ABSTRACT
OBJECTIVE:
Critical colonization or local infection is very common in chronic wounds, but clinically problematic. Because therapeutic options for these conditions are limited in number and efficacy, the study authors tested a new acid-oxidizing solution (AOS [Nexodyn]; APR Applied Pharma Research S.A., Balerna, Switzerland) to determine its ancillary antimicrobial properties and potential support for wound healing.
DESIGN AND SETTING:
This open-label clinical case series was conducted with a prospective, single-arm design at the Federal County Hospital in Bregenz, Austria.
PATIENTS:
In the study, 30 patients with critically colonized or locally infected chronic leg ulcers of any origin were included.
INTERVENTIONS:
The AOS was applied on each leg ulcer at every dressing change for 35 days.
MAIN OUTCOME MEASURES:
The tolerability and performance of the AOS were assessed by evaluating the ulcer characteristics and comparing them with those at baseline. The clinical course of wounds was analyzed using standard measures for bioburden, local infection, pain, pH, and wound healing.
MAIN RESULTS:
Application of the solution was well tolerated, and no adverse events were recorded. In all patients, local infection was overcome, and wound bed pH and wound area decreased significantly. In addition, patient pain levels decreased to a level where interventions were not required after study day 7. In 37% of all patients, a complete resolution of chronic ulcers was achieved by the end of the study period.
CONCLUSION:
According to these results, the AOS seems to be a valid and highly tolerable treatment to support wound healing in locally infected ulcers. Nevertheless, larger controlled cohort studies are needed to substantiate these findings.
KEYWORDS: acid-oxidizing solution, bioburden, chronic ulcers, critically colonized wounds, local infection, pH, ulcers, wound microenvironment
INTRODUCTION
Chronic leg or foot ulcers are chronic wounds below the knee that persist for more than 6 weeks.1 They are the most prevalent type of chronic ulcers and are typically caused by venous insufficiency or arterial occlusion syndrome.2,3 Affecting between 0.6% and 3% of the general population in developed countries, the prevalence has increased to more than 5% in people older than 80 years.4,5 In the United Kingdom, treatment costs for chronic wounds have been estimated at £2.3 to £3.1 billion6 and account for an estimated $6 to $15 billion annually in the United States.7
Critical colonization by pathogens and infection of chronic wounds present a dual problem for healthcare workers. On the one hand, local infections result in delayed wound healing with a high impact on quality of life, leading to increased exudate, pain and discomfort, delays in returning to a normal daily routine, and exacerbating concerns about amputation. On the other hand, critically and locally infected wounds are a potential source of dangerous systemic infections. This is particularly relevant for immunocompromised patients or for grossly contaminated wounds.8 Further, chronic wounds are often accompanied by microbial bioburden or biofilm. Bioburden, which is typically abundant, polymicrobial, and extremely diverse, acts as a significant barrier to healing for all chronic wounds.9
With demographic changes and a higher incidence of chronic ulcers, providers must consider increased treatment costs and nursing care.8,10,11 In clinical practice, managing chronic infections is a key part of treating chronic wounds that requires a range of different products from antiseptics to specific dressings.
Antibiotics or antiseptic wound cleansing solutions are the standard of care in the treatment of locally infected wounds. However, the products currently on the market show only limited efficacy in promoting the healing of venous leg ulcers.12 In recent years, iodine-based wound products and silver-containing dressings have been widely used to clean and control local infections.13–16 Although the antibacterial effects of nanocrystalline silver are well known,17 local infections often cannot be controlled with silver-based dressings alone. Further, some in vitro studies indicate that silver-based dressings may be cytotoxic.18–20 In a recent international consensus,21 the appropriate use of silver-containing dressings was discussed on the basis of 2 Cochrane reviews and a high-profile randomized controlled trial.22–24 Because of this controversy,25–28 the experts set guidelines for appropriate use of silver dressings based on wound characteristics. For clinical practice, further research is needed to prove the effectiveness of other antimicrobial and antiseptic wound cleansing products, and a high demand exists for an easily applicable, active topical treatment to control chronic wound infection.
For the treatment of chronic wounds, such as lower leg and vascular ulcers, as well as diabetic foot and pressure ulcers, postsurgical wounds, burns, and other lesions, a highly pure (>95% of free chlorine species) hypochlorous acid–based acid-oxidizing solution (AOS [Nexodyn]; APR Applied Pharma Research S.A., Balerna, Switzerland) has been developed.29 In preclinical tests, the AOS has shown a favorable tolerability profile without acute toxicity and no significant signs of eye, mucosa, or skin irritation or mutagenic or sensitizing properties.30
The aim of this clinical case series was to assess the local tolerability, safety, and performance of the AOS together with application of nonadherent absorbent dressings in the treatment of critically colonized and locally infected leg ulcers.
METHODS
This prospective, single-arm, open-label clinical case series was conducted at the Central Ambulance of Wound Care, Department of Nursing, Federal County Hospital in Bregenz, Austria, between March and December 2015. The study was approved according to the Austrian Medical Devices Law in compliance with the ethical guidelines of the 1975 Declaration of Helsinki (ethical committee no. EK-2-2015/0002, approved April 13, 2015).
The wound characteristics (records) of all patients receiving the AOS were documented and analyzed at each assessment using a case report form. Baseline characteristics of each wound were assessed at start of treatment (day 0). Ulcer characteristics were then evaluated at day 3 and every 7 days (± 2) until the end of the study at day 35, for a total of 6 assessments per participant.
