Supplemental Digital Content is available in the text
Keywords: chronic hepatitis C, co-infection, glomerular filtration, HIV, kidney function, sustained virologic response
Abstract
Objective:
To examine the impact of sustained virologic response (SVR) and illicit (injection and noninjection) drug use on kidney function among hepatitis C virus (HCV) and HIV co-infected individuals.
Design:
Longitudinal observational cohort study of HCV-HIV co-infected patients.
Methods:
Data from 1631 patients enrolled in the Canadian Co-Infection Cohort between 2003 and 2016 were analyzed. Patients who achieved SVR were matched 1 : 2 with chronically infected patients using time-dependent propensity scores. Linear regression with generalized estimating equations was used to model differences in estimated glomerular filtration rates (eGFR) between chronic HCV-infected patients and those achieving SVR. The relationship between illicit drug use and eGFR was explored in patients who achieved SVR.
Results:
We identified 384 co-infected patients who achieved SVR (53% treated with interferon-free antiviral regimens) and 768 propensity-score matched patients with chronic HCV infection. Most patients were men (78%) and white (87%), with a median age of 51 years (interquartile range: 45–56). During 1767 person-years of follow-up, 4041 eGFR measurements were available for analysis. Annual rates of decline in eGFR were similar between patients with SVR [−1.32 (ml/min per 1.73 m2)/year, 95% confidence interval (CI) −1.75 to −0.90] and chronic infection [−1.19 (ml/min per 1.73 m2) per year, 95% CI −1.55 to −0.84]. Among SVR patients, recent injection cocaine use was associated with rapid eGFR decline [−2.16 (ml/min per 1.73 m2)/year, 95% CI −4.17 to −0.16].
Conclusion:
SVR did not reduce the rate of kidney function decline among HCV–HIV co-infected patients. Increased risk of chronic kidney disease in co-infection may not be related to persistent HCV replication but to ongoing injection cocaine use.
Introduction
Due to shared routes of transmission, chronic hepatitis C virus (HCV) is prevalent among HIV-infected populations [1]. It is estimated that as many as 4.4 million people are co-infected globally, with the highest prevalence (>80%) observed among HIV-infected people who inject drugs [2]. Compared with HCV mono-infection, HIV co-infected patients have lower spontaneous clearance rates, higher HCV viral loads, and accelerated progression of HCV-associated liver disease [3,4]. In the era of combination antiretroviral therapy (cART), rates of liver-related outcomes, including cirrhosis and hepatocellular carcinoma are increased in the setting of HCV–HIV co-infection, contributing to excess mortality in this population [5,6].
With increasing life expectancy, chronic kidney disease (CKD) has emerged as an important cause of morbidity in the HIV-infected population [7]. Without timely identification, HIV-infected individuals with CKD are at increased risk of cardiovascular disease and premature mortality [8,9]. Although the cause of CKD in HIV-infected patients on cART is likely multifactorial, and includes exposure to nephrotoxic antiretroviral agents [10], greater prevalence of traditional CKD risk factors [11], immunosuppression [12], and host/genetic factors [13], HCV co-infection has been identified as a leading risk factor for CKD and end-stage renal disease (ESRD) in this population [14–16]. These observations support the current view of chronic HCV as a systemic illness that is associated with extrahepatic comorbidities in both the general and HIV-infected population [17,18]. Recent evidence has suggested an association between illicit drug use and CKD, though separating the effect of viral replication and substance use has been difficult [19–21].
The benefits of successful HCV treatment in HCV–HIV co-infected individuals include both a reduction in all-cause and liver-related mortality and improvement in quality of life [22,23]. There has, however, been comparatively less research on the role of viral cure on extrahepatic chronic illness, including CKD. There is evidence suggesting a possible protective effect from interferon-based treatment on incident CKD/ESRD in HCV mono-infected populations in Asia [24,25]. These studies have been limited by small numbers of clinical events, lack of laboratory confirmation of a sustained virologic response (SVR) to treatment, and lack of adjustment for potential confounders, such as substance use and other risk behaviours. As such, the findings may not be generalizable to HIV-infected populations in North America and Europe. Furthermore, there is currently no data evaluating risk factors for CKD progression among co-infected patients who achieve SVR, a population who will become more numerous as new direct-acting antivirals (DAAs) are rolled-out.
We sought to determine whether SVR reduces short-term rates of kidney function decline in HCV–HIV co-infected Canadians and to evaluate the contributions of ongoing illicit cocaine and opiate use on kidney function decline among co-infected patients who achieved SVR.
Patients and methods
Canadian Co-Infection Cohort
Data were obtained from the Canadian Co-Infection Cohort (CCC), a large multicentre prospective study, which has enrolled HCV–HIV co-infected patients from 18 hospital and community-based clinics across Canada. The cohort has been described previously [26]. Briefly, all participants complete a baseline questionnaire to provide demographic and risk factor information and study coordinators collect information on HIV and HCV treatment histories and clinical comorbidities. Questionnaire and clinical data is updated biannually. The study was approved by the community advisory committee of the Canadian Institutes of Health Research – Canadian HIV Trials Network and by all institutional ethics boards of participating centres. Written informed consent was obtained for all patients.
Inclusion criteria
We included all co-infected patients with chronic HCV who were enrolled between January 2003 and December 2016. Patients were excluded if they had evidence of spontaneous HCV clearance or had previously achieved an SVR prior to enrollment.
Study design
To examine the association between SVR and kidney function, we employed a longitudinal cohort study design with incidence-density sampling. We created a base cohort with all chronically infected patients and defined a risk set on each calendar date a patient achieved an SVR (the index date). Each risk set contained chronic HCV patients who had a study visit +/−30 days of the index date. Patients who would eventually develop SVR were eligible to be included in control risk sets for other SVR patients before they initiated therapy. Patients who developed SVR were excluded from all exposure risk sets and patients who failed HCV treatment during the study period remained classified as chronically infected.
