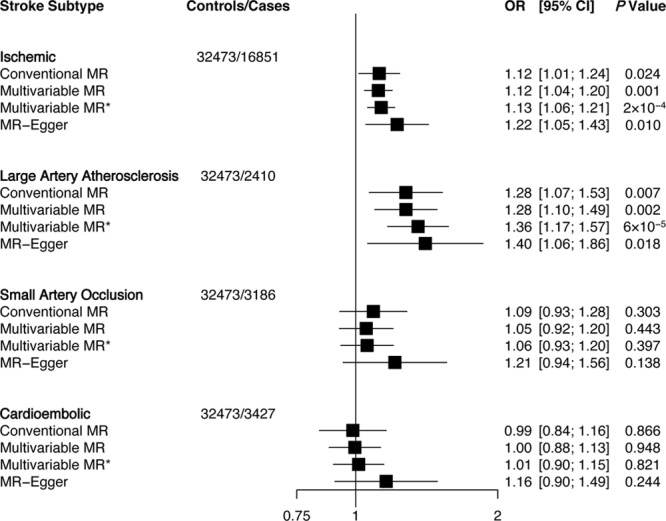
Association of low-density lipoprotein cholesterol with ischemic stroke and subtypes using different Mendelian randomization (MR) analyses. Odds ratio (OR) of ischemic stroke per 1-SD increase in each lipid trait. Conventional MR estimates were derived from 2-sample MR that forces the intercept of the slope line to zero and does not account for pleiotropy. Multivariable MR adjusts for other lipid traits and MR-Egger adjusts for unbalanced pleiotropy. *Multivariable MR analysis using summary estimates of 343 single nucleotide polymorphisms that adjusts for lipid traits, body mass index (BMI), waist hip ratio adjusted for BMI, fasting plasma glucose, and fasting plasma insulin. CI indicates confidence interval.
Genetically predicted elevations in HDLC levels were associated with lower risk of small artery occlusion stroke (OR: 0.79; 95% CI: 0.67–0.93; per 1-SD elevation of HDLC) using conventional MR (Figure 2). Similar associations were observed using multivariable MR. MR-Egger showed no evidence of pleiotropic bias (P intercept=0.33). Multivariable MR analyses showed a weaker evidence of association between HDLC and ischemic stroke as it did not pass Bonferroni correction. In addition, the MR-Egger estimate showed a null association (OR: 1.01; 95% CI: 0.87–1.18). No associations were observed for HDLC with large artery atherosclerosis or cardioembolic strokes. Finally, genetically elevated triglycerides did not associate with ischemic stroke or any of its subtypes (Figure 3).
Figure 2.
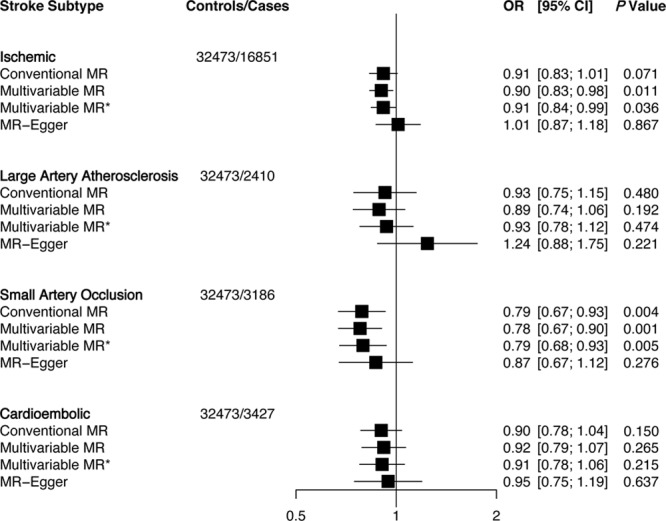
Association of high-density lipoprotein cholesterol with ischemic stroke and subtypes using different Mendelian randomization (MR) analyses. Odds ratio (OR) of ischemic stroke per 1-SD increase in each lipid trait. Conventional MR estimates were derived from 2-sample MR that forces the intercept of the slope line to zero and does not account for pleiotropy. Multivariable MR adjusts for other lipid traits and MR-Egger adjusts for unbalanced pleiotropy. *Multivariable MR analysis using summary estimates of 343 single nucleotide polymorphisms that adjust for lipid traits, body mass index (BMI), waist hip ratio adjusted for BMI, fasting plasma glucose, and fasting plasma insulin. CI indicates confidence interval.