Patients
According to the study plan, patients had to meet the following inclusion criteria: age between 18 and 95 years, presence of a critically colonized or locally infected chronic leg ulcer of any origin (onset at least 6 weeks before enrollment), a wound area of up to 20 × 10 cm, and no visible exposure of tendon or bone. In cases of multiple wounds per patient, only 1 study wound was examined, chosen based on size and suitable localization.
Patients were excluded if their wounds were acute or showed a greater than 60% presence of necrotic eschar. In addition, pregnant and breastfeeding women were excluded from the study. Patients taking ongoing systemic antibiotic therapy or who used these drugs within 3 weeks before onset of the study were excluded. Further exclusion criteria included allergy or intolerance to any of the components of the AOS, as well as participation in any clinical trial up to 1 month prior to the start of the study. All patients consented to anonymous patient data collection, including pictures.
Treatment
In the 35-day study, condition of the ulcers (wound size, local infection, pH value, etc) were assessed at the initiation visit (day 0; baseline) by a single investigator.
Ulcer treatment in all patients was conducted as follows: First, the ulcers were cleaned with a dry gauze directly after removal of the dressing. Second, the AOS was liberally sprayed to cover the whole wound. After 2 minutes, ulcers were cleaned again with a sterile gauze. The AOS was administered for a second time. Then, Adaptic Nonadhering Dressing (KCI Medizinprodukte GmbH/An Acelity Company, Wiesbaden, Germany), which was soaked with the AOS, and a sterile, highly absorbent all-purpose dressing (Vliwazell; Lohmann and Rauscher, Rengsdorf, Germany) were applied to the wound surface. Adaptic is a primary wound contact dressing composed of knitted cellulose acetate fabric and impregnated with a petrolatum emulsion.
All patients with venous ulcers were treated with a compression therapy in addition to the application of the AOS. Patients with ulcers of other origins did not receive any further therapy.
As long as the wound was critically colonized or locally infected, a daily dressing change was conducted. In wounds without infection, a dressing change was performed every other day, and dressing changes over the weekend were delayed to the first working day.
Evaluation of Study Parameters: Primary Outcome
The tolerability of the application of the AOS was assessed as the primary outcome parameter of the current study. Therefore, the following parameters were evaluated at every visit and compared with baseline ulcer characteristics: no problems, new development/intensification of erythema, maceration, blisters, or congestion of exudate.
At study start and at each designated visit, a global evaluation of the patient’s acceptance of the AOS immediately after product application was evaluated by each patient using 2 qualitative 4-point scales, one for comfort (1 = very comfortable, 2 = slightly comfortable, 3 = slightly uncomfortable, and 4 = very uncomfortable) and the other for pain perception (1 = relief sensation [such as pain relief and cooling effect], 2 = partial relief sensation, 3 = slight pain sensation, and 4 = pain sensation).
Evaluation of Study Parameters: Secondary Outcomes
Secondary outcome measures included a number of clinically relevant parameters, such as clinical signs of infection, dynamics of pain, dynamics of the bioburden coating the wound, wound size reduction and healing, patient acceptance, and device management.
For the evaluation of the wound size, the digital planimetry software program PictZar (BioVisual Technologies LLC, Elmwood Park, New Jersey) was used.
Critical colonization/local infection was diagnosed by the investigator on clinical grounds using the well-established criteria of (1) impaired fragile granulation tissue, (2) more exudate, (3) more pain, and (4) impaired wound healing.31–33 The severity of the clinical picture was graded according to a scoring system, which has been successfully used in other antimicrobial trials,34 with a scale from 1 to 10 (1 = no signs, 10 = maximal signs).
Wound coverings were evaluated using the percentage of bioburden load covering the wound surface as measured by PictZar.
Dynamics of pain were evaluated before each dressing change by asking the patients about their pain levels for the whole interval since the last visit. For this, a visual analog scale ranging from 0 to 10 (0 = no pain, 10 = worst imaginable pain) was used.
Dynamics of pH values were addressed by putting a probe of pH 7.0 adjusted pH meter (HI99181; Hanna Instruments, Villafranca Padovana, Italy) into the center of the wound.
Wound healing was defined by the dynamics of the wound area, which was also measured with PictZar.
The overall usability and convenience (eg, ease of use, handling, cleanliness, time needed for wound treatment, and utilization of accessory resources such as gauzes, tissues, or other devices) in association with the AOS were evaluated at the last visit by the caregiver using the following 4-point scale: 1 = excellent overall convenience, 2 = good overall convenience, 3 = fair overall convenience, and 4 = poor overall convenience.
Patients with completely healed wounds stopped subsequent planned visits for wound control. A wound area of 0 was inputted for these patients thereafter. All other parameters (local infection, bioburden, pH, pain score, comfort, and application pain perception) were not provided and remained missing. Inputting best values for these parameters for healed wounds was not performed, but it can be assumed that they would have further improved results from visit 4 to visit 6.
Statistical Analysis
Continuous data were described as mean ± SD in case of normally distributed data and with median (minimum-maximum) otherwise. Categorical data were described by counts and percentages. A nonparametric Friedman test was used to detect changes over time for blocked continuous variables (local infection, pain, bioburden, pH, and wound size). Nonparametric partial Spearman correlations (rs) were calculated to assess the association between continuous variables adjusted for effects of different visit times. Linear mixed models with repeated measures were calculated to assess signs of local infection and wound size by time under treatment, baseline pH measurement, and the corresponding baseline value before start of treatment, which was signs of local infection or wound size, respectively. Dependency between repeated measures for each patient was statistically modeled by a first-order autoregressive variance-covariance matrix.
Statistical analyses were conducted by using SAS software (version 9.4; SAS Institute Inc, Cary, North Carolina). All P values are 2 sided, and P ≤ .05 was considered statistically significant.