Definitions
SVR was defined by either an undetectable qualitative polymerase chain reaction (e.g. Amplicor HCV Test v2.0) or quantitative (e.g. Abbott Real Time HCV) HCV viral load below the assay's lower limit of detection, obtained at least 12 weeks after the end of HCV treatment. Estimated glomerular filtration rates (eGFR) were calculated using the serum creatinine-based Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [27]. Alcohol consumption and illicit (injection and noninjection) cocaine and opiate substance use was obtained by self-report at each visit. Hazardous alcohol consumption was assessed using the Alcohol Use Disorders Identification Test (AUDIT-C) score [28]. Duration of HCV infection was estimated as the earliest date of starting to use injection drugs or date of first positive serologic test. Hypertension was defined by clinical diagnosis or two consecutive visits with SBP at least 140 mmHg or DBP at least 90 mmHg. Diabetes was defined by clinical diagnosis or two consecutive visits with either fasting serum glucose at least 7 mmol/l or random serum glucose at least 11.1 mmol/l. Liver fibrosis was measured using the aspartate aminotransferase-to-platelet ratio index (APRI) with values at least 1.5 indicative of significant fibrosis (≥ F2) [29].
Statistical analysis
SVR patients were matched with chronically infected patients using time-dependent propensity scores [30]. Conditional logistic regression was used to calculate the probability of developing SVR given time-varying patient characteristics at each risk set defined on the index date [31]. We matched patients on the logit of the propensity score using the nearest neighbor algorithm to select two chronically infected patients. Matching was performed with replacement and no caliper was used. The use of time-dependent propensity score matching ensures that patients who developed SVR during the interferon-based and interferon-free eras were matched with comparable patients who could have been treated during the same time period but were ultimately not. The propensity score model included potential confounders of the SVR and CKD relationship and independent predictors of kidney function [32]. Covariates included age, sex, aboriginal and African/Caribbean ethnicity, injection and noninjection cocaine and opiate use in the last 6 months, hazardous alcohol use, CD4+ T-cell count (linear and quadratic term), detectable HIV RNA (≥ 50 copies/ml), current or previous tenofovir disoproxil fumarate or protease inhibitor use, duration of HCV infection, hypertension, diabetes, significant liver fibrosis, and baseline eGFR. Balance of covariate distributions was assessed by comparing standardized mean differences [33]. For all patients in the matched sample, analysis follow-up time began on the index date and person-time was censored at the earliest of death, last available kidney function measurement, HCV treatment initiation (if a matched patient would develop SVR), or 31 December 2016.
In our primary analysis, we used a population-averaged linear model fit with generalized estimating equations (GEE) to compare annual rates of eGFR decline between SVR and chronically infected patients [34]. The difference in eGFR slopes between the two groups was measured with an interaction term between exposure status and linear time in three separate models. First, we fit an ‘unadjusted’ model, which only accounts for differences in measured baseline characteristics in our propensity score-matched sample. Second, we accounted for residual imbalances in baseline characteristics by including both linear and quadratic terms for the propensity score in the GEE model. Lastly, we included time-updated covariates to estimate the direct effect of SVR on eGFR that is not mediated by changing values of other variables that may be modified by SVR status. We assumed an exchangeable correlation structure and robust standard errors were used to account for repeated longitudinal data and the matching with replacement.
In our secondary analysis, we examined the association between illicit drug use and annual rates of kidney function decline among HIV-infected patients who achieved SVR using a similar population-averaged linear model. Differences in eGFR slopes, adjusted for age, sex, ethnicity, CD4+ T-cell count, detectable HIV viral load, HCV duration and liver fibrosis, were estimated with an interaction term between each drug and linear time. Short-term kidney function trajectories between those who used injection or noninjection cocaine and opiates, as compared with nonusers, were estimated in a reference 50-year-old, male, Caucasian HIV-infected patient who achieved SVR and was infected with HCV for 20 years, had no liver fibrosis, an undetectable HIV viral load, and a CD4+ T-cell count at least 500 cells/μl. Statistical analyses were performed using STATA version 14.1 (College Station, Texas, USA).
Results
Of the 1726 co-infected patients who were enrolled in the CCC since 2003, we excluded 42 spontaneous clearers and 53 patients who were previously treated and developed SVR (Supplemental Figure). Characteristics of the base cohort at enrollment, as well as those with spontaneous clearance and previous SVR are shown in Supplemental Table 1. During the study period, 562 patients were treated for chronic HCV. Of those treated, a treatment response was not available for 53 patients (9%), most of whom had just recently completed therapy (42/53; 79%). Of the 509 patients with known treatment responses, 45 (9%) had not yet completed any posttreatment study follow-up. Of the 457 patients with an available treatment result and posttreatment follow-up, 384 (84%) developed SVR.
Measured baseline covariate balance between patients who achieved SVR (n = 384) and propensity score-matched chronically infected patients (n = 768) are shown in Table 1. Covariates appeared balanced between the two populations, with no substantial imbalances between the two study groups. More than half of all patients in the SVR group were treated with an all-oral DAA regimen (202/384; 53%) and 70% had HCV genotype 1 (268/384).
Table 1.