Figure 3.
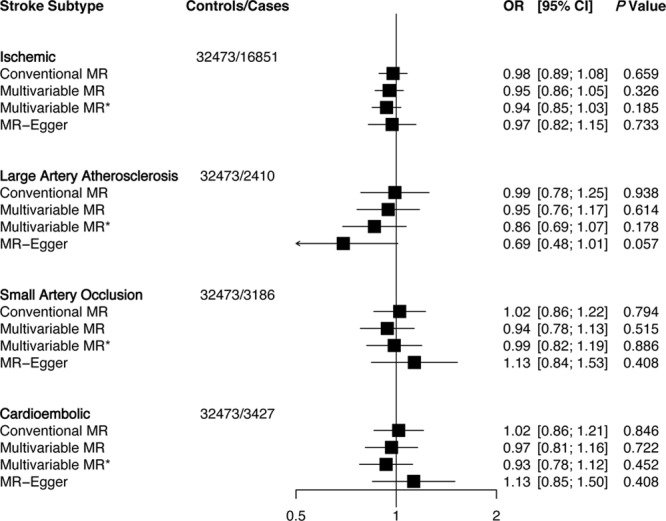
Association of triglycerides with ischemic stroke and subtypes using different Mendelian randomization (MR) analyses. Odds ratio (OR) of ischemic stroke per 1-SD increase in each lipid trait. Conventional MR estimates were derived from 2-sample MR that forces the intercept of the slope line to zero and does not account for pleiotropy. Multivariable MR adjusts for other lipid traits and MR-Egger adjusts for unbalanced pleiotropy. *Multivariable MR analysis using summary estimates of 343 single nucleotide polymorphisms that adjust for lipid traits, body mass index (BMI), waist hip ratio adjusted for BMI, fasting plasma glucose, and fasting plasma insulin. CI indicates confidence interval.
In sensitivity analyses, MR-Pleiotropy Residual Sum and Outlier test showed outlier pleiotropy between LDLC and ischemic stroke. After excluding outlier SNPs, LDLC remained associated with ischemic stroke (OR: 1.14; 95% CI: 1.06–1.24; P=0.0009; Table IV in the online-only Data Supplement). The 2-stage Steiger filtering resulted in 66- and 61-SNP LDLC instruments (explaining 5.9% and 5.6% of LDLC variance, respectively) that indicated direct association between LDLC and ischemic stroke (P=0.0001). Excluding SNPs associated with HDLC and triglycerides resulted in a 55-SNP instrument (explaining 3.6% of LDLC variance), which showed direct association with ischemic stroke (OR: 1.20; 95% CI: 1.08–1.34). All LDLC instruments after Steiger filtering and exclusion of HDLC and triglycerides SNPs showed direct association with large artery atherosclerosis stroke with ORs ranging between 1.32 and 1.36 using conventional MR.
MR-Pleiotropy Residual Sum and Outlier test showed outlier pleiotropy between HDLC and small artery atherosclerosis stroke (Table V in the online-only Data Supplement). Removal of outlier SNPs did not change our results (OR: 0.81; 95% CI: 0.70–0.93; P=0.003). Two-stage Steiger filtering resulted in 76- and 50-SNP HDLC instruments (explaining 4.9% and 4.2% of HDLC variance, respectively), which also showed nominal associations (P=0.014 and 0.010, respectively). Excluding LDLC- and triglycerides-associated SNPs resulted in a 50-SNP instrument (explaining 1.9% of HDLC variance) that did not show a significant association (OR: 0.82; 95% CI: 0.65–1.04; P=0.11).