RESULTS
Demographics of Patients and Characteristics of Target Ulcers
Seventeen (56.67%) of the 30 patients included in the clinical case series with locally infected chronic leg ulcers were male, and 13 (43.33%) were female. The median age was 66.3 years (range, 34.6–80.2 years; Table 1). Ulcers were of different etiology: 13 venous, 4 arterial, 12 mixed, and 1 diabetic (Table 1). At baseline, the mean local infection score, which ranged from 1 (no signs) to 10 (maximal signs), was 7.90 (SD, 1.52). The mean pain score (using the visual analog scale) reached 7.96 (SD, 1.35). At the start of the study, 65% of the patients’ wounds (13 of 20 patients) were completely covered with bioburden. Only 1 patient (5%) had no bioburden, and 2 patients (10%) had between 1% and 2% of the wound area covered. The mean pH value was 9.25 (SD, 0.61), and the median wound area was 3.06 cm2 (0.49–32.79 cm2).
Table 1.
PATIENT AND DISEASE CHARACTERISTICS AT START OF THE STUDY (N = 30)

Tolerability and Adverse Events
For safety analysis, clinical data from all 30 patients were included. All patients (100%) tolerated the application of the AOS at all visits and at all 171 dressing changes. Further, no adverse or serious adverse events were reported during the study period. As previously described, acceptance of the AOS was evaluated at each designated visit; immediately after application of the AOS, 70% of all patients answered that they felt comfortable, and 30% of the patients felt very comfortable with the product’s application across the study period. All patients reported either partial or full pain relief with the application of the AOS during their visits. Whereas 13.3% of the patients reported full relief at visit 1, at visit 6 the proportion of patients with full relief increased to 50%. In all cases (100%), caregivers said the AOS had good overall convenience, which was assessed by a global evaluation of the product’s usability at the last visit.
Representative wound images from study patients reveal the beneficial effect of integrating the AOS into treatment (Figure 1). Within 3 and 4 weeks, respectively, all infected ulcers had improved significantly.
Figure 1.
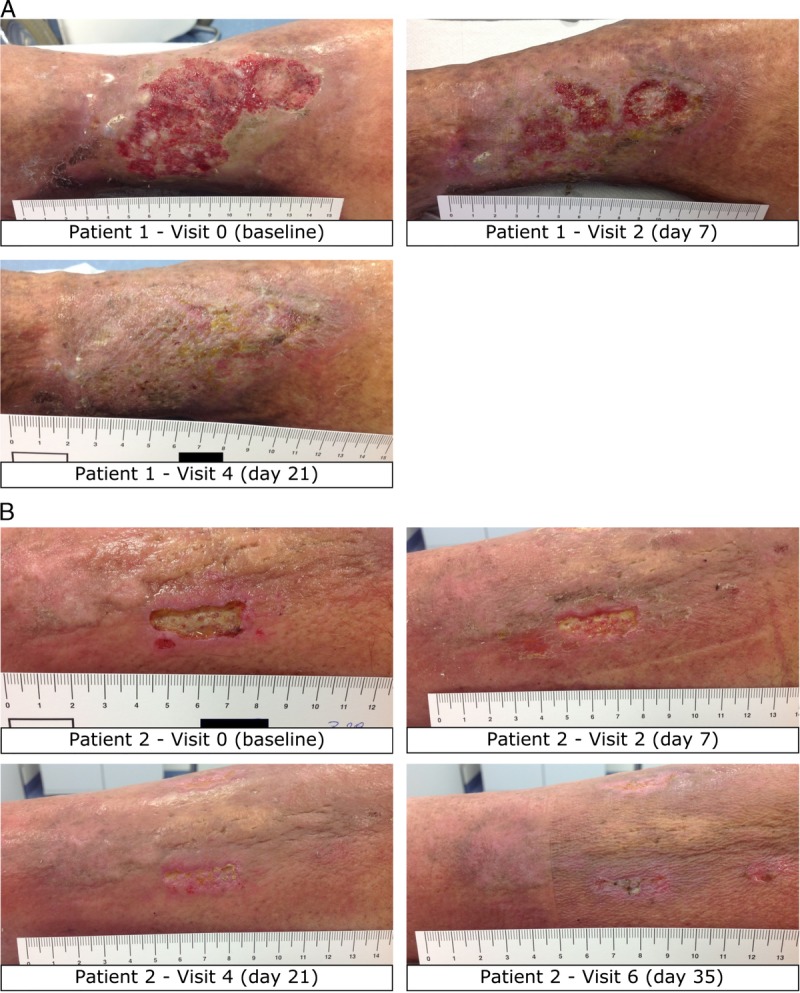
COURSE OF HEALING IN 2 REPRESENTATIVE STUDY PATIENTS RECEIVING TREATMENT INCLUDING THE AOS
Infection Parameters
The addition of the AOS to the described dressings led to a considerable improvement of the clinical signs of local infection from a median score of 8 at baseline to a median score of 1.5 at visit 6 (P < .0001; Figure 2, Table 2). In addition, the AOS significantly decreased the presence of local bioburden covering the wound from median baseline levels of 100% (0%–100%) to 13.55% (0%–81%) at visit 5 (day 28 ± 2; P = .0009; Table 2 and Figure 3). The decreased local infection score and diminished percentages of bioburden were accompanied by a noteworthy reduction in wound pH values. At the start of the study, the ulcers showed a highly alkaline pH (9.25 ± 0.61). Mean pH decreased significantly (P < .0001) over time, with ulcers showing an almost neutral pH value (7.68 ± 0.71) by visit 5 (day 28 ± 2; Table 2 and Figure 4).