Comparison of baseline characteristics in the propensity score-matched sample.
| Sustained virologic response (n = 384) | Chronic infection (n = 768) | Standardized mean difference | |
| Median age (IQR) (years) | 51 (45–55) | 51 (45–55) | 0 |
| Female | 80 (21%) | 169 (22%) | 0.03 |
| African/Caribbean ethnicity | 17 (4%) | 24 (3%) | 0.07 |
| Aboriginal ethnicity | 35 (9%) | 75 (10%) | 0.02 |
| Injection cocaine use | 43 (11%) | 94 (12%) | 0.03 |
| Injection opiate use | 46 (12%) | 90 (12%) | 0.01 |
| Noninjection cocaine use | 61 (16%) | 142 (18%) | 0.07 |
| Noninjection opiate use | 58 (15%) | 128 (17%) | 0.04 |
| Hazardous alcohol consumption | 64 (17%) | 141 (18%) | 0.04 |
| Median current CD4+ count (IQR) (cells/μl) | 530 (360–740) | 530 (330–760) | 0.01 |
| Median nadir CD4+ count (IQR) (cells/μl) | 190 (90–300) | 190 (90–325) | 0.09 |
| Detectable HIV RNA at least 50 copies/ml | 39 (10%) | 72 (9%) | 0.03 |
| Prior AIDS-defining events | 98 (26%) | 226 (29%) | 0.09 |
| Current tenofovir disoproxil fumarate exposure | 223 (58%) | 430 (56%) | 0.04 |
| Ever tenofovir disoproxil fumarate exposure | 267 (70%) | 533 (69%) | 0 |
| Current protease inhibitor exposurea | 112 (29%) | 216 (28%) | 0.02 |
| Ever protease inhibitor exposurea | 178 (46%) | 354 (46%) | 0.01 |
| Median duration of HCV infection (IQR) (years) | 21 (12–30) | 21 (12–29) | 0.02 |
| Hypertension | 57 (15%) | 119 (15%) | 0.02 |
| Diabetes | 28 (7%) | 50 (7%) | 0.03 |
| Liver fibrosis (APRI ≥ 1.5) | 156 (41%) | 348 (45%) | 0.09 |
| Median eGFR (IQR) (ml/min per 1.73 m2) | 91 (74–104) | 91 (74–104) | 0.01 |
| Recent hospitalization in the last 6 months | 41 (11%) | 100 (13%) | 0.07 |
Values are n (%), unless otherwise indicated. APRI, aspartate aminotransferase-to-platelet ratio index; eGFR, estimated glomerular filtration rate; HCV, hepatitis C virus; IQR, interquartile range.
aLopinavir/ritonavir or atazanavir, with or without ritonavir.
In the primary analysis, we included 1152 HCV–HIV co-infected participants who were followed for a total of 1767 person-years of follow-up (PYFU). A total of 4041 post-SVR eGFR measurements were available for analysis. The mean follow-up time was 1.6 years in the SVR group and 1.5 years in the chronically infected group. Annual rates of decline in eGFR were similar between SVR [−1.32 (ml/min per 1.73 m2)/year, 95% confidence interval (CI) −1.75 to −0.90] and chronically infected patients [−1.19 (ml/min per 1.73 m2)/year, 95% CI −1.55 to −0.84; Table 2]. Our results were similar after additionally adjusting for the propensity score to account for measured baseline covariate imbalances. After including time-dependent covariates in the outcome model, rates of eGFR decline were slightly attenuated, though there remained no statistically or clinically significant difference between the two groups.
Table 2.
Annual rates of eGFR decline [(ml/min per 1.73 m2)/year] between sustained virologic response and chronic hepatitis C virus patients in the propensity score-matched sample (n = 1152).
| ΔeGFR [(ml/min per 1.73 m2)/year; 95% CI]a | |||
| Model 1b | Model 2c | Model 3d | |
| SVR | −1.32 (−1.75 to −0.90) | −1.41 (−1.72 to −1.11) | −1.26 (−1.70 to −0.83) |
| Chronic HCV | −1.19 (−1.55 to −0.84) | −1.28 (−1.53 to −1.02) | −1.16 (−1.54 to −0.79) |
| Difference | −0.13 (−0.68 to 0.42) | −0.14 (−0.54 to 0.26) | −0.10 (−0.67 to 0.47) |
CI, confidence interval; eGFR, estimated glomerular filtration rate; HCV, hepatitis C virus; SVR, sustained virologic response.
aRobust standard errors.
bUnadjusted propensity score-matched sample.
cPropensity score-matched sample with additional adjustment for propensity score using linear and quadratic term.
dPropensity score-matched sample with additional adjustment for time-updated covariates: injection and noninjection drug use, hazardous alcohol consumption, CD4+ cell count, detectable HIV RNA, current use of tenofovir disoproxil fumarate, current use of atazanavir or lopinavir, hypertension, diabetes, and liver fibrosis.
Among the 384 patients who achieved SVR, 43 (11%) and 46 (12%) patients reported recent injection cocaine and injection opiate use within the last 6 months, respectively, and 61 (16%) and 58 (15%) reported recent noninjection crack/cocaine and opiate use, respectively. After SVR, the adjusted annual rate of change in eGFR was faster among active injection cocaine users [−2.16 (ml/min per 1.73 m2)/year, 95% CI −4.17 to −0.16], as compared with nonusers of any illicit drug [−0.67 (ml/min per 1.73 m2)/year, 95% CI −1.14 to −0.20] but was not statistically significant [difference: −1.49 (ml/min per 1.73 m2)/year, 95% CI −3.49 to 0.51]. No similar accentuation in decline was observed among the other illicit drugs (Supplemental Table 2). Analyses of eGFR trajectories demonstrated that in the reference population, after 5 years, injection cocaine users will have a lower eGFR (76 ml/min per 1.73 m2, 95% CI 68–83), compared with nonusers of any illicit drug (86 ml/min per 1.73 m2, 95% CI 84–88). There were no significant differences in eGFR trajectories between other illicit drug users and nonusers (Fig. 1).