Finally, an LDLC-lowering instrument created by SNPs in the LDLR gene supported a causal role of LDLC in ischemic (OR: 0.78; 95% CI: 0.63–0.96; per 1-SD lower LDLC) and large artery stroke (OR: 0.66; 95% CI: 0.45–0.98). Although, the HMGCR LDLC instrument showed lower risk of ischemic stroke (OR: 0.70; 95% CI: 0.50–0.99), no association was observed with large artery stroke, but a strong association was observed with small artery occlusion stroke (OR: 0.41; 95% CI: 0.21–0.81). The PCSK9 LDLC instrument showed an unexpected higher risk of cardioembolic stroke with lower LDLC but no association with all ischemic or the other stroke subtypes. The NPC1L1 instrument showed lower risk of ischemic (OR: 0.61; 95% CI: 0.37–0.99), large artery atherosclerosis (OR: 0.18; 95% CI: 0.06–0.53), and small artery occlusion (OR: 0.22; 95% CI: 0.08–0.56) strokes. Finally, a CETP instrument for higher HDLC suggested lower risk of ischemic (OR: 0.94; 95% CI: 0.88–1.00) and small artery occlusion stroke (OR: 0.88; 95% CI: 0.78–1.00; Figure I in the online-only Data Supplement).
Discussion
This MR study provides evidence for a direct relationship between LDLC and ischemic stroke that is likely driven by an association with large artery atherosclerosis stroke. There was no evidence of association of LDLC with small artery occlusion or cardioembolic stroke. In addition, results from this study provide evidence for an inverse association between HDLC and small artery occlusion stroke. Finally, this study does not provide any support for association of genetically higher triglycerides with ischemic, large artery atherosclerosis, small artery occlusion, or cardioembolic strokes.
Observational studies have provided discrepant results concerning the relationship of LDLC, HDLC, and triglycerides with ischemic stroke. Most but not all observational studies support a direct association between elevated total and LDLC and ischemic stroke.1,26,27 In addition, most studies report an inverse relationship between HDLC and ischemic stroke27–30 and a direct relationship between triglycerides and ischemic stroke.1,27–31 However, most observational studies have not performed subtyping of ischemic stroke into different pathophysiological stroke subtypes. Few studies have shown direct association between total cholesterol and large artery atherosclerosis stroke.32 However, the association between LDLC and small artery occlusion stroke has not been consistent among studies.1,33,34 Randomized controlled trials (RTCs) have provided evidence for a causal association between LDLC and ischemic stroke while such evidence is lacking for HDLC and triglycerides. Statins have consistently shown benefits in terms of cardiovascular risk reduction including stroke.2,35 However, there is insufficient evidence from HDLC and triglyceride-targeted trials concerning if elevation of HDLC or lowering of triglycerides decreases the risk of ischemic stroke. In a meta-analysis of clinical trials, none of 3 HDLC raising agents reduced ischemic stroke risk in patients treated with statins, which could potentially be related to off-target effects by these drugs.5
Clinical trials have previously shown that statins, and more recently PCSK9 inhibitors, provide comparable risk reduction in both ischemic stroke and CAD.2,36 Attributable to a lifelong genetic exposure to lower LDLC, MR studies have demonstrated higher risk reduction in CAD compared with RCTs (≈70% versus ≈25% per 1 mmol/L lower LDLC).2,6,36 Given the comparable effects of LDL lowering drugs in RCTs, one would expect MR studies with LDLC to provide a similar magnitude of risk increase for ischemic stroke as earlier shown for CAD. However, our study indicated only a 12% increased risk of ischemic stroke and 28% increased risk of large artery atherosclerosis stroke with 1 mmol/L higher LDLC. Although the MR-Egger estimates provided larger estimates (22% and 40%, respectively), they remained below the expected effect of lifelong exposure to elevated LDLC. One explanation for lower estimates could lie in the pathophysiological heterogeneity of ischemic stroke. In addition, it may be partially attributed to differences in the characteristics of the stroke cases in RCTs compared with those in our study or other MR studies. It is likely that lipid-lowering RCTs are enriched for lipid-related cardiovascular disease and that the incident stroke cases may carry more of a large artery atherosclerosis phenotype, that is, patients with higher risk by LDLC in our study compared with any ischemic stroke. Finally, prolonged exposure to elevated LDLC could have different consequences in stroke versus CAD.