Figure 2.
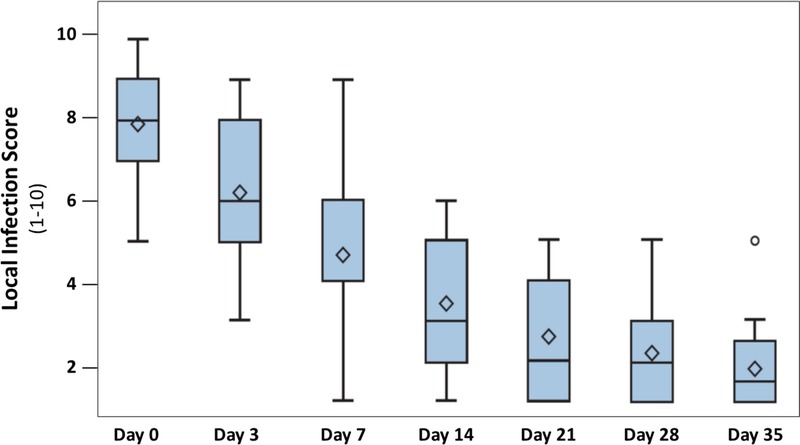
CHANGE IN LOCAL INFECTION SCORE
Data are presented in box plots where the lower and upper borders of the box represent lower and upper quartiles of the data distribution, respectively. Diamonds represent mean values, and the bars in the box, the median of the data. Over time, the local infection score decreased steadily and significantly (P < .0001, Friedman test). Between day 21 (visit 4) and 35 (visit 6), chronic ulcers had healed in 37% of patients.
Table 2.
PERFORMANCE PARAMETERS OF CHRONIC ULCERS IN PATIENTS TREATED WITH AOS FOR 35 DAYS
Figure 3.
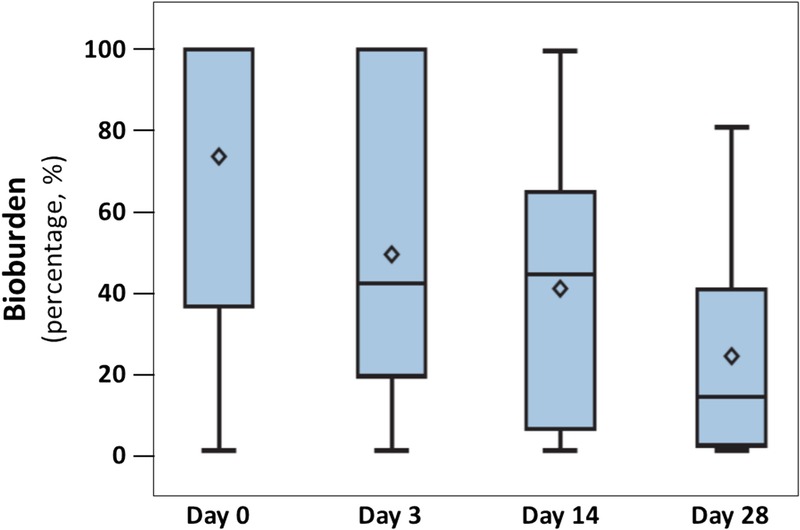
CHANGE IN BIOBURDEN
Data are presented by box plots where the lower and upper borders of the box represent lower and upper quartiles of the data distribution, respectively. Diamonds represent mean values, and the bars in the box, the median of the data. Over time, the percentage of bioburden covering the wound decreased steadily and significantly (P = .0009, Friedman test).
Figure 4.
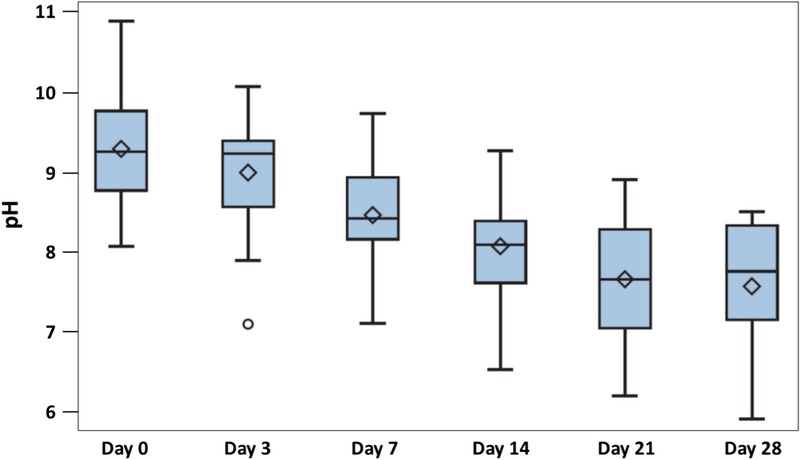
CHANGE IN PH VALUE
Data are presented by box plots where the lower and upper borders of the box represent lower and upper quartiles of the data distribution, respectively. Diamonds represent mean values, and the bars in the box, the median of the data. Over time, the pH values measured on the wound beds decreased steadily and significantly (P < .0001, Friedman test).
The effects of treatment on the local infection score from day 3 onward were modeled based on the elapsed treatment time and baseline measurements of local infection and pH. Only baseline infection score (P = .0002) and elapsed time (P < .0001) showed a significant association with local infection score after the start of treatment. The higher the initial infection score, the higher the infection score after treatment; however, the more time that elapsed, the smaller the documented infection score was.