Fig. 1.
Kidney function trajectories in the reference population, by illicit drug use, among patients with sustained virologic response (n = 384).
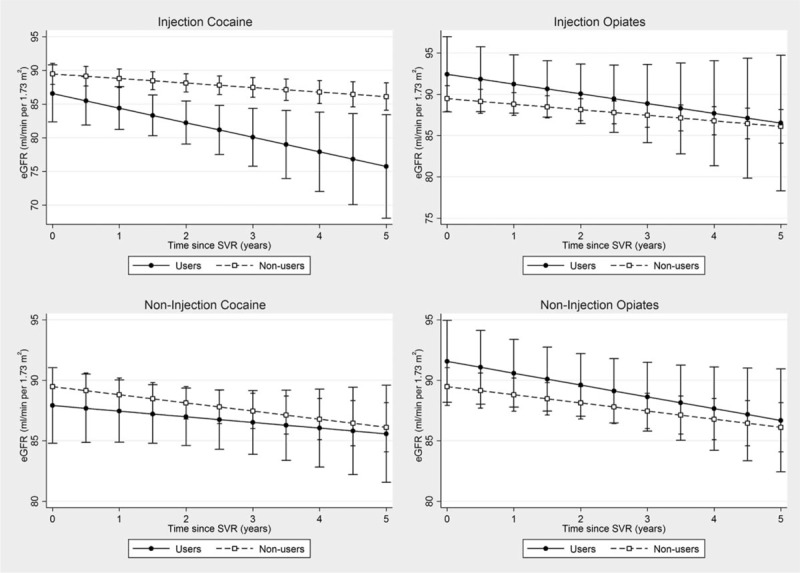
eGFR, estimated glomerular filtration rate; SVR, sustained virologic response.
Discussion
In this analysis of HCV–HIV co-infected patients who were treated in both the interferon and interferon-free eras, we found that annual rates of change in eGFR were similar between those who were successfully treated and comparably matched patients who were chronically infected. These findings suggest that there is no immediate benefit of successful HCV treatment for kidney function and that HCV cure may not reduce the overall incidence of CKD in co-infected patients.
Our results differ from previous reports in cohort studies of HIV-negative populations. In a clinical cohort of 650 HCV mono-infected patients with cirrhosis treated with interferon in Japan, SVR was associated with a 63% reduction in CKD incidence [24]. Given this population was cirrhotic, this finding may be explained by the impact of SVR on liver disease progression, which reduces the risk of hepatorenal syndrome, and not specifically an effect of HCV elimination on renal function [35]. A large administrative-linked, population-based study in Taiwan reported that initiation of HCV treatment was associated with an 85% decline in ESRD incidence compared with untreated HCV-infected individuals [25,36]. This study excluded patients with advanced liver disease and specific comorbidities, such as those with psychiatric diagnoses, which would be a contraindication to interferon-based treatment and did not control for lifestyle risk factors, such as alcohol and drug use, which would be less common in people who initiate therapy with interferon. The protective association of interferon treatment may, therefore, be overestimated, though because of the large effect, further investigation is warranted.
Our findings, however, are consistent with two recent cohort studies examining the association between HCV viral eradication and CKD in co-infected populations treated in the interferon era. In an analysis of 340 HCV–HIV co-infected patients treated in Italy, Leone et al.[37] report that achieving a SVR was not associated with a shorter time to incident CKD. Similarly, an analysis of 2503 co-infected patients in the Swiss HIV Cohort Study found no evidence that successful treatment with interferon was associated with a reduced risk of ESRD, as compared with those who were chronically infected [38]. In contrast, a recent Spanish cohort study, which directly compared 1625 HCV–HIV co-infected patients with SVR to those who failed treatment in the interferon-era, reported a clinically significant protective effect of SVR on CKD [39]. The latter study, however, did not adjust for baseline eGFR and if low eGFR is an important predictor for failing to achieve SVR, then the non-SVR group would have shorter time to CKD and the protective effect of successful treatment may be overestimated [24].
HCV has been implicated as an important risk factor for metabolic, cardiovascular, kidney, and neurological extrahepatic manifestations [17,40]. Although the pathophysiologic effect of HCV on extrahepatic comorbidities are not completely understood, they are likely related to a combination of direct HCV effects, such as viral replication in extrahepatic cells, or immune activation resulting in chronic inflammation, and indirect effects such as drug and alcohol use, poor nutrition, and HIV-related issues [23]. For some comorbidities, such as diabetes, wherever HCV viral replication is known to directly affect insulin-signaling pathways[17], the effect of successful treatment has resulted in a reduction of clinical outcomes observed in several epidemiologic studies [38,39,41]. For other extrahepatic comorbidities, such as cardiovascular disease and CKD, the mechanisms are likely multifaceted and HCV viral eradication alone may not reduce the incidence of all clinical outcomes. For example, on the one hand, chronic HCV has been associated with favourable lipid profiles [42], but has also been associated with greater prevalence of atherogenic risk factors [43]. In epidemiologic studies, SVR has been strongly associated with a significant reduction in cerebrovascular events and acute coronary syndrome in a HCV mono-infected population [25,44], but produced conflicting results in HIV co-infected populations [38,39]. For kidney diseases, it is clear that HCV treatment reduces complications associated with mixed cryoglobulinemia, which is associated with the development of membranoproliferative glomerular nephritis (MPGN) disease, though it is less clear if HCV treatment has an impact on kidney function in patients without any underlying renal diseases [45].