As discussed above, our results suggest that genetic contribution of LDLC-related mechanisms in large artery stroke may be of lower magnitude compared with CAD despite sharing an atherosclerotic pathogenic origin. In fact, a recent study reported that a PCSK9 loss-of-function variant was not associated with ischemic nor large artery atherosclerosis stroke.37 Similarly, no association between lower LDLC by the PCSK9 variants and ischemic stroke or large artery atherosclerosis stroke was observed in our study. In addition, our study indicated a weaker effect of LDLC lowering through HMGCR and NPC1L1 variants on ischemic stroke (OR: 0.70 and 0.61 per 1 mmol/L lower LDLC) compared with previously reported effect by the same instruments on CAD (OR: ≈0.50 per 1 mmol/L lower LDLC).8 In line with these observations, previous GWAS studies have indicated enrichment of lipid pathways in CAD but not in ischemic stroke pathogenesis.37,38
Our study does not provide support for a causal relationship between LDLC and small artery occlusion or cardioembolic stroke. However, it is important to remember that our results cannot exclude a weaker causal association. We were 80% powered to detect a minimum OR of 1.2 for both of these outcomes, and lack of evidence in these MR analyses could be a consequence of insufficient statistical power. In contrast, the lower risk of ischemic stroke by HMGCR-mediated lower LDLC in our study appeared to be solely mediated through its effect on small artery occlusion. This is in contrast to the J-STARS trial that found a lower risk of large artery atherosclerosis but not small artery occlusion or cardioembolic stroke among individuals in the pravastatin arm of the trial.4 However, both the HMGCR instrument and the J-STARS trial were underpowered to detect associations with stroke subtypes, and therefore these results should not be taken as conclusive evidence. Finally, our study indicated a larger effect of NPC1L1-mediated lower LDLC on ischemic, large artery atherosclerosis, and small artery occlusion, which is in line with reported larger risk reduction of ischemic stroke (21%) compared with myocardial infarction (13%) by ezetimibe in the IMPROVE-IT trial (Improved Reduction of Outcomes: Vytorin Efficacy International Trial).39
The relationship between HDLC and ischemic stroke seems to be less clear as compared with LDLC in our study. Estimates from conventional and multivariable MR indicated nominal evidence for a weak inverse association, which vanished in the MR-Egger analysis. However, the MR-Egger intercept was not significant, and the MR-Egger analysis is less powerful compared with the inverse-variance–weighted methods.12 A recent Framingham study investigated the role of 47 HDLC SNPs in ischemic stroke and reported no association. However, that study included 301 ischemic stroke cases compared with 16 851 in the present study.40 Evidence from previous MR studies does not support a causal role between HDLC and CAD,6,41 which is also consistent with our results of no association between genetically elevated HDLC and large artery atherosclerosis stroke. However, our study suggests an inverse association between HDLC and small artery occlusion stroke supported by both conventional and multivariable MR analyses but not by the less-powered MR-Egger. In addition, CETP-mediated higher HDLC provided some evidence for lower risk of small artery occlusion stroke. This needs to be further investigated in future MR studies with larger numbers and MRI-confirmed cases of small artery occlusion stroke. The putative role of HDLC in small artery occlusion or lacunar strokes has been reported in few previous studies. Higher serum HDLC has previously been found to associate with higher cerebral vasculature CO2 reactivity that reflects the function of smaller intracerebral arteries.42 There is also some evidence that HDLC may affect endothelial dysfunction or brain soluble amyloid levels, which are probable pathogenic mechanisms in small artery occlusion stroke.43–45
Our study does not support a causal role of triglycerides in ischemic stroke. This in contrast to recent MR studies that support a direct causal role between triglycerides and CAD.6,7,46 Indeed, these results provide further support for a differential role of lipids in stroke and CAD, as discussed above. Our results are in line with those from clinical trials that have not been able to provide evidence that triglyceride lowering would affect stroke risk. In addition, our study does not show any direct causal relationship between triglycerides and large artery atherosclerosis stroke even though it shares common pathogenic mechanisms with CAD.