Ulcer Healing and Wound Size
By the end of the study period, 11 (36.67%) of the treated 30 chronic ulcers had healed completely (Table 2). Further, no reinfection of any of the examined ulcers was observed during the study period. In general, the treatment regimen led to a highly significant decrease in wound size (P < .0001; Figure 5). At the beginning of the study, the wound size was a median 3.06 cm2 (0.49–32.79 cm2), which decreased to a median of 0.59 cm2 (0–15.25 cm2) at the end of the study (Table 2). Interestingly, the decreased wound size correlated significantly with the diminished pH value of the wound (partial Spearman correlation coefficient adjusted by visit time; rs = 0.1957, P = .0108). Further, a strong and highly significant correlation between the pH change and the successful control of infection was detected (rs = 0.6960 adjusted for visit time; P < .0001).
Figure 5.
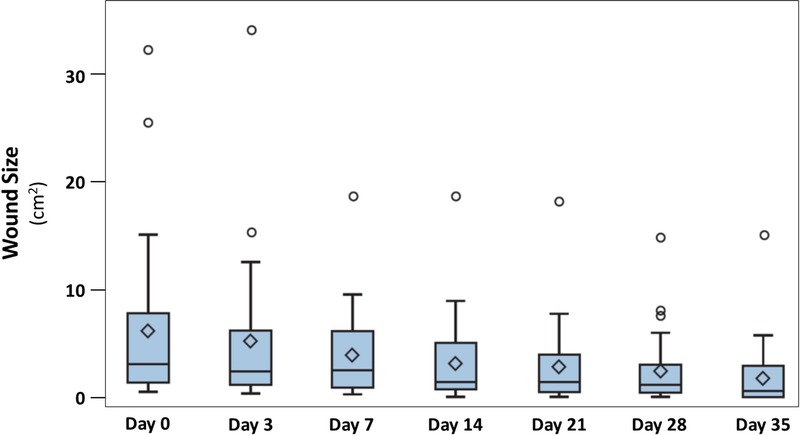
CHANGE IN WOUND SIZE
Data are presented by box plots where the lower and upper borders of the box represent lower and upper quartiles of the data distribution, respectively. Diamonds represent mean values, and the bars in the box, the median of the data. Over time, wound area decreased steadily and significantly (P < .0001, Friedman test).
Similar effects were observed for the influence of treatment on wound size. Baseline wound size (P < .0001) and elapsed treatment time (P = .0038) were significantly associated with wound size during treatment, whereas baseline pH did not reveal a significant effect.
Pain
Wound-associated pain levels decreased significantly over the study duration (P < .0001; Figure 6). At baseline, patient pain level was a median 8 (6–10). Pain perception significantly and steadily decreased over the study period, reaching a median value of 1 (1–5) at visit 6 (day 35 ± 2; Table 2).
Figure 6.
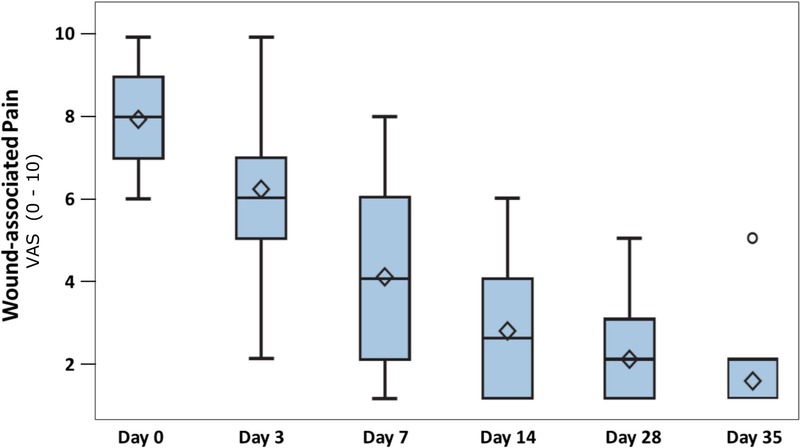
CHANGE IN WOUND-ASSOCIATED PAIN
Data are presented by box plots where the lower and upper borders of the box represent lower and upper quartiles of the data distribution, respectively. Diamonds represent mean values, and the bars in the box, the median of the data. Over time, the wound-associated pain decreased steeply and significantly (P < .0001, Friedman test).
DISCUSSION
In this clinical case series, an AOS was used to control local infections in chronic leg ulcers of any origin. Even without the additional application of antiseptics, local infections were resolved at the latest by day 28 of the study. Applied only with commonly used inert gauzes, the solution substantially contributed to a reduced local infection score and a diminished percentage of bioburden covering the wound as determined by the investigator.
Swabs or microbiologic analyses to determine bacterial load and composition were not performed within this study for several reasons. First, signs of clinical local infections were clearly defined by well-established criteria,31–33 as well as the use of a widely accepted scoring system.34 Another rationale is that it is not the bacterial load but the clinical outcome that determines the progression of wound healing.35 A third important aspect is that although the germs within a wound are detectable with standard microbiologic methods, no conclusion can be drawn regarding their pathogenicity, which is essential information.
One possible pathogenic mechanism underlying the observed infection reduction is a normalization of the alkaline pH value of the chronic wounds (mean, 9.25 ± 0.61) to a neutral pH value (mean, 7.68 ± 0.71) induced by the use of the highly acidic solution. In general, the correlation between skin surface pH and differential bacterial colonization patterns is well known, and the prevention or halting of colonization by lowering the pH in wounds with acidifying agents has been demonstrated in several investigations for different bacterial strains.36–38 Under normal conditions, the skin forms an acidic milieu as a functional physiologic barrier to control bacterial growth.39,40 However, within chronic dermal lesions, the skin’s acidic milieu is disturbed by the body’s internal pH. This enables the growth of the most relevant human-pathogenic bacteria.38–42
The strong impact of a decreased pH on the bacteria can be explained by different effects. On the one hand, structural changes in proteins have been observed in human-pathogenic strains such as staphylococci.43 Further, it can perturb bacterial transmembrane pH gradients36 and have an effect on proton-regulated protein expression pathways.43,44
In accordance with recent literature demonstrating the usefulness of acidic solutions in controlling bacterial growth,38,45 these results provide convincing evidence for the beneficial effect of acidification of the chronic wound milieu. This aspect is also supported by a strong correlation between the pH change and the successful control of locally infected ulcers treated with the AOS.