There are several factors that may explain our null findings. First, given that most co-infected patients have been infected with HCV for more than two decades, it is possible that successful HCV treatment may have a limited impact on the kidney after HCV replication has continued unabated during this long period. This is unlikely, however, as most patients had normal kidney function (≥90 ml/min per 1.73 m2) at the time of SVR, suggesting that HCV itself may not be an important contributor to CKD in HIV-infected populations and that other exposures such as cocaine use may explain the increased risk of chronic renal impairment [20]. Second, it may be possible that SVR may have a stronger effect on kidney function among subpopulations of patients who are more likely to rapidly progress to ESRD after developing CKD, such as HIV-infected African Americans, who carry the APOL1 or MYH9 genetic variants, or patients with cirrhosis or diabetes [46]. We were underpowered, however, to test this hypothesis in our study.
Few studies have examined the relative contributions of HCV viremia, lifestyle factors, and alternative mechanisms to the development of CKD in patients with chronic viral infections [19–21]. We reported earlier that frequent and cumulative exposure to injection crack/cocaine was associated with chronic renal impairment in HCV–HIV co-infected patients, independent of HCV viral replication [20]. Garg et al.[19] have also reported an association between crack/cocaine use and acute kidney injury among a small cohort of chronic HCV-infected patients. Recently, in a large cohort of HCV mono-infected veterans and matched controls, Rogal et al.[21] found that both alcohol and drug dependence, as measured with administrative billing codes, were associated with an increased risk of CKD. We extend these findings to HIV co-infected patients who achieved SVR and note the increasing role that substance use, namely injection cocaine, has on short-term kidney function. Whether these effects persist after long-term substance use or whether kidney function normalizes after cessation remains to be investigated. This is the first study to examine the effect that lifestyle risk factors have on kidney function after SVR, and we encourage the development of further long-term studies assessing the impact of substance use on CKD and other extrahepatic illnesses in HIV or HCV-infected populations [23].
Our analysis adds to the developing body of literature on extrahepatic outcomes following successful HCV treatment in the co-infected population and has several strengths. First, we included a large number of patients who were treated with DAAs, which have replaced interferon-based therapy for both treatment-naive and treatment-experienced chronic HCV patients, regardless of genotype. Compared with previous research in this area, our study population is, then, more generalizable to those co-infected patients initiating care in low-incidence settings. Second, our use of time-dependent propensity scores reduces the impact that unmeasured confounding, which varies with time would have on influencing both why patients would initiate HCV treatment and subsequent changes in their kidney function. This is particularly important, as our research encompasses several distinct HCV treatment periods, with an increasing number of HCV–HIV co-infected patients with comorbid conditions initiating treatment. Lastly, our use of routinely measured eGFR values allows us to model annual rates of change in kidney function, which are clinically relevant for those treating HIV-infected patients on stable antiretroviral therapy. Furthermore, the use of repeated eGFR measurements allow us to increase statistical power, as compared with previous analyses, and reduce biases associated with irregular eGFR measurements leading to CKD diagnosis in patients at risk for the outcome.
Our study has several potential limitations. First, we were limited by a relatively short follow-up, particularly among those who were recently treated with new DAA regimens. Therefore, we had insufficient follow-up time to allow incident CKD events to occur. Second, we could not directly compare SVR patients with patients who failed treatment, given the small numbers of co-infected patients who failed (n = 73). Comparison of these two groups would provide a less biased estimate of the effect of SVR on kidney function, as it would control for factors related to treatment initiation, but such analyses have become increasingly difficult as HCV treatment becomes much more effective. Our method of using time-dependent propensity scores with incidence density sampling provided a matched sample with comparable baseline characteristics. It is possible, however, that unmeasured characteristics, such as poor nutrition or the use of concomitant nephrotoxic medications, may have been more prevalent in the chronically infected group and these factors may also have influenced eGFR [47] however, the groups were well balanced with respect to HIV disease stage, comorbid conditions, and recent hospitalizations suggesting exposure to such medications would likewise be balanced. Furthermore, we were unable to assess if urinary biomarkers of kidney disease, such as proteinuria, were differentially distributed between our study groups. Lastly, we relied on patient self-report to assess illicit drug-use exposures. Although this may be affected by social desirability biases, nurses and clinicians maintain good relationships with study participants in the CCC, yielding little incentive to falsely reporting data.
In conclusion, we found that SVR was not associated with a short-term reduction in the rate of change in eGFR among HCV–HIV co-infected patients receiving clinical care. Regardless, HCV treatment should continue to be promoted, given its clear benefits in reducing the risk of liver-disease progression, premature mortality, and HCV transmission. For co-infected individuals who achieved SVR, monitoring eGFR in those who continue to use cocaine and referral to substance-use services would be beneficial, given their higher risk of experiencing kidney injury over time.