The main advantages of our study include the large number of ischemic stroke cases and the availability of data on ischemic stroke subtypes. In addition, we have used the most up-to-date summary-level genetic data on lipids and other cardiometabolic traits. Finally, we have used several methods to correct for a possible pleiotropic bias (multivariable MR, MR-Egger, Steiger filtering, and exclusion of pleiotropic SNPs). However, our study still has several limitations. Although the number of ischemic stroke cases was relatively large, the numbers of ischemic stroke subtypes were still relatively low, and therefore lack of evidence in some of our MR analyses could be a consequence of insufficient statistical power. However, most estimates were consistent using different MR approaches, which indicates that the observed associations are not likely to be by chance observations. Finally, we cannot rule out bias because of population stratification. However, all the SNP trait and SNP disease estimates were obtained from predominantly studies of European ancestry, which indicates that the cases, controls, and lipid measurements were obtained from comparable or similar populations.
Our results suggest that elevated LDLC levels increase the risk for ischemic stroke, indicating that further LDLC reduction is likely to result in further risk reduction in ischemic stroke. Our study further suggests that the LDLC-lowering effect may be of particular importance for risk reduction of large artery atherosclerosis stroke. However, elevated triglycerides do not increase the risk for ischemic stroke or any of its subtypes, indicating that future triglyceride-targeted therapies may not lead to beneficial effects in terms of decreasing the risk of ischemic stroke although they will likely lead to beneficial coronary effects.46 Finally, our results provide some evidence of lower small artery occlusion stroke risk by elevated HDLC, but this needs to be confirmed by adequately powered future studies.
Sources of Funding
This work was supported by the European Research Council (consolidator grant no. 649021, Dr Orho-Melander), the Swedish Research Council, the Swedish Heart and Lung Foundation, the Region Skåne, the Swedish Diabetes Foundation, the Novo Nordisk Foundation, the Albert Påhlsson Foundation, and the Linneus Foundation for the Lund University Diabetes Center. This work was, in part, supported by a senior Investigator award from the National Institute for Health Research (Dr Markus), by infrastructural support provided by the Cambridge University Trust National Institute for Health Research Biomedical Research Centre (Drs Markus and Traylor), and a British Heart Foundation Programme Grant (RG/16/4/32218). This research was supported, in part, by award number U01NS069208 from the National Institute of Neurological Disorders and Stroke. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health.
Disclosures
None.
Supplementary Material
Footnotes
The online-only Data Supplement is available with this article at http://stroke.ahajournals.org/lookup/suppl/doi:10.1161/STROKEAHA.117.019653/-/DC1.
References
- 1.Yaghi S, Elkind MS. Lipids and cerebrovascular disease: research and practice. Stroke. 2015;46:3322–3328. doi: 10.1161/STROKEAHA.115.011164. doi: 10.1161/STROKEAHA.115.011164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, et al. Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amarenco P, Bogousslavsky J, Callahan A, 3rd, Goldstein LB, Hennerici M, Rudolph AE, et al. Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–559. doi: 10.1056/NEJMoa061894. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- 4.Hosomi N, Nagai Y, Kohriyama T, Ohtsuki T, Aoki S, Nezu T, et al. J-STARS Collaborators. The Japan Statin Treatment Against Recurrent Stroke (J-STARS): a multicenter, randomized, open-label, parallel-group study. EBioMedicine. 2015;2:1071–1078. doi: 10.1016/j.ebiom.2015.08.006. doi: 10.1016/j.ebiom.