Approximately 37% of all patients in this clinical case series showed complete ulcer healing during or at the end of the study. This observation could be explained by different properties of the AOS. First, because of the active cleansing properties of the AOS, wound-associated infections were eliminated, accompanied by a reduction in wound pH values. Through this mechanism, an important prerequisite for wound healing was fulfilled. This finding is in accordance with results demonstrated by Tsukada et al46 showing a restoration of the low pH acid mantle in patients with pressure injuries as wounds progress toward healing.
However, the pH value not only influences the bacterial colonization of a wound, but also plays a pivotal role in the highly coordinated physiologic wound healing process.47 Within the 3 overlapping major phases of wound healing (inflammation, proliferation, and remodeling), pH value changes are supposed to have a substantial impact on the function of the different types of cells and enzymes involved. For example, under normal conditions, the pH gradient progresses from an alkaline to a neutral wound milieu and then becomes acidic at the beginning of the inflammation phase.47,48 This initial physiologic acidosis is important for the induction of wound healing. Further, the activity of enzymes, such as the matrix metalloproteinases) and their opponent, tissue inhibitors, is strongly regulated by pH changes. The physiologic balance between enzymes responsible for tissue degradation and those promoting tissue reassembly is a prerequisite for successful wound healing. However, in chronic wounds, this balance is lost, resulting in a domination of catabolic processes inhibiting the wound healing progression.39,49,50 Another aspect is the favored oxygen release in an acidic environment that supports tissue oxygenation. This is important to the enhanced energy metabolism of regenerating cells within a wound, as well as resistance to infections.51–53 Based on all of this information, study authors hypothesize that AOS application together with inert gauzes was sufficient to normalize the pH value of chronic wounds and influence biochemical reactions critical for physiologic wound healing.38–40,42,47,48
In addition, these data are in line with the results of a recently published in vitro study in reconstructed human epidermis showing that an AOS could induce morphologic changes to the extracellular matrix of biofilms, resulting in the facilitated release and elimination of bacteria from the extracellular matrix.30 However, because of missing data regarding bioburden, these data should be considered with caution.
A preliminary clinical experience in patients (n = 20) treated with the AOS and standard treatments for 6 weeks confirms the beneficial effects. Overall, a significant reduction versus baseline of the wound size was demonstrated, while a complete healing of chronic wounds was seen in 25% of the patients.54 As presented in this study, the AOS significantly reduced the percentage of bioburden covering the wound, and therefore, this newly developed and well-tolerated medical innovation could play a key role in the management of critically infected ulcers.
Limitations
This clinical case series was designed as a single-arm study to examine the safety and performance of the AOS together with inert gauzes in a pilot test. Therefore, the presented study has a prospective observational design, asking mainly for tolerability and a basic proof of concept, especially for patients within a daily routine. In this pilot study, it made sense to restrict wound size to up to 20 × 10 cm, which explains why few patients with small wound areas were treated.
Because no comparator or control groups exist in this study, results obtained reveal a clear tendency of the characteristics of the treatment device used, but yield no highly robust evidence. In this respect, study authors found that application of the AOS together with inert gauzes facilitated wound healing significantly. However, to determine whether the low pH of the AOS was the decisive factor causing the decreased wound pH, a double-blind randomized controlled trial comparing topical agents with different pH values should be conducted. In all patients included in this study, a beneficial safety and performance profile of the AOS could be seen. Nevertheless, to fully understand the wide range of reactions and benefits, larger patient cohorts in randomized controlled comparative trials are needed.
CONCLUSIONS
This prospective, open-label clinical study was conducted to assess ancillary antimicrobial properties of a new AOS and its support of wound healing. The tolerability and effectiveness profile was assessed by evaluating the clinical course of wounds using standard measures for bioburden, local infection, pain, pH, and wound healing. According to these results, the addition of an AOS seems to be a valid and highly tolerable method to support wound healing in locally infected ulcers. Nevertheless, larger controlled cohort studies are needed to substantiate these findings.
Footnotes
Acknowledgments: The authors thank Mrs Ramona Lundt and Mrs Andrea Rathmann-Schmitz, PhD, for their assistance in preparing the manuscript for publication and Ms Gaia Piraccini at APR Applied Pharma Research S.A. for her review and comments.
This study was financially supported by APR Applied Pharma Research S.A. (Balerna, Switzerland), and study authors were provided Nexodyn free of charge. Dr Strohal works as a consultant for APR Applied Pharma Research S.A.
The other authors declare that they have no conflicts of interest.