Acknowledgements
The Site Investigators of the Canadian Co-Infection Cohort (CTN222) are: Drs Lisa Barrett, MD, QEII Health Science Center for Clinical Research, Halifax, NS; Jeff Cohen, MD, Windsor Regional Hospital Metropolitan Campus, Windsor, Ontario; Brian Conway, MD, PENDER Downtown Infectious Diseases Clinic, Vancouver, British Columbia; Curtis Cooper, MD, The Ottawa Hospital Research Institute, Ottawa, Ontario; Pierre Côté, MD, Clinique du Quartier Latin, Montréal, Québec; Joseph Cox, MD, MSc, MUHC IDTC-Montréal General Hospital, Montréal, Québec; John Gill, MD, Southern Alberta HIV Clinic, Calgary, Alberta; Shariq Haider, MD, McMaster University Medical Centre – SIS Clinic, Hamilton, Ontario; Mark Hull, MD, MHSc, BC Centre for Excellence in HIV/AIDS, Vancouver, British Columbia; Marina Klein, MD, MSc, McGill University Health Centre, Division of Infectious Diseases and Chronic Viral Illness Service, Montreal, Québec; Julio Montaner, MD, BC Centre for Excellence in HIV/AIDS and the University of British Columbia, Vancouver, British Columbia; Neora Pick, MD, Oak Tree Clinic, Children's and Women's Health Centre of British Columbia, University of British Columbia, Vancouver, British Columbia; Anita Rachlis, MD, Sunnybrook &Women's College Health Sciences Centre, Toronto, Ontario; Danielle Rouleau, MD, Centre Hospitalier de l’Université de Montréal, Montréal, Québec; Roger Sandre, MD, Health Sciences North - The HAVEN/Hemophilia Program, Sudbury, Ontario; Aida Sadr, MD, Native BC Health Center, St-Paul's Hospital, Vancouver, British Columbia; Mark Tyndall, MD, ScD, Department of Medicine, Infectious Diseases Division, University of Ottawa, Ottawa, Ontario; Marie-Louise Vachon, MD, Centre Hospitalier Universitaire de Québec, Québec, Québec; Steve Sanche, MD, SHARE University of Saskatchewan, Saskatoon, Sakatchewan; Sharon Walmsley, MD, MSc, University Health Network, Toronto, Ontario; Alex Wong, MD, Regina Qu’Appelle Health Region, Regina General Hospital, Regina, Sakatchewan.
We thank all study coordinators and nurses for their assistance with study coordination, participant recruitment, and care.
Author contributions: As corresponding author, M.B.K. helped design and supervised the study and had full access to all the data and took responsibility for the integrity of the data and the accuracy of the data analysis. C.R. was responsible for managing the data and conducted all the analyses with critical input from J.C., M.-L.V., S.S., and E.E.M.M. C.R. and M.B.K. drafted the manuscript. J.C., M.-L.V., V.M.-L., S.L.M., C.C., M.J.G., M.H., and M.B.K. recruited and followed participants. All of the authors participated in critical revision and have seen and approved the final manuscript and have participated sufficiently in the work to take public responsibility for its content.
Financial support: The study was supported through grant support from the Fonds de recherche en santé – Québec, Réseau SIDA/maladies infectieuses (FRQ-S); the Canadian Institutes of Health Research (CIHR MOP-79529); and the Canadian Institutes of Health Research Canadian HIV Trials Network (CTN222).
Conflicts of interest
None of the authors feel in conflict of interest with regards to this study and there was no pharmaceutical industry support to conduct this study. Carmine Rossi, Sahar Saeed, Erica Moodie have no conflicts of interest to declare. Marina Klein has received research grants for investigator-initiated trials from Merck and ViiV Healthcare; consulting fees from ViiV Healthcare, Bristol-Meyers Squibb, Merck, Gilead and AbbVie. Joseph Cox received consulting fees from Bristol-Meyers Squibb; grants from ViiV Healthcare and Gilead; and payment for lectures from Merck. Marie-Louise Vachon has received consulting fees from Boehringer Ingelheim and Merck; consulting fees and lecture honoraria from Janssen Pharmaceuticals, Gilead, Hoffman–La Roche and Vertex Pharmaceuticals; and speaker fees from Gilead. Valérie Martel- Laferrière reports consulting fees from Merck and Gilead; grant from Gilead; and lecture fees from AbbVie, Merck and Gilead. Sharon Walmsley received grants, consulting fees, lecture fees, nonfinancial support and fees for the development of educational presentations from Merck, ViiV Healthcare, GlaxoSmithKline, Pfizer, Gilead, Abbvie, Bristol-Myers Squibb and Janssen. Curtis Cooper reports consulting fees from AbbVie, Gilead, and Merck; and grants from AbbVie and Gilead. John Gill received personal fees for being a member of the national advisory boards of Abbvie, Gilead, Merck, Janssen, and ViiV Healthcare. Mark Hull, received grant support from National Institute on Drug Abuse, and honoraria from (speaking engagements and/or consultancy) AbbVie, Bristol Myers Squibb, Gilead, Merck, Ortho-Janssen, and ViiV. Salary awards were obtained from the FRQ-S (Chercheur National career award to M.B.K.) and CanHepC Postdoctoral fellowship (C.R.).
Data were presented previously as a poster at the 26th Annual Canadian Conference on HIV/AIDS Research in Montréal, Canada (6–9 April 2017) and the 4th International HIV/Viral Hepatitis Co-infection Meeting in Paris, France (22–23 July 2017).