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keene D, Price C, Shun-Shin MJ, Francis DP. Effect on cardiovascular risk of high density lipoprotein targeted drug treatments niacin, fibrates, and CETP inhibitors: meta-analysis of randomised controlled trials including 117,411 patients. BMJ. 2014;349:g4379. doi: 10.1136/bmj.g4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White J, Swerdlow DI, Preiss D, Fairhurst-Hunter Z, Keating BJ, Asselbergs FW, et al. Association of lipid fractions with risks for coronary artery disease and diabetes. JAMA Cardiol. 2016;1:692–699. doi: 10.1001/jamacardio.2016.1884. doi: 10.1001/jamacardio.2016.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Do R, Willer CJ, Schmidt EM, Sengupta S, Gao C, Peloso GM, et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat Genet. 2013;45:1345–1352. doi: 10.1038/ng.2795. doi: 10.1038/ng.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ference BA, Robinson JG, Brook RD, Catapano AL, Chapman MJ, Neff DR, et al. Variation in PCSK9 and HMGCR and risk of cardiovascular disease and diabetes. N Engl J Med. 2016;375:2144–2153. doi: 10.1056/NEJMoa1604304. doi: 10.1056/NEJMoa1604304. [DOI] [PubMed] [Google Scholar]
- 9.Holmes MV, Asselbergs FW, Palmer TM, Drenos F, Lanktree MB, Nelson CP, et al. UCLEB C; onsortium. Mendelian randomization of blood lipids for coronary heart disease. Eur Heart J. 2015;36:539–550. doi: 10.1093/eurheartj/eht571. doi: 10.1093/eurheartj/eht571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ference BA, Yoo W, Alesh I, Mahajan N, Mirowska KK, Mewada A, et al. Effect of long-term exposure to lower low-density lipoprotein cholesterol beginning early in life on the risk of coronary heart disease: a Mendelian randomization analysis. J Am Coll Cardiol. 2012;60:2631–2639. doi: 10.1016/j.jacc.2012.09.017. doi: 10.1016/j.jacc.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 11.Burgess S, Thompson SG. Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am J Epidemiol. 2015;181:251–260. doi: 10.1093/aje/kwu283. doi: 10.1093/aje/kwu283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44:512–525. doi: 10.1093/ije/dyv080. doi: 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willer CJ, Schmidt EM, Sengupta S, Peloso GM, Gustafsson S, Kanoni S, et al. Global Lipids Genetics Consortium. Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013;45:1274–1283. doi: 10.1038/ng.2797. doi: 10.1038/ng.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.NINDS Stroke Genetics Network (SiGN), International Stroke Genetics Consortium (ISGC) Loci associated with ischaemic stroke and its subtypes (SiGN): a genome-wide association study. Lancet Neurol. 2016;15:174–184. doi: 10.1016/S1474-4422(15)00338-5. doi: 10.1016/S1474-4422(15)00338-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 16.Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37:658–665. doi: 10.1002/gepi.21758. doi: 10.1002/gepi.21758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burgess S, Dudbridge F, Thompson SG. Re: “Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects”. Am J Epidemiol. 2015;181:290–291. doi: 10.1093/aje/kwv017. doi: 10.1093/aje/kwv017. [DOI] [PubMed] [Google Scholar]
- 18.Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, et al. LifeLines Cohort Study; ADIPOGen Consortium; AGEN-BMI Working Group; CARDIOGRAMplusC4D Consortium; CKDGen Consortium; GLGC; ICBP; MAGIC Investigators; MuTHER Consortium; MIGen Consortium; PAGE Consortium; ReproGen Consortium; GENIE Consortium; International Endogene Consortium. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206. doi: 10.1038/nature14177. doi: 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shungin D, Winkler TW, Croteau-Chonka DC, Ferreira T, Locke AE, Mägi R, et al. ADIPOGen Consortium; CARDIOGRAMplusC4D Consortium; CKDGen Consortium; GEFOS Consortium; GENIE Consortium; GLGC; ICBP; International Endogene Consortium; LifeLines Cohort Study; MAGIC Investigators; MuTHER Consortium; PAGE Consortium; ReproGen Consortium. New genetic loci link adipose and insulin biology to body fat distribution. Nature. 