REFERENCES
- 1.Kahle B, Hermanns HJ, Gallenkemper G. Evidence-based treatment of chronic leg ulcers. Dtsch Arztebl Int 2011;108(14):231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valencia IC, Falabella A, Kirsner RS, Eaglstein WH. Chronic venous insufficiency and venous leg ulceration. J Am Acad Dermatol 2001;44(3):401-21. [DOI] [PubMed] [Google Scholar]
- 3.Richmond NA, Maderal AD, Vivas AC. Evidence-based management of common chronic lower extremity ulcers. Dermatol Ther 2013;26(3):187-96. [DOI] [PubMed] [Google Scholar]
- 4.Rayner R, Carville K, Keaton J, Prentice J, Santamaria N. Leg ulcers: atypical presentations and associated comorbidities. Wound Pract Res 2009;17(4):168-85. [Google Scholar]
- 5.Agale SV. Chronic leg ulcers: epidemiology, aetiopathogenesis, and management. Ulcers 2013;2013:1-9. [Google Scholar]
- 6.Posnett J, Franks PJ. The burden of chronic wounds in the UK. Nurs Times 2008;104(3):44-5. [PubMed] [Google Scholar]
- 7.Markova A, Mostow EN. US skin disease assessment: ulcer and wound care. Dermatol Clin 2012;30(1):107-11, ix. [DOI] [PubMed] [Google Scholar]
- 8.White RJ, Cooper R, Kingsley A. Wound colonization and infection: the role of topical antimicrobials. Br J Nurs 2001;10(9):563-78. [DOI] [PubMed] [Google Scholar]
- 9.Jones CE, Kennedy JP. Treatment options to manage wound biofilm. Adv Wound Care 2012;1(3):120-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marola S, Ferrarese A, Solej M, Enrico S, Nano M, Martino V. Management of venous ulcers: state of the art. Int J Surg 2016;33 Suppl 1:S132-4. [DOI] [PubMed] [Google Scholar]
- 11.Powers JG, Higham C, Broussard K, Phillips TJ. Wound healing and treating wounds: chronic wound care and management. J Am Acad Dermatol 2016;74(4):607-25. [DOI] [PubMed] [Google Scholar]
- 12.O’Meara S, Al-Kurdi D, Ologun Y, Ovington LG, Martyn-St James M, Richardson R. Antibiotics and antiseptics for venous leg ulcers. Cochrane Database Syst Rev 2014;(1):CD003557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forlee M, Rossington A, Searle R. A prospective, open, multicentre study to evaluate a new gelling fibre dressing containing silver in the management of venous leg ulcers. Int Wound J 2014;11(4):438-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gunal O, Tuncel U, Turan A, Barut S, Kostakoglu N. The use of vacuum-assisted closure and GranuFoam Silver® dressing in the management of diabetic foot ulcer. Surg Infect (Larchmt) 2015;16(5):558-65. [DOI] [PubMed] [Google Scholar]
- 15.Saez-Martin LC, Garcia-Martinez L, Roman-Curto C, Sanchez-Hernandez MV, Suarez-Fernandez RM. Negative pressure and nanocrystalline silver dressings for nonhealing ulcer: a randomized pilot study. Wound Repair Regen 2015;23(6):948-52. [DOI] [PubMed] [Google Scholar]
- 16.Woo KY, Coutts PM, Sibbald RG. A randomized controlled trial to evaluate an antimicrobial dressing with silver alginate powder for the management of chronic wounds exhibiting signs of critical colonization. Adv Skin Wound Care 2012;25:503-8. [DOI] [PubMed] [Google Scholar]
- 17.Lansdown ABG. Silver I: its antibacterial properties and mechanism of action. J Wound Care 2002;11(4):6. [DOI] [PubMed] [Google Scholar]
- 18.Zou SB, Yoon WY, Han SK, Jeong SH, Cui ZJ, Kim WK. Cytotoxicity of silver dressings on diabetic fibroblasts. Int Wound J 2013;10(3):306-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsang KK, Kwong EW, Woo KY, To TS, Chung JW, Wong TK. The anti-inflammatory and antibacterial action of nanocrystalline silver and manuka honey on the molecular alternation of diabetic foot ulcer: a comprehensive literature review. Evid Based Complement Alternat Med 2015;2015:218283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim TH, Kim M, Park HS, Shin US, Gong MS, Kim HW. Size-dependent cellular toxicity of silver nanoparticles. J Biomed Mater Res 2012;100(4):1033-43. [DOI] [PubMed] [Google Scholar]
- 21.Leaper D. Appropriate use of silver dressings in wounds: international consensus document. Int Wound J 2012;9(5):461-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michaels J, Campbell B, King B, Palfreyman S, Shackley P, Stevenson M. Randomized controlled trial and cost - effectiveness analysis of silver - donating antimicrobial dressings for venous leg ulcers (VULCAN trial). Br J Surg 2009;96(10):1147-56. [DOI] [PubMed] [Google Scholar]
- 23.Vermeulen H, van Hattem JM, Storm-Versloot MN, Ubbink DT. Topical silver for treating infected wounds. Cochrane Database Syst Rev 2007;(1):CD005486. [DOI] [PubMed] [Google Scholar]
- 24.Storm-Versloot MN, Vos CG, Ubbink DT, Vermeulen H. Topical silver for preventing wound infection. Cochrane Database Syst Rev 2010;(3):CD006478. [DOI] [PubMed] [Google Scholar]
- 25.Lansdown AB. A pharmacological and toxicological profile of silver as an antimicrobial agent in medical devices. Adv Pharmacol Sci 2010;2010:910686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burd A, Kwok CH, Hung SC, et al. A comparative study of the cytotoxicity of silver-based dressings in monolayer cell, tissue explant, and animal models. Wound Repair Regen 2007;15(1):94-104. [DOI] [PubMed] [Google Scholar]