Supplementary Material
References
- 1.Peters L, Klein MB. Epidemiology of hepatitis C virus in HIV-infected patients. Curr Opin HIV AIDS 2015; 10:297–302. [DOI] [PubMed] [Google Scholar]
- 2.Platt L, Easterbrook P, Gower E, McDonald B, Sabin K, McGowan C, et al. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis 2016; 16:797–808. [DOI] [PubMed] [Google Scholar]
- 3.Seaberg EC, Witt MD, Jacobson LP, Detels R, Rinaldo CR, Margolick JB, et al. Spontaneous clearance of the hepatitis C virus among men who have sex with men. Clin Infect Dis 2015; 61:1381–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lo Re V, 3rd, Kallan MJ, Tate JP, Localio AR, Lim JK, Goetz MB, et al. Hepatic decompensation in antiretroviral-treated patients co-infected with HIV and hepatitis C virus compared with hepatitis C virus-monoinfected patients: a cohort study. Ann Intern Med 2014; 160:369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Limketkai BN, Mehta SH, Sutcliffe CG, Higgins YM, Torbenson MS, Brinkley SC, et al. Relationship of liver disease stage and antiviral therapy with liver-related events and death in adults coinfected with HIV/HCV. JAMA 2012; 308:370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein MB, Rollet-Kurhajec K, Moodie EEM, Yaphe S, Tyndall M, Walmsley S, et al. Mortality in HIV-hepatitis C co-infected patients enrolled in the Canadian Co-infection Cohort Study compared to the general Canadian population (2003–2013). AIDS 2014; 28:1957–1965. [DOI] [PubMed] [Google Scholar]
- 7.Lucas GM, Ross MJ, Stock PG, Shlipak MG, Wyatt CM, Gupta SK, et al. HIV Medicine Association of the Infectious Diseases Society of America. Clinical practice guideline for the management of chronic kidney disease in patients infected with HIV: 2014 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014; 59:e96–e138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi AI, Li Y, Deeks SG, Grunfeld C, Volberding PA, Shlipak MG. Association between kidney function and albuminuria with cardiovascular events in HIV-infected persons. Circulation 2010; 121:651–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryom L, Lundgren JD, Ross M, Kirk O, Law M, Morlat P, et al. D:A:D Study Group. Renal impairment and cardiovascular disease in HIV-positive individuals: the D:A:D study. J Infect Dis 2016; 214:1212–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mocroft A, Lundgren JD, Ross M, Fux CA, Reiss P, Moranne O, et al. Cumulative and current exposure to potentially nephrotoxic antiretrovirals and development of chronic kidney disease in HIV-positive individuals with a normal baseline estimated glomerular filtration rate: a prospective international cohort study. Lancet HIV 2016; 3:e23–e32. [DOI] [PubMed] [Google Scholar]
- 11.Achhra AC, Mocroft A, Ross MJ, Ryom L, Lucas GM, Furrer H, et al. International Network for Strategic Initiatives in Global HIV Trials (INSIGHT) START Study Group. Kidney disease in antiretroviral-naive HIV-positive adults with high CD4 counts: prevalence and predictors of kidney disease at enrolment in the INSIGHT Strategic Timing of AntiRetroviral Treatment (START) trial. HIV Med 2015; 16 suppl 1:55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Sadr WM, Lundgren J, Neaton JD, Gordin F, Abrams D, Arduino RC, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med 2006; 355:2283–2296. [DOI] [PubMed] [Google Scholar]
- 13.Jotwani V, Shlipak MG, Scherzer R, Parekh RS, Kao WH, Bennett M, et al. APOL1 genotype and glomerular and tubular kidney injury in women with HIV. Am J Kidney Dis 2015; 65:889–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peters L, Grint D, Lundgren JD, Rockstroh JK, Soriano V, Reiss P, et al. Hepatitis C virus viremia increases the incidence of chronic kidney disease in HIV-infected patients. AIDS 2012; 26:1917–1926. [DOI] [PubMed] [Google Scholar]
- 15.Jotwani V, Li Y, Grunfeld C, Choi AI, Shlipak MG. Risk factors for ESRD in HIV-infected individuals: traditional and HIV-related factors. Am J Kidney Dis 2012; 59:628–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossi C, Raboud J, Walmsley S, Cooper C, Antoniou T, Burchell AN, et al. Canadian Observational Cohort (CANOC) Collaboration. Hepatitis C co-infection is associated with an increased risk of incident chronic kidney disease in HIV-infected patients initiating combination antiretroviral therapy. BMC Infect Dis 2017; 17:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Negro F, Forton D, Craxi A, Sulkowski MS, Feld JJ, Manns MP. Extrahepatic morbidity and mortality of chronic hepatitis C. Gastroenterology 2015; 149:1345–1360. [DOI] [PubMed] [Google Scholar]
- 18.Soriano V, Berenguer J. Extrahepatic comorbidities associated with hepatitis C virus in HIV-infected patients. Curr Opin HIV AIDS 2015; 10:309–315. [DOI] [PubMed] [Google Scholar]
- 19.Garg S, Hoenig M, Edwards EM, Bliss C, Heeren T, Tumilty S, et al. Incidence and predictors of acute kidney injury in an urban cohort of subjects with HIV and hepatitis C virus coinfection. AIDS Patient Care STDS 2011; 25:135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rossi C, Cox J, Cooper C, Martel-Laferriere V, Walmsley S, Gill J, et al. Frequent injection cocaine use increases the risk of renal impairment among hepatitis C and HIV co-infected patients. AIDS 2016; 30:1403–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rogal SS, Yan P, Rimland D, Lo Re V, 3rd, Al-Rowais H, Fried L, et al. Electronically Retrieved Cohort of HCV Infected Veterans Study Group. Incidence and progression of chronic kidney disease after hepatitis C seroconversion: results from ERCHIVES. Dig Dis Sci 2016; 61:930–936. [DOI] [PubMed] [Google Scholar]