2015;518:187–196. doi: 10.1038/nature14132. doi: 10.1038/nature14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott RA, Lagou V, Welch RP, Wheeler E, Montasser ME, Luan J, et al. DIAbetes Genetics Replication and Meta-analysis (DIAGRAM) Consortium. Large-scale association analyses identify new loci influencing glycemic traits and provide insight into the underlying biological pathways. Nat Genet. 2012;44:991–1005. doi: 10.1038/ng.2385. doi: 10.1038/ng.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hemani G, Tilling K, Davey Smith G. Correction: orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. 2017;13:e1007149. doi: 10.1371/journal.pgen.1007081. doi: 10.1371/journal.pgen.1007149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yavorska OO, Burgess S. MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int J Epidemiol. 2017;46:1734–1739. doi: 10.1093/ije/dyx034. doi: 10.1093/ije/dyx034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verbanck M, Chen C-Y, Neale B, Do R. Widespread pleiotropy confounds causal relationships between complex traits and diseases inferred from Mendelian randomization [published online ahead of print June 30, 2017]. BioRxiv. doi: 10.1101/157552. https://www.biorxiv.org/content/early/2017/06/30/157552. [Google Scholar]
- 24.Ference BA, Kastelein JJP, Ginsberg HN, Chapman MJ, Nicholls SJ, Ray KK, et al. Association of genetic variants related to CETP inhibitors and statins with lipoprotein levels and cardiovascular risk. JAMA. 2017;318:947–956. doi: 10.1001/jama.2017.11467. doi: 10.1001/jama.2017.11467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brion MJ, Shakhbazov K, Visscher PM. Calculating statistical power in Mendelian randomization studies. Int J Epidemiol. 2013;42:1497–1501. doi: 10.1093/ije/dyt179. doi: 10.1093/ije/dyt179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurth T, Everett BM, Buring JE, Kase CS, Ridker PM, Gaziano JM. Lipid levels and the risk of ischemic stroke in women. Neurology. 2007;68:556–562. doi: 10.1212/01.wnl.0000254472.41810.0d. doi: 10.1212/01.wnl.0000254472.41810.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shahar E, Chambless LE, Rosamond WD, Boland LL, Ballantyne CM, McGovern PG, et al. Atherosclerosis Risk in Communities Study. Plasma lipid profile and incident ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2003;34:623–631. doi: 10.1161/01.STR.0000057812.51734.FF. doi: 10.1161/01.STR.0000057812.51734.FF. [DOI] [PubMed] [Google Scholar]
- 28.Lindenstrøm E, Boysen G, Nyboe J. Influence of total cholesterol, high density lipoprotein cholesterol, and triglycerides on risk of cerebrovascular disease: the Copenhagen City Heart Study. BMJ. 1994;309:11–15. doi: 10.1136/bmj.309.6946.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sacco RL, Benson RT, Kargman DE, Boden-Albala B, Tuck C, Lin IF, et al. High-density lipoprotein cholesterol and ischemic stroke in the elderly: the Northern Manhattan Stroke Study. JAMA. 2001;285:2729–2735. doi: 10.1001/jama.285.21.2729. [DOI] [PubMed] [Google Scholar]
- 30.Amarenco P, Labreuche J, Touboul PJ. High-density lipoprotein-cholesterol and risk of stroke and carotid atherosclerosis: a systematic review. Atherosclerosis. 2008;196:489–496. doi: 10.1016/j.atherosclerosis.2007.07.033. doi: 10.1016/j.atherosclerosis.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 31.Labreuche J, Touboul PJ, Amarenco P. Plasma triglyceride levels and risk of stroke and carotid atherosclerosis: a systematic review of the epidemiological studies. Atherosclerosis. 2009;203:331–345. doi: 10.1016/j.atherosclerosis.2008.08.040. doi: 10.1016/j.atherosclerosis.2008.08.040. [DOI] [PubMed] [Google Scholar]
- 32.Tirschwell DL, Smith NL, Heckbert SR, Lemaitre RN, Longstreth WT, Jr, Psaty BM. Association of cholesterol with stroke risk varies in stroke subtypes and patient subgroups. Neurology. 2004;63:1868–1875. doi: 10.1212/01.wnl.0000144282.42222.da. [DOI] [PubMed] [Google Scholar]