- 27.Leaper DJ. Silver dressings: their role in wound management. Int Wound J 2006;3(4):282-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olson ME, Wright JB, Lam K, Burrell RE. Healing of porcine donor sites covered with silver-coated dressings. Eur J Surg 2000;166(6):486-9. [DOI] [PubMed] [Google Scholar]
- 29.Nexodyn®. Nexodyn® AOS (AOS). APR Applied Pharma Research S.A., Balerna, Switzerland. [Google Scholar]
- 30.D’Atanasio N, Capezzone de Joannon A, Mangano G, et al. A new acid-oxidizing solution: assessment of its role on methicillin-resistant Staphylococcus aureus (MRSA) biofilm morphological changes. Wounds 2015;27(10):265-73. [PubMed] [Google Scholar]
- 31.Ayello EA, Dowsett C, Schultz GS, et al. TIME heals all wounds. Nursing 2004;34(4):36-41. [DOI] [PubMed] [Google Scholar]
- 32.Kingsley A. The wound infection continuum and its application to clinical practice. Ostomy Wound Manage 2003;49(7A Suppl):1-7. [PubMed] [Google Scholar]
- 33.Sibbald RG, Browne AC, Coutts P, Queen D. Screening evaluation of an ionized nanocrystalline silver dressing in chronic wound care. Ostomy Wound Manage 2001;47(10):38-43. [PubMed] [Google Scholar]
- 34.Hammerle G, Strohal R. Efficacy and cost-effectiveness of octenidine wound gel in the treatment of chronic venous leg ulcers in comparison to modern wound dressings. Int Wound J 2016;13(2):182-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kallstrom G. Are quantitative bacterial wound cultures useful? J Clin Microbiol 2014;52(8):2753-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bojar RA, Cunliffe WJ, Holland KT. Disruption of the transmembrane pH gradient-a possible mechanism for the antibacterial action of azelaic acid in Propionibacterium acnes and Staphylococcus epidermidis. J Antimicrob Chemother 1994;34(3):321-30. [DOI] [PubMed] [Google Scholar]
- 37.Leveen HH, Falk G, Borek B, et al. Chemical acidification of wounds. An adjuvant to healing and the unfavorable action of alkalinity and ammonia. Ann Surg 1973;178(6):745-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schreml S, Szeimies RM, Karrer S, Heinlin J, Landthaler M, Babilas P. The impact of the pH value on skin integrity and cutaneous wound healing. J Eur Acad Dermatol Venereol 2010;24(4):373-8. [DOI] [PubMed] [Google Scholar]
- 39.Schneider LA, Korber A, Grabbe S, Dissemond J. Influence of pH on wound-healing: a new perspective for wound-therapy? Arch Dermatol Res 2007;298:413-20. [DOI] [PubMed] [Google Scholar]
- 40.Jones EM, Cochrane CA, Percival SL. The Effect of pH on the extracellular matrix and biofilms. Adv Wound Care 2015;4(7):431-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas LV, Wimpenny JW, Davis JG. Effect of three preservatives on the growth of Bacillus cereus, Vero cytotoxigenic Escherichia coli and Staphylococcus aureus, on plates with gradients of pH and sodium chloride concentration. Int J Food Microbiol 1993;17(4):289-301. [DOI] [PubMed] [Google Scholar]
- 42.Dissemond J. Die Bedeutung des pH-Wertes für die Wundheilung. HARTMANN WundForum; 2006;1:15-9. [Google Scholar]
- 43.Kumaran D, Eswaramoorthy S, Furey W, Sax M, Swaminathan S. Structure of staphylococcal enterotoxin C2 at various pH levels. Acta Crystallogr D Biol Crystallogr 2001;57(Pt 9):1270-5. [DOI] [PubMed] [Google Scholar]
- 44.Shimokawa N, Kumaki I, Takayama K. MafG-2 is a novel Maf protein that is expressed by stimulation of extracellular H(+). Cell Signal 2001;13(11):835-9. [DOI] [PubMed] [Google Scholar]
- 45.Kurabayashi H, Tamura K, Machida I, Kubota K. Inhibiting bacteria and skin pH in hemiplegia: effects of washing hands with acidic mineral water. Am J Phys Med Rehabil 2002;81(1):40-6. [DOI] [PubMed] [Google Scholar]
- 46.Tsukada K, Tokunaga K, Iwama T, Mishima Y. The pH changes of pressure ulcers related to the healing process of wounds. Wounds 1992;4(1):16-20. [Google Scholar]
- 47.Nagoba BS, Suryawanshi NM, Wadher B, Selkar S. Acidic environment and wound healing: a review. Wounds 2015;27(1):5-11. [Google Scholar]
- 48.Gethin G. The significance of surface pH in chronic wounds. Wounds UK 2007;3:52-5. [Google Scholar]
- 49.Bullen EC, Longaker MT, Updike DL, et al. Tissue inhibitor of metalloproteinases-1 is decreased and activated gelatinases are increased in chronic wounds. J Invest Dermatol 1995;104(2):236-40. [DOI] [PubMed] [Google Scholar]
- 50.Trengove NJ, Stacey MC, MacAuley S, et al. Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair Regen 1999;7(6):442-52. [DOI] [PubMed] [Google Scholar]
- 51.Gordillo GM, Sen CK. Revisiting the essential role of oxygen in wound healing. Am J Surg 2003;186(3):259-63. [DOI] [PubMed] [Google Scholar]
- 52.Rodriguez PG, Felix FN, Woodley DT, Shim EK. The role of oxygen in wound healing: a review of the literature. Dermatol Surg 2008;34(9):1159-69. [DOI] [PubMed] [Google Scholar]
- 53.Sen CK. Wound healing essentials: let there be oxygen. Wound Repair Regen 2009;17(1):1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ricci E. The management of chronic ulcers with an acidoxidising solution. J Wound Care 2016;25(8):443-50. [DOI] [PubMed] [Google Scholar]