- 22.Yeung MW, Young J, Moodie E, Rollet-Kurhajec KC, Schwartzman K, Greenaway C, et al. Changes in quality of life, healthcare use, and substance use in HIV/hepatitis C coinfected patients after hepatitis C therapy: a prospective cohort study. HIV Clin Trials 2015; 16:100–110. [DOI] [PubMed] [Google Scholar]
- 23.Lo Re V. Extrahepatic complications of hepatitis C virus infection in HIV and the impact of successful antiviral treatment. Clin Infect Dis 2017; 64:498–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arase Y, Suzuki F, Kawamura Y, Suzuki Y, Kobayashi M, Matsumoto N, et al. Development rate of chronic kidney disease in hepatitis C virus patients with advanced fibrosis after interferon therapy. Hepatol Res 2011; 41:946–954. [DOI] [PubMed] [Google Scholar]
- 25.Hsu YC, Ho HJ, Huang YT, Wang HH, Wu MS, Lin JT, et al. Association between antiviral treatment and extrahepatic outcomes in patients with hepatitis C virus infection. Gut 2015; 64:495–503. [DOI] [PubMed] [Google Scholar]
- 26.Klein MB, Rollet KC, Saeed S, Cox J, Potter M, Cohen J, et al. HIV and hepatitis C virus coinfection in Canada: challenges and opportunities for reducing preventable morbidity and mortality. HIV Med 2013; 14:10–20. [DOI] [PubMed] [Google Scholar]
- 27.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Arch Intern Med 1998; 158:1789–1795. [DOI] [PubMed] [Google Scholar]
- 29.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003; 38:518–526. [DOI] [PubMed] [Google Scholar]
- 30.Lu B. Propensity score matching with time-dependent covariates. Biometrics 2005; 61:721–728. [DOI] [PubMed] [Google Scholar]
- 31.Suissa S, Moodie EE, Dell’Aniello S. Prevalent new-user cohort designs for comparative drug effect studies by time-conditional propensity scores. Pharmacoepidemiol Drug Saf 2017; 26:459–468. [DOI] [PubMed] [Google Scholar]
- 32.Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Sturmer T. Variable selection for propensity score models. Am J Epidemiol 2006; 163:1149–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009; 28:3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leffondre K, Boucquemont J, Tripepi G, Stel VS, Heinze G, Dunkler D. Analysis of risk factors associated with renal function trajectory over time: a comparison of different statistical approaches. Nephrol Dial Transplant 2015; 30:1237–1243. [DOI] [PubMed] [Google Scholar]
- 35.Russ KB, Stevens TM, Singal AK. Acute kidney injury in patients with cirrhosis. J Clin Transl Hepatol 2015; 3:195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsu Y-C, Lin J-T, Ho HJ, Kao Y-H, Huang Y-T, Hsiao N-W, et al. Antiviral treatment for hepatitis C virus infection is associated with improved renal and cardiovascular outcomes in diabetic patients. Hepatology 2014; 59:1293–1302. [DOI] [PubMed] [Google Scholar]
- 37.Leone S, Prosperi M, Costarelli S, Nasta P, Maggiolo F, Di Giambenedetto S, et al. Incidence and predictors of cardiovascular disease, chronic kidney disease, and diabetes in HIV/HCV-coinfected patients who achieved sustained virological response. Eur J Clin Microbiol Infect Dis 2016; 35:1511–1520. [DOI] [PubMed] [Google Scholar]
- 38.Kovari H, Rauch A, Kouyos R, Rougemont M, Cavassini M, Schmid P, et al. Swiss HIV Cohort Study. Hepatitis C infection and the risk of nonliver-related morbidity and mortality in HIV-positive persons in the Swiss HIV Cohort Study. Clin Infect Dis 2017; 64:490–497. [DOI] [PubMed] [Google Scholar]
- 39.Berenguer J, Rodriguez-Castellano E, Carrero A, Von Wichmann MA, Montero M, Galindo MJ, et al. GESIDA HIV/HCV Cohort Study Group. Eradication of HCV and nonliver-related non-AIDS-related events in HIV/HCV coinfection. Hepatology 2017; 66:344–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Younossi Z, Park H, Henry L, Adeyemi A, Stepanova M. Extrahepatic manifestations of hepatitis C: a meta-analysis of prevalence, quality of life, and economic burden. Gastroenterology 2016; 150:1599–1608. [DOI] [PubMed] [Google Scholar]
- 41.Arase Y, Suzuki F, Suzuki Y, Akuta N, Kobayashi M, Kawamura Y, et al. Sustained virological response reduces incidence of onset of type 2 diabetes in chronic hepatitis C. Hepatology 2009; 49:739–744. [DOI] [PubMed] [Google Scholar]
- 42.Dai CY, Chuang WL, Ho CK, Hsieh MY, Huang JF, Lee LP, et al. Associations between hepatitis C viremia and low serum triglyceride and cholesterol levels: a community-based study. J Hepatol 2008; 49:9–16. [DOI] [PubMed] [Google Scholar]
- 43.Ishizaka N, Ishizaka Y, Yamkado M. Atherosclerosis as a possible extrahepatic manifestation of chronic hepatitis C virus infection. Clin Med Insights Cardiol 2014; 8:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hsu CS, Kao JH, Chao YC, Lin HH, Fan YC, Huang CJ, et al. Interferon-based therapy reduces risk of stroke in chronic hepatitis C patients: a population-based cohort study in Taiwan. Aliment Pharmacol Ther 2013; 38:415–423. [DOI] [PubMed] [Google Scholar]
- 45.Gragnani L, Fognani E, Piluso A, Boldrini B, Urraro T, Fabbrizzi A, et al. MaSVE Study Group. Long-term effect of HCV eradication in patients with mixed cryoglobulinemia: a prospective, controlled, open-label, cohort study. Hepatology 2015; 61:1145–1153. [DOI] [PubMed] [Google Scholar]
- 46.Abraham AG, Althoff KN, Jing Y, Estrella MM, Kitahata MM, Wester CW, et al. North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of the International Epidemiologic Databases to Evaluate AIDS (IeDEA). End-stage renal disease among HIV-infected adults in North America. Clin Infect Dis 2015; 60:941–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crews DC, Kuczmarski MF, Grubbs V, Hedgeman E, Shahinian VB, Evans MK, et al. Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team. Effect of food insecurity on chronic kidney disease in lower-income Americans. Am J Nephrol 2014; 39:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