- 33.Imamura T, Doi Y, Arima H, Yonemoto K, Hata J, Kubo M, et al. LDL cholesterol and the development of stroke subtypes and coronary heart disease in a general Japanese population: the Hisayama study. Stroke. 2009;40:382–388. doi: 10.1161/STROKEAHA.108.529537. doi: 10.1161/STROKEAHA.108.529537. [DOI] [PubMed] [Google Scholar]
- 34.Amarenco P, Labreuche J, Elbaz A, Touboul PJ, Driss F, Jaillard A, et al. GENIC Investigators. Blood lipids in brain infarction subtypes. Cerebrovasc Dis. 2006;22:101–108. doi: 10.1159/000093237. doi: 10.1159/000093237. [DOI] [PubMed] [Google Scholar]
- 35.Heart Protection Study Collaborative Group. MRC/BHF heart protection study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. doi: 10.1016/S0140-6736(02)09327-3. [Google Scholar]
- 36.Taylor F, Ward K, Moore TH, Burke M, Davey Smith G, Casas JP, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2011:CD004816. doi: 10.1002/14651858.CD004816.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hopewell JC, Malik R, Valdés-Márquez E, Worrall BB, Collins R METASTROKE Collaboration of the ISGC. Differential effects of PCSK9 variants on risk of coronary disease and ischaemic stroke. Eur Heart J. 2018;39:354–359. doi: 10.1093/eurheartj/ehx373. doi: 10.1093/eurheartj/ehx373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malik R, Traylor M, Pulit SL, Bevan S, Hopewell JC, Holliday EG, et al. ISGC Analysis Group; METASTROKE collaboration; Wellcome Trust Case Control Consortium 2 (WTCCC2); NINDS Stroke Genetics Network (SiGN) Low-frequency and common genetic variation in ischemic stroke: the METASTROKE collaboration. Neurology. 2016;86:1217–1226. doi: 10.1212/WNL.0000000000002528. doi: 10.1212/WNL.0000000000002528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al. IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. doi: 10.1056/NEJMoa1410489. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 40.Pikula A, Beiser AS, Wang J, Himali JJ, Kelly-Hayes M, Kase CS, et al. Lipid and lipoprotein measurements and the risk of ischemic vascular events: Framingham Study. Neurology. 2015;84:472–479. doi: 10.1212/WNL.0000000000001202. doi: 10.1212/WNL.0000000000001202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, et al. Plasma HDL cholesterol and risk of myocardial infarction: a Mendelian randomisation study. Lancet. 2012;380:572–580. doi: 10.1016/S0140-6736(12)60312-2. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bakker SL, de Leeuw FE, Koudstaal PJ, Hofman A, Breteler MM. Cerebral CO2 reactivity, cholesterol, and high-density lipoprotein cholesterol in the elderly. Neurology. 2000;54:987–989. doi: 10.1212/wnl.54.4.987. [DOI] [PubMed] [Google Scholar]
- 43.Knottnerus IL, Ten Cate H, Lodder J, Kessels F, van Oostenbrugge RJ. Endothelial dysfunction in lacunar stroke: a systematic review. Cerebrovasc Dis. 2009;27:519–526. doi: 10.1159/000212672. doi: 10.1159/000212672. [DOI] [PubMed] [Google Scholar]
- 44.Riwanto M, Landmesser U. High density lipoproteins and endothelial functions: mechanistic insights and alterations in cardiovascular disease. J Lipid Res. 2013;54:3227–3243. doi: 10.1194/jlr.R037762. doi: 10.1194/jlr.R037762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robert J, Stukas S, Button E, Cheng WH, Lee M, Fan J, et al. Reconstituted high-density lipoproteins acutely reduce soluble brain Aβ levels in symptomatic APP/PS1 mice. Biochim Biophys Acta. 2016;1862:1027–1036. doi: 10.1016/j.bbadis.2015.10.005. doi: 10.1016/j.bbadis.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 46.Crosby J, Peloso GM, Auer PL, et al. TG and HDL Working Group of the Exome Sequencing Project, National Heart, Lung, and Blood Institute. Loss-of-function mutations in APOC3. triglycerides, and coronary disease. N Engl J Med. 2014;371:22–31. doi: 10.1056/NEJMoa1307095. doi: 10.1056/NEJMoa1307095. [DOI] [PMC free article] [PubMed] [Google Scholar]


